Accuracy of Machine Learning Algorithms for the Classification of Molecular Features of Gliomas on MRI: A Systematic Literature Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Guidelines and Registration
2.2. Search Strategy, Inclusion Criteria, and Exclusion Criteria
2.3. Statistical Analysis
3. Results
3.1. Review of the Included Studies
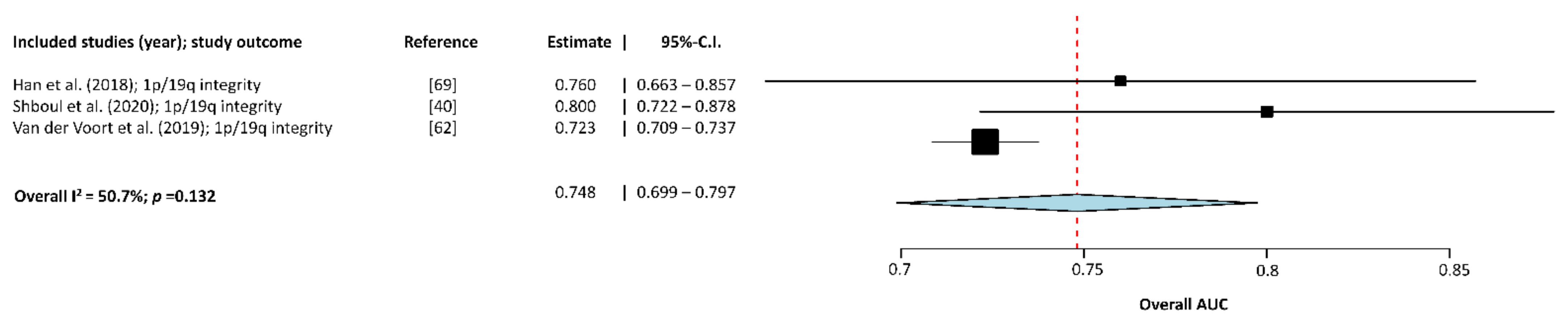
3.2. Meta-Analysis of the Included Studies
3.3. Testing for Publication Bias
4. Discussion
4.1. Implementation of Computer-Aided Approaches in Future Medicine
4.2. Clinical Relevance of Computer-Aided Diagnosis
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Koshy, M.; Villano, J.L.; Dolecek, T.A.; Howard, A.; Mahmood, U.; Chmura, S.J.; Weichselbaum, R.R.; McCarthy, B.J. Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J. Neurooncol. 2012, 107, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015. Neuro Oncol. 2018, 20, iv1–iv86. [Google Scholar] [CrossRef] [Green Version]
- Abd-Ellah, M.K.; Awad, A.I.; Khalaf, A.A.M.; Hamed, H.F.A. A review on brain tumor diagnosis from MRI images: Practical implications, key achievements, and lessons learned. Magn. Reson. Imaging 2019, 61, 300–318. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Lecavalier-Barsoum, M.; Quon, H.; Abdulkarim, B. Adjuvant treatment of anaplastic oligodendrogliomas and oligoastrocytomas. Cochrane Database Syst. Rev. 2014, 2014, CD007104. [Google Scholar] [CrossRef] [PubMed]
- Ohgaki, H.; Kleihues, P. Genetic pathways to primary and secondary glioblastoma. Am. J. Pathol. 2007, 170, 1445–1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riemenschneider, M.J.; Jeuken, J.W.; Wesseling, P.; Reifenberger, G. Molecular diagnostics of gliomas: State of the art. Acta Neuropathol. 2010, 120, 567–584. [Google Scholar] [CrossRef] [Green Version]
- Simon, M.; Hosen, I.; Gousias, K.; Rachakonda, S.; Heidenreich, B.; Gessi, M.; Schramm, J.; Hemminki, K.; Waha, A.; Kumar, R. TERT promoter mutations: A novel independent prognostic factor in primary glioblastomas. Neuro Oncol. 2015, 17, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Narang, J.; Jain, R.; Scarpace, L.; Saksena, S.; Schultz, L.R.; Rock, J.P.; Rosenblum, M.; Patel, S.C.; Mikkelsen, T. Tumor vascular leakiness and blood volume estimates in oligodendrogliomas using perfusion CT: An analysis of perfusion parameters helping further characterize genetic subtypes as well as differentiate from astroglial tumors. J. Neuro Oncol. 2011, 102, 287–293. [Google Scholar] [CrossRef]
- Saito, T.; Yamasaki, F.; Kajiwara, Y.; Abe, N.; Akiyama, Y.; Kakuda, T.; Takeshima, Y.; Sugiyama, K.; Okada, Y.; Kurisu, K. Role of perfusion-weighted imaging at 3 T in the histopathological differentiation between astrocytic and oligodendroglial tumors. Eur. J. Radiol. 2012, 81, 1863–1869. [Google Scholar] [CrossRef]
- Lapointe, S.; Perry, A.; Butowski, N.A. Primary brain tumours in adults. Lancet 2018, 392, 432–446. [Google Scholar] [CrossRef]
- Rudie, J.D.; Rauschecker, A.M.; Bryan, R.N.; Davatzikos, C.; Mohan, S. Emerging Applications of Artificial Intelligence in Neuro-Oncology. Radiology 2019, 290, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Kalra, M.; Orton, C.G. Machine learning will transform radiology significantly within the next 5 years. Med. Phys. 2017, 44, 2041–2044. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Summers, R.M. Machine learning and radiology. Med. Image Anal. 2012, 16, 933–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Six, O. The Ultimate Guide to AI in Radiology. Available online: https://www.quantib.com/the-ultimate-guide-to-ai-in-radiology (accessed on 5 July 2020).
