Prospective Evaluation of Quality of Life and Functional Outcomes after Carbon Ion Radiotherapy for Inoperable Bone and Soft Tissue Sarcomas
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Carbon Ion Radiotherapy
2.3. Functional Outcome and Quality of Life Assessment
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
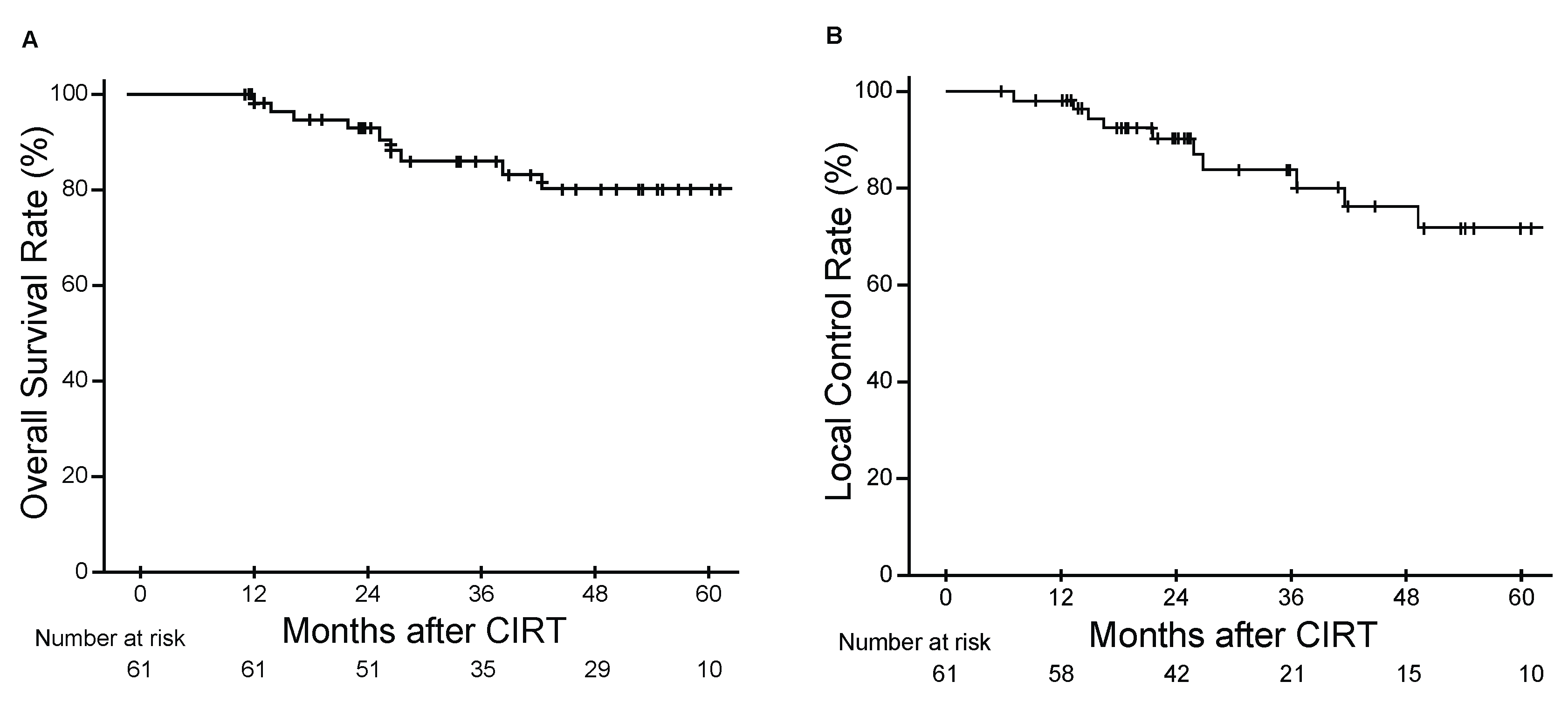
3.2. Clinical Efficacy and Safety
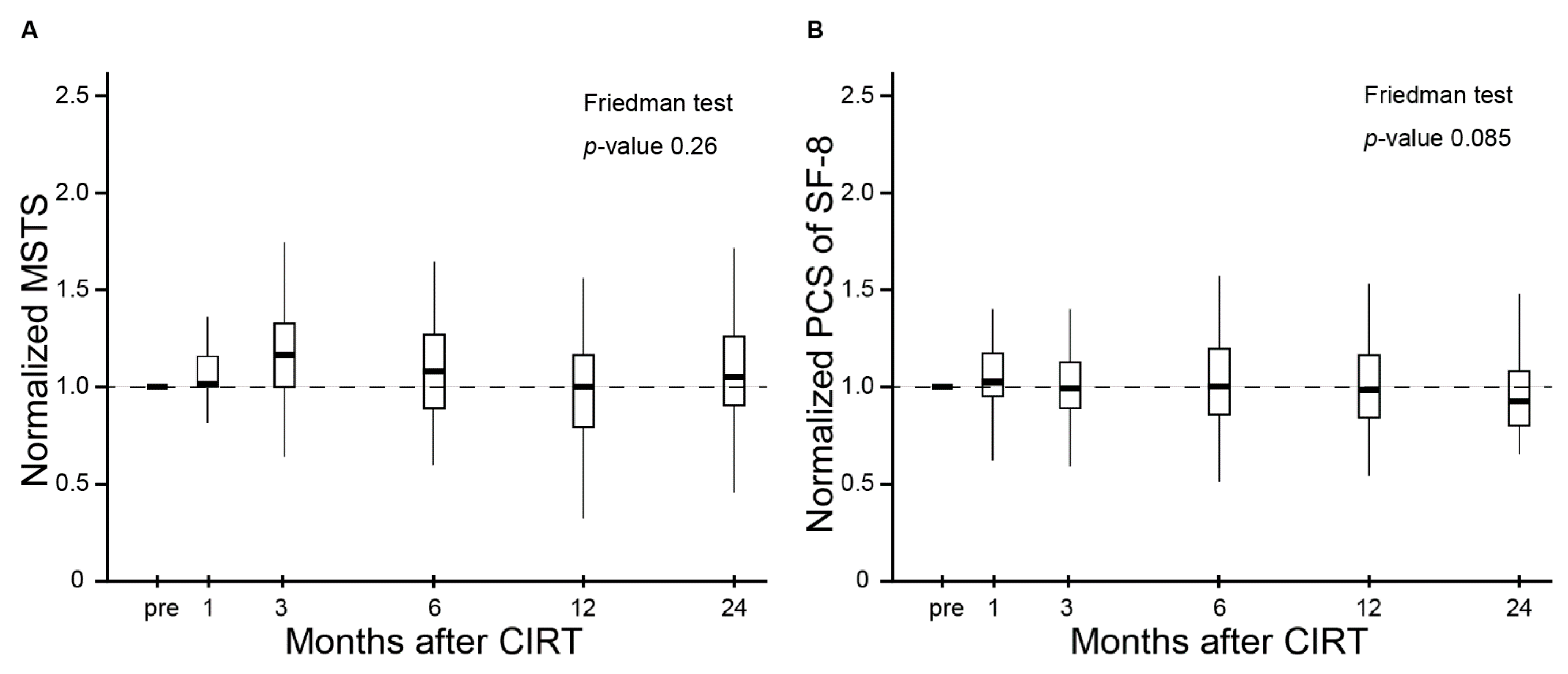
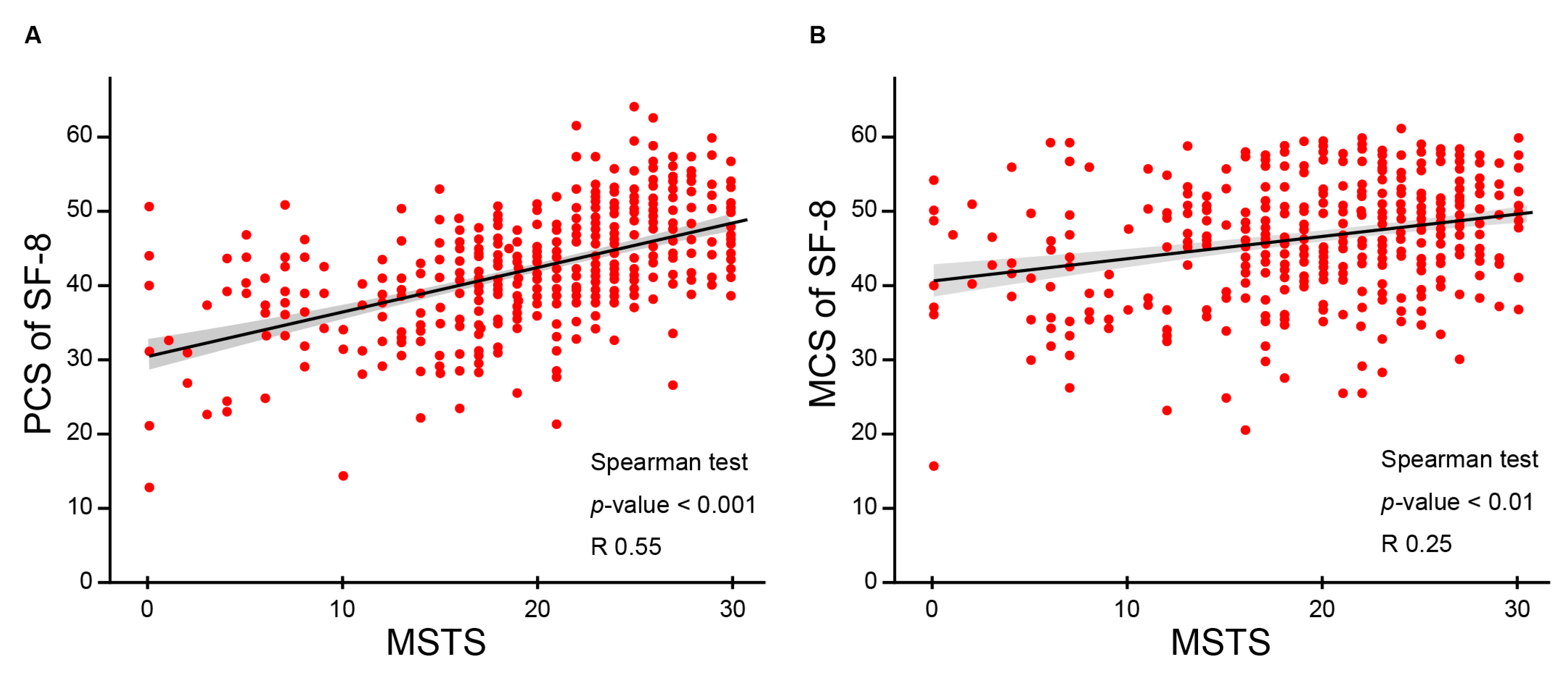
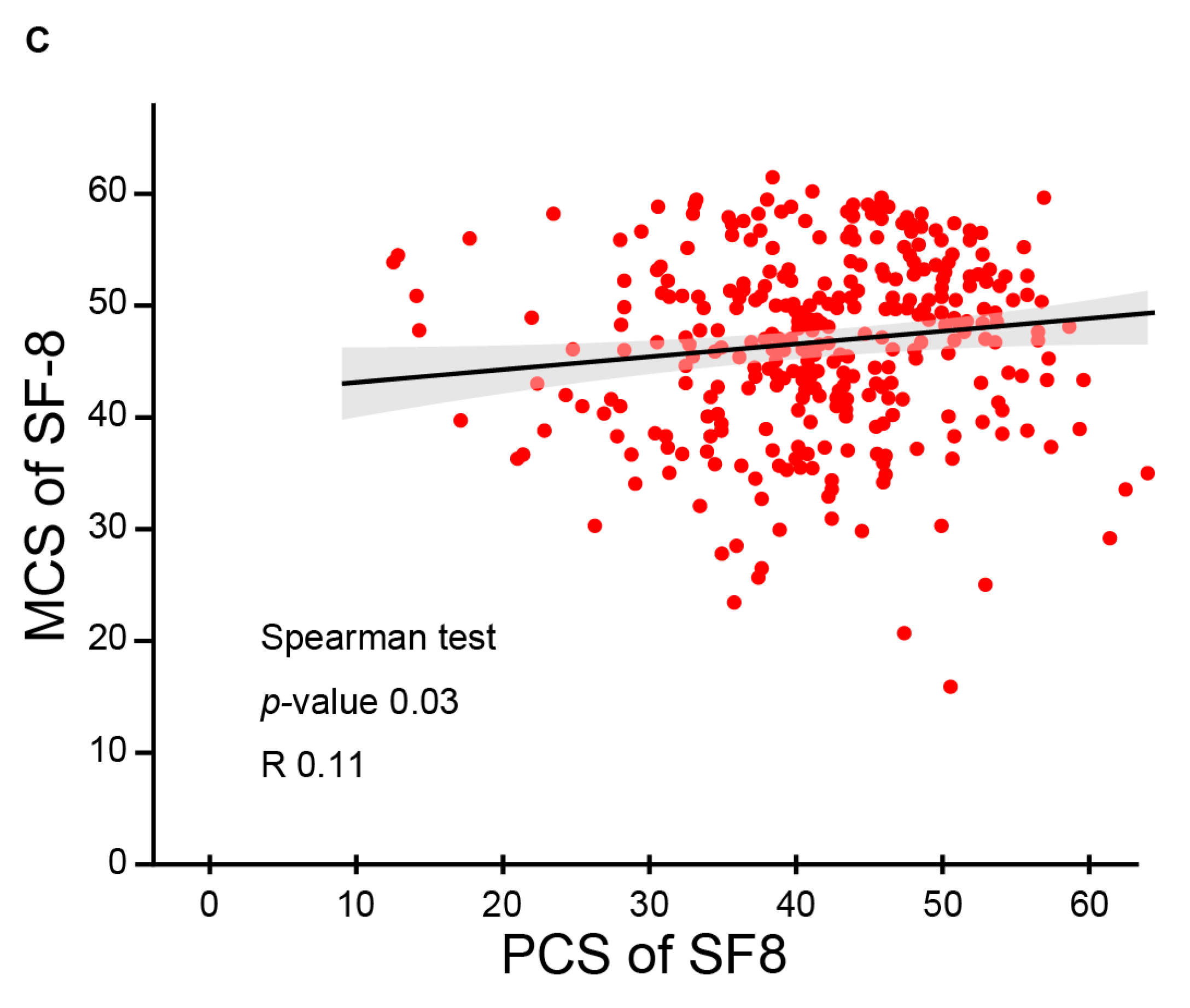
3.3. QOL and Functional Outcomes Following CIRT
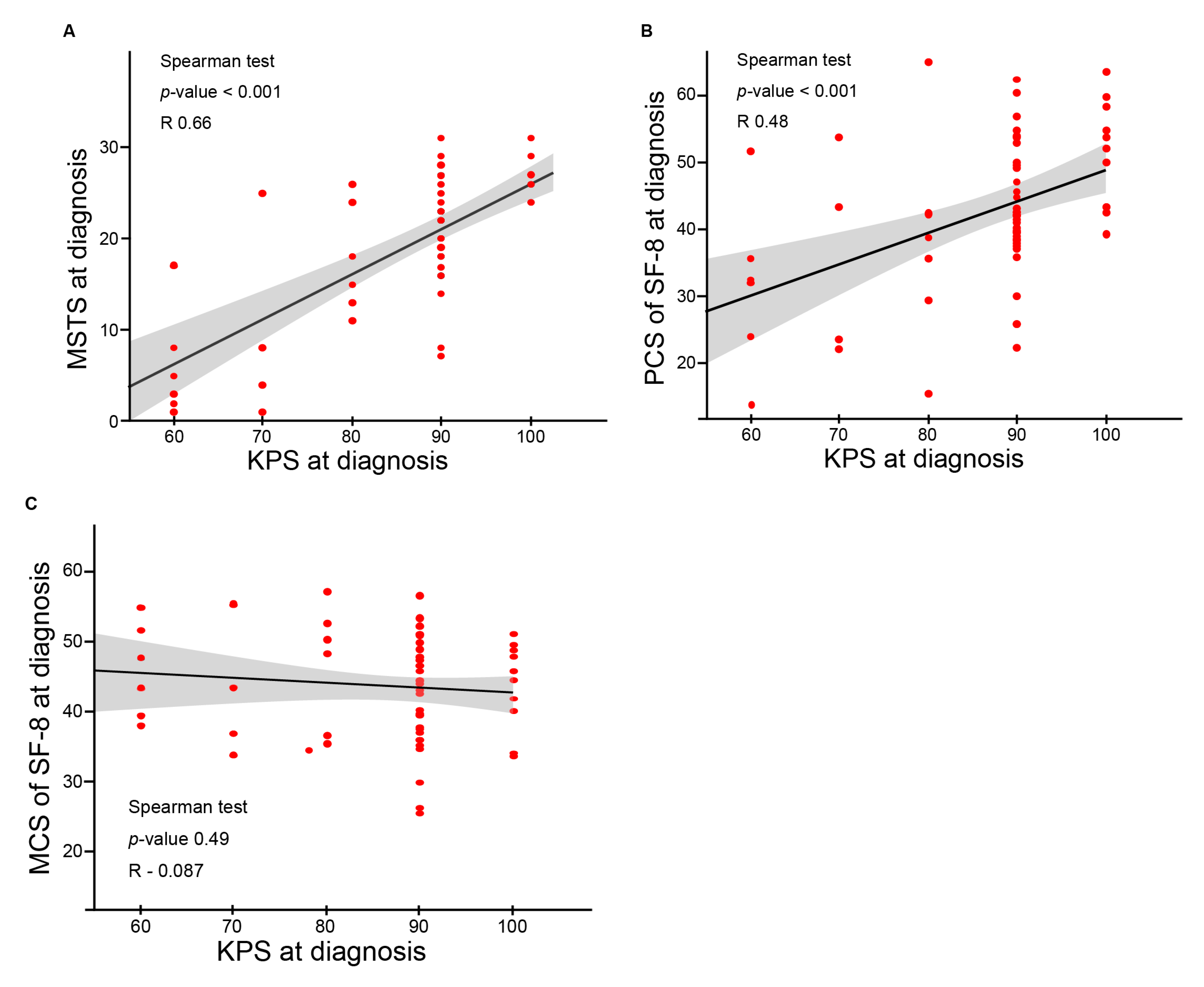
3.4. Identification of Predictors of Functional Outcomes and QOL by Uni- and Multivariate Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schöffski, P.; Cornillie, J.; Wozniak, A.; Li, H.; Hompes, D. Soft Tissue Sarcoma: An Update on Systemic Treatment Options for Patients with Advanced Disease. Oncol. Res. Treat. 2014, 37, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Stiller, C.; Trama, A.; Serraino, D.; Rossi, S.; Navarro, C.; Chirlaque, M.; Casali, P.G. Descriptive epidemiology of sarcomas in Europe: Report from the RARECARE project. Eur. J. Cancer 2013, 49, 684–695. [Google Scholar] [CrossRef]
- Larrier, N.A.; Czito, B.G.; Kirsch, D.G. Radiation Therapy for Soft Tissue Sarcoma. Surg. Oncol. Clin. N. Am. 2016, 25, 841–860. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, N.; Colterjohn, N.; Farrokhyar, F.; Tozer, R.; Figueredo, A.; Ghert, M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 2008, 113, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Enneking, W.F.; Spanier, S.S.; Malawer, M.M. The effect of the anatomic setting on the results of surgical procedures for soft parts sarcoma of the thigh. Cancer 1981, 47, 1005–1022. [Google Scholar] [CrossRef]
- Ness, K.K.; Hudson, M.M.; Ginsberg, J.P.; Nagarajan, R.; Kaste, S.C.; Marina, N.; Whitton, J.; Robison, L.L.; Gurney, J.G. Physical Performance Limitations in the Childhood Cancer Survivor Study Cohort. J. Clin. Oncol. 2009, 27, 2382–2389. [Google Scholar] [CrossRef] [PubMed]
