Childhood Cancer: Occurrence, Treatment and Risk of Second Primary Malignancies
Abstract
Simple Summary
Abstract
1. Introduction
2. Cancer Therapies and Risks of Second Primary Malignancies
2.1. External Beam Radiation Therapy
2.2. Chemotherapy
2.3. Targeted and Immune-Based Therapies
3. Childhood Cancer and Second Primary Malignancies
3.1. Hematologic Malignancies
3.1.1. Acute Leukemia
Acute Lymphoblastic Leukemia
Acute Myeloid Leukemia
Acute Leukemia as Second Primary Malignancy
3.1.2. Non-Hodgkin Lymphoma
3.1.3. Hodgkin Lymphoma
3.1.4. Hematopoietic Stem Cell Transplantation and Risk of Second Primary Malignancies
3.2. Solid Tumors
3.2.1. Brain Tumors
Medulloblastoma
- Gliomas
Ependymoma
- Low-Grade Gliomas
- High-Grade Gliomas
- Diffuse Intrinsic Pontine Glioma
Late Sequela and Second Primary Malignancies after Treatment of Pediatric Brain Cancer
3.2.2. Sarcomas
Rhabdomyosarcoma
Nonrhabdomyosarcoma
Osteosarcoma
Ewing Sarcoma
3.2.3. Other Tumor Entities
Neuroblastoma
Wilms Tumor
Retinoblastoma
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D-CRT | 3-Dimensional conformal radiotherapy |
| ABVD | Adriamycin [doxorubicin], bleomycin, vinblastine, and dacarbazine |
| ACT | Adaptive T-cell therapy |
| ACVR1 | Activin A receptor type I |
| AIEOP | Assoziazione Italiana Ematologica Oncologia Pediatrica |
| AKT | Protein kinase B |
| ALK | Anaplastic lymphoma kinase |
| ALL | Acute lymphoblastic leukemia |
| AML | Acute myeloid leukemia |
| AML1 | Acute myeloid leukemia 1 |
| ASPL | Alveolar soft part sarcoma locus |
| AT | Ataxia-telangiectasia |
| ATRX | Chromatin remodeler ATP-dependent helicase |
| BCL2 | B-cell lymphoma 2 |
| BCOR | BCL-6 corepressor |
| BET | Bromodomain and extra-terminal motif |
| BFM | Berlin-Frankfurt-Muenster |
| BLM | Bloom syndrome |
| BRAF | v-raf murine sarcoma viral oncogene homolog B |
| BRCA2 | Breast cancer 2 |
| BTK | Bruton tyrosine kinase |
| CAR | Chimeric antigen receptor |
| CASP8 | Caspase 8 |
| CBFB | Core-binding factor subunit beta |
| CD | Cluster of differentiation |
| CDC5L | Cell division cycle 5-like |
| CDK | Cyclin-dependent kinase |
| CDKN2 | Cyclin-dependent kinase inhibitor |
| CHK1 | Checkpoint kinase 1 |
| CHK2 | Checkpoint kinase 2 |
| CLL | Chronic lymphocytic leukemia |
| CNS | Central nervous system |
| CT | Chemotherapy |
| CML | Chronic myelogenous leukemia |
| CTLA-4 | Cytotoxic T lymphocyte antigen-4 |
| CTNNB1 | Catenin beta-1 |
| CXCL12 | C-X-C motif chemokine 12 |
| CXCR4 | C-X-C chemokine receptor type 4 |
| CYP | Cytochrome P450 |
| DNA | Deoxyribonucleic acid |
| DNA-PK | DNA-dependent protein kinase |
| DIPG | Diffuse intrinsic pontine glioma |
| DS | Down syndrome |
| EBV | Epstein-Barr-Virus |
| EBRT | External beam radiation therapy |
| EGFR | Epidermal growth factor receptor |
| ERCC | Excision repair cross-complementing |
| ERG | ETS-related gene |
| ERK | Extracellular signal-regulated kinase |
| EPN | Ependymoma |
| ES | Ewing sarcoma |
| EZH2 | Enhancer of zeste homolog 2 |
| FA | Fanconi anemia |
| FBXW7 | F-box/WD repeat-containing protein 7 |
| FGFR | Fibroblast growth factor receptor |
| FLI-1 | Leukemia integration 1 transcription factor |
| FLT3 | fms like tyrosine kinase 3 |
| FOXO1 | Forkhead box protein O1 |
| GCCR | German Childhood Cancer Registry |
| GST | Glutathione s-transferase |
| HDAC | Histone deacetylase |
| HER2 | Human epidermal growth factor receptor 2 |
| HGG | High-grade glioma |
| HIC1 | Hypermethylated in cancer 1 |
| HL | Hodgkin lymphoma |
| HSCT | Hematopoietic stem cell transplantation |
| ICI | Immune checkpoint inhibitor |
| IDH | Isocitrate dehydrogenase isozymes |
| IFN | Interferon |
| IGF1R | Insulin-like growth factor 1 receptor |
| IGRT | Image-guided radiotherapy |
| IKZF1 | Ikaros family zinc finger protein 1 |
| IL-2 | Interleukin 2 |
| IMRT | Intensity-modulated radiotherapy |
| IR | Ionizing radiation |
| ITD | Internal tandem duplication |
| IVA | Ifosfamide, vincristine, and actinomycin D |
| JAK2 | Janus kinase 2 |
| KRAS | Kirsten rat sarcoma viral oncogene |
| KIT | Tyrosine-protein kinase KIT |
| LFS | Li–Fraumeni syndrome |
| LGG | Low-grade gliomas |
| LIG4 | DNA ligase 4 |
| LSD1 | Lysine-specific demethylase 1 |
| MAPK | Mitogen-activated protein kinase |
| MB | Medulloblastoma |
| MDS | Myelodysplastic syndrome |
| MET | Mesenchymal-epithelial transition factor |
| MGMT | O6-methylguanine DNA methyltransferase |
| MLL | Mixed-lineage leukemia |
| MOPP | Mustargen [mechlorethamine], oncovin [vincristine], procarbazine, and prednisone |
| MSH | MutS homolog |
| MYC | bHLH transcription factor |
| mTOR | Mechanistic target of rapamycin |
| NB | Neuroblastoma |
| NBS | Nijmegen breakage syndrome |
| NF | Neurofibroma |
| NF1 | Neurofibromatosis 1 |
| NF2 | Neurofibromatosis 2 |
| NF-kB | Nuclear factor ‘kappa-light-chain-enhancer’ of activated B-cells |
| NHL | Non-Hodgkin lymphoma |
| NKX2.2 | Homeobox protein Nkx-2.2 |
| NQO1 | Nicotinamide adenine dinucleotide phosphate: quinone oxidoreductase |
| NRMS | Nonrhabdomyosarcoma |
| NTRK | Neurotrophic receptor tyrosine kinase |
| OP/EPA | Vincristine, prednisone, procarbazine/etoposide, and doxorubicin |
| OPPA/COPP | Cyclophosphamide, vincristine, prednisone, and procarbazine |
| OS | Osteosarcoma |
| OTX2 | Orthodenticle homeobox 2 |
| PALB2 | Partner and localizer of BRCA2 |
| PAPPA | Pregnancy-associated plasma protein A |
| PARP | Poly(ADP-ribose)-polymerase |
| PAX3 | Paired box gene 3 |
| PD-1 | Programmed cell death protein 1 |
| PDGFR | Platelet-derived growth factor receptor |
| PD-L1 | Programmed cell death protein ligand 1 |
| PI3K | Phosphatidylinositol 3-kinase |
| PIK3CA | Phosphatidylinositol-4,5-bisphosphate 3 |
| PIM1 | Proto-oncogene serine/threonine-protein kinase Pim-1 |
| PMP22 | Peripheral myelin protein 22 |
| PRIM1 | DNA primase small subunit |
| PTCH1 | Patched homolog 1 |
| PTEN | Phosphatase and tensin homolog |
| PVT1 | Plasmacytoma variant translocation 1 |
| RAG1 | Recombination activating gene 1 |
| RARA | Retinoic acid receptor alpha |
| RAS | Rat Sarcoma viral oncogene |
| RASSF1A | Ras association domain-containing |
| RB | Retinoblastoma |
| RMS | Rhabdomyosarcoma |
| RNA | Ribonucleic acid |
| RR | Relative risk |
| R/R | Relapsed/refractory |
| RUNX1 | Runt-related transcription factor 1 |
| SBRT | Stereotactic body radiotherapy |
| SEER | Surveillance, epidemiology and end results |
| SHH | Sonic hedgehog |
| SIR | Standardized incidence ratio |
| SKP2 | S-phase kinase-associated protein 2 |
| SMARC | SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily |
| SMR | Standardized mortality ratio |
| SPM | Second primary malignancy |
| STS | Soft tissue sarcoma |
| SUFU | Suppressor of fused homolog |
| TFE3 | Transcription factor E3 |
| TMZ | Temozolomide |
| TNF | Tumor necrosis factor |
| TRK | Tyrosine receptor kinase |
| TP53 | Tumor suppressor protein 53 |
| TPMT | Thiopurine methyltransferase |
| TPCV | Thioguanine, procarbazine, lomustine, and vincristine |
| TSC | Tuberous sclerosis complex |
| VAC | Vincristine, actinomycin D, and cyclophosphamide |
| VACD | Vincristine, cyclophosphamide, actinomycin D, and doxorubicin |
| VEGF | Vascular endothelial growth factor |
| WRN | Werner syndrome |
| XPD | Xeroderma pigmentosum group D gene |
| XRCC1 | X-ray cross-complementing factor 1 |
| YAP1 | Yes-associated protein 1 |
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Oeffinger, K.C.; Hudson, M.M. Long-term complications following childhood and adolescent cancer: Foundations for providing risk-based health care for survivors. CA Cancer J. Clin. 2004, 54, 208–236. [Google Scholar] [CrossRef]
- Travis, L.B.; Ng, A.K.; Allan, J.M.; Pui, C.H.; Kennedy, A.R.; Xu, X.G.; Purdy, J.A.; Applegate, K.; Yahalom, J.; Constine, L.S.; et al. Second malignant neoplasms and cardiovascular disease following radiotherapy. J. Natl. Cancer Inst. 2012, 104, 357–370. [Google Scholar] [CrossRef]
- de Vries, S.; Schaapveld, M.; Janus, C.P.M.; Daniëls, L.A.; Petersen, E.J.; van der Maazen, R.W.M.; Zijlstra, J.M.; Beijert, M.; Nijziel, M.R.; Verschueren, K.M.S.; et al. Long-term cause-specific mortality in hodgkin lymphoma patients. J. Natl. Cancer Inst. 2020. [Google Scholar] [CrossRef]
- Curtis, R.E.; Freedman, D.M.; Ron, E.; Ries, L.A.G.; Hacker, D.G.; Edwards, B.K.; Tucker, M.A.; Fraumeni, J.F.J. New Malignancies among Cancer Survivors: SEER Cancer Registries, 1973–2000; No. 05-5302; National Cancer Institute: Bethesda, MD, USA, 2006.
- Steliarova-Foucher, E.; Colombet, M.; Ries, L.A.G.; Moreno, F.; Dolya, A.; Bray, F.; Hesseling, P.; Shin, H.Y.; Stiller, C.A. International incidence of childhood cancer, 2001–2010: A population-based registry study. Lancet Oncol. 2017, 18, 719–731. [Google Scholar] [CrossRef]
- Autier, P. Increasing incidence of cancer in children and competing risks. Lancet Oncol. 2018, 19, 1136–1137. [Google Scholar] [CrossRef]
- Bhakta, N.; Force, L.M.; Allemani, C.; Atun, R.; Bray, F.; Coleman, M.P.; Steliarova-Foucher, E.; Frazier, A.L.; Robison, L.L.; Rodriguez-Galindo, C.; et al. Childhood cancer burden: A review of global estimates. Lancet Oncol. 2019, 20, e42–e53. [Google Scholar] [CrossRef]
- Ries, L.A.G.; Melbert, D.; Krapcho, M. SEER Cancer Statistics Review, 1975–2004, National Cancer Institute. Available online: http://seer.cancer.gov/csr/1975_2004/ (accessed on 20 January 2021).
- Hudson, M.M.; Neglia, J.P.; Woods, W.G.; Sandlund, J.T.; Pui, C.H.; Kun, L.E.; Robison, L.L.; Green, D.M. Lessons from the past: Opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies. Pediatr. Blood Cancer 2012, 58, 334–343. [Google Scholar] [CrossRef]
- Rossig, C.; Juergens, H.; Schrappe, M.; Moericke, A.; Henze, G.; von Stackelberg, A.; Reinhardt, D.; Burkhardt, B.; Woessmann, W.; Zimmermann, M.; et al. Effective childhood cancer treatment: The impact of large scale clinical trials in Germany and Austria. Pediatr. Blood Cancer 2013, 60, 1574–1581. [Google Scholar] [CrossRef]
- Friedman, D.L.; Whitton, J.; Leisenring, W.; Mertens, A.C.; Hammond, S.; Stovall, M.; Donaldson, S.S.; Meadows, A.T.; Robison, L.L.; Neglia, J.P. Subsequent neoplasms in 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 2010, 102, 1083–1095. [Google Scholar] [CrossRef]
- National Cancer Institute. Late Effects of Treatment for Childhood Cancer (PDQ)–Health Professional Version. Available online: https://www.cancer.gov/types/childhood-cancers/late-effects-hp-pdq (accessed on 26 January 2021).
- Delaney, G.; Jacob, S.; Featherstone, C.; Barton, M. The role of radiotherapy in cancer treatment: Estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 2005, 104, 1129–1137. [Google Scholar] [CrossRef]
- Rothkamm, K.; Löbrich, M. Misrepair of radiation-induced DNA double-strand breaks and its relevance for tumorigenesis and cancer treatment (review). Int. J. Oncol. 2002, 21, 433–440. [Google Scholar] [CrossRef]
- Dörr, W.; Herrmann, T. Cancer induction by radiotherapy: Dose dependence and spatial relationship to irradiated volume. J. Radiol. Prot. 2002, 22, A117–A121. [Google Scholar] [CrossRef] [PubMed]
- Dörr, W.; Herrmann, T. Second primary tumors after radiotherapy for malignancies. Treatment-related parameters. Strahlenther. Onkol. 2002, 178, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Meadows, A.T.; Baum, E.; Fossati-Bellani, F.; Green, D.; Jenkin, R.D.; Marsden, B.; Nesbit, M.; Newton, W.; Oberlin, O.; Sallan, S.G.; et al. Second malignant neoplasms in children: An update from the Late Effects Study Group. J. Clin. Oncol. 1985, 3, 532–538. [Google Scholar] [CrossRef]
- Wall, B.F.; Haylock, R.; Jansen, J.T.M.; Hillier, M.C.; Hart, D.; Shrimpton, P.C. Radiation Risks from Medical X-ray Examinations as a Function of the Age and Sex of the Patient. Health Prot. Agencycentre Radiat. Chem. Environ. Hazards 2011. Available online: https://www.researchgate.net/publication/265259419_Radiation_Risks_from_Medical_X-ray_Examinations_as_a_Function_of_the_Age_and_Sex_of_the_Patient (accessed on 25 November 2020).