- Kononenko, I. Machine learning for medical diagnosis: History, state of the art and perspective. Artif. Intell. Med. 2001, 23, 89–109. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, A.V.; Blears, E.E.; Ross, E.; Lall, R.R.; Ortega-Barnett, J. Machine learning applications for the differentiation of primary central nervous system lymphoma from glioblastoma on imaging: A systematic review and meta-analysis. Neurosurg. Focus 2018, 45, E5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Huang, Y.; Song, Y.; Xie, D.; Hu, M.; Qiu, H.; Chu, J. Diagnostic accuracy and potential covariates for machine learning to identify IDH mutations in glioma patients: Evidence from a meta-analysis. Eur. Radiol. 2020, 30, 4664–4674. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Faes, L.; Kale, A.U.; Wagner, S.K.; Fu, D.J.; Bruynseels, A.; Mahendiran, T.; Moraes, G.; Shamdas, M.; Kern, C.; et al. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: A systematic review and meta-analysis. Lancet Digit. Health 2019, 1, E271–E297. [Google Scholar] [CrossRef]
- Sakai, K.; Yamada, K. Machine learning studies on major brain diseases: 5-year trends of 2014–2018. Jpn. J. Radiol. 2019, 37, 34–72. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, G.S.; Moons, K.G.M. Reporting of artificial intelligence prediction models. Lancet 2019, 393, 1577–1579. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Wallace, B.C.; Dahabreh, I.J.; Trikalinos, T.A.; Lau, J.; Trow, P.; Schmid, C.H. Closing the Gap between Methodologists and End-Users: R as a Computational Back-End. J. Stat. Softw. 2012, 49, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 1. [Google Scholar] [CrossRef] [Green Version]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Arita, H.; Kinoshita, M.; Kawaguchi, A.; Takahashi, M.; Narita, Y.; Terakawa, Y.; Tsuyuguchi, N.; Okita, Y.; Nonaka, M.; Moriuchi, S.; et al. Lesion location implemented magnetic resonance imaging radiomics for predicting IDH and TERT promoter mutations in grade II/III gliomas. Sci. Rep. 2018, 8, 11773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, H.; Erson-Omay, E.Z.; Li, X.; Gunel, M.; Moliterno, J.; Fulbright, R.K. A quantitative model based on clinically relevant MRI features differentiates lower grade gliomas and glioblastoma. Eur. Radiol. 2020, 30, 3073–3082. [Google Scholar] [CrossRef] [PubMed]
- Carver, E.; Snyder, J.; Griffith, B.; Wen, N. Quantitative image feature analysis in diffuse glioma—A valuable MR imaging biomarker for preoperative idh mutation classification. Neuro Oncol. 2019, 21, vi61. [Google Scholar] [CrossRef]
- Cui, G.; Jeong, J.; Lei, Y.; Wang, T.; Dong, X.; Liu, T.; Curran, W.; Mao, H.; Yang, X. Machine-learning-based classification of low-grade and high-grade glioblastoma using radiomic features in multiparametric MRI. Med. Phys. 2018, 45, e617. [Google Scholar] [CrossRef]
- Li, Y.M.; Liu, X.; Qian, Z.H.; Sun, Z.Y.; Xu, K.B.; Wang, K.; Fan, X.; Zhang, Z.; Li, S.W.; Wang, Y.Y.; et al. Genotype prediction of ATRX mutation in lower-grade gliomas using an MRI radiomics signature. Eur. Radiol. 2018, 28, 2960–2968. [Google Scholar] [CrossRef]
- Li, Y.M.; Qian, Z.H.; Xu, K.B.; Wang, K.; Fan, X.; Li, S.W.; Jiang, T.; Liu, X.; Wang, Y.Y. MRI features predict p53 status in lower-grade gliomas via a machine-learning approach. Neuroimage Clin. 2018, 17, 306–311. [Google Scholar] [CrossRef]
- Hwan-Ho, C.; Hyunjin, P. Classification of low-grade and high-grade glioma using multi-modal image radiomics features. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Korea, 11–15 July 2017; pp. 3081–3084. [Google Scholar] [CrossRef]
- Jiang, C.; Kong, Z.; Liu, S.; Feng, S.; Zhang, Y.; Zhu, R.; Chen, W.; Wang, Y.; Lyu, Y.; You, H.; et al. Fusion Radiomics Features from Conventional MRI Predict MGMT Promoter Methylation Status in Lower Grade Gliomas. Eur. J. Radiol. 2019, 121, 108714. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Kong, Z.; Zhang, Y.; Liu, S.; Liu, Z.; Chen, W.; Liu, P.; Liu, D.; Wang, Y.; Lyu, Y.; et al. Conventional magnetic resonance imaging-based radiomic signature predicts telomerase reverse transcriptase promoter mutation status in grade II and III gliomas. Neuroradiology 2020, 62, 803–813. [Google Scholar] [CrossRef]
- Matsui, Y.; Maruyama, T.; Nitta, M.; Saito, T.; Tsuzuki, S.; Tamura, M.; Kusuda, K.; Fukuya, Y.; Asano, H.; Kawamata, T.; et al. Prediction of lower-grade glioma molecular subtypes using deep learning. J. Neurooncol. 2020, 146, 321–327. [Google Scholar] [CrossRef]
- Mzoughi, H.; Njeh, I.; Wali, A.; Slima, M.B.; BenHamida, A.; Mhiri, C.; Mahfoudhe, K.B. Deep Multi-Scale 3D Convolutional Neural Network (CNN) for MRI Gliomas Brain Tumor Classification. J. Digit. Imaging 2020, 33, 903–915. [Google Scholar] [CrossRef]