- Imai, R.; Kamada, T.; Araki, N.; Abe, S.; Iwamoto, Y.; Ozaki, T.; Kanehira, C.; Kaya, M.; Takahashi, K.; Chuman, H.; et al. Carbon Ion Radiation Therapy for Unresectable Sacral Chordoma: An Analysis of 188 Cases. Int. J. Radiat. Oncol. 2016, 95, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Imai, R.; Kamada, T.; Araki, N.; Abe, S.; Iwamoto, Y.; Ozaki, T.; Chuman, H.; Hiraga, H.; Hiruma, T.; Kameda, N.; et al. Carbon ion radiotherapy for unresectable localized axial soft tissue sarcoma. Cancer Med. 2018, 7, 4308–4314. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Imai, R.; Kamada, T.; Maruyama, K.; Tsuji, H.; Tsujii, H.; Shioyama, Y.; Honda, H.; Isu, K. Working Group for Bone and Soft Tissue Sarcomas Impact of carbon ion radiotherapy for primary spinal sarcoma. Cancer 2013, 119, 3496–3503. [Google Scholar] [CrossRef]
- Outani, H.; Hamada, K.; Imura, Y.; Oshima, K.; Sotobori, T.; Demizu, Y.; Kakunaga, S.; Joyama, S.; Imai, R.; Okimoto, T.; et al. Comparison of clinical and functional outcome between surgical treatment and carbon ion radiotherapy for pelvic chondrosarcoma. Int. J. Clin. Oncol. 2015, 21, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Crompton, J.G.; Ogura, K.; Bernthal, N.M.; Kawai, A.; Eilber, F.C. Local Control of Soft Tissue and Bone Sarcomas. J. Clin. Oncol. 2018, 36, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Ciernik, I.F.; Niemierko, A.; Harmon, D.C.; Ba, W.K.; Chen, Y.-L.; Yock, T.I.; Ebb, D.H.; Choy, E.; Raskin, K.A.; Liebsch, N.; et al. Proton-based radiotherapy for unresectable or incompletely resected osteosarcoma. Cancer 2011, 117, 4522–4530. [Google Scholar] [CrossRef] [PubMed]
- Leiser, D.; Calaminus, G.; Malyapa, R.; Bojaxhiu, B.; Albertini, F.; Kliebsch, U.; Mikroutsikos, L.; Morach, P.; Bolsi, A.; Walser, M.; et al. Tumour control and Quality of Life in children with rhabdomyosarcoma treated with pencil beam scanning proton therapy. Radiother. Oncol. 2016, 120, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.; Orecchia, R.; Loeffler, J.S. Charged-particle therapy in cancer: Clinical uses and future perspectives. Nat. Rev. Clin. Oncol. 