- Arain, A.; Herman, T.; Matthiesen, C. Late Effects of Radiation Therapy in Pediatric Cancer Survivors. J. Okla. State Med. Assoc. 2015, 108, 129–135. [Google Scholar]
- Jairam, V.; Roberts, K.B.; Yu, J.B. Historical trends in the use of radiation therapy for pediatric cancers: 1973–2008. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, e151–e155. [Google Scholar] [CrossRef] [PubMed]
- Hennewig, U.; Kaatsch, P.; Blettner, M.; Spix, C. Local radiation dose and solid second malignant neoplasms after childhood cancer in Germany: A nested case-control study. Radiat. Environ. Biophys. 2014, 53, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.J.; Curtis, R.E.; Hall, E.J.; Ron, E. Second malignancies in prostate carcinoma patients after radiotherapy compared with surgery. Cancer 2000, 88, 398–406. [Google Scholar] [CrossRef]
- Boice, J.D., Jr.; Day, N.E.; Andersen, A.; Brinton, L.A.; Brown, R.; Choi, N.W.; Clarke, E.A.; Coleman, M.P.; Curtis, R.E.; Flannery, J.T.; et al. Second cancers following radiation treatment for cervical cancer. An international collaboration among cancer registries. J. Natl. Cancer Inst. 1985, 74, 955–975. [Google Scholar] [CrossRef]
- Brenner, D.J.; Doll, R.; Goodhead, D.T.; Hall, E.J.; Land, C.E.; Little, J.B.; Lubin, J.H.; Preston, D.L.; Preston, R.J.; Puskin, J.S.; et al. Cancer risks attributable to low doses of ionizing radiation: Assessing what we really know. Proc. Natl. Acad. Sci. USA 2003, 100, 13761–13766. [Google Scholar] [CrossRef]
- von Koppenfels, R.; Thiede, G. Mehrfachmalignome. Strahlenther 1973, 146, 619–632. [Google Scholar]
- Warren, S.; Gates, O. Multiple primary malignant tumors, a survey of the literature and statistical study. Am. J. Cancer 1932, 16, 1358–1414. [Google Scholar]
- Bucci, M.K.; Bevan, A.; Roach, M., III. Advances in radiation therapy: Conventional to 3D, to IMRT, to 4D, and beyond. CA Cancer J. Clin. 2005, 55, 117–134. [Google Scholar] [CrossRef]
- Ruben, J.D.; Lancaster, C.M.; Jones, P.; Smith, R.L. A comparison of out-of-field dose and its constituent components for intensity-modulated radiation therapy versus conformal radiation therapy: Implications for carcinogenesis. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1458–1464. [Google Scholar] [CrossRef]
- Abo-Madyan, Y.; Aziz, M.H.; Aly, M.M.; Schneider, F.; Sperk, E.; Clausen, S.; Giordano, F.A.; Herskind, C.; Steil, V.; Wenz, F.; et al. Second cancer risk after 3D-CRT, IMRT and VMAT for breast cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2014, 110, 471–476. [Google Scholar] [CrossRef]
- Hall, E.J.; Wuu, C.S. Radiation-induced second cancers: The impact of 3D-CRT and IMRT. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 83–88. [Google Scholar] [CrossRef]
- Durante, M.; Orecchia, R.; Loeffler, J.S. Charged-particle therapy in cancer: Clinical uses and future perspectives. Nat. Rev. Clin. Oncol. 2017, 14, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.; Paganetti, H. Nuclear physics in particle therapy: A review. Rep. Prog. Phys. Phys. Soc. 2016, 79, 096702. [Google Scholar] [CrossRef] [PubMed]
- Jermann, M. Particle therapy statistics in 2014. Int. J. Pract. Ther. 2015, 2, 50–54. [Google Scholar] [CrossRef]
- Combs, S.E. Does Proton Therapy Have a Future in CNS Tumors? Curr. Treat. Opt. Neurol. 2017, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Breneman, J.C.; Donaldson, S.S.; Constine, L.; Merchant, T.; Marcus, K.; Paulino, A.C.; Followill, D.; Mahajan, A.; Laack, N.; Esiashvili, N.; et al. The Children’s Oncology Group Radiation Oncology Discipline: 15 Years of Contributions to the Treatment of Childhood Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 860–874. [Google Scholar] [CrossRef]
- Hall, E.J. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 1–7. [Google Scholar] [CrossRef]
- Stokkevåg, C.H.; Schneider, U.; Muren, L.P.; Newhauser, W. Radiation-induced cancer risk predictions in proton and heavy ion radiotherapy. Phys. Med. 2017, 42, 259–262. [Google Scholar] [CrossRef]
- Leroy, R.; Benahmed, N.; Hulstaert, F.; Van Damme, N.; De Ruysscher, D. Proton Therapy in Children: A Systematic Review of Clinical Effectiveness in 15 Pediatric Cancers. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 267–278. [Google Scholar] [CrossRef]
- Yock, T.I.; Caruso, P.A. Risk of second cancers after photon and proton radiotherapy: A review of the data. Health Phys. 2012, 103, 577–585. [Google Scholar] [CrossRef]
- Veldeman, L.; Madani, I.; Hulstaert, F.; De Meerleer, G.; Mareel, M.; De Neve, W. Evidence behind use of intensity-modulated radiotherapy: A systematic review of comparative clinical studies. Lancet Oncol. 2008, 9, 367–375. [Google Scholar] [CrossRef]
- Larionova, I.; Cherdyntseva, N.; Liu, T.; Patysheva, M.; Rakina, M.; Kzhyshkowska, J. Interaction of tumor-associated macrophages and cancer chemotherapy. Oncoimmunology 2019, 8, 1596004. [Google Scholar] [CrossRef]
- Turcotte, L.M.; Liu, Q.; Yasui, Y.; Henderson, T.O.; Gibson, T.M.; Leisenring, W.; Arnold, M.A.; Howell, R.M.; Green, D.M.; Armstrong, G.T.; et al. Chemotherapy and Risk of Subsequent Malignant Neoplasms in the Childhood Cancer Survivor Study Cohort. J. Clin. Oncol. 2019, 37, 3310–3319. [Google Scholar] [CrossRef] [PubMed]
- Gururangan, S. Late effects of chemotherapy. Cancer Treat. Res. 2009, 150, 43–65. [Google Scholar] [CrossRef] [PubMed]
- Florescu, M.; Cinteza, M.; Vinereanu, D. Chemotherapy-induced Cardiotoxicity. Maedica 2013, 8, 59–67. [Google Scholar] [PubMed]
- Lazăr, D.R.; Farcaş, A.D.; Blag, C.; Neaga, A.; Zdrenghea, M.T.; Căinap, C.; Lazăr, F.L.; Stef, A.; Căinap, S.S. Cardiotoxicity: A Major Setback in Childhood Leukemia Treatment. Dis. Markers 2021, 2021, 8828410. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Davies, S.M.; Robison, L.L. Leukemia. In Multiple Primary Cancers; Neugut, A.I., Meadows, A.T., Robinson, E., Eds.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 1999; pp. 257–276. [Google Scholar]
- Leone, G.; Voso, M.T.; Sica, S.; Morosetti, R.; Pagano, L. Therapy related leukemias: Susceptibility, prevention and treatment. Leuk. Lymphoma 2001, 41, 255–276. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, M.M.; Wilson, L.M.; Stovall, M.A.; Marsden, H.B.; Potok, M.H.; Kingston, J.E.; Chessells, J.M. Epipodophyllotoxins, alkylating agents, and radiation and risk of secondary leukaemia after childhood cancer. BMJ 1992, 304, 951–958. [Google Scholar] [CrossRef]
- Rheingold, S.R.; Neugut, A.I.; Meadows, A.T. Therapy-Related Secondary Cancers. In Holland-Frei Cancer Medicine, 6th ed.; Kufe, D.W., Pollock, R.E., Weichselbaum, R.R., Eds.; PMPH-USA: Shelton, CT, USA, 2003. [Google Scholar]
- Henderson, T.O.; Moskowitz, C.S.; Chou, J.F.; Bradbury, A.R.; Neglia, J.P.; Dang, C.T.; Onel, K.; Novetsky Friedman, D.; Bhatia, S.; Strong, L.C.; et al. Breast Cancer Risk in Childhood Cancer Survivors Without a History of Chest Radiotherapy: A Report From the Childhood Cancer Survivor Study. J. Clin. Oncol. 2016, 34, 910–918. [Google Scholar] [CrossRef]
- Swerdlow, A.J.; Higgins, C.D.; Smith, P.; Cunningham, D.; Hancock, B.W.; Horwich, A.; Hoskin, P.J.; Lister, T.A.; Radford, J.A.; Rohatiner, A.Z.; et al. Second cancer risk after chemotherapy for Hodgkin’s lymphoma: A collaborative British cohort study. J. Clin. Oncol. 2011, 29, 4096–4104. [Google Scholar] [CrossRef]
- Morton, L.M.; Dores, G.M.; Curtis, R.E.; Lynch, C.F.; Stovall, M.; Hall, P.; Gilbert, E.S.; Hodgson, D.C.; Storm, H.H.; Johannesen, T.B.; et al. Stomach cancer risk after treatment for hodgkin lymphoma. J. Clin. Oncol. 2013, 31, 3369–3377. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.S.; Curtis, R.E.; Hauptmann, M.; Kleinerman, R.A.; Lynch, C.F.; Stovall, M.; Smith, S.A.; Weathers, R.; Andersson, M.; Dores, G.M.; et al. Stomach Cancer Following Hodgkin Lymphoma, Testicular Cancer and Cervical Cancer: A Pooled Analysis of Three International Studies with a Focus on Radiation Effects. Radiat. Res. 2017, 187, 186–195. [Google Scholar] [CrossRef]
- Dores, G.M.; Curtis, R.E.; van Leeuwen, F.E.; Stovall, M.; Hall, P.; Lynch, C.F.; Smith, S.A.; Weathers, R.E.; Storm, H.H.; Hodgson, D.C.; et al. Pancreatic cancer risk after treatment of Hodgkin lymphoma. Ann. Oncol. 2014, 25, 2073–2079. [Google Scholar] [CrossRef]
- Metayer, C.; Lynch, C.F.; Clarke, E.A.; Glimelius, B.; Storm, H.; Pukkala, E.; Joensuu, T.; van Leeuwen, F.E.; van’t Veer, M.B.; Curtis, R.E.; et al. Second cancers among long-term survivors of Hodgkin’s disease diagnosed in childhood and adolescence. J. Clin. Oncol. 2000, 18, 2435–2443. [Google Scholar] [CrossRef]
- de Vathaire, F.; Haddy, N.; Allodji, R.S.; Hawkins, M.; Guibout, C.; El-Fayech, C.; Teinturier, C.; Oberlin, O.; Pacquement, H.; Diop, F.; et al. Thyroid Radiation Dose and Other Risk Factors of Thyroid Carcinoma Following Childhood Cancer. J. Clin. Endocrinol. Metab. 2015, 100, 4282–4290. [Google Scholar] [CrossRef]
- Nottage, K.; McFarlane, J.; Krasin, M.J.; Li, C.; Srivastava, D.; Robison, L.L.; Hudson, M.M. Secondary colorectal carcinoma after childhood cancer. J. Clin. Oncol. 2012, 30, 2552–2558. [Google Scholar] [CrossRef]
- Hawkins, M.M.; Wilson, L.M.; Burton, H.S.; Potok, M.H.; Winter, D.L.; Marsden, H.B.; Stovall, M.A. Radiotherapy, alkylating agents, and risk of bone cancer after childhood cancer. J. Natl. Cancer Inst. 1996, 88, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Tucker, M.A.; D’Angio, G.J.; Boice, J.D., Jr.; Strong, L.C.; Li, F.P.; Stovall, M.; Stone, B.J.; Green, D.M.; Lombardi, F.; Newton, W.; et al. Bone sarcomas linked to radiotherapy and chemotherapy in children. N. Engl. J. Med. 1987, 317, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Henderson, T.O.; Whitton, J.; Stovall, M.; Mertens, A.C.; Mitby, P.; Friedman, D.; Strong, L.C.; Hammond, S.; Neglia, J.P.; Meadows, A.T.; et al. Secondary sarcomas in childhood cancer survivors: A report from the Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 2007, 99, 300–308. [Google Scholar] [CrossRef]
- Travis, L.B.; Curtis, R.E.; Glimelius, B.; Holowaty, E.J.; Van Leeuwen, F.E.; Lynch, C.F.; Hagenbeek, A.; Stovall, M.; Banks, P.M.; Adami, J.; et al. Bladder and kidney cancer following cyclophosphamide therapy for non-Hodgkin’s lymphoma. J. Natl. Cancer Inst. 1995, 87, 524–530. [Google Scholar] [CrossRef]
- Birdwell, S.H.; Hancock, S.L.; Varghese, A.; Cox, R.S.; Hoppe, R.T. Gastrointestinal cancer after treatment of Hodgkin’s disease. Int. J. Radiat. Oncol. Biol. Phys. 1997, 37, 67–73. [Google Scholar] [CrossRef]
- Seibel, N.L.; Janeway, K.; Allen, C.E.; Chi, S.N.; Cho, Y.J.; Glade Bender, J.L.; Kim, A.; Laetsch, T.W.; Irwin, M.S.; Takebe, N.; et al. Pediatric oncology enters an era of precision medicine. Curr. Probl. Cancer 2017, 41, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Paret, C.; Russo, A.; Otto, H.; Mayer, A.; Zahnreich, S.; Wagner, W.; Samuel, D.; Scharnhorst, D.; Solomon, D.A.; Dhall, G.; et al. Personalized therapy: CNS HGNET-BCOR responsiveness to arsenic trioxide combined with radiotherapy. Oncotarget 2017, 8, 114210–114225. [Google Scholar] [CrossRef]
- Jones, D.T.W.; Banito, A.; Grünewald, T.G.P.; Haber, M.; Jäger, N.; Kool, M.; Milde, T.; Molenaar, J.J.; Nabbi, A.; Pugh, T.J.; et al. Molecular characteristics and therapeutic vulnerabilities across paediatric solid tumours. Nat. Rev. Cancer 2019, 19, 420–438. [Google Scholar] [CrossRef]
- Ferrari, A.; Casanova, M.; Massimino, M.; Sultan, I. Peculiar features and tailored management of adult cancers occurring in pediatric age. Expert Rev. Anticancer Ther. 2010, 10, 1837–1851. [Google Scholar] [CrossRef]
- Kattner, P.; Strobel, H.; Khoshnevis, N.; Grunert, M.; Bartholomae, S.; Pruss, M.; Fitzel, R.; Halatsch, M.E.; Schilberg, K.; Siegelin, M.D.; et al. Compare and contrast: Pediatric cancer versus adult malignancies. Cancer Metastasis Rev. 2019, 38, 673–682. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Baudino, T.A. Targeted Cancer Therapy: The Next Generation of Cancer Treatment. Curr. Drug Discov. Technol. 2015, 12, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Biau, J.; Chautard, E.; Verrelle, P.; Dutreix, M. Altering DNA Repair to Improve Radiation Therapy: Specific and Multiple Pathway Targeting. Front. Oncol. 2019, 9, 1009. [Google Scholar] [CrossRef] [PubMed]
- Mackall, C.L.; Merchant, M.S.; Fry, T.J. Immune-based therapies for childhood cancer. Nat. Rev. Clin. Oncol. 2014, 11, 693–703. [Google Scholar] [CrossRef]
- Ragoonanan, D.; Khazal, S.J.; Abdel-Azim, H.; McCall, D.; Cuglievan, B.; Tambaro, F.P.; Ahmad, A.H.; Rowan, C.M.; Gutierrez, C.; Schadler, K.; et al. Diagnosis, grading and management of toxicities from immunotherapies in children, adolescents and young adults with cancer. Nat. Rev. Clin. Oncol. 2021. [Google Scholar] [CrossRef]
- Kroschinsky, F.; Stölzel, F.; von Bonin, S.; Beutel, G.; Kochanek, M.; Kiehl, M.; Schellongowski, P. New drugs, new toxicities: Severe side effects of modern targeted and immunotherapy of cancer and their management. Crit. Care 2017, 21, 89. [Google Scholar] [CrossRef]
- Zahnreich, S.; Mayer, A.; Loquai, C.; Grabbe, S.; Schmidberger, H. Radiotherapy with BRAF inhibitor therapy for melanoma: Progress and possibilities. Future Oncol. 2016, 12, 95–106. [Google Scholar] [CrossRef]
- Kennedy, L.B.; Salama, A.K.S. A review of cancer immunotherapy toxicity. CA Cancer J. Clin. 2020, 70, 86–104. [Google Scholar] [CrossRef]
- Crotty, E.; Downey, K.; Ferrerosa, L.; Flores, C.; Hegde, B.; Raskin, S.; Hwang, E.; Vitanza, N.; Okada, H. Considerations when treating high-grade pediatric glioma patients with immunotherapy. Expert Rev. Neurother. 2021, 21, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, K.; Elkrief, A.; Calabrese, C.; Lapointe, R.; Hudson, M.; Routy, B.; Miller, W.H., Jr.; Calabrese, L. Moving towards personalized treatments of immune-related adverse events. Nat. Rev. Clin. Oncol. 2020, 17, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Shah, N.N.; Qin, H.; Yates, B.; Su, L.; Shalabi, H.; Raffeld, M.; Ahlman, M.A.; Stetler-Stevenson, M.; Yuan, C.; Guo, S.; et al. Clonal expansion of CAR T cells harboring lentivector integration in the CBL gene following anti-CD22 CAR T-cell therapy. Blood Adv. 2019, 3, 2317–2322. [Google Scholar] [CrossRef] [PubMed]
- Cornetta, K.; Duffy, L.; Feldman, S.A.; Mackall, C.L.; Davila, M.L.; Curran, K.J.; Junghans, R.P.; Tang, J.Y.; Kochenderfer, J.N.; O’Cearbhaill, R.; et al. Screening Clinical Cell Products for Replication Competent Retrovirus: The National Gene Vector Biorepository Experience. Mol. Ther. Methods Clin. Dev. 2018, 10, 371–378. [Google Scholar] [CrossRef]
- Gardner, R.; Wu, D.; Cherian, S.; Fang, M.; Hanafi, L.A.; Finney, O.; Smithers, H.; Jensen, M.C.; Riddell, S.R.; Maloney, D.G.; et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood 2016, 127, 2406–2410. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Long Term Followup after Administration of Human Gene Therapy Products: Guidance for Industry. 2020. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/long-term-follow-after-administration-human-gene-therapy-products (accessed on 8 March 2021).
- Shalabi, H.; Gust, J.; Taraseviciute, A.; Wolters, P.L.; Leahy, A.B.; Sandi, C.; Laetsch, T.W.; Wiener, L.; Gardner, R.A.; Nussenblatt, V.; et al. Beyond the storm-subacute toxicities and late effects in children receiving CAR T cells. Nat. Rev. Clin. Oncol. 2021. [Google Scholar] [CrossRef]
- Galluzzi, L.; Humeau, J.; Buqué, A.; Zitvogel, L.; Kroemer, G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2020, 17, 725–741. [Google Scholar] [CrossRef]
- Weichselbaum, R.R.; Liang, H.; Deng, L.; Fu, Y.X. Radiotherapy and immunotherapy: A beneficial liaison? Nat. Rev. Clin. Oncol. 2017, 14, 365–379. [Google Scholar] [CrossRef]
- Rodriguez-Galindo, C.; Friedrich, P.; Morrissey, L.; Frazier, L. Global challenges in pediatric oncology. Curr. Opin. Pediatrics 2013, 25, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Kaatsch, P.; Spix, C. German Childhood Cancer Registry—Annual Report, 2011 (1980–2010). Available online: https://www.kinderkrebsregister.de/dkkr-gb/latest-publications/annual-reports/annual-report-2011.html?L=1 (accessed on 13 February 2021).
- Abraho, R.; Ribeiro, R.C.; Brunson, A.; Keegan, T.H.M. The burden of second primary cancers among childhood cancer survivors. Ann. Cancer Epidemiol. 2020, 4. [Google Scholar] [CrossRef]
- Grobner, S.N.; Worst, B.C.; Weischenfeldt, J.; Buchhalter, I.; Kleinheinz, K.; Rudneva, V.A.; Johann, P.D.; Balasubramanian, G.P.; Segura-Wang, M.; Brabetz, S.; et al. The landscape of genomic alterations across childhood cancers. Nature 2018, 555, 321–327. [Google Scholar] [CrossRef]
- Brodeur, G.M.; Nichols, K.E.; Plon, S.E.; Schiffman, J.D.; Malkin, D. Pediatric Cancer Predisposition and Surveillance: An Overview, and a Tribute to Alfred G. Knudson Jr. Clin. Cancer Res. 2017, 23, e1–e5. [Google Scholar] [CrossRef]
- Zhang, J.; Walsh, M.F.; Wu, G.; Edmonson, M.N.; Gruber, T.A.; Easton, J.; Hedges, D.; Ma, X.; Zhou, X.; Yergeau, D.A.; et al. Germline Mutations in Predisposition Genes in Pediatric Cancer. N. Engl. J. Med. 2015, 373, 2336–2346. [Google Scholar] [CrossRef] [PubMed]
- Mody, R.J.; Wu, Y.M.; Lonigro, R.J.; Cao, X.; Roychowdhury, S.; Vats, P.; Frank, K.M.; Prensner, J.R.; Asangani, I.; Palanisamy, N.; et al. Integrative Clinical Sequencing in the Management of Refractory or Relapsed Cancer in Youth. JAMA 2015, 314, 913–925. [Google Scholar] [CrossRef]
- Parsons, D.W.; Roy, A.; Yang, Y.; Wang, T.; Scollon, S.; Bergstrom, K.; Kerstein, R.A.; Gutierrez, S.; Petersen, A.K.; Bavle, A.; et al. Diagnostic Yield of Clinical Tumor and Germline Whole-Exome Sequencing for Children with Solid Tumors. JAMA Oncol. 2016, 2, 616–624. [Google Scholar] [CrossRef] [PubMed]
- McBride, K.A.; Ballinger, M.L.; Killick, E.; Kirk, J.; Tattersall, M.H.; Eeles, R.A.; Thomas, D.M.; Mitchell, G. Li-Fraumeni syndrome: Cancer risk assessment and clinical management. Nat. Rev. Clin. Oncol. 2014, 11, 260–271. [Google Scholar] [CrossRef]
- Agaimy, A.; Foulkes, W.D. Hereditary SWI/SNF complex deficiency syndromes. Semin. Diagn. Pathol. 2018, 35, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Kuhlen, M.; Taeubner, J.; Brozou, T.; Wieczorek, D.; Siebert, R.; Borkhardt, A. Family-based germline sequencing in children with cancer. Oncogene 2019, 38, 1367–1380. [Google Scholar] [CrossRef]
- Jongmans, M.C.; Loeffen, J.L.; Waanders, E.; Hoogerbrugge, P.M.; Ligtenberg, M.J.; Kuiper, R.P.; Hoogerbrugge, N. Recognition of genetic predisposition in pediatric cancer patients: An easy-to-use selection tool. Eur. J. Med. Genet. 2016, 59, 116–125. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Sorrell, A.D.; Espenschied, C.R.; Culver, J.O.; Weitzel, J.N. Tumor protein p53 (TP53) testing and Li-Fraumeni syndrome: Current status of clinical applications and future directions. Mol. Diagn. Ther. 2013, 17, 31–47. [Google Scholar] [CrossRef]
- Kappel, S.; Janschek, E.; Wolf, B.; Rudas, M.; Teleky, B.; Jakesz, R.; Kandioler, D. TP53 germline mutation may affect response to anticancer treatments: Analysis of an intensively treated Li-Fraumeni family. Breast Cancer Res. Treat. 2015, 151, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Heymann, S.; Delaloge, S.; Rahal, A.; Caron, O.; Frebourg, T.; Barreau, L.; Pachet, C.; Mathieu, M.C.; Marsiglia, H.; Bourgier, C. Radio-induced malignancies after breast cancer postoperative radiotherapy in patients with Li-Fraumeni syndrome. Radiat. Oncol. 2010, 5, 104. [Google Scholar] [CrossRef] [PubMed]
- Travis, L.B.; Fosså, S.D.; Schonfeld, S.J.; McMaster, M.L.; Lynch, C.F.; Storm, H.; Hall, P.; Holowaty, E.; Andersen, A.; Pukkala, E.; et al. Second cancers among 40,576 testicular cancer patients: Focus on long-term survivors. J. Natl. Cancer Inst. 2005, 97, 1354–1365. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Voronova, N.V.; Chistiakov, P.A. Genetic variations in DNA repair genes, radiosensitivity to cancer and susceptibility to acute tissue reactions in radiotherapy-treated cancer patients. Acta Oncol. 2008, 47, 809–824. [Google Scholar] [CrossRef]
- Kühne, M.; Riballo, E.; Rief, N.; Rothkamm, K.; Jeggo, P.A.; Löbrich, M. A double-strand break repair defect in ATM-deficient cells contributes to radiosensitivity. Cancer Res. 2004, 64, 500–508. [Google Scholar] [CrossRef]
- Palumbo, E.; Piotto, C.; Calura, E.; Fasanaro, E.; Groff, E.; Busato, F.; El Khouzai, B.; Rigo, M.; Baggio, L.; Romualdi, C.; et al. Individual Radiosensitivity in Oncological Patients: Linking Adverse Normal Tissue Reactions and Genetic Features. Front. Oncol. 2019, 9, 987. [Google Scholar] [CrossRef]
- Allan, J.M.; Travis, L.B. Mechanisms of therapy-related carcinogenesis. Nat. Rev. Cancer 2005, 5, 943–955. [Google Scholar] [CrossRef]
- Allan, J.M.; Rabkin, C.S. Genetic susceptibility to iatrogenic malignancy. Pharmacogenomics 2005, 6, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Allan, J.M. Genetic susceptibility to radiogenic cancer in humans. Health Phys. 2008, 95, 677–686. [Google Scholar] [CrossRef]
- Barnard, D.R.; Lange, B.; Alonzo, T.A.; Buckley, J.; Kobrinsky, J.N.; Gold, S.; Neudorf, S.; Sanders, J.; Burden, L.; Woods, W.G. Acute myeloid leukemia and myelodysplastic syndrome in children treated for cancer: Comparison with primary presentation. Blood 2002, 100, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Sather, H.N.; Pabustan, O.B.; Trigg, M.E.; Gaynon, P.S.; Robison, L.L. Low incidence of second neoplasms among children diagnosed with acute lymphoblastic leukemia after 1983. Blood 2002, 99, 4257–4264. [Google Scholar] [CrossRef]
- Carret, A.S.; Tabori, U.; Crooks, B.; Hukin, J.; Odame, I.; Johnston, D.L.; Keene, D.L.; Freeman, C.; Bouffet, E. Outcome of secondary high-grade glioma in children previously treated for a malignant condition: A study of the Canadian Pediatric Brain Tumour Consortium. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2006, 81, 33–38. [Google Scholar] [CrossRef]
- Robison, L.L.; Mertens, A. Second tumors after treatment of childhood malignancies. Hematol. Oncol. Clin. N. Am. 1993, 7, 401–415. [Google Scholar] [CrossRef]
- Löning, L.; Zimmermann, M.; Reiter, A.; Kaatsch, P.; Henze, G.; Riehm, H.; Schrappe, M. Secondary neoplasms subsequent to Berlin-Frankfurt-Münster therapy of acute lymphoblastic leukemia in childhood: Significantly lower risk without cranial radiotherapy. Blood 2000, 95, 2770–2775. [Google Scholar] [CrossRef]
- Neglia, J.P.; Friedman, D.L.; Yasui, Y.; Mertens, A.C.; Hammond, S.; Stovall, M.; Donaldson, S.S.; Meadows, A.T.; Robison, L.L. Second malignant neoplasms in five-year survivors of childhood cancer: Childhood cancer survivor study. J. Natl. Cancer Inst. 2001, 93, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, H.C.; Hawkins, M.M.; Stiller, C.A.; Winter, D.L.; Marsden, H.B.; Stevens, M.C. Long-term population-based risks of second malignant neoplasms after childhood cancer in Britain. Br. J. Cancer 2004, 91, 1905–1910. [Google Scholar] [CrossRef]
- Cardous-Ubbink, M.C.; Heinen, R.C.; Bakker, P.J.; van den Berg, H.; Oldenburger, F.; Caron, H.N.; Voute, P.A.; van Leeuwen, F.E. Risk of second malignancies in long-term survivors of childhood cancer. Eur. J. Cancer 2007, 43, 351–362. [Google Scholar] [CrossRef] [PubMed]
- MacArthur, A.C.; Spinelli, J.J.; Rogers, P.C.; Goddard, K.J.; Phillips, N.; McBride, M.L. Risk of a second malignant neoplasm among 5-year survivors of cancer in childhood and adolescence in British Columbia, Canada. Pediatr. Blood Cancer 2007, 48, 453–459. [Google Scholar] [CrossRef] [PubMed]
- German Childhood Cancer Registry. Annual Report 1019. 2019. Available online: https://www.kinderkrebsregister.de/dkkr/ergebnisse/jahresberichte.html (accessed on 15 February 2021).