- Sasaki, T.; Kinoshita, M.; Fujita, K.; Fukai, J.; Hayashi, N.; Uematsu, Y.; Okita, Y.; Nonaka, M.; Moriuchi, S.; Uda, T.; et al. Radiomics and MGMT promoter methylation for prognostication of newly diagnosed glioblastoma. Sci. Rep. 2019, 9, 14435. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Kinoshita, M.; Fujita, K.; Arita, H.; Uda, T.; Tsuyuguchi, H.; Hayashi, N.; Fukai, J.; Uematsu, Y.; Mori, K.; et al. Radiomics of glioblastoma for predicting mgmt promotor methylation status and prognosis. Neuro Oncol. 2018, 20, vi192. [Google Scholar] [CrossRef]
- Shboul, Z.A.; Chen, J.; Iftekharuddin, K.M. Prediction of Molecular Mutations in Diffuse Low-Grade Gliomas using MR Imaging Features. Sci. Rep. 2020, 10, 3711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Pang, P.; Lou, L.; Feng, Q.; Ding, Z.; Zhou, J. Radiomic prediction models for the level of Ki-67 and p53 in glioma. J. Int. Med. Res. 2020, 48, 300060520914466. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zhang, S.T.; Wei, J.W.; Dong, D.; Wang, X.C.; Yang, G.Q.; Tian, J.; Zhang, H. A radiomics nomogram may improve the prediction of IDH genotype for astrocytoma before surgery. Eur. Radiol. 2019, 29, 3325–3337. [Google Scholar] [CrossRef]
- Tian, H.; Wu, H.; Wu, G.; Xu, G. Noninvasive Prediction of TERT Promoter Mutations in High-Grade Glioma by Radiomics Analysis Based on Multiparameter MRI. BioMed Res. Int. 2020, 2020, 3872314. [Google Scholar] [CrossRef]
- Xi, Y.B.; Guo, F.; Xu, Z.L.; Li, C.; Wei, W.; Tian, P.; Liu, T.T.; Liu, L.; Chen, G.; Ye, J.; et al. Radiomics signature: A potential biomarker for the prediction of MGMT promoter methylation in glioblastoma. J. Magn. Reson. Imaging 2018, 47, 1380–1387. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, C.; Zhao, F.; Tian, Z.; Wang, J.; Ma, X.; Xu, J. Radiomics-Based Machine Learning Technology Enables Better Differentiation Between Glioblastoma and Anaplastic Oligodendroglioma. Front. Oncol. 2019, 9, 1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahammed Muneer, K.V.; Rajendran, V.R. Glioma Tumor Grade Identification Using Artificial Intelligent Techniques. J. Med. Syst. 2019, 43, 113. [Google Scholar] [CrossRef] [PubMed]
- Bakas, S.; Rathore, S.; Nasrallah, M.; Akbari, H.; Binder, Z.; Ha, S.M.; Mamourian, E.; Morrissette, J.; O’Rourke, D.; Davatzikos, C. Non-invasive in vivo signature of IDH1 mutational status in high grade glioma, from clinically-acquired multi-parametric magnetic resonance imaging, using multivariate machine learning. Neuro Oncol. 2018, 20, vi184–vi185. [Google Scholar] [CrossRef] [Green Version]
- Bangalore Yogananda, C.G.; Shah, B.R.; Vejdani-Jahromi, M.; Nalawade, S.S.; Murugesan, G.K.; Yu, F.F.; Pinho, M.C.; Wagner, B.C.; Mickey, B.; Patel, T.R.; et al. A novel fully automated MRI-based deep-learning method for classification of IDH mutation status in brain gliomas. Neuro Oncol. 2020, 22, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Citak-Er, F.; Firat, Z.; Kovanlikaya, I.; Ture, U.; Ozturk-Isik, E. Machine-learning in grading of gliomas based on multi-parametric magnetic resonance imaging at 3T. Comput. Biol. Med. 2018, 99, 154–160. [Google Scholar] [CrossRef]
- De Looze, C.; Beausang, A.; Cryan, J.; Loftus, T.; Buckley, P.G.; Farrell, M.; Looby, S.; Reilly, R.; Brett, F.; Kearney, H. Machine learning: A useful radiological adjunct in determination of a newly diagnosed glioma’s grade and IDH status. J. Neurooncol. 2018, 139, 491–499. [Google Scholar] [CrossRef]
- Gates, E.D.H.; Lin, J.S.; Weinberg, J.S.; Prabhu, S.S.; Hamilton, J.; Hazle, J.D.; Fuller, G.N.; Baladandayuthapani, V.; Fuentes, D.T.; Schellingerhout, D. Imaging-Based Algorithm for the Local Grading of Glioma. AJNR Am. J. Neuroradiol. 2020, 41, 400–407. [Google Scholar] [CrossRef]
- Inano, R.; Oishi, N.; Kunieda, T.; Arakawa, Y.; Yamao, Y.; Shibata, S.; Kikuchi, T.; Fukuyama, H.; Miyamoto, S. Voxel-based clustered imaging by multiparameter diffusion tensor images for glioma grading. Neuroimage Clin. 2014, 5, 396–407. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Jung, S.Y.; Park, J.E.; Jo, Y.; Park, S.Y.; Nam, S.J.; Kim, J.H.; Kim, H.S. Diffusion- and perfusion-weighted MRI radiomics model may predict isocitrate dehydrogenase (IDH) mutation and tumor aggressiveness in diffuse lower grade glioma. Eur. Radiol. 2020, 30, 2142–2151. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, J.; Kim, S.T.; Shin, H.M.; You, H.J.; Choi, J.W.; Seol, H.J.; Nam, D.H.; Lee, J.I.; Kong, D.S. Prediction of IDH1 Mutation Status in Glioblastoma Using Machine Learning Technique Based on Quantitative Radiomic Data. World Neurosurg. 2019, 125, e688–e696. [Google Scholar] [CrossRef]