2017, 14, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Rettig, E.M.; D’Souza, G.; Thompson, C.B.; Koch, W.M.; Eisele, D.W.; Fakhry, C. Health-related quality of life before and after head and neck squamous cell carcinoma: Analysis of the Surveillance, Epidemiology, and End Results-Medicare Health Outcomes Survey linkage. Cancer 2016, 122, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- Gotay, C.C.; Kawamoto, C.T.; Bottomley, A.; Efficace, F. The Prognostic Significance of Patient-Reported Outcomes in Cancer Clinical Trials. J. Clin. Oncol. 2008, 26, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Dierselhuis, E.F.; Goulding, K.A.; Stevens, M.; Jutte, P.C. Intralesional treatment versus wide resection for central low-grade chondrosarcoma of the long bones. Cochrane Database Syst. Rev. 2019, 3, CD010778. [Google Scholar] [CrossRef]
- Rivard, J.D.; Puloski, S.S.; Temple, W.J.; Fyfe, A.; Kwan, M.; Schachar, N.; Kurien, E.; Lanuke, K.; Mack, L.A. Quality of Life, Functional Outcomes, and Wound Complications in Patients with Soft Tissue Sarcomas Treated with Preoperative Chemoradiation: A Prospective Study. Ann. Surg. Oncol. 2015, 22, 2869–2875. [Google Scholar] [CrossRef] [PubMed]
- Kask, G.; Barner-Rasmussen, I.; Repo, J.P.; Kjäldman, M.; Kilk, K.; Blomqvist, C.; Tukiainen, E.J. Functional Outcome Measurement in Patients with Lower-Extremity Soft Tissue Sarcoma: A Systematic Literature Review. Ann. Surg. Oncol. 2019, 26, 4707–4722. [Google Scholar] [CrossRef] [PubMed]
- Kanai, T.; Endo, M.; Minohara, S.; Miyahara, N.; Koyama-Ito, H.; Tomura, H.; Matsufuji, N.; Futami, Y.; Fukumura, A.; Hiraoka, T.; et al. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int. J. Radiat. Oncol. 1999, 44, 201–210. [Google Scholar] [CrossRef]
- Enneking, W.F.; Dunham, W.; Gebhardt, M.C.; Malawar, M.; Pritchard, D.J. A system for the functhiona evaluation of reconstructive prodedures after surgical treatment of tumors. Clin. Orthop. Relat. Res. 1993, 286, 241–246. [Google Scholar]