- Greaves, M. Molecular genetics, natural history and the demise of childhood leukaemia. Eur. J. Cancer 1999, 35, 1941–1953. [Google Scholar] [CrossRef]
- Savaşan, S.; Taub, J.W.; Ravindranath, Y. Down syndrome and leukemia--an overview of cytogenetic and molecular events. Turk. J. Pediatr. 1997, 39, 519–531. [Google Scholar]
- Buffler, P.A.; Kwan, M.L.; Reynolds, P.; Urayama, K.Y. Environmental and genetic risk factors for childhood leukemia: Appraising the evidence. Cancer Investig. 2005, 23, 60–75. [Google Scholar] [CrossRef]
- Zelent, A.; Greaves, M.; Enver, T. Role of the TEL-AML1 fusion gene in the molecular pathogenesis of childhood acute lymphoblastic leukaemia. Oncogene 2004, 23, 4275–4283. [Google Scholar] [CrossRef]
- Conter, V.; Valsecchi, M.G.; Buldini, B.; Parasole, R.; Locatelli, F.; Colombini, A.; Rizzari, C.; Putti, M.C.; Barisone, E.; Lo Nigro, L.; et al. Early T-cell precursor acute lymphoblastic leukaemia in children treated in AIEOP centres with AIEOP-BFM protocols: A retrospective analysis. Lancet Haematol. 2016, 3, e80–e86. [Google Scholar] [CrossRef]
- Schrappe, M.; Valsecchi, M.G.; Bartram, C.R.; Schrauder, A.; Panzer-Grümayer, R.; Möricke, A.; Parasole, R.; Zimmermann, M.; Dworzak, M.; Buldini, B.; et al. Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: Results of the AIEOP-BFM-ALL 2000 study. Blood 2011, 118, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Maloney, K.W.; Gore, L. Agents in Development for Childhood Acute Lymphoblastic Leukemia. Paediatr. Drugs 2018, 20, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Pinnix, C.C.; Yahalom, J.; Specht, L.; Dabaja, B.S. Radiation in Central Nervous System Leukemia: Guidelines from the International Lymphoma Radiation Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Piccaluga, P.P.; Arpinati, M.; Candoni, A.; Laterza, C.; Paolini, S.; Gazzola, A.; Sabattini, E.; Visani, G.; Pileri, S.A. Surface antigens analysis reveals significant expression of candidate targets for immunotherapy in adult acute lymphoid leukemia. Leuk. Lymphoma 2011, 52, 325–327. [Google Scholar] [CrossRef]
- Inaba, H.; Pui, C.H. Immunotherapy in pediatric acute lymphoblastic leukemia. Cancer Metastasis Rev. 2019, 38, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.G.; Li, Y.; Payne-Turner, D.; Harvey, R.C.; Yang, Y.L.; Pei, D.; McCastlain, K.; Ding, L.; Lu, C.; Song, G.; et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N. Engl. J. Med. 2014, 371, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.L.; Brown, P.A. Treatment of pediatric acute lymphoblastic leukemia. Pediatr. Clin. N. Am. 2015, 62, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Hunger, S.P.; Mullighan, C.G. Acute Lymphoblastic Leukemia in Children. N. Engl. J. Med. 2015, 373, 1541–1552. [Google Scholar] [CrossRef]
- Neglia, J.P.; Meadows, A.T.; Robison, L.L.; Kim, T.H.; Newton, W.A.; Ruymann, F.B.; Sather, H.N.; Hammond, G.D. Second neoplasms after acute lymphoblastic leukemia in childhood. N. Engl. J. Med. 1991, 325, 1330–1336. [Google Scholar] [CrossRef]
- Neglia, J.P.; Robison, L.L.; Stovall, M.; Liu, Y.; Packer, R.J.; Hammond, S.; Yasui, Y.; Kasper, C.E.; Mertens, A.C.; Donaldson, S.S.; et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 2006, 98, 1528–1537. [Google Scholar] [CrossRef]
- Schmiegelow, K.; Levinsen, M.F.; Attarbaschi, A.; Baruchel, A.; Devidas, M.; Escherich, G.; Gibson, B.; Heydrich, C.; Horibe, K.; Ishida, Y.; et al. Second malignant neoplasms after treatment of childhood acute lymphoblastic leukemia. J. Clin. Oncol. 2013, 31, 2469–2476. [Google Scholar] [CrossRef]
- Hijiya, N.; Hudson, M.M.; Lensing, S.; Zacher, M.; Onciu, M.; Behm, F.G.; Razzouk, B.I.; Ribeiro, R.C.; Rubnitz, J.E.; Sandlund, J.T.; et al. Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. JAMA 2007, 297, 1207–1215. [Google Scholar] [CrossRef]
- Pui, C.H. Childhood leukemias. N. Engl. J. Med. 1995, 332, 1618–1630. [Google Scholar] [CrossRef]
- Ertz-Archambault, N.; Kelemen, K. Relapse and cytogenetic evolution in myeloid neoplasms. Panminerva Med. 2017, 59, 308–319. [Google Scholar] [CrossRef]
- Klco, J.M.; Mullighan, C.G. Advances in germline predisposition to acute leukaemias and myeloid neoplasms. Nat. Rev. Cancer 2021, 21, 122–137. [Google Scholar] [CrossRef]
- Rasche, M.; Zimmermann, M.; Borschel, L.; Bourquin, J.P.; Dworzak, M.; Klingebiel, T.; Lehrnbecher, T.; Creutzig, U.; Klusmann, J.H.; Reinhardt, D. Successes and challenges in the treatment of pediatric acute myeloid leukemia: A retrospective analysis of the AML-BFM trials from 1987 to 2012. Leukemia 2018, 32, 2167–2177. [Google Scholar] [CrossRef]
- Zwaan, C.M.; Kolb, E.A.; Reinhardt, D.; Abrahamsson, J.; Adachi, S.; Aplenc, R.; De Bont, E.S.; De Moerloose, B.; Dworzak, M.; Gibson, B.E.; et al. Collaborative Efforts Driving Progress in Pediatric Acute Myeloid Leukemia. J. Clin. Oncol. 2015, 33, 2949–2962. [Google Scholar] [CrossRef]
- Abbott, B.L.; Rubnitz, J.E.; Tong, X.; Srivastava, D.K.; Pui, C.H.; Ribeiro, R.C.; Razzouk, B.I. Clinical significance of central nervous system involvement at diagnosis of pediatric acute myeloid leukemia: A single institution’s experience. Leukemia 2003, 17, 2090–2096. [Google Scholar] [CrossRef] [PubMed]
- Maakaron, J.E.; Rogosheske, J.; Long, M.; Bachanova, V.; Mims, A.S. CD33-Targeted Therapies: Beating the Disease or Beaten to Death? J. Clin. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Tarlock, K.; Alonzo, T.A.; Gerbing, R.B.; Raimondi, S.C.; Hirsch, B.A.; Sung, L.; Pollard, J.A.; Aplenc, R.; Loken, M.R.; Gamis, A.S.; et al. Gemtuzumab Ozogamicin Reduces Relapse Risk in FLT3/ITD Acute Myeloid Leukemia: A Report from the Children’s Oncology Group. Clin. Cancer Res. 2016, 22, 1951–1957. [Google Scholar] [CrossRef]
- Perl, A.E.; Martinelli, G.; Cortes, J.E.; Neubauer, A.; Berman, E.; Paolini, S.; Montesinos, P.; Baer, M.R.; Larson, R.A.; Ustun, C.; et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N. Engl. J. Med. 2019, 381, 1728–1740. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; Marcucci, G.; et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef]
- Leung, W.; Ribeiro, R.C.; Hudson, M.; Tong, X.; Srivastava, D.K.; Rubnitz, J.E.; Sandlund, J.T.; Razzouk, B.I.; Evans, W.E.; Pui, C.H. Second malignancy after treatment of childhood acute myeloid leukemia. Leukemia 2001, 15, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dai, Y.; Zheng, T.; Ma, S. Risk Factors of Non-Hodgkin Lymphoma. Expert Opin. Med. Diagn. 2011, 5, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Reiter, A.; Schrappe, M.; Tiemann, M.; Ludwig, W.D.; Yakisan, E.; Zimmermann, M.; Mann, G.; Chott, A.; Ebell, W.; Klingebiel, T.; et al. Improved treatment results in childhood B-cell neoplasms with tailored intensification of therapy: A report of the Berlin-Frankfurt-Münster Group Trial NHL-BFM 90. Blood 1999, 94, 3294–3306. [Google Scholar] [PubMed]
- Reiter, A.; Schrappe, M.; Parwaresch, R.; Henze, G.; Müller-Weihrich, S.; Sauter, S.; Sykora, K.W.; Ludwig, W.D.; Gadner, H.; Riehm, H. Non-Hodgkin’s lymphomas of childhood and adolescence: Results of a treatment stratified for biologic subtypes and stage—A report of the Berlin-Frankfurt-Münster Group. J. Clin. Oncol. 1995, 13, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Anoop, P.; Sankpal, S.; Stiller, C.; Tewari, S.; Lancaster, D.L.; Khabra, K.; Taj, M.M. Outcome of childhood relapsed or refractory mature B-cell non-Hodgkin lymphoma and acute lymphoblastic leukemia. Leuk. Lymphoma 2012, 53, 1882–1888. [Google Scholar] [CrossRef] [PubMed]
- Rigaud, C.; Auperin, A.; Jourdain, A.; Haouy, S.; Couec, M.L.; Aladjidi, N.; Gandemer, V.; Lambliotte, A.; Plat, G.; Landman-Parker, J.; et al. Outcome of relapse in children and adolescents with B-cell non-Hodgkin lymphoma and mature acute leukemia: A report from the French LMB study. Pediatr. Blood Cancer 2019, 66, e27873. [Google Scholar] [CrossRef]
- Griffin, T.C.; Weitzman, S.; Weinstein, H.; Chang, M.; Cairo, M.; Hutchison, R.; Shiramizu, B.; Wiley, J.; Woods, D.; Barnich, M.; et al. A study of rituximab and ifosfamide, carboplatin, and etoposide chemotherapy in children with recurrent/refractory B-cell (CD20+) non-Hodgkin lymphoma and mature B-cell acute lymphoblastic leukemia: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2009, 52, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Minard-Colin, V.; Aupérin, A.; Pillon, M.; Burke, G.A.A.; Barkauskas, D.A.; Wheatley, K.; Delgado, R.F.; Alexander, S.; Uyttebroeck, A.; Bollard, C.M.; et al. Rituximab for High-Risk, Mature B-Cell Non-Hodgkin’s Lymphoma in Children. N. Engl. J. Med. 2020, 382, 2207–2219. [Google Scholar] [CrossRef] [PubMed]
- Dunleavy, K.; Pittaluga, S.; Maeda, L.S.; Advani, R.; Chen, C.C.; Hessler, J.; Steinberg, S.M.; Grant, C.; Wright, G.; Varma, G.; et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N. Engl. J. Med. 2013, 368, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Harker-Murray, P.D.; Pommert, L.; Barth, M.J. Novel Therapies Potentially Available for Pediatric B-Cell Non-Hodgkin Lymphoma. J. Natl. Compr. Cancer Netw. 2020, 18, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, M.J.; Hochberg, J.; Bjornard, K.L.; Brinkman, T.M. Long-term survivors of childhood, adolescent and young adult non-Hodgkin lymphoma. Br. J. Haematol. 2019, 185, 1099–1110. [Google Scholar] [CrossRef]
- Moser, O.; Zimmermann, M.; Meyer, U.; Klapper, W.; Oschlies, I.; Schrappe, M.; Attarbaschi, A.; Mann, G.; Niggli, F.; Spix, C.; et al. Second malignancies after treatment of childhood non-Hodgkin lymphoma: A report of the Berlin-Frankfurt-Muenster study group. Haematologica 2020. [Google Scholar] [CrossRef]
- Piccaluga, P.P.; Agostinelli, C.; Gazzola, A.; Tripodo, C.; Bacci, F.; Sabattini, E.; Sista, M.T.; Mannu, C.; Sapienza, M.R.; Rossi, M.; et al. Pathobiology of hodgkin lymphoma. Adv. Hematol. 2011, 2011, 920898. [Google Scholar] [CrossRef]
- Crump, C.; Sundquist, K.; Sieh, W.; Winkleby, M.A.; Sundquist, J. Perinatal and family risk factors for Hodgkin lymphoma in childhood through young adulthood. Am. J. Epidemiol. 2012, 176, 1147–1158. [Google Scholar] [CrossRef]
- Schellong, G.; Pötter, R.; Brämswig, J.; Wagner, W.; Prott, F.J.; Dörffel, W.; Körholz, D.; Mann, G.; Rath, B.; Reiter, A.; et al. High cure rates and reduced long-term toxicity in pediatric Hodgkin’s disease: The German-Austrian multicenter trial DAL-HD-90. The German-Austrian Pediatric Hodgkin’s Disease Study Group. J. Clin. Oncol. 1999, 17, 3736–3744. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, J. Standard therapy of advanced Hodgkin lymphoma. Hematol. Am. Soc. Hematol. Educ. Program 2009, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Rancea, M.; von Tresckow, B.; Monsef, I.; Engert, A.; Skoetz, N. High-dose chemotherapy followed by autologous stem cell transplantation for patients with relapsed or refractory Hodgkin lymphoma: A systematic review with meta-analysis. Crit. Rev. Oncol./Hematol. 2014, 92, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Green, M.R.; Monti, S.; Rodig, S.J.; Juszczynski, P.; Currie, T.; O’Donnell, E.; Chapuy, B.; Takeyama, K.; Neuberg, D.; Golub, T.R.; et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood 2010, 116, 3268–3277. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, F.E.; Ng, A.K. Long-term risk of second malignancy and cardiovascular disease after Hodgkin lymphoma treatment. Hematol. Am. Soc. Hematol. Educ. Program 2016, 2016, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Schellong, G.; Riepenhausen, M.; Bruch, C.; Kotthoff, S.; Vogt, J.; Bölling, T.; Dieckmann, K.; Pötter, R.; Heinecke, A.; Brämswig, J.; et al. Late valvular and other cardiac diseases after different doses of mediastinal radiotherapy for Hodgkin disease in children and adolescents: Report from the longitudinal GPOH follow-up project of the German-Austrian DAL-HD studies. Pediatr. Blood Cancer 2010, 55, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.S.; Stovall, M.; Gospodarowicz, M.; Van Leeuwen, F.E.; Andersson, M.; Glimelius, B.; Joensuu, T.; Lynch, C.F.; Curtis, R.E.; Holowaty, E.; et al. Lung cancer after treatment for Hodgkin’s disease: Focus on radiation effects. Radiat. Res. 2003, 159, 161–173. [Google Scholar] [CrossRef]
- Ng, A.K.; van Leeuwen, F.E. Hodgkin lymphoma: Late effects of treatment and guidelines for surveillance. Semin. Hematol. 2016, 53, 209–215. [Google Scholar] [CrossRef]
- van Leeuwen, F.E.; Klokman, W.J.; Stovall, M.; Dahler, E.C.; van’t Veer, M.B.; Noordijk, E.M.; Crommelin, M.A.; Aleman, B.M.; Broeks, A.; Gospodarowicz, M.; et al. Roles of radiation dose, chemotherapy, and hormonal factors in breast cancer following Hodgkin’s disease. J. Natl. Cancer Inst. 2003, 95, 971–980. [Google Scholar] [CrossRef]
- Travis, L.B.; Gospodarowicz, M.; Curtis, R.E.; Clarke, E.A.; Andersson, M.; Glimelius, B.; Joensuu, T.; Lynch, C.F.; van Leeuwen, F.E.; Holowaty, E.; et al. Lung cancer following chemotherapy and radiotherapy for Hodgkin’s disease. J. Natl. Cancer Inst. 2002, 94, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, A.J.; Schoemaker, M.J.; Allerton, R.; Horwich, A.; Barber, J.A.; Cunningham, D.; Lister, T.A.; Rohatiner, A.Z.; Vaughan Hudson, G.; Williams, M.V.; et al. Lung cancer after Hodgkin’s disease: A nested case-control study of the relation to treatment. J. Clin. Oncol. 2001, 19, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
- Preston, D.L.; Kusumi, S.; Tomonaga, M.; Izumi, S.; Ron, E.; Kuramoto, A.; Kamada, N.; Dohy, H.; Matsuo, T.; Matsui, T.; et al. Cancer incidence in atomic bomb survivors. Part III. Leukemia, lymphoma and multiple myeloma, 1950–1987. Radiat. Res. 1994, 137, S68–S97. [Google Scholar] [CrossRef]
- Darby, S.C.; Doll, R.; Gill, S.K.; Smith, P.G. Long term mortality after a single treatment course with X-rays in patients treated for ankylosing spondylitis. Br. J. Cancer 1987, 55, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Pui, C.H.; Crist, W.M.; Look, A.T. Biology and clinical significance of cytogenetic abnormalities in childhood acute lymphoblastic leukemia. Blood 1990, 76, 1449–1463. [Google Scholar] [CrossRef]
- Aplan, P.D. Chromosomal translocations involving the MLL gene: Molecular mechanisms. DNA Repair 2006, 5, 1265–1272. [Google Scholar] [CrossRef][Green Version]
- Pui, C.H.; Evans, W.E. A 50-year journey to cure childhood acute lymphoblastic leukemia. Semin. Hematol. 2013, 50, 185–196. [Google Scholar] [CrossRef]
- Meadows, A.T.; Friedman, D.L.; Neglia, J.P.; Mertens, A.C.; Donaldson, S.S.; Stovall, M.; Hammond, S.; Yasui, Y.; Inskip, P.D. Second neoplasms in survivors of childhood cancer: Findings from the Childhood Cancer Survivor Study cohort. J. Clin. Oncol. 2009, 27, 2356–2362. [Google Scholar] [CrossRef] [PubMed]
- Pui, C.H.; Carroll, W.L.; Meshinchi, S.; Arceci, R.J. Biology, risk stratification, and therapy of pediatric acute leukemias: An update. J. Clin. Oncol. 2011, 29, 551–565. [Google Scholar] [CrossRef]
- Dores, G.M.; Devesa, S.S.; Curtis, R.E.; Linet, M.S.; Morton, L.M. Acute leukemia incidence and patient survival among children and adults in the United States, 2001–2007. Blood 2012, 119, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Creutzig, U.; Zimmermann, M.; Ritter, J.; Reinhardt, D.; Hermann, J.; Henze, G.; Jürgens, H.; Kabisch, H.; Reiter, A.; Riehm, H.; et al. Treatment strategies and long-term results in paediatric patients treated in four consecutive AML-BFM trials. Leukemia 2005, 19, 2030–2042. [Google Scholar] [CrossRef]
- Creutzig, U.; Zimmermann, M.; Dworzak, M.N.; Ritter, J.; Schellong, G.; Reinhardt, D. Development of a curative treatment within the AML-BFM studies. Klin. Padiatr. 2013, 225 (Suppl. 1), S79–S86. [Google Scholar] [CrossRef]
- Greenlee, R.T.; Hill-Harmon, M.B.; Murray, T.; Thun, M. Cancer statistics, 2001. CA Cancer J. Clin. 2001, 51, 15–36. [Google Scholar] [CrossRef] [PubMed]