- Li, Y.; Liang, Y.; Sun, Z.; Xu, K.; Fan, X.; Li, S.; Zhang, Z.; Jiang, T.; Liu, X.; Wang, Y. Radiogenomic analysis of PTEN mutation in glioblastoma using preoperative multi-parametric magnetic resonance imaging. Neuroradiology 2019, 61, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Y.; Yu, J.; Guo, Y.; Cao, W. Deep Learning based Radiomics (DLR) and its usage in noninvasive IDH1 prediction for low grade glioma. Sci. Rep. 2017, 7, 5467. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.M.; Weng, R.C.; Cheng, S.J.; Wang, H.J.; Hsieh, K.L. Computer-aided diagnosis of isocitrate dehydrogenase genotypes in glioblastomas from radiomic patterns. Medicine 2020, 99, e19123. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.F.; Hsu, F.T.; Hsieh, K.L.; Kao, Y.J.; Cheng, S.J.; Hsu, J.B.; Tsai, P.H.; Chen, R.J.; Huang, C.C.; Yen, Y.; et al. Machine Learning-Based Radiomics for Molecular Subtyping of Gliomas. Clin. Cancer Res. 2018, 24, 4429–4436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathore, S.; MacLean, N.; Akbari, H.; Shukla, G.; Bagley, S. TMOD-40. In Vivo Evaluation of O6-Methylguanine-DNA-Methyltransferase (MGMT) Promoter Methylation Status for De Novo Glioblastoma Patients Using Deep Learning Features. Neuro Oncol. 2019, 21, vi271–vi272. [Google Scholar] [CrossRef]
- Shofty, B.; Artzi, M.; Ben Bashat, D.; Liberman, G.; Haim, O.; Kashanian, A.; Bokstein, F.; Blumenthal, D.T.; Ram, Z.; Shahar, T. MRI radiomics analysis of molecular alterations in low-grade gliomas. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Tongtong, L.; Guoqing, W.; Jinhua, Y.; Yi, G.; Yuanyuan, W.; Zhifeng, S.; Liang, C. A mRMRMSRC feature selection method for radiomics approach. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Korea, 11–15 July 2017; pp. 616–619. [Google Scholar] [CrossRef]
- Van der Voort, S.R.; Incekara, F.; Wijnenga, M.M.J.; Kapas, G.; Gardeniers, M.; Schouten, J.W.; Starmans, M.P.A.; Nandoe Tewarie, R.; Lycklama, G.J.; French, P.J.; et al. Predicting the 1p/19q Codeletion Status of Presumed Low-Grade Glioma with an Externally Validated Machine Learning Algorithm. Clin. Cancer Res. 2019, 25, 7455–7462. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Chang, K.; Ramkissoon, S.; Tanguturi, S.; Bi, W.L.; Reardon, D.A.; Ligon, K.L.; Alexander, B.M.; Wen, P.Y.; Huang, R.Y. Multimodal MRI features predict isocitrate dehydrogenase genotype in high-grade gliomas. Neuro Oncol. 2017, 19, 109–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Tian, Q.; Wang, L.; Liu, Y.; Li, B.; Liang, Z.; Gao, P.; Zheng, K.; Zhao, B.; Lu, H. Radiomics Strategy for Molecular Subtype Stratification of Lower-Grade Glioma: Detecting IDH and TP53 Mutations Based on Multimodal MRI. J. Magn. Reson. Imaging 2018, 48, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xiao, J.; Wu, S.; Lv, F.; Gong, J.; Jiang, L.; Yu, R.; Luo, T. Deep Convolutional Radiomic Features on Diffusion Tensor Images for Classification of Glioma Grades. J. Digit. Imaging 2020, 33, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Chang, K.; Bai, H.X.; Xiao, B.; Su, C.; Bi, W.L.; Zhang, P.J.; Senders, J.T.; Vallières, M.; Kavouridis, V.K.; et al. Machine learning reveals multimodal MRI patterns predictive of isocitrate dehydrogenase and 1p/19q status in diffuse low- and high-grade gliomas. J. Neurooncol. 2019, 142, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.; Bakas, S.; Nasrallah, M.P.; Bagley, S.J.; Akbari, H.; Ha, S.M.; Mamourian, E.C.; Watt, C.D.; Davatzikos, C. Non-invasive determination of the O6-methylguanine-DNA-methyltransferase (MGMT) promoter methylation status in glioblastoma (GBM) using magnetic resonance imaging (MRI). J. Clin. Oncol. Conf. 2018, 36, 2051. [Google Scholar] [CrossRef]
- Bonte, S.; Goethals, I.; Van Holen, R. Automated grade prediction of glioma patients based on magnetic resonance imaging and a random forests approach. Neuro Oncol. 2016, 18, iv38. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Xie, Z.; Zang, Y.; Zhang, S.; Gu, D.; Zhou, M.; Gevaert, O.; Wei, J.; Li, C.; Chen, H.; et al. Non-invasive genotype prediction of chromosome 1p/19q co-deletion by development and validation of an MRI-based radiomics signature in lower-grade gliomas. J. Neurooncol. 2018, 140, 297–306. [Google Scholar] [CrossRef]
- Kinoshita, M.; Arita, H.; Kawaguchi, A.; Takahashi, M.; Fukai, J.; Ishibashi, K.; Tsuyuguchi, N.; Okita, Y.; Narita, Y.; Ichimura, K.; et al. MRI based radiogenomics for glioma. Neuroradiology 2018, 60, 1118–1119. [Google Scholar] [CrossRef]
- Park, C.J.; Choi, Y.S.; Park, Y.W.; Ahn, S.S.; Kang, S.G.; Chang, J.H.; Kim, S.H.; Lee, S.K. Diffusion tensor imaging radiomics in lower-grade glioma: Improving subtyping of isocitrate dehydrogenase mutation status. Neuroradiology 2020, 62, 319–326. [Google Scholar] [CrossRef]