- Iwata, S.; Uehara, K.; Ogura, K.; Akiyama, T.; Shinoda, Y.; Yonemoto, T.; Kawai, A. Reliability and Validity of a Japanese-language and Culturally Adapted Version of the Musculoskeletal Tumor Society Scoring System for the Lower Extremity. Clin. Orthop. Relat. Res. 2016, 474, 2044–2052. [Google Scholar] [CrossRef] [PubMed]
- Uehara, K.; Ogura, K.; Akiyama, T.; Shinoda, Y.; Iwata, S.; Kobayashi, E.; Tanzawa, Y.; Yonemoto, T.; Kawano, H.; Kawai, A. Reliability and Validity of the Musculoskeletal Tumor Society Scoring System for the Upper Extremity in Japanese Patients. Clin. Orthop. Relat. Res. 2017, 475, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E.; Kosinski, M.; Dewey, J.E.; Gandek, B. How to Score and Interpret Single-Item Health Status Measures: A Manual for Users of the SF-8 Health Survey; QualityMetric, Inc.: Lincoln, RI, USA, 2001; Volume 15, p. 5. [Google Scholar]
- Fukuhara, S.; Bito, S.; Green, J.; Hsiao, A.; Kurokawa, K. Translation, Adaptation, and Validation of the SF-36 Health Survey for Use in Japan. J. Clin. Epidemiol. 1998, 51, 1037–1044. [Google Scholar] [CrossRef]
- Nishida, Y.; Kamada, T.; Imai, R.; Tsukushi, S.; Yamada, Y.; Sugiura, H.; Shido, Y.; Wasa, J.; Ishiguro, N. Clinical Outcome of Sacral Chordoma With Carbon Ion Radiotherapy Compared With Surgery. Int. J. Radiat. Oncol. 2011, 79, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.E. Sarcoma and metastatic carcinoma. J. Surg. Oncol. 2000, 73, 39–46. [Google Scholar] [CrossRef]
- Shirai, K.; Saitoh, J.-I.; Musha, A.; Abe, T.; Kobayashi, D.; Takahashi, T.; Tamaki, T.; Kawamura, H.; Takayasu, Y.; Shino, M.; et al. Prospective observational study of carbon-ion radiotherapy for non-squamous cell carcinoma of the head and neck. Cancer Sci. 2017, 108, 2039–2044. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.; O’Sullivan, B.; Bell, R.; Turcotte, R.; Catton, C.; Wunder, J.; Chabot, P.; Hammond, A.; Benk, V.; Isler, M.; et al. Function and Health Status Outcomes in a Randomized Trial Comparing Preoperative and Postoperative Radiotherapy in Extremity Soft Tissue Sarcoma. J. Clin. Oncol. 2002, 20, 4472–4477. [Google Scholar] [CrossRef]