- Creutzig, U.; Ritter, J.; Zimmermann, M.; Reinhardt, D.; Hermann, J.; Berthold, F.; Henze, G.; Jürgens, H.; Kabisch, H.; Havers, W.; et al. Improved treatment results in high-risk pediatric acute myeloid leukemia patients after intensification with high-dose cytarabine and mitoxantrone: Results of Study Acute Myeloid Leukemia-Berlin-Frankfurt-Münster 93. J. Clin. Oncol. 2001, 19, 2705–2713. [Google Scholar] [CrossRef]
- Meadows, A.T. Second malignant neoplasms in childhood cancer survivors. J. Assoc. Pediatr. Oncol. Nurses 1989, 6, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.M.; Teitell, M.A. Second malignancy after treatment of pediatric Hodgkin disease. J. Pediatr. Hematol. Oncol. 2005, 27, 28–36. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Granfeldt Østgård, L.S.; Medeiros, B.C.; Sengeløv, H.; Nørgaard, M.; Andersen, M.K.; Dufva, I.H.; Friis, L.S.; Kjeldsen, E.; Marcher, C.W.; Preiss, B.; et al. Epidemiology and Clinical Significance of Secondary and Therapy-Related Acute Myeloid Leukemia: A National Population-Based Cohort Study. J. Clin. Oncol. 2015, 33, 3641–3649. [Google Scholar] [CrossRef] [PubMed]
- Guru Murthy, G.S.; Abedin, S. Myeloid malignancies after treatment for solid tumours. Best Pract. Res. Clin. Haematol. 2019, 32, 40–46. [Google Scholar] [CrossRef]
- Arseneau, J.C.; Sponzo, R.W.; Levin, D.L.; Schnipper, L.E.; Bonner, H.; Young, R.C.; Canellos, G.P.; Johnson, R.E.; DeVita, V.T. Nonlymphomatous malignant tumors complicating Hodgkin’s disease. Possible association with intensive therapy. N. Engl. J. Med. 1972, 287, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Pedersen-Bjergaard, J.; Philip, P.; Larsen, S.O.; Andersson, M.; Daugaard, G.; Ersbøll, J.; Hansen, S.W.; Hou-Jensen, K.; Nielsen, D.; Sigsgaard, T.C.; et al. Therapy-related myelodysplasia and acute myeloid leukemia. Cytogenetic characteristics of 115 consecutive cases and risk in seven cohorts of patients treated intensively for malignant diseases in the Copenhagen series. Leukemia 1993, 7, 1975–1986. [Google Scholar] [PubMed]
- Abdelhameed, A.; Pond, G.R.; Mitsakakis, N.; Brandwein, J.; Chun, K.; Gupta, V.; Kamel-Reid, S.; Lipton, J.H.; Minden, M.D.; Schimmer, A.; et al. Outcome of patients who develop acute leukemia or myelodysplasia as a second malignancy after solid tumors treated surgically or with strategies that include chemotherapy and/or radiation. Cancer 2008, 112, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Nardi, V.; Winkfield, K.M.; Ok, C.Y.; Niemierko, A.; Kluk, M.J.; Attar, E.C.; Garcia-Manero, G.; Wang, S.A.; Hasserjian, R.P. Acute myeloid leukemia and myelodysplastic syndromes after radiation therapy are similar to de novo disease and differ from other therapy-related myeloid neoplasms. J. Clin. Oncol. 2012, 30, 2340–2347. [Google Scholar] [CrossRef]
- Pedersen-Bjergaard, J.; Pedersen, M.; Roulston, D.; Philip, P. Different genetic pathways in leukemogenesis for patients presenting with therapy-related myelodysplasia and therapy-related acute myeloid leukemia. Blood 1995, 86, 3542–3552. [Google Scholar] [CrossRef]
- Pedersen-Bjergaard, J.; Rowley, J.D. The balanced and the unbalanced chromosome aberrations of acute myeloid leukemia may develop in different ways and may contribute differently to malignant transformation. Blood 1994, 83, 2780–2786. [Google Scholar] [CrossRef] [PubMed]
- Carli, P.M.; Sgro, C.; Parchin-Geneste, N.; Isambert, N.; Mugneret, F.; Girodon, F.; Maynadié, M. Increase therapy-related leukemia secondary to breast cancer. Leukemia 2000, 14, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.K.; Johansson, B.; Larsen, S.O.; Pedersen-Bjergaard, J. Chromosomal abnormalities in secondary MDS and AML. Relationship to drugs and radiation with specific emphasis on the balanced rearrangements. Haematologica 1998, 83, 483–488. [Google Scholar] [PubMed]
- Ahuja, H.G.; Felix, C.A.; Aplan, P.D. The t(11;20)(p15;q11) chromosomal translocation associated with therapy-related myelodysplastic syndrome results in an NUP98-TOP1 fusion. Blood 1999, 94, 3258–3261. [Google Scholar] [CrossRef]
- Sabattini, E.; Bacci, F.; Sagramoso, C.; Pileri, S.A. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: An overview. Pathologica 2010, 102, 83–87. [Google Scholar]
- Sandlund, J.T.; Downing, J.R.; Crist, W.M. Non-Hodgkin’s lymphoma in childhood. N. Engl. J. Med. 1996, 334, 1238–1248. [Google Scholar] [CrossRef]
- Kaatsch, P.; Spix, C. German Childhood Cancer Registry, Annual Report 2003. 2004. Available online: https://www.unimedizin-mainz.de/index.php?id=22658 (accessed on 18 February 2021).
- Percy, C.L.; Smith, M.A.; Linet, M.; Lynn, A.; Ries, L.A.G.; Friedman, D. Lymphomas and reticuloendothelial neoplasms. In Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995; National Cancer Institute: Bethesda, MD, USA, 1999; pp. 35–49. [Google Scholar]
- Burkhardt, B.; Zimmermann, M.; Oschlies, I.; Niggli, F.; Mann, G.; Parwaresch, R.; Riehm, H.; Schrappe, M.; Reiter, A. The impact of age and gender on biology, clinical features and treatment outcome of non-Hodgkin lymphoma in childhood and adolescence. Br. J. Haematol. 2005, 131, 39–49. [Google Scholar] [CrossRef]
- Salzburg, J.; Burkhardt, B.; Zimmermann, M.; Wachowski, O.; Woessmann, W.; Oschlies, I.; Klapper, W.; Wacker, H.H.; Ludwig, W.D.; Niggli, F.; et al. Prevalence, clinical pattern, and outcome of CNS involvement in childhood and adolescent non-Hodgkin’s lymphoma differ by non-Hodgkin’s lymphoma subtype: A Berlin-Frankfurt-Munster Group Report. J. Clin. Oncol. 2007, 25, 3915–3922. [Google Scholar] [CrossRef]
- Reiter, A.; Schrappe, M.; Ludwig, W.D.; Tiemann, M.; Parwaresch, R.; Zimmermann, M.; Schirg, E.; Henze, G.; Schellong, G.; Gadner, H.; et al. Intensive ALL-type therapy without local radiotherapy provides a 90% event-free survival for children with T-cell lymphoblastic lymphoma: A BFM group report. Blood 2000, 95, 416–421. [Google Scholar] [PubMed]
- Reiter, A.; Schrappe, M.; Tiemann, M.; Parwaresch, R.; Zimmermann, M.; Yakisan, E.; Dopfer, R.; Bucsky, P.; Mann, G.; Gadner, H.; et al. Successful treatment strategy for Ki-1 anaplastic large-cell lymphoma of childhood: A prospective analysis of 62 patients enrolled in three consecutive Berlin-Frankfurt-Munster group studies. J. Clin. Oncol. 1994, 12, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Woessmann, W.; Seidemann, K.; Mann, G.; Zimmermann, M.; Burkhardt, B.; Oschlies, I.; Ludwig, W.D.; Klingebiel, T.; Graf, N.; Gruhn, B.; et al. The impact of the methotrexate administration schedule and dose in the treatment of children and adolescents with B-cell neoplasms: A report of the BFM Group Study NHL-BFM95. Blood 2005, 105, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Minard-Colin, V.; Brugières, L.; Reiter, A.; Cairo, M.S.; Gross, T.G.; Woessmann, W.; Burkhardt, B.; Sandlund, J.T.; Williams, D.; Pillon, M.; et al. Non-Hodgkin Lymphoma in Children and Adolescents: Progress Through Effective Collaboration, Current Knowledge, and Challenges Ahead. J. Clin. Oncol. 2015, 33, 2963–2974. [Google Scholar] [CrossRef]
- Smith, M.A.; Altekruse, S.F.; Adamson, P.C.; Reaman, G.H.; Seibel, N.L. Declining childhood and adolescent cancer mortality. Cancer 2014, 120, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Bluhm, E.C.; Ronckers, C.; Hayashi, R.J.; Neglia, J.P.; Mertens, A.C.; Stovall, M.; Meadows, A.T.; Mitby, P.A.; Whitton, J.A.; Hammond, S.; et al. Cause-specific mortality and second cancer incidence after non-Hodgkin lymphoma: A report from the Childhood Cancer Survivor Study. Blood 2008, 111, 4014–4021. [Google Scholar] [CrossRef]
- Landmann, E.; Oschlies, I.; Zimmermann, M.; Moser, O.; Graf, N.; Suttorp, M.; Greiner, J.; Reiter, A. Secondary non-Hodgkin lymphoma (NHL) in children and adolescents after childhood cancer other than NHL. Br. J. Haematol. 2008, 143, 387–394. [Google Scholar] [CrossRef]
- Barista, I.; Varan, A.; Ozyar, E. Bimodal age distribution in Hodgkin’s disease and nasopharyngeal carcinoma. Med. Hypotheses 2007, 68, 1421. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Holmqvist, A.S.; Chen, Y.; Berano Teh, J.; Sun, C.; Birch, J.M.; van den Bos, C.; Diller, L.R.; Dilley, K.; Ginsberg, J.; Martin, L.T.; et al. Risk of solid subsequent malignant neoplasms after childhood Hodgkin lymphoma-Identification of high-risk populations to guide surveillance: A report from the Late Effects Study Group. Cancer 2019, 125, 1373–1383. [Google Scholar] [CrossRef]
- Travis, L.B.; Hill, D.A.; Dores, G.M.; Gospodarowicz, M.; van Leeuwen, F.E.; Holowaty, E.; Glimelius, B.; Andersson, M.; Wiklund, T.; Lynch, C.F.; et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA 2003, 290, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, C.S.; Chou, J.F.; Wolden, S.L.; Bernstein, J.L.; Malhotra, J.; Novetsky Friedman, D.; Mubdi, N.Z.; Leisenring, W.M.; Stovall, M.; Hammond, S.; et al. Breast cancer after chest radiation therapy for childhood cancer. J. Clin. Oncol. 2014, 32, 2217–2223. [Google Scholar] [CrossRef] [PubMed]
- Salloum, E.; Doria, R.; Schubert, W.; Zelterman, D.; Holford, T.; Roberts, K.B.; Farber, L.R.; Kiehl, R.K.; Cardinale, J.; Cooper, D.L. Second solid tumors in patients with Hodgkin’s disease cured after radiation or chemotherapy plus adjuvant low-dose radiation. J. Clin. Oncol. 1996, 14, 2435–2443. [Google Scholar] [CrossRef]
- Koontz, B.F.; Kirkpatrick, J.P.; Clough, R.W.; Prosnitz, R.G.; Gockerman, J.P.; Moore, J.O.; Prosnitz, L.R. Combined-modality therapy versus radiotherapy alone for treatment of early-stage Hodgkin’s disease: Cure balanced against complications. J. Clin. Oncol. 2006, 24, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.M.; Nathan, P.C.; Hodgson, D.C.; Jenkin, D.; Weitzman, S.; Grant, R.M.; Manson, D.; Bross, A.; Doyle, J.J.; Danjoux, C.; et al. Survival and late effects in children with Hodgkin’s lymphoma treated with MOPP/ABV and low-dose, extended-field irradiation. J. Clin. Oncol. 2006, 24, 5735–5741. [Google Scholar] [CrossRef]
- O’Brien, M.M.; Donaldson, S.S.; Balise, R.R.; Whittemore, A.S.; Link, M.P. Second malignant neoplasms in survivors of pediatric Hodgkin’s lymphoma treated with low-dose radiation and chemotherapy. J. Clin. Oncol. 2010, 28, 1232–1239. [Google Scholar] [CrossRef]
- Bhatia, S.; Robison, L.L.; Oberlin, O.; Greenberg, M.; Bunin, G.; Fossati-Bellani, F.; Meadows, A.T. Breast cancer and other second neoplasms after childhood Hodgkin’s disease. N. Engl. J. Med. 1996, 334, 745–751. [Google Scholar] [CrossRef]
- Beaty, O., III; Hudson, M.M.; Greenwald, C.; Luo, X.; Fang, L.; Wilimas, J.A.; Thompson, E.I.; Kun, L.E.; Pratt, C.B. Subsequent malignancies in children and adolescents after treatment for Hodgkin’s disease. J. Clin. Oncol. 1995, 13, 603–609. [Google Scholar] [CrossRef]
- Wolden, S.L.; Lamborn, K.R.; Cleary, S.F.; Tate, D.J.; Donaldson, S.S. Second cancers following pediatric Hodgkin’s disease. J. Clin. Oncol. 1998, 16, 536–544. [Google Scholar] [CrossRef]
- Sankila, R.; Garwicz, S.; Olsen, J.H.; Döllner, H.; Hertz, H.; Kreuger, A.; Langmark, F.; Lanning, M.; Möller, T.; Tulinius, H. Risk of subsequent malignant neoplasms among 1,641 Hodgkin’s disease patients diagnosed in childhood and adolescence: A population-based cohort study in the five Nordic countries. Association of the Nordic Cancer Registries and the Nordic Society of Pediatric Hematology and Oncology. J. Clin. Oncol. 1996, 14, 1442–1446. [Google Scholar] [CrossRef] [PubMed]
- Tucker, M.A. Solid second cancers following Hodgkin’s disease. Hematol. Oncol. Clin. N. Am. 1993, 7, 389–400. [Google Scholar] [CrossRef]
- Dores, G.M.; Metayer, C.; Curtis, R.E.; Lynch, C.F.; Clarke, E.A.; Glimelius, B.; Storm, H.; Pukkala, E.; van Leeuwen, F.E.; Holowaty, E.J.; et al. Second malignant neoplasms among long-term survivors of Hodgkin’s disease: A population-based evaluation over 25 years. J. Clin. Oncol. 2002, 20, 3484–3494. [Google Scholar] [CrossRef] [PubMed]
- Jenkin, D.; Greenberg, M.; Fitzgerald, A. Second malignant tumours in childhood Hodgkin’s disease. Med. Pediatr. Oncol. 1996, 26, 373–379. [Google Scholar] [CrossRef]
- Meadows, A.T.; Obringer, A.C.; Marrero, O.; Oberlin, O.; Robison, L.; Fossati-Bellani, F.; Green, D.; Voûte, P.A.; Morris-Jones, P.; Greenberg, M.; et al. Second malignant neoplasms following childhood Hodgkin’s disease: Treatment and splenectomy as risk factors. Med. Pediatr. Oncol. 1989, 17, 477–484. [Google Scholar] [CrossRef]
- Bhatia, S.; Yasui, Y.; Robison, L.L.; Birch, J.M.; Bogue, M.K.; Diller, L.; DeLaat, C.; Fossati-Bellani, F.; Morgan, E.; Oberlin, O.; et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin’s disease: Report from the Late Effects Study Group. J. Clin. Oncol. 2003, 21, 4386–4394. [Google Scholar] [CrossRef] [PubMed]
- Dörffel, W.; Riepenhausen, M.; Lüders, H.; Brämswig, J. Late Effects Following Treatment of Hodgkin Lymphoma During Childhood and Adolescence. Results of the Hodgkin Lymphoma Late Effects Research Project. Klin. Padiatr. 2016, 228, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Schaapveld, M.; Aleman, B.M.; van Eggermond, A.M.; Janus, C.P.; Krol, A.D.; van der Maazen, R.W.; Roesink, J.; Raemaekers, J.M.; de Boer, J.P.; Zijlstra, J.M.; et al. Second Cancer Risk Up to 40 Years after Treatment for Hodgkin’s Lymphoma. N. Engl. J. Med. 2015, 373, 2499–2511. [Google Scholar] [CrossRef]
- Choi, Y.; Diefenbach, C.S. Advances in Therapy for Relapsed or Refractory Hodgkin Lymphoma. Curr. Oncol. Rep. 2020, 22, 6. [Google Scholar] [CrossRef]
- Baker, K.S.; DeFor, T.E.; Burns, L.J.; Ramsay, N.K.; Neglia, J.P.; Robison, L.L. New malignancies after blood or marrow stem-cell transplantation in children and adults: Incidence and risk factors. J. Clin. Oncol. 2003, 21, 1352–1358. [Google Scholar] [CrossRef]
- Ljungman, P.; Urbano-Ispizua, A.; Cavazzana-Calvo, M.; Demirer, T.; Dini, G.; Einsele, H.; Gratwohl, A.; Madrigal, A.; Niederwieser, D.; Passweg, J.; et al. Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: Definitions and current practice in Europe. Bone Marrow Transplant. 2006, 37, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Ricardi, U.; Filippi, A.R.; Biasin, E.; Ciammella, P.; Botticella, A.; Franco, P.; Corrias, A.; Vassallo, E.; Ragona, R.; Fagioli, F. Late toxicity in children undergoing hematopoietic stem cell transplantation with TBI-containing conditioning regimens for hematological malignancies. Strahlenther. Onkol. 2009, 185 (Suppl. 2) (Suppl. 2), 17–20. [Google Scholar] [CrossRef]
- Darrington, D.L.; Vose, J.M.; Anderson, J.R.; Bierman, P.J.; Bishop, M.R.; Chan, W.C.; Morris, M.E.; Reed, E.C.; Sanger, W.G.; Tarantolo, S.R.; et al. Incidence and characterization of secondary myelodysplastic syndrome and acute myelogenous leukemia following high-dose chemoradiotherapy and autologous stem-cell transplantation for lymphoid malignancies. J. Clin. Oncol. 1994, 12, 2527–2534. [Google Scholar] [CrossRef]
- Park, S.; Brice, P.; Noguerra, M.E.; Simon, D.; Rousselot, P.; Kerneis, Y.; Morel, P.; Marolleau, J.P.; Gisselbrecht, C. Myelodysplasias and leukemias after autologous stem cell transplantation for lymphoid malignancies. Bone Marrow Transplant. 2000, 26, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Socié, G.; Curtis, R.E.; Deeg, H.J.; Sobocinski, K.A.; Filipovich, A.H.; Travis, L.B.; Sullivan, K.M.; Rowlings, P.A.; Kingma, D.W.; Banks, P.M.; et al. New malignant diseases after allogeneic marrow transplantation for childhood acute leukemia. J. Clin. Oncol. 2000, 18, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Curtis, R.E.; Rowlings, P.A.; Deeg, H.J.; Shriner, D.A.; Socíe, G.; Travis, L.B.; Horowitz, M.M.; Witherspoon, R.P.; Hoover, R.N.; Sobocinski, K.A.; et al. Solid cancers after bone marrow transplantation. N. Engl. J. Med. 1997, 336, 897–904. [Google Scholar] [CrossRef]
- Mehta, P.A.; Davies, S.M.; Leemhuis, T.; Myers, K.; Kernan, N.A.; Prockop, S.E.; Scaradavou, A.; O’Reilly, R.J.; Williams, D.A.; Lehmann, L.; et al. Radiation-free, alternative-donor HCT for Fanconi anemia patients: Results from a prospective multi-institutional study. Blood 2017, 129, 2308–2315. [Google Scholar] [CrossRef]