- Park, Y.W.; Choi, Y.S.; Ahn, S.S.; Chang, J.H.; Kim, S.H.; Lee, S.K. Radiomics MRI Phenotyping with Machine Learning to Predict the Grade of Lower-Grade Gliomas: A Study Focused on Nonenhancing Tumors. Korean J. Radiol. 2019, 20, 1381–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Meng, J.; Yu, Q.; Li, P.; Fu, S. Radiomics-based machine learning methods for isocitrate dehydrogenase genotype prediction of diffuse gliomas. J. Cancer Res. Clin. Oncol. 2019, 145, 543–550. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.S.; Feng, X.L.; Hu, Y.C.; Han, Y.; Tian, Q.; Sun, Y.Z.; Zhang, J.; Ge, X.W.; Cheng, S.C.; Li, X.L.; et al. Better efficacy in differentiating WHO grade II from III oligodendrogliomas with machine-learning than radiologist’s reading from conventional T1 contrast-enhanced and fluid attenuated inversion recovery images. BMC Neurol. 2020, 20, 48. [Google Scholar] [CrossRef]
- Chang, K.; Bai, H.X.; Zhou, H.; Su, C.; Bi, W.L.; Agbodza, E.; Kavouridis, V.K.; Senders, J.T.; Boaro, A.; Beers, A.; et al. Residual Convolutional Neural Network for the Determination of IDH Status in Low- and High-Grade Gliomas from MR Imaging. Clin. Cancer Res. 2018, 24, 1073–1081. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Kamdar, M.R. MRI to MGMT: Predicting methylation status in glioblastoma patients using convolutional recurrent neural networks. Pac. Symp. Biocomput. 2018, 23, 331–342. [Google Scholar]
- Li, Z.C.; Bai, H.; Sun, Q.; Li, Q.; Liu, L.; Zou, Y.; Chen, Y.; Liang, C.; Zheng, H. Multiregional radiomics features from multiparametric MRI for prediction of MGMT methylation status in glioblastoma multiforme: A multicentre study. Eur. Radiol. 2018, 28, 3640–3650. [Google Scholar] [CrossRef]
- Li, Z.C.; Bai, H.; Sun, Q.; Zhao, Y.; Lv, Y.; Zhou, J.; Liang, C.; Chen, Y.; Liang, D.; Zheng, H. Multiregional radiomics profiling from multiparametric MRI: Identifying an imaging predictor of IDH1 mutation status in glioblastoma. Cancer Med. 2018, 7, 5999–6009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, S.; Zhang, R.; Liang, D.; Song, T.; Ai, T.; Xia, C.; Xia, L.; Wang, Y. Multimodal 3D DenseNet for IDH Genotype Prediction in Gliomas. Genes 2018, 9, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, S.; Tanaka, S.; Takahashi, M.; Yamazawa, E.; Hana, T.; Kitagawa, Y.; Takayanagi, S.; Takahashi, W.; Nakamoto, T.; Haga, A.; et al. Visualization of judgment basis of CNN to grading glioma. Neuro Oncol. 2019, 21, vi163. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, L.F.; Zhang, X.; Han, Y.; Nan, H.Y.; Hu, Y.C.; Hu, B.; Yan, S.L.; Zhang, J.; Cheng, D.L.; et al. Glioma Grading on Conventional MR Images: A Deep Learning Study With Transfer Learning. Front. Neurosci. 2018, 12, 804. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, W.; Nakamoto, T.; Akihiro, H.; Satoshi, T.; Shota, T.; Aoki, S.; Kiritoshi, T.; Ogita, M.; Yamashita, H.; Nakagawa, K. MRI-based radiogenomics analysis of 1p/19q codeletion in grade II and III gliomas. Radiother. Oncol. 2019, 133, S1057–S1058. [Google Scholar] [CrossRef]
- Wei, J.; Yang, G.; Hao, X.; Gu, D.; Tan, Y.; Wang, X.; Dong, D.; Zhang, S.; Wang, L.; Zhang, H.; et al. A multi-sequence and habitat-based MRI radiomics signature for preoperative prediction of MGMT promoter methylation in astrocytomas with prognostic implication. Eur. Radiol. 2019, 29, 877–888. [Google Scholar] [CrossRef] [Green Version]
- Batchala, P.P.; Muttikkal, T.J.E.; Donahue, J.H.; Patrie, J.T.; Schiff, D.; Fadul, C.E.; Mrachek, E.K.; Lopes, M.B.; Jain, R.; Patel, S.H. Neuroimaging-Based Classification Algorithm for Predicting 1p/19q-Codeletion Status in IDH-Mutant Lower Grade Gliomas. AJNR Am. J. Neuroradiol. 2019, 40, 426–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Shi, Z.; Lian, Y.; Li, Z.; Liu, T.; Gao, Y.; Wang, Y.; Chen, L.; Mao, Y. Noninvasive IDH1 mutation estimation based on a quantitative radiomics approach for grade II glioma. Eur. Radiol. 2017, 27, 3509–3522. [Google Scholar] [CrossRef]
- Clark, V.E.; Cahill, D.P. Extent of Resection Versus Molecular Classification: What Matters When? Neurosurg. Clin. N. Am. 2019, 30, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Dubbink, H.J.; Atmodimedjo, P.N.; Kros, J.M.; French, P.J.; Sanson, M.; Idbaih, A.; Wesseling, P.; Enting, R.; Spliet, W.; Tijssen, C.; et al. Molecular classification of anaplastic oligodendroglioma using next-generation sequencing: A report of the prospective randomized EORTC Brain Tumor Group 26951 phase III trial. Neuro Oncol. 2016, 18, 388–400. [Google Scholar] [CrossRef]
- Sotoudeh, H.; Shafaat, O.; Bernstock, J.D.; Brooks, M.D.; Elsayed, G.A.; Chen, J.A.; Szerip, P.; Chagoya, G.; Gessler, F.; Sotoudeh, E.; et al. Artificial Intelligence in the Management of Glioma: Era of Personalized Medicine. Front. Oncol. 2019, 9, 768. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, A.P.; Liong, R.; Koppen, J.; Murthy, S.V.; Lasocki, A. Noninvasive Determination of IDH and 1p19q Status of Lower-grade Gliomas Using MRI Radiomics: A Systematic Review. AJNR Am. J. Neuroradiol. 2021, 42, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Van den Bent, M.J. Interobserver variation of the histopathological diagnosis in clinical trials on glioma: A clinician’s perspective. Acta Neuropathol. 2010, 120, 297–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Leeuwen, K.G.; Schalekamp, S.; Rutten, M.; van Ginneken, B.; de Rooij, M. Artificial intelligence in radiology: 100 commercially available products and their scientific evidence. Eur. Radiol. 2021, 31, 3797–3804. [Google Scholar] [CrossRef] [PubMed]