- Davidge, K.; Bell, R.; Ferguson, P.; Turcotte, R.; Wunder, J.; Davis, A.M. Patient expectations for surgical outcome in extremity soft tissue sarcoma. J. Surg. Oncol. 2009, 100, 375–381. [Google Scholar] [CrossRef]
- Janssen, S.J.; Pereira, N.R.P.; Raskin, K.A.; Ferrone, M.L.; Hornicek, F.J.; van Dijk, C.N.; Lozano-Calderón, S.A.; Schwab, J.H. A comparison of questionnaires for assessing physical function in patients with lower extremity bone metastases. J. Surg. Oncol. 2016, 114, 691–696. [Google Scholar] [CrossRef]
- Davis, A.M.; O’Sullivan, B.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Hammond, A.; Benk, V.; Kandel, R. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother. Oncol. 2005, 75, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Q.; Eisenberg, B.L.; Kane, J.M.; Li, X.A.; Lucas, D.; Petersen, I.A.; Delaney, T.F.; Freeman, C.R.; Finkelstein, S.E.; et al. Significant Reduction of Late Toxicities in Patients With Extremity Sarcoma Treated With Image-Guided Radiation Therapy to a Reduced Target Volume: Results of Radiation Therapy Oncology Group RTOG-0630 Trial. J. Clin. Oncol. 2015, 33, 2231–2238. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, J.S.; Durante, M. Charged particle therapy—optimization, challenges and future directions. Nat. Rev. Clin. Oncol. 2013, 10, 411–424. [Google Scholar] [CrossRef]
- Sharma, S.; Takyar, S.; Manson, S.C.; Powell, S.; Penel, N. Efficacy and safety of pharmacological interventions in second- or later-line treatment of patients with advanced soft tissue sarcoma: A systematic review. BMC Cancer 2013, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- Coens, C.; Van Der Graaf, W.T.A.; Blay, J.-Y.; Chawla, S.P.; Judson, I.; Sanfilippo, R.; Manson, S.C.; Hodge, R.A.; Marreaud, S.; Prins, J.B.; et al. Health-related quality-of-life results from PALETTE: A randomized, double-blind, phase 3 trial of pazopanib versus placebo in patients with soft tissue sarcoma whose disease has progressed during or after prior chemotherapy-a European Organization for res. Cancer 2015, 121, 2933–2941. [Google Scholar] [CrossRef] [PubMed]