- Stepensky, P.; Shapira, M.Y.; Balashov, D.; Trakhtman, P.; Skorobogatova, E.; Rheingold, L.; Brooks, R.; Revel-Vilk, S.; Weintraub, M.; Stein, J.; et al. Bone marrow transplantation for Fanconi anemia using fludarabine-based conditioning. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2011, 17, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Río, P.; Navarro, S.; Wang, W.; Sánchez-Domínguez, R.; Pujol, R.M.; Segovia, J.C.; Bogliolo, M.; Merino, E.; Wu, N.; Salgado, R.; et al. Successful engraftment of gene-corrected hematopoietic stem cells in non-conditioned patients with Fanconi anemia. Nat. Med. 2019, 25, 1396–1401. [Google Scholar] [CrossRef]
- Bishop, A.J.; McDonald, M.W.; Chang, A.L.; Esiashvili, N. Infant brain tumors: Incidence, survival, and the role of radiation based on Surveillance, Epidemiology, and End Results (SEER) Data. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Rickert, C.H.; Probst-Cousin, S.; Gullotta, F. Primary intracranial neoplasms of infancy and early childhood. Child’s Nerv. Syst. 1997, 13, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-Oncol. 2019, 21, v1–v100. [Google Scholar] [CrossRef] [PubMed]
- McKinney, P.A. Central nervous system tumours in children: Epidemiology and risk factors. Bioelectromagnetics 2005, S60–S68. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Pui, C.H.; Gajjar, A.J.; Kane, J.R.; Qaddoumi, I.A.; Pappo, A.S. Challenging issues in pediatric oncology. Nat. Rev. Clin. Oncol. 2011, 8, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.T. Long-term survivors of childhood central nervous system malignancies: The experience of the Childhood Cancer Survivor Study. Eur. J. Paediatr. Neurol. 2010, 14, 298–303. [Google Scholar] [CrossRef]
- Szalontay, L.; Khakoo, Y. Medulloblastoma: An Old Diagnosis with New Promises. Curr. Oncol. Rep. 2020, 22, 90. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kumar, V.; McGuire, T.; Coulter, D.W.; Sharp, J.G.; Mahato, R.I. Challenges and Recent Advances in Medulloblastoma Therapy. Trends Pharmacol. Sci. 2017, 38, 1061–1084. [Google Scholar] [CrossRef]
- Merchant, T.E.; Kun, L.E.; Krasin, M.J.; Wallace, D.; Chintagumpala, M.M.; Woo, S.Y.; Ashley, D.M.; Sexton, M.; Kellie, S.J.; Ahern, V.; et al. Multi-institution prospective trial of reduced-dose craniospinal irradiation (23.4 Gy) followed by conformal posterior fossa (36 Gy) and primary site irradiation (55.8 Gy) and dose-intensive chemotherapy for average-risk medulloblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 782–787. [Google Scholar] [CrossRef]
- Packer, R.J.; Gajjar, A.; Vezina, G.; Rorke-Adams, L.; Burger, P.C.; Robertson, P.L.; Bayer, L.; LaFond, D.; Donahue, B.R.; Marymont, M.H.; et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J. Clin. Oncol. 2006, 24, 4202–4208. [Google Scholar] [CrossRef]
- Sklar, C.A.; Constine, L.S. Chronic neuroendocrinological sequelae of radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1113–1121. [Google Scholar] [CrossRef]
- Uday, S.; Murray, R.D.; Picton, S.; Chumas, P.; Raju, M.; Chandwani, M.; Alvi, S. Endocrine sequelae beyond 10 years in survivors of medulloblastoma. Clin. Endocrinol. 2015, 83, 663–670. [Google Scholar] [CrossRef]
- Packer, R.J.; Zhou, T.; Holmes, E.; Vezina, G.; Gajjar, A. Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: Results of Children’s Oncology Group trial A9961. Neuro-Oncol. 2013, 15, 97–103. [Google Scholar] [CrossRef]
- Hoff, K.V.; Hinkes, B.; Gerber, N.U.; Deinlein, F.; Mittler, U.; Urban, C.; Benesch, M.; Warmuth-Metz, M.; Soerensen, N.; Zwiener, I.; et al. Long-term outcome and clinical prognostic factors in children with medulloblastoma treated in the prospective randomised multicentre trial HIT’91. Eur. J. Cancer 2009, 45, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, T.J.; Aguilera, D.; Castellino, R.C. The rationale for targeted therapies in medulloblastoma. Neuro-Oncol. 2014, 16, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Reuther, F.J.; Löhler, J.; Herms, J.; Hugo, H.H.; Schindler, C.; Leithäuser, F.; Melzner, I.; Möller, P.; Scheil, S. Low incidence of SV40-like sequences in ependymal tumours. J. Pathol. 2001, 195, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.R.; O’Donnell, C.C.; Curry, W.T.; Bove, C.M.; MacCollin, M.; Nunes, F.P. Spinal ependymomas in neurofibromatosis Type 2: A retrospective analysis of 55 patients. J. Neurosurg. Spine 2011, 14, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Vitanza, N.A.; Partap, S. Pediatric Ependymoma. J. Child Neurol. 2016, 31, 1354–1366. [Google Scholar] [CrossRef]
- Kilday, J.P.; Rahman, R.; Dyer, S.; Ridley, L.; Lowe, J.; Coyle, B.; Grundy, R. Pediatric ependymoma: Biological perspectives. Mol. Cancer Res. Mcr. 2009, 7, 765–786. [Google Scholar] [CrossRef] [PubMed]
- Merchant, T.E.; Li, C.; Xiong, X.; Kun, L.E.; Boop, F.A.; Sanford, R.A. Conformal radiotherapy after surgery for paediatric ependymoma: A prospective study. Lancet Oncol. 2009, 10, 258–266. [Google Scholar] [CrossRef]
- Swanson, E.L.; Amdur, R.J.; Morris, C.G.; Galloway, T.J.; Marcus, R.B., Jr.; Pincus, D.W.; Smith, A. Intracranial ependymomas treated with radiotherapy: Long-term results from a single institution. J. Neuro-Oncol. 2011, 102, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, S.M.; Sethi, R.; Lavally, B.; Yeap, B.Y.; Marcus, K.J.; Caruso, P.; Pulsifer, M.; Huang, M.; Ebb, D.; Tarbell, N.J.; et al. Proton radiotherapy for pediatric central nervous system ependymoma: Clinical outcomes for 70 patients. Neuro-Oncol. 2013, 15, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Toescu, S.M.; Aquilina, K. Current and Emerging Methods of Management of Ependymoma. Curr. Oncol. Rep. 2019, 21, 78. [Google Scholar] [CrossRef]
- Delgado-López, P.D.; Corrales-García, E.M.; Alonso-García, E.; García-Leal, R.; González-Rodrigálvarez, R.; Araus-Galdós, E.; Martín-Alonso, J. Central nervous system ependymoma: Clinical implications of the new molecular classification, treatment guidelines and controversial issues. Clin. Transl. Oncol. 2019, 21, 1450–1463. [Google Scholar] [CrossRef] [PubMed]
- Merchant, T.E.; Bendel, A.E.; Sabin, N.D.; Burger, P.C.; Shaw, D.W.; Chang, E.; Wu, S.; Zhou, T.; Eisenstat, D.D.; Foreman, N.K.; et al. Conformal Radiation Therapy for Pediatric Ependymoma, Chemotherapy for Incompletely Resected Ependymoma, and Observation for Completely Resected, Supratentorial Ependymoma. J. Clin. Oncol. 2019, 37, 974–983. [Google Scholar] [CrossRef]
- Fotakopoulos, G.; Vagkopoulos, K.; Gatos, C.; Kotlia, P.; Brotis, A. Spinal cord ependymomas and the appearance of other de novo tumors: A systematic review. J. Med. Case Rep. 2014, 8, 438. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rogers, H.A.; Kilday, J.P.; Mayne, C.; Ward, J.; Adamowicz-Brice, M.; Schwalbe, E.C.; Clifford, S.C.; Coyle, B.; Grundy, R.G. Supratentorial and spinal pediatric ependymomas display a hypermethylated phenotype which includes the loss of tumor suppressor genes involved in the control of cell growth and death. Acta Neuropathol. 2012, 123, 711–725. [Google Scholar] [CrossRef]
- Rousseau, E.; Ruchoux, M.M.; Scaravilli, F.; Chapon, F.; Vinchon, M.; De Smet, C.; Godfraind, C.; Vikkula, M. CDKN2A, CDKN2B and p14ARF are frequently and differentially methylated in ependymal tumours. Neuropathol. Appl. Neurobiol. 2003, 29, 574–583. [Google Scholar] [CrossRef]
- Pollack, I.F.; Agnihotri, S.; Broniscer, A. Childhood brain tumors: Current management, biological insights, and future directions. J. Neurosurg. Pediatr. 2019, 23, 261–273. [Google Scholar] [CrossRef]
- Benesch, M.; Eder, H.G.; Sovinz, P.; Raith, J.; Lackner, H.; Moser, A.; Urban, C. Residual or recurrent cerebellar low-grade glioma in children after tumor resection: Is re-treatment needed? A single center experience from 1983 to 2003. Pediatr. Neurosurg. 2006, 42, 159–164. [Google Scholar] [CrossRef]
- Banerjee, A.; Jakacki, R.I.; Onar-Thomas, A.; Wu, S.; Nicolaides, T.; Young Poussaint, T.; Fangusaro, J.; Phillips, J.; Perry, A.; Turner, D.; et al. A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: A Pediatric Brain Tumor Consortium (PBTC) study. Neuro-Oncol. 2017, 19, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Pfister, S.; Janzarik, W.G.; Remke, M.; Ernst, A.; Werft, W.; Becker, N.; Toedt, G.; Wittmann, A.; Kratz, C.; Olbrich, H.; et al. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J. Clin. Investig. 2008, 118, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Sievert, A.J.; Lang, S.S.; Boucher, K.L.; Madsen, P.J.; Slaunwhite, E.; Choudhari, N.; Kellet, M.; Storm, P.B.; Resnick, A.C. Paradoxical activation and RAF inhibitor resistance of BRAF protein kinase fusions characterizing pediatric astrocytomas. Proc. Natl. Acad. Sci. USA 2013, 110, 5957–5962. [Google Scholar] [CrossRef]
- Krueger, D.A.; Care, M.M.; Holland, K.; Agricola, K.; Tudor, C.; Mangeshkar, P.; Wilson, K.A.; Byars, A.; Sahmoud, T.; Franz, D.N. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N. Engl. J. Med. 2010, 363, 1801–1811. [Google Scholar] [CrossRef] [PubMed]
- Bandopadhayay, P.; Bergthold, G.; London, W.B.; Goumnerova, L.C.; Morales La Madrid, A.; Marcus, K.J.; Guo, D.; Ullrich, N.J.; Robison, N.J.; Chi, S.N.; et al. Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: An analysis of the Surveillance Epidemiology and End Results (SEER) database. Pediatr. Blood Cancer 2014, 61, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Parsons, D.W.; Jones, S.; Zhang, X.; Lin, J.C.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Siu, I.M.; Gallia, G.L.; et al. An integrated genomic analysis of human glioblastoma multiforme. Science 2008, 321, 1807–1812. [Google Scholar] [CrossRef]
- Network, C.G.A.R. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008, 455, 1061–1068. [Google Scholar] [CrossRef]
- Gallia, G.L.; Rand, V.; Siu, I.M.; Eberhart, C.G.; James, C.D.; Marie, S.K.; Oba-Shinjo, S.M.; Carlotti, C.G.; Caballero, O.L.; Simpson, A.J.; et al. PIK3CA gene mutations in pediatric and adult glioblastoma multiforme. Mol. Cancer Res. 2006, 4, 709–714. [Google Scholar] [CrossRef]
- Pollack, I.F.; Finkelstein, S.D.; Burnham, J.; Holmes, E.J.; Hamilton, R.L.; Yates, A.J.; Finlay, J.L.; Sposto, R. Age and TP53 mutation frequency in childhood malignant gliomas: Results in a multi-institutional cohort. Cancer Res. 2001, 61, 7404–7407. [Google Scholar]
- Newcomb, E.W.; Alonso, M.; Sung, T.; Miller, D.C. Incidence of p14ARF gene deletion in high-grade adult and pediatric astrocytomas. Hum. Pathol. 2000, 31, 115–119. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Mistry, M.; Zhukova, N.; Merico, D.; Rakopoulos, P.; Krishnatry, R.; Shago, M.; Stavropoulos, J.; Alon, N.; Pole, J.D.; Ray, P.N.; et al. BRAF mutation and CDKN2A deletion define a clinically distinct subgroup of childhood secondary high-grade glioma. J. Clin. Oncol. 2015, 33, 1015–1022. [Google Scholar] [CrossRef]
- Toll, S.A.; Tran, H.N.; Cotter, J.; Judkins, A.R.; Tamrazi, B.; Biegel, J.A.; Dhall, G.; Robison, N.J.; Waters, K.; Patel, P.; et al. Sustained response of three pediatric BRAF(V600E) mutated high-grade gliomas to combined BRAF and MEK inhibitor therapy. Oncotarget 2019, 10, 551–557. [Google Scholar] [CrossRef]
- Laetsch, T.W.; DuBois, S.G.; Mascarenhas, L.; Turpin, B.; Federman, N.; Albert, C.M.; Nagasubramanian, R.; Davis, J.L.; Rudzinski, E.; Feraco, A.M.; et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: Phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 2018, 19, 705–714. [Google Scholar] [CrossRef]
- Project, I.C.G.C.P.T. Recurrent MET fusion genes represent a drug target in pediatric glioblastoma. Nat. Med. 2016, 22, 1314–1320. [Google Scholar] [CrossRef]
- Brennan, C.W.; Verhaak, R.G.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The somatic genomic landscape of glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef] [PubMed]
- Bouffet, E.; Larouche, V.; Campbell, B.B.; Merico, D.; de Borja, R.; Aronson, M.; Durno, C.; Krueger, J.; Cabric, V.; Ramaswamy, V.; et al. Immune Checkpoint Inhibition for Hypermutant Glioblastoma Multiforme Resulting From Germline Biallelic Mismatch Repair Deficiency. J. Clin. Oncol. 2016, 34, 2206–2211. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, T.M.; Krasin, M.J.; Liu, W.; Armstrong, G.T.; Ojha, R.P.; Sadighi, Z.S.; Gupta, P.; Kimberg, C.; Srivastava, D.; Merchant, T.E.; et al. Long-Term Neurocognitive Functioning and Social Attainment in Adult Survivors of Pediatric CNS Tumors: Results From the St Jude Lifetime Cohort Study. J. Clin. Oncol. 2016, 34, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Fullerton, H.J.; Stratton, K.; Leisenring, W.; Weathers, R.E.; Stovall, M.; Armstrong, G.T.; Goldsby, R.E.; Packer, R.J.; Sklar, C.A.; et al. Radiation, atherosclerotic risk factors, and stroke risk in survivors of pediatric cancer: A report from the Childhood Cancer Survivor Study. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Tsui, K.; Gajjar, A.; Li, C.; Srivastava, D.; Broniscer, A.; Wetmore, C.; Kun, L.E.; Merchant, T.E.; Ellison, D.W.; Orr, B.A.; et al. Subsequent neoplasms in survivors of childhood central nervous system tumors: Risk after modern multimodal therapy. Neuro-Oncol. 2015, 17, 448–456. [Google Scholar] [CrossRef]
- Karremann, M.; Krämer, N.; Hoffmann, M.; Wiese, M.; Beilken, A.; Corbacioglu, S.; Dilloo, D.; Driever, P.H.; Scheurlen, W.; Kulozik, A.; et al. Haematological malignancies following temozolomide treatment for paediatric high-grade glioma. Eur. J. Cancer 2017, 81, 1–8. [Google Scholar] [CrossRef]
- Neyns, B.; Cordera, S.; Joosens, E.; Pouratian, N. Non-Hodgkin’s lymphoma in patients with glioma treated with temozolomide. J. Clin. Oncol. 2008, 26, 4518–4519. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gupta, D.; Mohanti, B.K.; Thulkar, S.; Dwary, A.; Goyal, S.; Muzumder, S.; Das, P. Non-Hodgkin lymphoma following temozolomide. Pediatr. Blood Cancer 2009, 53, 661–662. [Google Scholar] [CrossRef] [PubMed]
- Greene, M.H.; Boice, J.D., Jr.; Strike, T.A. Carmustine as a cause of acute nonlymphocytic leukemia. N. Engl. J. Med. 1985, 313, 579. [Google Scholar] [PubMed]
- Perry, J.R.; Brown, M.T.; Gockerman, J.P. Acute leukemia following treatment of malignant glioma. J. Neuro-Oncol. 1998, 40, 39–46. [Google Scholar] [CrossRef]
- Hoffman, L.M.; Veldhuijzen van Zanten, S.E.M.; Colditz, N.; Baugh, J.; Chaney, B.; Hoffmann, M.; Lane, A.; Fuller, C.; Miles, L.; Hawkins, C.; et al. Clinical, Radiologic, Pathologic, and Molecular Characteristics of Long-Term Survivors of Diffuse Intrinsic Pontine Glioma (DIPG): A Collaborative Report From the International and European Society for Pediatric Oncology DIPG Registries. J. Clin. Oncol. 2018, 36, 1963–1972. [Google Scholar] [CrossRef]
- Hargrave, D.; Bartels, U.; Bouffet, E. Diffuse brainstem glioma in children: Critical review of clinical trials. Lancet Oncol. 2006, 7, 241–248. [Google Scholar] [CrossRef]
- Janssens, G.O.; Jansen, M.H.; Lauwers, S.J.; Nowak, P.J.; Oldenburger, F.R.; Bouffet, E.; Saran, F.; Kamphuis-van Ulzen, K.; van Lindert, E.J.; Schieving, J.H.; et al. Hypofractionation vs conventional radiation therapy for newly diagnosed diffuse intrinsic pontine glioma: A matched-cohort analysis. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 315–320. [Google Scholar] [CrossRef]
- Mueller, S.; Jain, P.; Liang, W.S.; Kilburn, L.; Kline, C.; Gupta, N.; Panditharatna, E.; Magge, S.N.; Zhang, B.; Zhu, Y.; et al. A pilot precision medicine trial for children with diffuse intrinsic pontine glioma-PNOC003: A report from the Pacific Pediatric Neuro-Oncology Consortium. Int. J. Cancer 2019, 145, 1889–1901. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; de Blank, P.M.; Kruchko, C.; Petersen, C.M.; Liao, P.; Finlay, J.L.; Stearns, D.S.; Wolff, J.E.; Wolinsky, Y.; Letterio, J.J.; et al. Alex’s Lemonade Stand Foundation Infant and Childhood Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007–2011. Neuro-Oncol. 2015, 16 (Suppl. 10), x1–x36. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Rouse, C.; Chen, Y.; Dowling, J.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro-Oncol. 2014, 16 (Suppl. 4), iv1-63. [Google Scholar] [CrossRef]