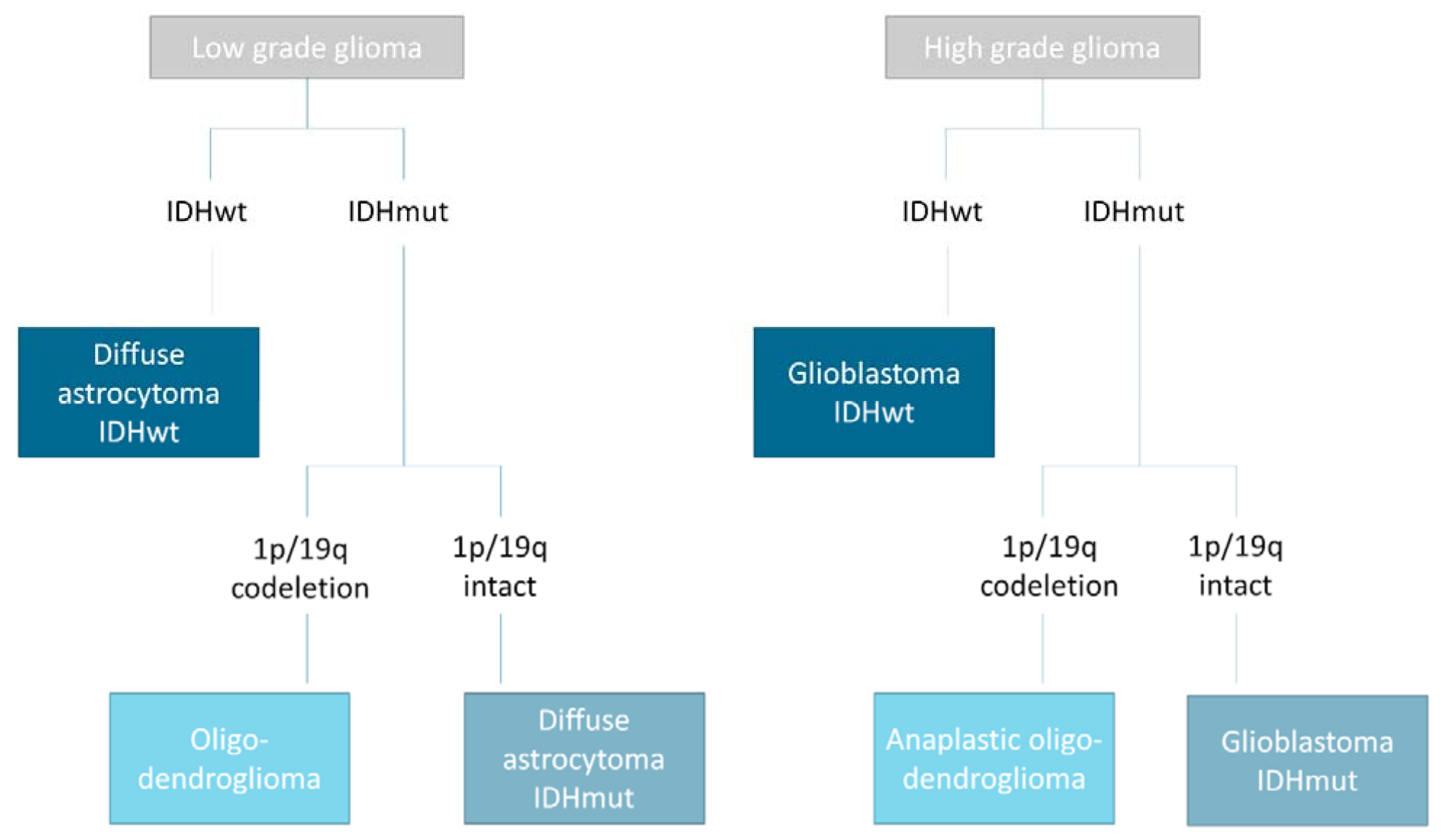




| Training Set | Validation Set | Performance * | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First Author (Year of Publication) (Reference) | N | Age (Mean ± SD) | Gender (Male-Female) | N | Validated on an Independent External Dataset? | Input Imaging Data | MLA Method | Target Condition | Sensitivity | Specificity | AUC (±SD) | Accuracy |
| Ahammed Muneer (2019) [46] | 389 | NR | NR | 168 | No | T2w images; tumor segmentation | Deep CNN | Glioma grade | 92.72 | 98.13 | NR | 94.64 |
| Arita (2018) [27] | 111 | NR | NR | 58 | No | T2w-based VOI segmentation and T1w, T2w, FLAIR, and T1w +c images | Lasso and Elastic-Net Regularized Generalized Linear Model | IDH genotype | NR | NR | NR | 87 |
| Bakas (2018) [47] | 86 | NR | NR | NR | No | T1w, T2w, FLAIR, T1w +c images; DTI series and DSC-PWI series | Multivariate machine learning model with a Random Forests algorithm | IDH genotype | 66.7 | 92.9 | NR | 88.4 |
| Bangalore Yogananda (2020) [48] | 214 | NR | NR | 214 | No | T2w, FLAIR, and T1w +c images | 3D Dense-UNet: T2-Net | IDH genotype | 97 | 98 | 0.98 ± 0.146 | 97.14 |
| 3D Dense-UNet: TS-Net | IDH genotype | 98 | 97 | 0.99 ± 0.146 | 97.12 | |||||||
| Batchala (2019) [84] | 102 | NR | 50–52 | 106 | No | T1w, T2w, FLAIR, and T1w +c images; DSC-PWI series | Multivariate model | 1p/19q integrity | NR | NR | NR | 81.1 |
| Bonte (2016) [68] | 274 | NR | NR | NR | No | BraTS-data (T1w, T2w, FLAIR, and T1w +c images) | Random Forests algorithm | LGG/HGG | 95.5 | 79.5 | NR | 92.3 |
| Cao (2020) [28] | 141 | NR | 74–67 | 88 | No | T1w, T2w, FLAIR, and T1w +c images | Lasso and Elastic-Net Regularized Generalized Linear Model with Support vector machine classifier | LGG/HGG | NR | NR | 0.915 ± 0.356 | NR |
| Carver (2019) [29] | 78 | NR | NR | 50 | Yes | T1w, T2w, FLAIR, and T1w +c images | Lasso and Elastic-Net Regularized Generalized Linear Model | IDH genotype | NR | NR | NR | 74 |
| Chang (2018) [77] | 1188 | NR | NR | 153 | No | T1w, T2w, FLAIR, and T1w +c images | Residual CNN model | IDH genotype | NR | NR | 0.93 | 83.0 |
| Citak-Er (2018) [49] | 43 | 49.5 ± 12.8 | 25–18 | NR | No | T1w, T2w, DW images; DTI series, DSC-PWI series, and MRS | Support vector machine classifier with linear kernel and logistic regression with a Random Forests algorithm | LGG/HGG | 86.7 | 96.4 | NR | 93.0 |
| Cui (2018) [30] | 40 | NR | NR | NR | No | T1w, T2w, FLAIR, and T1w +c images; tumor segmentation | Lasso and Elastic-Net Regularized Generalized Linear Model | LGG/HGG | NR | NR | 0.84 | NR |
| De Looze (2018) [50] | 381 | NR | 251–130 | NR | No | Three VASARI criteria as assessed on T1w, T2w, FLAIR, and DW images | Random Forests model | IDH genotype | 81 | 77 | 0.88 | NR |
| Glioma grade II/III | 82 | 94 | 0.98 | NR | ||||||||
| Glioma grade II/IV | 100 | 100 | 1.0 | NR | ||||||||
| Glioma grade III/IV | 83 | 97 | 0.97 | NR | ||||||||
| Fan (2019) [45] | 126 | 46.8 | NR | NR | No | T1w +c images | Lasso and Elastic-Net Regularized Generalized Linear Model adopted into linear discriminant analysis and Support vector machine classifier | glioblastoma/anaplastic oligodendro-glioma | 100.0 | 91.0 | 0.923 | 93.8 |
| Gates (2020) [51] | 23 | NR | NR | NR | No | T2, ADC, CBV, and Ktrans | Random Forests algorithm | Glioma grade | NR | NR | NR | 96 |
| Han (2018) [76] | 117 | NR | NR | 21 | No | T1w, T2w, and FLAIR images | Recurrent CNN model | MGMT promoter methylation status | NR | NR | 0.54 | 53 |
| Han (2018) [71] | 184 | 41.67 | 120–64 | 93 | No | T2w images and T2w-based segmentation | Random Forests algorithm | 1p/19q integrity | 68.3 | 71.2 | 0.760 ± 0.477 | 70.0 |
| Hwan-Ho (2017) [33] | 108 | NR | NR | NR | No | BraTS-data (T1w, T2w, FLAIR, and T1w +c images) and BraTS-segmentation | Lasso and Elastic-Net Regularized Generalized Linear Model and logistic regression | Glioma grade | 88.89 | 90.74 | 0.8870 | 89.81 |
| Inano (2014) [52] | 33 | NR | 22–11 | 33 | No | DW images, FA-maps, first eigenvalue, second eigenvalue, third eigenvalue, MD-maps, and raw T2 signal with no diffusion-weighting | Support vector machine classifiers | Glioma grade | 84.8 | 74.5 | 0.912 ± 0.028 | 80.4 |
| Jiang (2019) [34] | 87 | 45.4 ± 13.1 | 43–44 | 35 | Yes | T2w and T1w +c images | Lasso regression model with fusion Radiomics model and Support vector machine classifier | MGMT promoter methylation status | 82.1 | 85.7 | 0.898 ± 0.323 | 88.6 |