- Gough, N.; Koffman, J.; Ross, J.R.; Riley, J.; Judson, I. Does palliative chemotherapy really palliate and are we measuring it correctly? A mixed methods longitudinal study of health related quality of life in advanced soft tissue sarcoma. PLoS ONE 2019, 14, e0210731. [Google Scholar] [CrossRef]
- Kamada, T.; Tsujii, H.; Blakely, E.A.; Debus, J.; De Neve, W.; Durante, M.; Jäkel, O.; Mayer, R.; Orecchia, R.; Pötter, R.; et al. Carbon ion radiotherapy in Japan: An assessment of 20 years of clinical experience. Lancet Oncol. 2015, 16, e93–e100. [Google Scholar] [CrossRef]
- Malouff, T.D.; Mahajan, A.; Krishnan, S.; Beltran, C.; Seneviratne, D.S.; Trifiletti, D.M. Carbon Ion Therapy: A Modern Review of an Emerging Technology. Front. Oncol. 2020, 10, 82. [Google Scholar] [CrossRef]
- Van Roij, J.; Fransen, H.; Van De Poll-Franse, L.; Zijlstra, M.; Raijmakers, N. Measuring health-related quality of life in patients with advanced cancer: A systematic review of self-administered measurement instruments. Qual. Life Res. 2018, 27, 1937–1955. [Google Scholar] [CrossRef]
- De Vita, A.; Mercatali, L.; Recine, F.; Pieri, F.; Riva, N.; Bongiovanni, A.; Liverani, C.; Spadazzi, C.; Miserocchi, G.; Amadori, D.; et al. Current classification, treatment options, and new perspectives in the management of adipocytic sarcomas. OncoTargets Ther. 2016, 9, 6233–6246. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Overall, n = 61 | Characteristics | Overall, n = 61 |
|---|---|---|---|
| Age (mean, range) | 62 (24–93) | ||
| Gender, n (%) | Type, n (%) | ||
| Female | 19 (31.1) | Bone tumor | 37 (60.7) |
| Male | 42 (68.9) | Soft tissue tumor | 24 (39.3) |
| KPS, n (%) | Histopathology, n (%) | ||
| 100 | 10 (16.4) | Chordoma | 28 (45.9) |
| 90 | 35 (57.4) | Chondrosarcoma | 7 (11.5) |
| 80 | 6 (9.8) | Liposarcoma | 7 (11.5) |
| 70 | 4 (6.6) | Pleomorphic sarcoma | 5 (8.2) |
| 60 | 6 (9.8) | Spindle cell sarcoma | 4 (6.6) |
| Stage (Bone tumor), n (%) | Fibromyxoid sarcoma | 3 (4.9) | |
| Stage 1A | 8 (13.1) | Sarcoma, NOS | 3 (4.9) |
| Stage 1B | 21 (34.5) | Fibrosarcoma | 2 (3.3) |