- Robinson, G.W.; Orr, B.A.; Wu, G.; Gururangan, S.; Lin, T.; Qaddoumi, I.; Packer, R.J.; Goldman, S.; Prados, M.D.; Desjardins, A.; et al. Vismodegib Exerts Targeted Efficacy Against Recurrent Sonic Hedgehog-Subgroup Medulloblastoma: Results from Phase II Pediatric Brain Tumor Consortium Studies PBTC-025B and PBTC-032. J. Clin. Oncol. 2015, 33, 2646–2654. [Google Scholar] [CrossRef]
- Broniscer, A.; Gajjar, A. Supratentorial high-grade astrocytoma and diffuse brainstem glioma: Two challenges for the pediatric oncologist. Oncologist 2004, 9, 197–206. [Google Scholar] [CrossRef]
- Amirian, E.S.; Armstrong, T.S.; Aldape, K.D.; Gilbert, M.R.; Scheurer, M.E. Predictors of survival among pediatric and adult ependymoma cases: A study using Surveillance, Epidemiology, and End Results data from 1973 to 2007. Neuroepidemiology 2012, 39, 116–124. [Google Scholar] [CrossRef]
- Pajtler, K.W.; Witt, H.; Sill, M.; Jones, D.T.; Hovestadt, V.; Kratochwil, F.; Wani, K.; Tatevossian, R.; Punchihewa, C.; Johann, P.; et al. Molecular Classification of Ependymal Tumors across All CNS Compartments, Histopathological Grades, and Age Groups. Cancer Cell 2015, 27, 728–743. [Google Scholar] [CrossRef]
- Messahel, B.; Ashley, S.; Saran, F.; Ellison, D.; Ironside, J.; Phipps, K.; Cox, T.; Chong, W.K.; Robinson, K.; Picton, S.; et al. Relapsed intracranial ependymoma in children in the UK: Patterns of relapse, survival and therapeutic outcome. Eur. J. Cancer 2009, 45, 1815–1823. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Gunther, J.R.; Mahajan, A.; Jo, E.; Paulino, A.C.; Adesina, A.M.; Jones, J.Y.; Ketonen, L.M.; Su, J.M.; Okcu, M.F.; et al. Progression-free survival of children with localized ependymoma treated with intensity-modulated radiation therapy or proton-beam radiation therapy. Cancer 2017, 123, 2570–2578. [Google Scholar] [CrossRef]
- MacDonald, S.M.; Laack, N.N.; Terezakis, S. Humbling Advances in Technology: Protons, Brainstem Necrosis, and the Self-Driving Car. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Pollack, I.F.; Jakacki, R.I.; Butterfield, L.H.; Okada, H. Ependymomas: Development of immunotherapeutic strategies. Expert Rev. Neurother. 2013, 13, 1089–1098. [Google Scholar] [CrossRef]
- Kandels, D.; Pietsch, T.; Bison, B.; Warmuth-Metz, M.; Thomale, U.W.; Kortmann, R.D.; Timmermann, B.; Hernáiz Driever, P.; Witt, O.; Schmidt, R.; et al. Loss of efficacy of subsequent nonsurgical therapy after primary treatment failure in pediatric low-grade glioma patients-Report from the German SIOP-LGG 2004 cohort. Int. J. Cancer 2020. [Google Scholar] [CrossRef]
- Bornhorst, M.; Frappaz, D.; Packer, R.J. Pilocytic astrocytomas. Handb. Clin. Neurol. 2016, 134, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Avery, R.A.; Hwang, E.I.; Jakacki, R.I.; Packer, R.J. Marked recovery of vision in children with optic pathway gliomas treated with bevacizumab. JAMA Ophthalmol. 2014, 132, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Qaddoumi, I.; Sultan, I.; Gajjar, A. Outcome and prognostic features in pediatric gliomas: A review of 6212 cases from the Surveillance, Epidemiology, and End Results database. Cancer 2009, 115, 5761–5770. [Google Scholar] [CrossRef] [PubMed]
- Paugh, B.S.; Qu, C.; Jones, C.; Liu, Z.; Adamowicz-Brice, M.; Zhang, J.; Bax, D.A.; Coyle, B.; Barrow, J.; Hargrave, D.; et al. Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J. Clin. Oncol. 2010, 28, 3061–3068. [Google Scholar] [CrossRef] [PubMed]
- Donson, A.M.; Addo-Yobo, S.O.; Handler, M.H.; Gore, L.; Foreman, N.K. MGMT promoter methylation correlates with survival benefit and sensitivity to temozolomide in pediatric glioblastoma. Pediatr. Blood Cancer 2007, 48, 403–407. [Google Scholar] [CrossRef]
- Buttarelli, F.R.; Massimino, M.; Antonelli, M.; Lauriola, L.; Nozza, P.; Donofrio, V.; Arcella, A.; Oliva, M.A.; Di Rocco, C.; Giangaspero, F. Evaluation status and prognostic significance of O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation in pediatric high grade gliomas. Child’s Nerv. Syst. 2010, 26, 1051–1056. [Google Scholar] [CrossRef]
- Srivastava, A.; Jain, A.; Jha, P.; Suri, V.; Sharma, M.C.; Mallick, S.; Puri, T.; Gupta, D.K.; Gupta, A.; Sarkar, C. MGMT gene promoter methylation in pediatric glioblastomas. Child’s Nerv. Syst. 2010, 26, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, C.K.; Park, S.H.; Wang, K.C.; Cho, B.K.; Kim, S.K. MGMT promoter gene methylation in pediatric glioblastoma: Analysis using MS-MLPA. Child’s Nerv. Syst. 2011, 27, 1877–1883. [Google Scholar] [CrossRef] [PubMed]
- DeNunzio, N.J.; Yock, T.I. Modern Radiotherapy for Pediatric Brain Tumors. Cancers 2020, 12, 1533. [Google Scholar] [CrossRef]
- Maule, M.; Scélo, G.; Pastore, G.; Brennan, P.; Hemminki, K.; Pukkala, E.; Weiderpass, E.; Olsen, J.H.; Tracey, E.; McBride, M.L.; et al. Risk of second malignant neoplasms after childhood central nervous system malignant tumours: An international study. Eur. J. Cancer 2008, 44, 830–839. [Google Scholar] [CrossRef]
- Cai, Y.; Cao, L.; Bao, X.; Xie, L. Second malignant neoplasms in childhood malignant brain tumour: A long-term population-based study. J. Paediatr. Child Health 2012, 48, 990–996. [Google Scholar] [CrossRef]
- Galloway, T.J.; Indelicato, D.J.; Amdur, R.J.; Swanson, E.L.; Smith, A.A.; Marcus, R.B., Jr. Second tumors in pediatric patients treated with radiotherapy to the central nervous system. Am. J. Clin. Oncol. 2012, 35, 279–283. [Google Scholar] [CrossRef]
- Jenkin, D.; Greenberg, M.; Hoffman, H.; Hendrick, B.; Humphreys, R.; Vatter, A. Brain tumors in children: Long-term survival after radiation treatment. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 445–451. [Google Scholar] [CrossRef]
- Galloway, T.J.; Indelicato, D.J.; Amdur, R.J.; Swanson, E.L.; Morris, C.G.; Marcus, R.B. Favorable outcomes of pediatric patients treated with radiotherapy to the central nervous system who develop radiation-induced meningiomas. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Broniscer, A.; Ke, W.; Fuller, C.E.; Wu, J.; Gajjar, A.; Kun, L.E. Second neoplasms in pediatric patients with primary central nervous system tumors: The St. Jude Children’s Research Hospital experience. Cancer 2004, 100, 2246–2252. [Google Scholar] [CrossRef] [PubMed]
- Malbari, F.; Lindsay, H. Genetics of Common Pediatric Brain Tumors. Pediatr. Neurol. 2020, 104, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, M.; Pędziwiatr, K.; Skowrońska-Gardas, A.; Perek-Polnik, M.; Perek, D.; Olasek, P. Second brain tumors following central nervous system radiotherapy in childhood. Br. J. Radiol. 2014, 87, 20140211. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Jin, M.C.; Koenig, J.; Gibbs, I.C.; Soltys, S.G.; Chang, S.D.; Li, G.; Hayden Gephart, M.; Hiniker, S.M.; Pollom, E.L. Stereotactic Radiosurgery for Pediatric and Adult Intracranial and Spinal Ependymomas. Stereotact. Funct. Neurosurg. 2019, 97, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chung, S.Y.; Han, J.W.; Kim, D.S.; Kim, J.; Moon, J.Y.; Yoon, H.I.; Suh, C.O. Treatment outcome of anaplastic ependymoma under the age of 3 treated by intensity-modulated radiotherapy. Radiat. Oncol. J. 2020, 38, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.; Lim, P.S.; Bojaxhiu, B.; Teske, C.; Baust, K.; Zepter, S.; Kliebsch, U.; Timmermann, B.; Calaminus, G.; Weber, D.C. Clinical outcomes and quality of life in children and adolescents with primary brain tumors treated with pencil beam scanning proton therapy. Pediatr. Blood Cancer 2020, e28465. [Google Scholar] [CrossRef]
- Wang, S.S.; Bandopadhayay, P.; Jenkins, M.R. Towards Immunotherapy for Pediatric Brain Tumors. Trends Immunol. 2019, 40, 748–761. [Google Scholar] [CrossRef]
- Dasgupta, R.; Rodeberg, D. Non-rhabdomyosarcoma. Semin. Pediatr. Surg. 2016, 25, 284–289. [Google Scholar] [CrossRef]
- Li, F.P.; Fraumeni, J.F., Jr. Rhabdomyosarcoma in children: Epidemiologic study and identification of a familial cancer syndrome. J. Natl. Cancer Inst. 1969, 43, 1365–1373. [Google Scholar] [PubMed]
- Rubino, C.; Shamsaldin, A.; Lê, M.G.; Labbé, M.; Guinebretière, J.M.; Chavaudra, J.; de Vathaire, F. Radiation dose and risk of soft tissue and bone sarcoma after breast cancer treatment. Breast Cancer Res. Treat. 2005, 89, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Penel, N.; Grosjean, J.; Robin, Y.M.; Vanseymortier, L.; Clisant, S.; Adenis, A. Frequency of certain established risk factors in soft tissue sarcomas in adults: A prospective descriptive study of 658 cases. Sarcoma 2008, 2008, 459386. [Google Scholar] [CrossRef] [PubMed]
- Lagrange, J.L.; Ramaioli, A.; Chateau, M.C.; Marchal, C.; Resbeut, M.; Richaud, P.; Lagarde, P.; Rambert, P.; Tortechaux, J.; Seng, S.H.; et al. Sarcoma after radiation therapy: Retrospective multiinstitutional study of 80 histologically confirmed cases. Radiation Therapist and Pathologist Groups of the Fédération Nationale des Centres de Lutte Contre le Cancer. Radiology 2000, 216, 197–205. [Google Scholar] [CrossRef]
- Brady, M.S.; Gaynor, J.J.; Brennan, M.F. Radiation-associated sarcoma of bone and soft tissue. Arch. Surg. 1992, 127, 1379–1385. [Google Scholar] [CrossRef]
- Bjerkehagen, B.; Smeland, S.; Walberg, L.; Skjeldal, S.; Hall, K.S.; Nesland, J.M.; Småstuen, M.C.; Fosså, S.D.; Saeter, G. Radiation-induced sarcoma: 25-year experience from the Norwegian Radium Hospital. Acta Oncol. 2008, 47, 1475–1482. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Makena, M.R.; Yavvari, S.; Kaur, M.; Pham, T.; Urias, E.; Panapitiya, N.; Al-Rahawan, M.M. Sarcoma as Second Cancer in a Childhood Cancer Survivor: Case Report, Large Population Analysis and Literature Review. Medicina 2020, 56, 224. [Google Scholar] [CrossRef]
- Lupo, P.J.; Danysh, H.E.; Plon, S.E.; Curtin, K.; Malkin, D.; Hettmer, S.; Hawkins, D.S.; Skapek, S.X.; Spector, L.G.; Papworth, K.; et al. Family history of cancer and childhood rhabdomyosarcoma: A report from the Children’s Oncology Group and the Utah Population Database. Cancer Med. 2015, 4, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.L.; Kadan-Lottick, N.S.; Whitton, J.; Mertens, A.C.; Yasui, Y.; Liu, Y.; Meadows, A.T.; Robison, L.L.; Strong, L.C. Increased risk of cancer among siblings of long-term childhood cancer survivors: A report from the childhood cancer survivor study. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1922–1927. [Google Scholar] [CrossRef][Green Version]
- Doros, L.; Yang, J.; Dehner, L.; Rossi, C.T.; Skiver, K.; Jarzembowski, J.A.; Messinger, Y.; Schultz, K.A.; Williams, G.; André, N.; et al. DICER1 mutations in embryonal rhabdomyosarcomas from children with and without familial PPB-tumor predisposition syndrome. Pediatr. Blood Cancer 2012, 59, 558–560. [Google Scholar] [CrossRef] [PubMed]
- DeBaun, M.R.; Tucker, M.A. Risk of cancer during the first four years of life in children from The Beckwith-Wiedemann Syndrome Registry. J. Pediatr. 1998, 132, 398–400. [Google Scholar] [CrossRef]
- Shern, J.F.; Chen, L.; Chmielecki, J.; Wei, J.S.; Patidar, R.; Rosenberg, M.; Ambrogio, L.; Auclair, D.; Wang, J.; Song, Y.K.; et al. Comprehensive genomic analysis of rhabdomyosarcoma reveals a landscape of alterations affecting a common genetic axis in fusion-positive and fusion-negative tumors. Cancer Discov. 2014, 4, 216–231. [Google Scholar] [CrossRef]
- Linardic, C.M. PAX3-FOXO1 fusion gene in rhabdomyosarcoma. Cancer Lett. 2008, 270, 10–18. [Google Scholar] [CrossRef]
- Wolden, S.L.; Anderson, J.R.; Crist, W.M.; Breneman, J.C.; Wharam, M.D., Jr.; Wiener, E.S.; Qualman, S.J.; Donaldson, S.S. Indications for radiotherapy and chemotherapy after complete resection in rhabdomyosarcoma: A report from the Intergroup Rhabdomyosarcoma Studies I to III. J. Clin. Oncol. 1999, 17, 3468–3475. [Google Scholar] [CrossRef]
- Miwa, S.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Igarashi, K.; Tsuchiya, H. Recent Advances and Challenges in the Treatment of Rhabdomyosarcoma. Cancers 2020, 12, 1758. [Google Scholar] [CrossRef]
- Arndt, C.A.; Stoner, J.A.; Hawkins, D.S.; Rodeberg, D.A.; Hayes-Jordan, A.A.; Paidas, C.N.; Parham, D.M.; Teot, L.A.; Wharam, M.D.; Breneman, J.C.; et al. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: Children’s oncology group study D9803. J. Clin. Oncol. 2009, 27, 5182–5188. [Google Scholar] [CrossRef] [PubMed]
- Koscielniak, E.; Harms, D.; Henze, G.; Jürgens, H.; Gadner, H.; Herbst, M.; Klingebiel, T.; Schmidt, B.F.; Morgan, M.; Knietig, R.; et al. Results of treatment for soft tissue sarcoma in childhood and adolescence: A final report of the German Cooperative Soft Tissue Sarcoma Study CWS-86. J. Clin. Oncol. 1999, 17, 3706–3719. [Google Scholar] [CrossRef]
- Paulino, A.C.; Simon, J.H.; Zhen, W.; Wen, B.C. Long-term effects in children treated with radiotherapy for head and neck rhabdomyosarcoma. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 1489–1495. [Google Scholar] [CrossRef]
- Punyko, J.A.; Mertens, A.C.; Gurney, J.G.; Yasui, Y.; Donaldson, S.S.; Rodeberg, D.A.; Raney, R.B.; Stovall, M.; Sklar, C.A.; Robison, L.L.; et al. Long-term medical effects of childhood and adolescent rhabdomyosarcoma: A report from the childhood cancer survivor study. Pediatr. Blood Cancer 2005, 44, 643–653. [Google Scholar] [CrossRef]
- Archer, N.M.; Amorim, R.P.; Naves, R.; Hettmer, S.; Diller, L.R.; Ribeiro, K.B.; Rodriguez-Galindo, C. An Increased Risk of Second Malignant Neoplasms After Rhabdomyosarcoma: Population-Based Evidence for a Cancer Predisposition Syndrome? Pediatr. Blood Cancer 2016, 63, 196–201. [Google Scholar] [CrossRef]
- Kenney, L.B.; Laufer, M.R.; Grant, F.D.; Grier, H.; Diller, L. High risk of infertility and long term gonadal damage in males treated with high dose cyclophosphamide for sarcoma during childhood. Cancer 2001, 91, 613–621. [Google Scholar] [CrossRef]
- Walterhouse, D.O.; Pappo, A.S.; Meza, J.L.; Breneman, J.C.; Hayes-Jordan, A.A.; Parham, D.M.; Cripe, T.P.; Anderson, J.R.; Meyer, W.H.; Hawkins, D.S. Shorter-duration therapy using vincristine, dactinomycin, and lower-dose cyclophosphamide with or without radiotherapy for patients with newly diagnosed low-risk rhabdomyosarcoma: A report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. J. Clin. Oncol. 2014, 32, 3547–3552. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.A. Molecular biology and cytogenetics of soft tissue sarcomas: Relevance for targeted therapies. Cancer Treat. Res. 2004, 120, 99–116. [Google Scholar] [CrossRef]
- Pratt, C.B.; Pappo, A.S.; Gieser, P.; Jenkins, J.J.; Salzbergdagger, A.; Neff, J.; Rao, B.; Green, D.; Thomas, P.; Marcus, R.; et al. Role of adjuvant chemotherapy in the treatment of surgically resected pediatric nonrhabdomyosarcomatous soft tissue sarcomas: A Pediatric Oncology Group Study. J. Clin. Oncol. 1999, 17, 1219. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.M.; O’Sullivan, B.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Hammond, A.; Benk, V.; Kandel, R.; et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2005, 75, 48–53. [Google Scholar] [CrossRef]
- Chen, C.; Dorado Garcia, H.; Scheer, M.; Henssen, A.G. Current and Future Treatment Strategies for Rhabdomyosarcoma. Front. Oncol. 2019, 9, 1458. [Google Scholar] [CrossRef]
- Kim, C.; Kim, E.K.; Jung, H.; Chon, H.J.; Han, J.W.; Shin, K.H.; Hu, H.; Kim, K.S.; Choi, Y.D.; Kim, S.; et al. Prognostic implications of PD-L1 expression in patients with soft tissue sarcoma. BMC Cancer 2016, 16, 434. [Google Scholar] [CrossRef]
- MarjaŃska, A.; Drogosiewicz, M.; Dembowska-BagiŃska, B.; PawiŃska-WĄsikowska, K.; Balwierz, W.; Bobeff, K.; MŁynarski, W.; Mizia-Malarz, A.; Raciborska, A.; Wysocki, M.; et al. Nivolumab for the Treatment of Advanced Pediatric Malignancies. Anticancer Res. 2020, 40, 7095–7100. [Google Scholar] [CrossRef] [PubMed]
- Gorlick, R. Current concepts on the molecular biology of osteosarcoma. Cancer Treat. Res. 2009, 152, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.J.; Khanna, C. Osteosarcoma Genetics and Epigenetics: Emerging Biology and Candidate Therapies. Crit. Rev. Oncog. 2015, 20, 173–197. [Google Scholar] [CrossRef] [PubMed]