| Jiang (2020) [35] | 83 | 45.5 ± 12.3 | 50–33 | 33 | Yes | T2w and T1w +c images | Lasso regression model with radiomics signature model and Support vector machine classifier | TERT promoter mutation status | 71.4 | 89.5 | 0.827 ± 0.470 | 84.8 |
| Kim (2020) [53] | 127 | NR | 68–59 | 28 | No | T1w, T2w, FLAIR, T1w +c, DW images; DSC-PWI series | Recursive feature elimination with Support vector machine, completed with a Random Forests algorithm and a logistic regression classifier | IDH genotype | 53.6 | 86.7 | 0.747 ± 0.228 | NR |
| Kinoshita (2018) [70] | 199 | NR | NR | NR | No | Conventional MR sequences (NOS) | Random Forests algorithm | Glioma grade | NR | NR | 0.711 | 64.5 |
| Lee (2019) [54] | 88 | NR | 47–41 | 35 | Yes | T1w, T2w, FLAIR, DW images; DSC-PWI series | Eight machine learning classifiers: K-Nearest Neighbors, Support vector classification, Decision Tree, Random Forest, AdaBoost, Naive Bayes, Linear Discriminant Analysis, and Gradient Boosting | IDH genotype | NR | NR | NR | 83.4 |
| Li (2019) [55] | 69 | 60.0 | 37–32 | 40 | Yes | T2w and T1w +c images | Support vector machine classifier with Support vector machine classifier | PTEN genotype | 86.7 | 70.0 | 0.787 | 82.5 |
| Li (2018) [32] | 63 | 43.6 | 25–38 | 91 | Yes | T2w images | Lasso regression model with Support vector machine classifier | ATRX genotype | 57.1 | 85.7 | 0.725 | 76.9 |
| Li (2018) [33] | 180 | 39.2 | 111–69 | 92 | No | T2w images | Lasso regression model with Support vector machine classifier | P53 status | 62.2 | 85.1 | 0.763 | 70.7 |
| Li (2017) [56] | 151 | 40.7 ± 10.8 | 81–70 | 151 | No | T1w and FLAIR images | CNN for segmentation followed by DLR model with Support vector machine classifier | IDH genotype | 94.38 | 86.67 | 0.9521 | 92.44 |
| Li (2018) [77] | 133 | 54.2 | 79–54 | 60 | No | T1w, T2w, FLAIR, and T1w +c images | Multiregional Radiomics model | MGMT-methylation | NR | NR | 0.88 | 80 |
| Li (2018) [78] | 118 | 53.6 | 70–48 | 107 | No | T1w, T2w, FLAIR, and T1w +c images | Multiregional Radiomics models | IDH genotype | 80 | 99 | 0.96 | 97 |
| Liang (2018) [79] | 167 | 52.4 ± 15.5 | NR | NR | No | BraTS-data (T1w, T2w, FLAIR, and T1w +c images) | Multimodal Three-Dimensional DenseNet | IDH genotype | 78.5 | 88.0 | 0.857 | 84.6 |
| Lo (2020) [57] | 39 | NR | 28–11 | NR | No | T1w +c images; processed by transformed ranklet images. | Logistic regression classifier | IDH genotype | 57 | 97 | NR | 90 |
| Lu (2018) [58] | 214 | NR | NR | 70 | Yes | T1w, T2w, FLAIR, T1w +c, and DW images (T2w and DW images were optional) | Three-level machine learning model | LGG/HGG | 82.5 | 90.5 | NR | 87.7 |
| Matsui (2020) [36] | 217 | 42 | 131–86 | NR | No | T1w, T2w, and FLAIR images | Lasso regression model with DLR model | Grading LGG | NR | NR | NR | 58.5 |
| Mzoughi (2020) [37] | 284 | NR | NR | 67 | Yes | T1w +c images | Lasso regression model with 3D CNN model with Support vector machine classifier | Glioma grade | NR | NR | NR | 96.4 |
| Park (2020) [71] | 168 | NR | NR | 168 | No | T2w, FLAIR, and T1w +c images | Random Forests algorithm | IDH genotype | NR | NR | 0.900 ± 0.298 | NR |
| Park (2019) [72] | 136 | 44.99 ± 12.94 | 65–71 | 99 | Yes | T2w, FLAIR, and T1w +c images; DTI series | Random Forests algorithm | Glioma grade | 72.6 | 60.4 | 0.72 ± 0.51 | 66.7 |
| Rathore (2019) [59] | 202 | NR | NR | NR | No | T1w, T2w, FLAIR, and T1w +c images. Data were sometimes complemented with DTI and DSC-PWI series | CNN adjusted with a Support vector machine classifier | IDH genotype | 83 | 86 | 0.85 | 85 |
| MGMT | 83 | 85 | 0.84 | 83 | ||||||||
| Rathore (2018) [67] | 111 | NR | NR | NR | No | T1w, T2w, FLAIR, and T1w +c images | Support Vector Machine model with a Random Forests algorithm | MGMT-methylation | 75.0 | 97.0 | 0.80 | 88.28 |
| Rathore (2019) [59] | 270 | NR | NR | NR | No | T1w, T2w, FLAIR, and T1w +c images; DTI and DSC-PWI series | Cross-validated sequential feature selection | MGMT-methylation | NR | NR | NR | 86.95 |
| Sasaki (2018) [39] | 207 | NR | NR | NR | No | T1w, T2w, FLAIR, and T1w +c images | Lasso regression model with supervised component principal analysis | MGMT-methylation | NR | NR | NR | 68 |
| Sasaki (2019) [38] | 201 | NR | NR | NR | No | T1w, T2w, and T1w +c images | Lasso regression model with supervised component principal analysis | MGMT-methylation | 67 | 66 | NR | 67 |
| Shboul (2020) [40] | 81 | NR | NR | 27 | No | T1w, T2w, FLAIR, and T1w +c images | Lasso regression model with supervised component principal analysis and multi-resolution fractal modeling | IDH genotype | 90 | 79 | 0.84 ± 0.156 | NR |
| 1p/19q integrity | 75 | 85 | 0.80 ± 0.208 | NR | ||||||||
| MGMT-methylation | 93 | 73 | 0.83 ± 0.208 | NR | ||||||||