| Stage 2A | 3 (4.9) | MPNST | 1 (1.6) |
| Stage 2B | 5 (8.2) | Rhabdomyosarcoma | 1 (1.6) |
| Stage (Soft tissue tumor), n (%) | Site, n (%) | ||
| Stage 1A | 3 (4.9) | Pelvis | 43 (70.5) |
| Stage 1B | 1 (1.6) | Limb | 10 (16.4) |
| Stage 2 | 3 (4.9) | Spine/paraspine | 5 (8.2) |
| Stage 3A | 12 (19.7) | Chest | 2 (3.3) |
| Stage 3B | 5 (8.2) | Retroperitoneum | 1 (1.6) |
| T classification (Bone tumor), n (%) | CIRT planning, n (%) | ||
| T1 | 11 (18.0) | Total dose (Gy) | 67.2 (64–70.4) |
| T2 | 26 (42.6) | GTV (cc) | 171.80 (78.90–928) |
| T classification (Soft tissue tumor), n (%) | Tumor grading, n (%) | ||
| T1 | 7 (11.5) | G1 | 28 |
| T2 | 13 (21.3) | G2 | 12 |
| T3 | 4 (6.6) | G3 | 15 |
| GX | 6 | ||
| Characteristics | p-Value | Characteristics | p-Value | ||||
|---|---|---|---|---|---|---|---|
| MSTS | PCS | MCS | MSTS | PCS | MCS | ||
| Age | 0.96 | 0.61 | 0.31 | Bone or soft tissue | 0.73 | 0.45 | 0.31 |
| Sex | 0.0085 | 0.014 | 0.32 | Pathology | |||
| KPS | <0.0001 | <0.0001 | 0.39 | Chordoma | 0.25 | 0.67 | 0.15 |
| Stage (Bone tumor) | Chondrosarcoma | 0.96 | 0.39 | 0.57 | |||
| Stage 1A | 0.044 | 0.39 | 0.80 | Liposarcoma | 0.52 | 0.12 | 0.81 |
| Stage 1B | 0.93 | 0.39 | 0.055 | Pleomorphic sarcoma | 0.26 | 0.34 | 0.0018 |
| Stage 2A | 0.22 | 0.34 | 0.52 | Spindle cell sarcoma | 0.71 | 0.0013 | 0.16 |
| Stage 2B | 0.0017 | 0.089 | 0.34 | Fibromyxoid sarcoma | 0.53 | 0.4 | 0.88 |
| Stage (Soft tissue tumor) | Sarcoma, NOS | 0.0023 | 0.19 | 0.78 | |||
| Stage 1A | 0.84 | 0.89 | 0.15 | Fibrosarcoma | 0.52 | 0.35 | 0.079 |
| Stage 1B | 0.84 | 0.19 | 0.55 | MPNST | 0.9 | 0.53 | 0.31 |
| Stage 2 | 0.66 | 0.081 | 0.67 | Rhabdomyosarcoma | 0.86 | 0.8 | 0.35 |
| Stage 3A | 0.50 | 0.49 | 0.70 | Site | |||
| Stage 3B | 0.18 | 0.35 | 0.26 | Pelvis | 0.74 | 0.29 | 0.32 |
| T classification (Bone tumor) | Limb | 0.21 | 0.95 | 0.07 | |||
| T1 | 0.26 | 0.16 | 0.58 | Spine/paraspine | 0.56 | 0.61 | 0.98 |
| T2 | 0.11 | 0.071 | 0.15 | Chest | 0.48 | 0.21 | 0.80 |
| T classification (Soft tissue tumor) | Retroperitoneum | 0.82 | 0.81 | 0.99 | |||