- Seidensaal, K.; Mattke, M.; Haufe, S.; Rathke, H.; Haberkorn, U.; Bougatf, N.; Kudak, A.; Blattmann, C.; Oertel, S.; Kirchner, M.; et al. The role of combined ion-beam radiotherapy (CIBRT) with protons and carbon ions in a multimodal treatment strategy of inoperable osteosarcoma. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2021. [Google Scholar] [CrossRef]
- Janeway, K.A.; Grier, H.E. Sequelae of osteosarcoma medical therapy: A review of rare acute toxicities and late effects. Lancet Oncol. 2010, 11, 670–678. [Google Scholar] [CrossRef]
- Lee, J.S.; DuBois, S.G.; Boscardin, W.J.; Wustrack, R.L.; Goldsby, R.E. Secondary malignant neoplasms among children, adolescents, and young adults with osteosarcoma. Cancer 2014, 120, 3987–3993. [Google Scholar] [CrossRef]
- Riggi, N.; Stamenkovic, I. The Biology of Ewing sarcoma. Cancer Lett. 2007, 254, 1–10. [Google Scholar] [CrossRef]
- Karimi, A.M.; Campbell, S.R.; Parsai, S.; Angelov, L.; Scott, J.; Qi, P.; Anderson, P.; Chao, S.T.; Murphy, E.S. Aggressive Local Control With Multisite Stereotactic Body Radiation in Metastatic Ewing Sarcoma: A Literature Review and Case Report. Anticancer Res. 2020, 40, 951–955. [Google Scholar] [CrossRef]
- Grünewald, T.G.P.; Cidre-Aranaz, F.; Surdez, D.; Tomazou, E.M.; de Álava, E.; Kovar, H.; Sorensen, P.H.; Delattre, O.; Dirksen, U. Ewing sarcoma. Nat. Rev. Dis. Primers 2018, 4, 5. [Google Scholar] [CrossRef]
- Gargallo, P.; Juan, A.; Yáñez, Y.; Dolz, S.; Segura, V.; Castel, V.; Cañete, A. Precision medicine in Ewing sarcoma: A translational point of view. Clin. Transl. Oncol. 2020, 22, 1440–1454. [Google Scholar] [CrossRef]
- Chang, T.C.; Carter, R.A.; Li, Y.; Li, Y.; Wang, H.; Edmonson, M.N.; Chen, X.; Arnold, P.; Geiger, T.L.; Wu, G.; et al. The neoepitope landscape in pediatric cancers. Genome Med. 2017, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J.; et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017, 9, 34. [Google Scholar] [CrossRef]
- Spurny, C.; Kailayangiri, S.; Jamitzky, S.; Altvater, B.; Wardelmann, E.; Dirksen, U.; Hardes, J.; Hartmann, W.; Rossig, C. Programmed cell death ligand 1 (PD-L1) expression is not a predominant feature in Ewing sarcomas. Pediatr. Blood Cancer 2018, 65. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
- Thanindratarn, P.; Dean, D.C.; Nelson, S.D.; Hornicek, F.J.; Duan, Z. Advances in immune checkpoint inhibitors for bone sarcoma therapy. J. Bone Oncol. 2019, 15, 100221. [Google Scholar] [CrossRef]
- Zöllner, S.K.; Amatruda, J.F.; Bauer, S.; Collaud, S.; de Álava, E.; DuBois, S.G.; Hardes, J.; Hartmann, W.; Kovar, H.; Metzler, M.; et al. Ewing Sarcoma-Diagnosis, Treatment, Clinical Challenges and Future Perspectives. J. Clin. Med. 2021, 10, 1685. [Google Scholar] [CrossRef]
- Marina, N.M.; Pappo, A.S.; Parham, D.M.; Cain, A.M.; Rao, B.N.; Poquette, C.A.; Pratt, C.B.; Greenwald, C.; Meyer, W.H. Chemotherapy dose-intensification for pediatric patients with Ewing’s family of tumors and desmoplastic small round-cell tumors: A feasibility study at St. Jude Children’s Research Hospital. J. Clin. Oncol. 1999, 17, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Meyer, W.H.; Kun, L.; Marina, N.; Roberson, P.; Parham, D.; Rao, B.; Fletcher, B.; Pratt, C.B. Ifosfamide plus etoposide in newly diagnosed Ewing’s sarcoma of bone. J. Clin. Oncol. 1992, 10, 1737–1742. [Google Scholar] [CrossRef]
- Sultan, I.; Rihani, R.; Hazin, R.; Rodriguez-Galindo, C. Second malignancies in patients with Ewing Sarcoma Family of Tumors: A population-based study. Acta Oncol. 2010, 49, 237–244. [Google Scholar] [CrossRef]
- Lagutina, I.; Conway, S.J.; Sublett, J.; Grosveld, G.C. Pax3-FKHR knock-in mice show developmental aberrations but do not develop tumors. Mol. Cell. Biol. 2002, 22, 7204–7216. [Google Scholar] [CrossRef]
- Crist, W.M.; Anderson, J.R.; Meza, J.L.; Fryer, C.; Raney, R.B.; Ruymann, F.B.; Breneman, J.; Qualman, S.J.; Wiener, E.; Wharam, M.; et al. Intergroup rhabdomyosarcoma study-IV: Results for patients with nonmetastatic disease. J. Clin. Oncol. 2001, 19, 3091–3102. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.C.; Rey, A.; Bouvet, N.; Ellershaw, C.; Flamant, F.; Habrand, J.L.; Marsden, H.B.; Martelli, H.; Sanchez de Toledo, J.; Spicer, R.D.; et al. Treatment of nonmetastatic rhabdomyosarcoma in childhood and adolescence: Third study of the International Society of Paediatric Oncology—SIOP Malignant Mesenchymal Tumor 89. J. Clin. Oncol. 2005, 23, 2618–2628. [Google Scholar] [CrossRef]
- Dantonello, T.M.; Int-Veen, C.; Schuck, A.; Seitz, G.; Leuschner, I.; Nathrath, M.; Schlegel, P.G.; Kontny, U.; Behnisch, W.; Veit-Friedrich, I.; et al. Survival following disease recurrence of primary localized alveolar rhabdomyosarcoma. Pediatr. Blood Cancer 2013, 60, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Malempati, S.; Hawkins, D.S. Rhabdomyosarcoma: Review of the Children’s Oncology Group (COG) Soft-Tissue Sarcoma Committee experience and rationale for current COG studies. Pediatr. Blood Cancer 2012, 59, 5–10. [Google Scholar] [CrossRef]
- Mansky, P.; Arai, A.; Stratton, P.; Bernstein, D.; Long, L.; Reynolds, J.; Chen, D.; Steinberg, S.M.; Lavende, N.; Hoffman, K.; et al. Treatment late effects in long-term survivors of pediatric sarcoma. Pediatr. Blood Cancer 2007, 48, 192–199. [Google Scholar] [CrossRef]
- Yoon, S.S.; Chen, Y.L.; Kirsch, D.G.; Maduekwe, U.N.; Rosenberg, A.E.; Nielsen, G.P.; Sahani, D.V.; Choy, E.; Harmon, D.C.; DeLaney, T.F. Proton-beam, intensity-modulated, and/or intraoperative electron radiation therapy combined with aggressive anterior surgical resection for retroperitoneal sarcomas. Ann. Surg. Oncol. 2010, 17, 1515–1529. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.L.; Fox, E.; Merchant, M.S.; Reid, J.M.; Kudgus, R.A.; Liu, X.; Minard, C.G.; Voss, S.; Berg, S.L.; Weigel, B.J.; et al. Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): A multicentre, open-label, single-arm, phase 1-2 trial. Lancet Oncol. 2020, 21, 541–550. [Google Scholar] [CrossRef]
- Merchant, M.S.; Wright, M.; Baird, K.; Wexler, L.H.; Rodriguez-Galindo, C.; Bernstein, D.; Delbrook, C.; Lodish, M.; Bishop, R.; Wolchok, J.D.; et al. Phase I Clinical Trial of Ipilimumab in Pediatric Patients with Advanced Solid Tumors. Clin. Cancer Res. 2016, 22, 1364–1370. [Google Scholar] [CrossRef]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef]
- Ozaki, T.; Flege, S.; Kevric, M.; Lindner, N.; Maas, R.; Delling, G.; Schwarz, R.; von Hochstetter, A.R.; Salzer-Kuntschik, M.; Berdel, W.E.; et al. Osteosarcoma of the pelvis: Experience of the Cooperative Osteosarcoma Study Group. J. Clin. Oncol. 2003, 21, 334–341. [Google Scholar] [CrossRef]
- Olsen, J.H.; Garwicz, S.; Hertz, H.; Jonmundsson, G.; Langmark, F.; Lanning, M.; Lie, S.O.; Moe, P.J.; Møller, T.; Sankila, R.; et al. Second malignant neoplasms after cancer in childhood or adolescence. Nordic Society of Paediatric Haematology and Oncology Association of the Nordic Cancer Registries. BMJ 1993, 307, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.L.; Boice, J.D., Jr.; Abramson, D.H.; Tarone, R.E.; Kleinerman, R.A.; Stovall, M.; Goldman, M.B.; Seddon, J.M.; Tarbell, N.; Fraumeni, J.F., Jr.; et al. Cancer incidence after retinoblastoma. Radiation dose and sarcoma risk. JAMA 1997, 278, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Newton, W.A., Jr.; Meadows, A.T.; Shimada, H.; Bunin, G.R.; Vawter, G.F. Bone sarcomas as second malignant neoplasms following childhood cancer. Cancer 1991, 67, 193–201. [Google Scholar] [CrossRef]
- Ozonoff, M.B. Paediatric Orthopaedic Radiology; WB Saunders: London, UK, 1992; pp. 604–689. [Google Scholar]
- Bechler, J.R.; Robertson, W.W., Jr.; Meadows, A.T.; Womer, R.B. Osteosarcoma as a second malignant neoplasm in children. J. Bone Jt. Surg. Am. Vol. 1992, 74, 1079–1083. [Google Scholar] [CrossRef]
- Ludwig, J.A. Ewing sarcoma: Historical perspectives, current state-of-the-art, and opportunities for targeted therapy in the future. Curr. Opin. Oncol. 2008, 20, 412–418. [Google Scholar] [CrossRef]
- Marina, N.M.; Liu, Q.; Donaldson, S.S.; Sklar, C.A.; Armstrong, G.T.; Oeffinger, K.C.; Leisenring, W.M.; Ginsberg, J.P.; Henderson, T.O.; Neglia, J.P.; et al. Longitudinal follow-up of adult survivors of Ewing sarcoma: A report from the Childhood Cancer Survivor Study. Cancer 2017, 123, 2551–2560. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.Y.; Hall, E.T. Second malignancies in Ewing sarcoma survivors. Cancer 2017, 123, 4075. [Google Scholar] [CrossRef]
- Ginsberg, J.P.; Goodman, P.; Leisenring, W.; Ness, K.K.; Meyers, P.A.; Wolden, S.L.; Smith, S.M.; Stovall, M.; Hammond, S.; Robison, L.L.; et al. Long-term survivors of childhood Ewing sarcoma: Report from the childhood cancer survivor study. J. Natl. Cancer Inst. 2010, 102, 1272–1283. [Google Scholar] [CrossRef]
- Schuck, A.; Ahrens, S.; Paulussen, M.; Kuhlen, M.; Könemann, S.; Rübe, C.; Winkelmann, W.; Kotz, R.; Dunst, J.; Willich, N.; et al. Local therapy in localized Ewing tumors: Results of 1058 patients treated in the CESS 81, CESS 86, and EICESS 92 trials. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 168–177. [Google Scholar] [CrossRef]
- Paulussen, M.; Ahrens, S.; Lehnert, M.; Taeger, D.; Hense, H.W.; Wagner, A.; Dunst, J.; Harms, D.; Reiter, A.; Henze, G.; et al. Second malignancies after ewing tumor treatment in 690 patients from a cooperative German/Austrian/Dutch study. Ann. Oncol. 2001, 12, 1619–1630. [Google Scholar] [CrossRef]
- Casey, D.L.; Meyers, P.A.; Alektiar, K.M.; Magnan, H.; Healey, J.H.; Boland, P.J.; Wolden, S.L. Ewing sarcoma in adults treated with modern radiotherapy techniques. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2014, 113, 248–253. [Google Scholar] [CrossRef]
- La, T.H.; Meyers, P.A.; Wexler, L.H.; Alektiar, K.M.; Healey, J.H.; Laquaglia, M.P.; Boland, P.J.; Wolden, S.L. Radiation therapy for Ewing’s sarcoma: Results from Memorial Sloan-Kettering in the modern era. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 544–550. [Google Scholar] [CrossRef]
- Rombi, B.; DeLaney, T.F.; MacDonald, S.M.; Huang, M.S.; Ebb, D.H.; Liebsch, N.J.; Raskin, K.A.; Yeap, B.Y.; Marcus, K.J.; Tarbell, N.J.; et al. Proton radiotherapy for pediatric Ewing’s sarcoma: Initial clinical outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Krailo, M.D.; Chen, Z.; Burden, L.; Askin, F.B.; Dickman, P.S.; Grier, H.E.; Link, M.P.; Meyers, P.A.; Perlman, E.J.; et al. Therapy-related myelodysplasia and acute myeloid leukemia after Ewing sarcoma and primitive neuroectodermal tumor of bone: A report from the Children’s Oncology Group. Blood 2007, 109, 46–51. [Google Scholar] [CrossRef]
- Morales, E.; Olson, M.; Iglesias, F.; Dahiya, S.; Luetkens, T.; Atanackovic, D. Role of immunotherapy in Ewing sarcoma. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef]
- Applebaum, M.A.; Goldsby, R.; Neuhaus, J.; DuBois, S.G. Clinical features and outcomes in patients with secondary Ewing sarcoma. Pediatr. Blood Cancer 2013, 60, 611–615. [Google Scholar] [CrossRef]
- Ishida, Y.; Maeda, M.; Adachi, S.; Rikiishi, T.; Sato, M.; Kawaguchi, H.; Manabe, A.; Tokuyama, M.; Hori, H.; Okamura, J.; et al. Secondary bone/soft tissue sarcoma in childhood cancer survivors: A nationwide hospital-based case-series study in Japan. Jpn. J. Clin. Oncol. 2018, 48, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Spunt, S.L.; Rodriguez-Galindo, C.; Fuller, C.E.; Harper, J.; Krasin, M.J.; Billups, C.A.; Khoury, J.D. Ewing sarcoma-family tumors that arise after treatment of primary childhood cancer. Cancer 2006, 107, 201–206. [Google Scholar] [CrossRef]
- Kim, G.E.; Beach, B.; Gastier-Foster, J.M.; Murata-Collins, J.L.; Rowland, J.M.; O’Donnell, R.J.; Goldsby, R.E. Ewing sarcoma as a second malignant neoplasm after acute lymphoblastic leukemia. Pediatr. Blood Cancer 2005, 45, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, F.; Grotzer, M.A.; Niggli, F.; Zimmermann, D.; Rushing, E.; Bode-Lesniewska, B. Ewing’s Sarcoma as a Second Malignancy in Long-Term Survivors of Childhood Hematologic Malignancies. Sarcoma 2016, 2016, 5043640. [Google Scholar] [CrossRef] [PubMed]
- Matthay, K.K.; Maris, J.M.; Schleiermacher, G.; Nakagawara, A.; Mackall, C.L.; Diller, L.; Weiss, W.A. Neuroblastoma. Nat. Rev. Dis. Primers 2016, 2, 16078. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, G.M.; Bagatell, R. Mechanisms of neuroblastoma regression. Nat. Rev. Clin. Oncol. 2014, 11, 704–713. [Google Scholar] [CrossRef]
- Friedman, D.N.; Henderson, T.O. Late Effects and Survivorship Issues in Patients with Neuroblastoma. Children 2018, 5, 107. [Google Scholar] [CrossRef] [PubMed]
- Applebaum, M.A.; Vaksman, Z.; Lee, S.M.; Hungate, E.A.; Henderson, T.O.; London, W.B.; Pinto, N.; Volchenboum, S.L.; Park, J.R.; Naranjo, A.; et al. Neuroblastoma survivors are at increased risk for second malignancies: A report from the International Neuroblastoma Risk Group Project. Eur. J. Cancer 2017, 72, 177–185. [Google Scholar] [CrossRef]
- Leslie, S.W.; Sajjad, H.; Murphy, P.B. Wilms Tumor. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Ko, E.Y.; Ritchey, M.L. Current management of Wilms’ tumor in children. J. Pediatr. Urol. 2009, 5, 56–65. [Google Scholar] [CrossRef]
- Davidoff, A.M. Wilms tumor. Adv. Pediatr. 2012, 59, 247–267. [Google Scholar] [CrossRef]
- McDermott, D.F.; Drake, C.G.; Sznol, M.; Choueiri, T.K.; Powderly, J.D.; Smith, D.C.; Brahmer, J.R.; Carvajal, R.D.; Hammers, H.J.; Puzanov, I.; et al. Survival, Durable Response, and Long-Term Safety in Patients with Previously Treated Advanced Renal Cell Carcinoma Receiving Nivolumab. J. Clin. Oncol. 2015, 33, 2013–2020. [Google Scholar] [CrossRef]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Breslow, N.E.; Lange, J.M.; Friedman, D.L.; Green, D.M.; Hawkins, M.M.; Murphy, M.F.; Neglia, J.P.; Olsen, J.H.; Peterson, S.M.; Stiller, C.A.; et al. Secondary malignant neoplasms after Wilms tumor: An international collaborative study. Int. J. Cancer 2010, 127, 657–666. [Google Scholar] [CrossRef]
- Knudson, A.G., Jr. Mutation and cancer: Statistical study of retinoblastoma. Proc. Natl. Acad. Sci. USA 1971, 68, 820–823. [Google Scholar] [CrossRef]
- Kaewkhaw, R.; Rojanaporn, D. Retinoblastoma: Etiology, Modeling, and Treatment. Cancers 2020, 12, 2304. [Google Scholar] [CrossRef]
- Fletcher, O.; Easton, D.; Anderson, K.; Gilham, C.; Jay, M.; Peto, J. Lifetime risks of common cancers among retinoblastoma survivors. J. Natl. Cancer Inst. 2004, 96, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Kleinerman, R.A.; Tucker, M.A.; Tarone, R.E.; Abramson, D.H.; Seddon, J.M.; Stovall, M.; Li, F.P.; Fraumeni, J.F., Jr. Risk of new cancers after radiotherapy in long-term survivors of retinoblastoma: An extended follow-up. J. Clin. Oncol. 2005, 23, 2272–2279. [Google Scholar] [CrossRef]
- MacCarthy, A.; Bayne, A.M.; Brownbill, P.A.; Bunch, K.J.; Diggens, N.L.; Draper, G.J.; Hawkins, M.M.; Jenkinson, H.C.; Kingston, J.E.; Stiller, C.A.; et al. Second and subsequent tumours among 1927 retinoblastoma patients diagnosed in Britain 1951-2004. Br. J. Cancer 2013, 108, 2455–2463. [Google Scholar] [CrossRef]
- Maris, J.M.; Hogarty, M.D.; Bagatell, R.; Cohn, S.L. Neuroblastoma. Lancet 2007, 369, 2106–2120. [Google Scholar] [CrossRef]
- Tas, M.L.; Nagtegaal, M.; Kraal, K.; Tytgat, G.A.M.; Abeling, N.; Koster, J.; Pluijm, S.M.F.; Zwaan, C.M.; de Keizer, B.; Molenaar, J.J.; et al. Neuroblastoma stage 4S: Tumor regression rate and risk factors of progressive disease. Pediatr. Blood Cancer 2020, 67, e28061. [Google Scholar] [CrossRef]
- Szychot, E.; Apps, J.; Pritchard-Jones, K. Wilms’ tumor: Biology, diagnosis and treatment. Transl. Pediatr. 2014, 3, 12–24. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, I.W.; Oldenburger, F.; Cardous-Ubbink, M.C.; Geenen, M.M.; Heinen, R.C.; de Kraker, J.; van Leeuwen, F.E.; van der Pal, H.J.; Caron, H.N.; Koning, C.C.; et al. Evaluation of late adverse events in long-term wilms’ tumor survivors. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 370–378. [Google Scholar] [CrossRef]