| ATRX genotype | 69 | 83 | 0.70 ± 0.468 | NR | ||||||||
| TERT promoter mutation status | 77 | 86 | 0.82 ± 0.208 | NR | ||||||||
| Shofty (2018) [60] | 47 | 37.7 ± 10.6 | 27–20 | NR | No | T2w, FLAIR, and T1w +c images | Ensemble Radiomic Classifier model with a Support vector machine classifier | 1p/19q integrity | 92 | 83 | 0.87 | 87 |
| Sun (2020) [41] | 92 | NR | NR | NR | No | T1w, T2w images | Lasso regression model with logistic regression models | P53 status | 100 | 40 | 0.709 | 81.3 |
| Takahashi (2019) [80] | 44 | NR | NR | 11 | No | DW (b1000 and b2000) images, ADC-maps, FA-maps, and MK-maps | Deep CNN model | Glioma grade | NR | NR | NR | 82 |
| Takahashi (2019) [82] | 38 | NR | NR | NR | No | T2w-based VOI segmentation | Logistic regression models | 1p/19q integrity | 69.7 | 73.3 | 0.736 | 71.1 |
| Tan (2019) [42] | 74 | 47.93 ± 13.28 | 45–29 | 31 | No | FLAIR and T1w +c images; ADC-maps | Radiomics Nomogram model | IDH genotype | 86.7 | 87.5 | 0.900 ± 0.116 | 87.1 |
| Tian (2020) [43] | 88 | NR | 53–35 | 38 | No | T1w, T2w, FLAIR, and T1w +c images; MRS | Lasso regression model with Radiomics Nomogram model | TERT promoter mutation status | 75.0 | 90.9 | 0.889 ± 0.335 | 84.2 |
| Tongtong (2017) [61] | 110 | NR | NR | NR | No | 3D FLAIR images | Support vector machine classifier with minimum redundancy, maximum relevance, and maximum sparse representation coefficient | IDH genotype | 88 | 79 | 0.90 | 85 |
| van der Voort (2019) [62] | 284 | NR | 161–123 | 129 | Yes | T2w and T1w +c images. Data were sometimes complemented with FLAIR images | Support vector machine classifier | 1p/19q integrity | 73.2 | 61.7 | 0.723 ± 0.084 | 69.3 |
| Wei (2019) [83] | 74 | NR | 42–32 | 31 | No | FLAIR and T1w +c images; ADC-maps | Fusion Radiomics model by logistic regression modelling | MGMT promoter methylation | 94.4 | 53.9 | 0.902 ± 0.305 | 77.4 |
| Wu (2019) [73] | 84 | 53.5 ± 15.0 | 67–59 | 42 | No | T1w, T2w, FLAIR, and T1w +c images | Random Forests algorithm | IDH genotype | NR | NR | 0.931 ± 0.233 | 89.5 |
| Xi (2018) [44] | 98 | NR | 55–43 | 20 | Yes | T1w, T2w, and T1w +c images | Lasso regression model with Support vector machine model | MGMT promoter methylation | 87.5 | 75.0 | NR | 80.0 |
| Yang (2018) [81] | 113 | NR | NR | NR | No | T1w, T2w, FLAIR, and T1w +c images | CNN model | LGG/HGG | NR | NR | NR | 86.7 |
| Yu (2017) [85] | 110 | 40.3 ± 11.3 | 54–56 | 30 | No | FLAIR images | Radiomics model | IDH genotype | 88 | 67 | 0.79 | 83 |
| Zhang (2017) [63] | 90 | 51.4 | 52–38 | 30 | No | T1w, T2w, FLAIR, T1w +c, and DW images | Random Forests algorithm | IDH genotype | NR | NR | 0.9231 | 89 |
| Zhang (2018) [64] | 73 | NR | NR | 30 | No | T1w, T2w, FLAIR, and T1w +c images | Support vector machine-based recursive feature elimination | IDH genotype | 85.0 | 70.0 | 0.792 | 80.0 |
| P53 status | 84.6 | 85.7 | 0.869 | 85.0 | ||||||||
| Zhang (2020) [65] | 108 | NR | 61–47 | NR | No | DTI series | CNN model with a Support vector machine classifier | LGG/HGG | 98 | 86 | 0.93 | 94 |
| Glioma grade III/IV | 98 | 100 | 0.99 | 98 | ||||||||
| Zhao (2020) [74] | 36 | 45.0 ± 14.4 | 19–17 | 36 | No | FLAIR and T1w +c images | Random Forests algorithm | Glioma grade II/III | 77.8 | 78.3 | 0.861 ± 0.240 | 78.1 |
| Zhou (2019) [66] | 538 | NR | 303–235 | 206 | Yes | FLAIR and T1w +c images | Random Forests algorithm with a Support vector machine classifier | IDH genotype | NR | NR | 0.919 ± 0.286 | NR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Kempen, E.J.; Post, M.; Mannil, M.; Kusters, B.; ter Laan, M.; Meijer, F.J.A.; Henssen, D.J.H.A. Accuracy of Machine Learning Algorithms for the Classification of Molecular Features of Gliomas on MRI: A Systematic Literature Review and Meta-Analysis. Cancers 2021, 13, 2606. https://doi.org/10.3390/cancers13112606
van Kempen EJ, Post M, Mannil M, Kusters B, ter Laan M, Meijer FJA, Henssen DJHA. Accuracy of Machine Learning Algorithms for the Classification of Molecular Features of Gliomas on MRI: A Systematic Literature Review and Meta-Analysis. Cancers. 2021; 13(11):2606. https://doi.org/10.3390/cancers13112606
Chicago/Turabian Stylevan Kempen, Evi J., Max Post, Manoj Mannil, Benno Kusters, Mark ter Laan, Frederick J. A. Meijer, and Dylan J. H. A. Henssen. 2021. "Accuracy of Machine Learning Algorithms for the Classification of Molecular Features of Gliomas on MRI: A Systematic Literature Review and Meta-Analysis" Cancers 13, no. 11: 2606. https://doi.org/10.3390/cancers13112606
APA Stylevan Kempen, E. J., Post, M., Mannil, M., Kusters, B., ter Laan, M., Meijer, F. J. A., & Henssen, D. J. H. A. (2021). Accuracy of Machine Learning Algorithms for the Classification of Molecular Features of Gliomas on MRI: A Systematic Literature Review and Meta-Analysis. Cancers, 13(11), 2606. https://doi.org/10.3390/cancers13112606