- Murphy, A.J.; Chen, X.; Pinto, E.M.; Williams, J.S.; Clay, M.R.; Pounds, S.B.; Cao, X.; Shi, L.; Lin, T.; Neale, G.; et al. Forty-five patient-derived xenografts capture the clinical and biological heterogeneity of Wilms tumor. Nat. Commun. 2019, 10, 5806. [Google Scholar] [CrossRef]
- Jain, J.; Sutton, K.S.; Hong, A.L. Progress Update in Pediatric Renal Tumors. Curr. Oncol. Rep. 2021, 23, 33. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.L.; Ness, K.K.; Neglia, J.P.; Hammond, S.; Shnorhavorian, M.; Leisenring, W.L.; Stovall, M.; Robison, L.L.; Armstrong, G.T. Renal carcinoma after childhood cancer: A report from the childhood cancer survivor study. J. Natl. Cancer Inst. 2013, 105, 504–508. [Google Scholar] [CrossRef]
- Argani, P.; Laé, M.; Ballard, E.T.; Amin, M.; Manivel, C.; Hutchinson, B.; Reuter, V.E.; Ladanyi, M. Translocation carcinomas of the kidney after chemotherapy in childhood. J. Clin. Oncol. 2006, 24, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- Hedgepeth, R.C.; Zhou, M.; Ross, J. Rapid development of metastatic Xp11 translocation renal cell carcinoma in a girl treated for neuroblastoma. J. Pediatr. Hematol./Oncol. 2009, 31, 602–604. [Google Scholar] [CrossRef] [PubMed]
- Dimaras, H.; Corson, T.W.; Cobrinik, D.; White, A.; Zhao, J.; Munier, F.L.; Abramson, D.H.; Shields, C.L.; Chantada, G.L.; Njuguna, F.; et al. Retinoblastoma. Nat. Rev. Dis. Primers 2015, 1, 15021. [Google Scholar] [CrossRef]
- Haddy, N.; Tartier, L.; Koscielny, S.; Adjadj, E.; Rubino, C.; Brugières, L.; Pacquement, H.; Diallo, I.; de Vathaire, F.; Averbeck, D.; et al. Repair of ionizing radiation-induced DNA damage and risk of second cancer in childhood cancer survivors. Carcinogenesis 2014, 35, 1745–1749. [Google Scholar] [CrossRef] [PubMed]
- Zahnreich, S.; Poplawski, A.; Hartel, C.; Eckhard, L.S.; Galetzka, D.; Hankeln, T.; Löbrich, M.; Marron, M.; Mirsch, J.; Ritter, S.; et al. Spontaneous and Radiation-Induced Chromosome Aberrations in Primary Fibroblasts of Patients with Pediatric First and Second Neoplasms. Front. Oncol. 2020, 10, 1338. [Google Scholar] [CrossRef] [PubMed]
- Brackmann, L.K.; Poplawski, A.; Grandt, C.L.; Schwarz, H.; Hankeln, T.; Rapp, S.; Zahnreich, S.; Galetzka, D.; Schmitt, I.; Grad, C.; et al. Comparison of time and dose dependent gene expression and affected pathways in primary human fibroblasts after exposure to ionizing radiation. Mol. Med. 2020, 26, 85. [Google Scholar] [CrossRef] [PubMed]
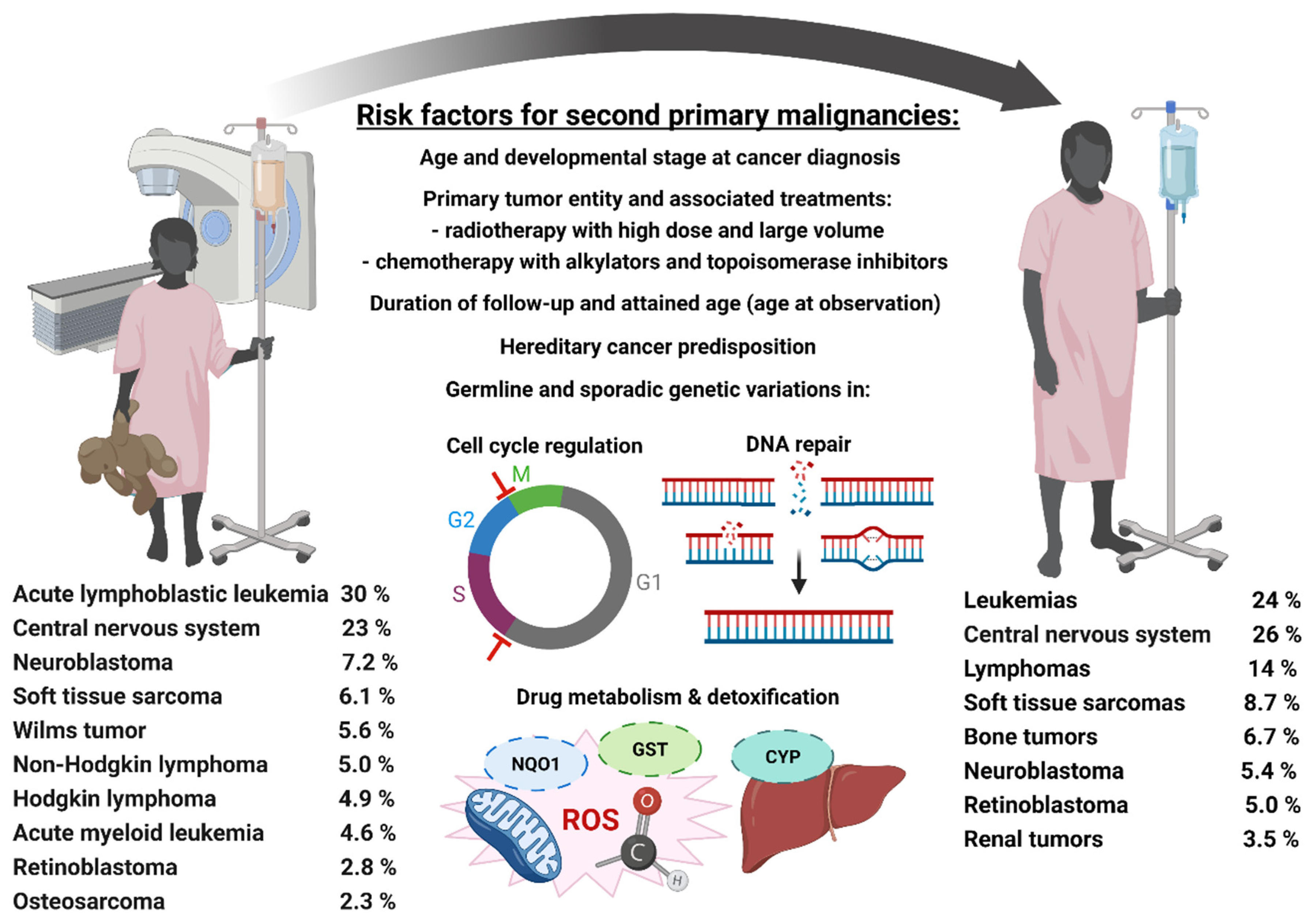
| Primary Hematological Tumor | Late Effects | ||||
|---|---|---|---|---|---|
| Entity | Predisposition and Risk Factors | Treatment | Non-Carcinogenic | Second Primary Malignancies | Risk Factors |
| Acute lymphoblastic leukemia | DS, FA, AT and Klinefelter syndrome [120,121] translocations causing TEL-AML1, MLL and MLLAF4 gene fusions [122,123] | CT (prednisone, cyclophosphamide, cytarabine, dexamethasone, etoposide, ifosfamide, methotrexate, doxorubicin, mercaptopurine, vincristine) intrathecal therapy [124,125,126] cranial or craniospinal EBRT [127] targeting CD19 and T-cell receptor-CD3 complex (blinatumomab), cytotoxic anti-CD22-calicheamicin conjugate (inotuzumab ozogamicin), anti-CD22 or -CD19 CAR-T cell-based therapies (e.g., with tisagenlecleucel), TRK inhibitors (dasatinib, ruxolitinib, crizotinib) [128,129,130] | growth, neurocognition [131,132,133,134,135] | meningioma, medulloblastoma, primary leukemia, lymphoma, thyroid, breast and bone cancer, soft tissue sarcoma, squamous cell carcinoma of the skin [12,133,136] | cranial EBRT [134] CT with alkylators (cyclophosphamide), topoisomerase II inhibitors (epipodophyllotoxins), high-dose therapy with methotrexate and mercaptopurine [135] |
| Acute myeloid leukemia | DS, NF, LFS, FA, and Klinefelter’s syndrome [137] viruses, IR, chemicals, or previous CT [137] loss or deletion of chromosome 5, 7, Y, and 9, t(8;21)(q22;q22), t(15;17)(q22;q11), or trisomy 8 and 21 [138] genetic alterations in TP53, RUNX1, IKZF1, and ETV6 [139] | CT (anthracyclines and cytarabine) [140,141] reduced EBRT [142] anti-CD33-calicheamicin conjugate (gemtuzumab ozogamicin), TRK inhibitors (sorafenib, midostaurin, gilteritinib) targeting FLT3-ITD TRK mutations [143,144,145,146] | endocrine abnormalities, cataracts, cardiac abnormalities, growth-hormone deficiency, hypothyroidism | Very rare due to death by any other cause mucoepidermoid carcinomas, supratentorial primitive neuroectodermal, ALL, NHL [147] | high dose CT cranial EBRT [147] |
| Non-Hodgkin lymphoma | infection and immune dysregulation [148] probably alterations in TNF and IL10, toll-like receptor or RAG1, LIG4, ERCC5, WRN, MGMT, and XRCC1 [148] | NHL-BFM and cranial EBRT if CNS involved plus CNS prophylaxis with intrathecal CT and methotextrat [149,150] rituximab targeting CD20 on B cells, antibody-drug conjugates (inotuzumab-ozogamicin, polatuzumab-vedotin, pinatuzumab-vedotin), CAR T-cell therapy (tisagenlecleucel), BTK inhibition (ibrutinib), T-cell-engaging antibody constructs (blinatumomab, mosunetuzumab) [151,152,153,154,155,156] | cardiac disease, pneumonia [157] | carcinomas, acute myeloid leukemia, lymphoid malignancies [157,158] | high doses of alkylators, topoisomerase II inhibitors, and anthracyclines [158] sex (female), lymphoblastic lymphoma, CNS involvement, AT, NBS, and constitutional mismatch repair deficiency [158] |
| Hodgkin lymphoma | Epstein-Barr-Virus, genetic factors, immune-related disorders, other infections, environmental exposures, familial predisposition [159,160] | involved-field/involved node EBRT CT (OP/EPA [vincristine, prednisone, procarbazine/etoposide, and doxorubicin] or OPPA/COPP [cyclophosphamide, vincristine, prednisone, and procarbazine]) [161] CD30-directed brentuximab vedotin, PD-1 (pembrolizumab, nivolumab) JAK2 inhibitors (itacitinib, ruxolitinib), CAR-T cells, histone deacetylases inhibitors(panobinostat), immunomodulatory drugs (lenalidomide), BTK inhibitors (ibrutinib), mTOR inhibitors (everolimus), CD25-directed antibody-drug conjugates (camidanlumab tesirine) [162,163,164] | pulmonary, dysfunction, endocrinopathies, thyroid dysfunction, infertility, neck muscle atrophy, persistent fatigue [165] | thyroid carcinoma, breast cancer, lung cancer, sarcoma, colorectal carcinoma, melanoma, cervix carcinoma [53,55,166,167,168,169] | EBRT, particularly chest exposures for breast cancer in females and lung cancer in males and alkylating drugs [55,167,170,171] |
| Primary Brain Tumor | Late Effects | ||||
|---|---|---|---|---|---|
| Entity | Predisposition and Risk Factors | Treatment | Non-Carcinogenic | SPM | Risk Factors |
| Medulloblastoma | Turcot syndrome, Gorlin syndrome, Rubinstein Taybi syndrome, LFS and FA alterations in WNT, SHH, MYC, PVT1, SMARCA4, OTX2, and abnormalities of chromosome 17 [248,249] | surgery and adjuvant craniospinal EBRT [250] CT with vincristine and cisplatin plus either lomustine or cyclophosphamide [251] SHH pathway-inhibitors (saridegib, erismodegib, or vismodegib) [249], targeting CDK4/6, c-Met, Wee1, PI3K/mTOR, EZH2, CHK1/2, or the BET bromodomain pathways [248], PD-1 inhibitors (pembrolizumab, nivolumab), monoclonal antibodies against CD40 (APX005M), PEP-CMV (cytomegalovirus) based vaccine trials for oncolytic viral therapy [248] | hypothyroidism, adrenocorticotropic, hormone deficiency, altered metabolism [252,253] | glioma, meningioma, thyroid carcinoma [254,255,256] | CT and craniospinal EBRT |
| Ependymoma | infection with SV40 virus, NF2, Turcot syndrome B, LFS [257,258,259] | maximal surgical resection and adjuvant EBRT [260,261,262] proton beam EBRT [260,263] CT with platinum derivatives, etoposide, cyclophosphamide, vincristine, and methotrexate, (so far not superior to adjuvant EBRT) [264], inhibition of ERBB1 and ERBB2 (lapatinib), interference with the NFκB pathway, inhibition of YAP1 [265] | neurocognitive impairment, neurologic deficits, neuroendocrine deficiency [266] | known for adults: pancreatic cancer, prostate cancer, Hodgkin lymphoma, intracranial meningioma, pulmonary adenocarcinoma, gastric cancer, astrocytoma [267] | intrinsic risk factors [267] hypermethylated phenotype causing silencing of CDKN2A, CDKN2B, HIC1, RASSF1A, CASP8, MGM, and TP73 [268,269] |
| Low-grade gliomas | NF1, tuberous sclerosis, complex germline mutations [270] | surgery with no adjuvant therapy after complete tumor resection [271] CT with carboplatin and vincristine, TPCV (thioguanine, procarbazine, lomustine, and vincristine), weekly vinblastine monotherapy, BRAF-inhibitors (vemurafenib, dabrafenib, trametinib), MEK-inhibitors (selumetinib), mTOR inhibitors (everolimus), VEGF-inhibitors (bevacizumab) [272,273,274,275] | neurocognitive function, neuroendocrine deficiency, vasculopathy | EBRT [276] | |
| High-grade gliomas | compromised TP53, CDKN2A, PI3K/TRK, and RB pathway [277,278,279,280,281] | radical surgery followed by focal EBRT with temozolomide [282] BRAF and MEK inhibitors, pan-TRK inhibitors, monoclonal anti-PD-1 antibody (nivolumab) in MMR deficient patients [283,284,285,286,287,288] | neurocognitive function, neuroendocrine function, vascular changes leading to increased stroke risk [289] | hematologic, meningioma, gliomas | EBRT [289,290,291] temozolomide and other alkylating drugs [292,293,294,295,296] |
| Diffuse intrinsic pontine gliomas | mutations in histone variants H3F3A or HIST1H3B [297] | focal EBRT [298,299] targeting alterations in H3, TP53, ACVR1, PIK3CA, FGFR1, or PDGFR [300] | almost universally fatal | ||
| Primary Sarcoma | Late Effects | ||||
|---|---|---|---|---|---|
| Entity | Predisposition and Risk Factors | Treatment | Non-Carcinogenic | SPM | Risk Factors |
| Rhabdo-myosarcoma | first-degree relatives of pediatric RMS patients, LFS, germline DICER1 mutations, Beckwith Weidemann syndrome, Costello syndrome, Noonan syndrome, and genetic aberrations in FGFR4, IGF1R, PDGFRA, ERBB2/4, MET, MDM2, CDK4, PIK3CA and BCOR expression of PAX-FOXO chimeric proteins [341,342,343,344,345,346] | surgery and/or EBRT [347,348] CT by VAC (vincristine, actinomycin D, and cyclophosphamide) or IVA (ifosfamide, vincristine, and actinomycin D) [349,350] | visual, endocrine, cardiopulmonary, neurosensory, neuromotor, neuroendocrine, dental, thyroid, cognitive [351,352] | sarcomas, bone tumors, breast or thyroid cancer, or skin cancer without melanoma [177] | EBRT [353] cyclophosphamide [354,355] |
| Nonrhabdo-myosarcoma | NF, LFS and Maffucci syndrome translocations causing SYT-SSX, ASPL-TFE3, and TLS-CHOP fusion previous exposure to IR [333,356] | CT with doxorubicin, vincristine, cyclophosphamide, and dactinomycin [357] EBRT [358] targeting of IGF-1R (cixutumumab), PDGFR (pazopanib, sorafenib), VEGF (pazopanib, bevacizumab, sorafenib), ALK (crizotinib), MET (crizotinib), mTOR (temsirolimus), PD-L1 (nivolumab), or CAR-T cells targeting NY-ESO-1 or HER2 [348,359] | subcutaneous fibrosis, lymphedema, joint stiffness, cardiac, skeletal, renal, infertility [358,359,360,361] | EBRT [353] cyclophosphamide [354,355] | |
| Osteosarcoma | RB, Rothmund-Thomson syndrome, LFS, and WRN [362] amplifications in CDC5L, MAPK7, MET, PIM1, PMP22, PRIM1, RUNX2, and VEGFA [363] | neoadjuvant CT comprising cisplatin, doxorubicin followed by high-dose methotrexate, and surgical removal of the tumor often followed by adjuvant CT conventional high dose EBRT rarely applied, ion-beam EBRT for inoperable osteosarcoma [364] | cardiac toxicity nephrotoxicity, neurotoxicity, hearing loss, infertility [365] | hematopoietic, soft tissue, thyroid respiratory system, breast [366] | EBRT and high dose CT [366] |
| Ewing Sarcoma | translocations causing EWS-FLI-1 or EWS-ERG gene fusions [367] | EBRT and radical surgery SBRT [368] multiagent CT with VACD (vincristine, cyclophosphamide, Actinomycin D, and doxorubicin) CT plus ifosfamide and etoposide [369] targeting EWSR1/FLI1 or EZH2 (tazemetostat), BET proteins (BRD2, BRD3, BRD4), LSD1, NKX2.2 (vorinostat), CDK4/6, EWSR1/FLI1 (trabectedin, lurbinectedin), the IGF1/IGF1R-axis (cituximab, figitumumab) combined with a mTor1-inhibitor (temsirolimus), ERK or HSP90, PARP (olaparib, talazoparib, niraparib), VEGFR (cediranib, egorafenib), or c-KIT and PDGFR (imatinib, regorafenib) [370] immunological signaling pathways compromising CXCR4-CXCL12 (AMD3100), intracellular antigens including WT1, XAGE-1, the EWS–FLI1 chimeric transcription factor, cancer vaccines based on PAX3- and EWS–FLI1, vigil immunotherapy, oncolytic viruses, T cell receptor therapy with anti-CD3/4-1BBL cells or CD8+ T cells targeting antigens like PAPPA, EZH2 or CHM1, CAR-T cells targeting LINGO, ROR1 or IGF-1R [371,372,373,374,375] | cardiac, pulmonary, bone growth, musculoskeletal, infertility [376] | acute myeloid leukemia, myelodysplastic syndromes, breast and thyroid cancer [377,378,379] | EBRT VACD plus etoposide and ifosfamide [377,378] |
| Primary tumor | Late Effects | ||||
|---|---|---|---|---|---|
| Entity | Predisposition and Risk Factors | Treatment | Non-Carcinogenic | SPM | Risk Factors |
| Neuroblastoma | alterations of MYCN, TP53, ALK, TEEBRT, and ATRX [412] | watch and wait for low-risk [412] CT comprising cyclophosphamide, doxorubicin, vincristine, cisplatin, etoposide, and prolonged exposure to oral etoposide, multiagent myeloablative regimens, surgery, EBRT, and immunotherapy for intermediate- and high-risk as well as few low-risk patients [413] | musculoskeletal, pulmonary, hearing loss, primary hypothyroidism, cardiovascular [414] | leukemia, sarcoma, carcinoma, or brain tumors [336,337,415] | intense CT and prolonged exposure to oral etoposide, EBRT and myeloablative therapy [336,337] alterations XRCC3 and MSH2 [415] |
| Wilms tumor | alterations of WT1, CTNNB1, WTX, TP53, and MYNC [416] syndromes including WAGR, Drash, Beckwith- Wiedemann, Sotos, Perlman, Edward’s, Frasier, BLM, LFS, and Simpson-Golabi- Behmel | combination of surgery and CT comprising vincristine, dactinomycin, and doxorubicin in children with localized tumors plus EBRT for high-risk patients in metastatic disease [417,418] TRK inhibitors and anti-PD-1-antibodies (nivolumab, pembrolizumab) for advanced treatment-refractory renal cell carcinoma might be implemented into therapeutic strategies for Wilms tumors [419,420] | cardiotoxicity, musculoskeletal, growth and development, radiogenic lung fibrosis [417,418,421] | bone and soft-tissue sarcomas, breast cancer, lymphoma, leukemia, melanoma [417] | anthracyclines (doxorubicin) and EBRT [417,418,421] |
| Retinoblastoma | mutation of RB1 [422] | enucleation, intravenous CT (melphalan with or without topotecan and/or carboplatin), focal therapy (laser therapy, cryotherapy), intra-arterial CT with focal therapy [423] | loss of vision in the affected eye, deformities in the bones around the eye, myocardial dysfunction, hypothyroidism [424,425] | leiomyosarcoma, osteosarcoma, melanoma, cancer of the lung, bladder, brain, and nasal cavities [424,425,426] | germline RB1 mutation and EBRT [424,425,426] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahnreich, S.; Schmidberger, H. Childhood Cancer: Occurrence, Treatment and Risk of Second Primary Malignancies. Cancers 2021, 13, 2607. https://doi.org/10.3390/cancers13112607
Zahnreich S, Schmidberger H. Childhood Cancer: Occurrence, Treatment and Risk of Second Primary Malignancies. Cancers. 2021; 13(11):2607. https://doi.org/10.3390/cancers13112607
Chicago/Turabian StyleZahnreich, Sebastian, and Heinz Schmidberger. 2021. "Childhood Cancer: Occurrence, Treatment and Risk of Second Primary Malignancies" Cancers 13, no. 11: 2607. https://doi.org/10.3390/cancers13112607
APA StyleZahnreich, S., & Schmidberger, H. (2021). Childhood Cancer: Occurrence, Treatment and Risk of Second Primary Malignancies. Cancers, 13(11), 2607. https://doi.org/10.3390/cancers13112607