| T1 | 0.73 | 0.57 | 0.39 | Local control | 0.41 | 0.72 | 0.31 |
| T2 | 0.52 | 0.36 | 0.85 | Total dose | 0.039 | 0.017 | 0.17 |
| T3 | 0.16 | 0.51 | 0.70 | GTV volume | 0.68 | 0.11 | 0.78 |
| Tumor grading | Base line score | <0.0001 | <0.0001 | <0.0001 | |||
| G1 | 0.14 | 0.82 | 0.60 | Late adverse event | 0.07 | 0.79 | 0.63 |
| G2 | 0.51 | 0.99 | 0.97 | (Grade 2 or more) | |||
| G3 | 0.41 | 0.93 | 0.11 | ||||
| GX | 0.19 | 0.80 | 0.23 | ||||
| MSTS | ||||||
| Characteristics | Univariate | Multivariate | ||||
| β | 95% CI | p | β | 95% CI | p | |
| Local control | 0.29 | −0.4, 0.99 | 0.41 | 0.21 | −0.34, 0.71 | 0.48 |
| Adverse event | 0.58 | −0.049, 1.2 | 0.07 | 0.3 | −0.15, 0.81 | 0.17 |
| GTV volume | −0.00015 | −0.0009, 0.00059 | 0.68 | −0.00033 | −0.00084, 0.00028 | 0.32 |
| KPS | −0.055 | −0.073, −0.037 | <0.0001 | −0.057 | −0.070, −0.034 | <0.0001 |
| Sex | −0.73 | −1.26, −0.19 | 0.0085 | 0.66 | 0.20, 1.1 | 0.0052 |
| Total dose | 0.12 | 0.0061, 0.23 | 0.039 | 0.00023 | −0.10, 0.093 | 0.93 |
| PCS of SF−8 | ||||||
| Characteristics | Univariate | Multivariate | ||||
| β | 95% CI | p | β | 95% CI | p | |
| Local control | 0.048 | −0.21, 0.31 | 0.72 | 0.037 | −0.20, 0.28 | 0.75 |
| Adverse event | 0.031 | −0.21, 0.27 | 0.79 | −0.027 | −0.24, 0.019 | 0.81 |
| GTV volume | 0.000066 | −0.00021, 0.00034 | 0.63 | −0.000029 | −0.0024, 0.00028 | 0.82 |
| KPS | −0.014 | −0.022, −0.0059 | 0.0008 | −0.013 | −0.020, −0.0042 | 0.0037 |
| Sex | −0.25 | −0.46, −0.054 | 0.014 | 0.21 | 0.0043, 0.41 | 0.045 |
| Total dose | 0.047 | 0.0053, 0.088 | 0.028 | 0.014 | −0.030, 0.057 | 0.54 |
| MCS of SF−8 | ||||||
| Characteristics | Univariate | Multivariate | ||||
| β | 95% CI | p | β | 95% CI | p | |
| Local control | −0.069 | −0.20, 0.064 | 0.31 | −0.068 | −0.20, 0.069 | 0.32 |
| Adverse event | 0.056 | −0.15, 0.093 | 0.63 | 0.05 | −0.14, 0.12 | 0.88 |
| GTV volume | −0.00003 | −0.00017, 0.0070 | 0.67 | −0.000022 | −0.000017, 0.00013 | 0.77 |
| KPS | 0.0026 | −0.0017, 0.0070 | 0.23 | 0.0026 | −0.0022, 0.0073 | 0.28 |
| Sex | 0.054 | −0.054, 0.16 | 0.32 | 0.057 | −0.062, 0.18 | 0.34 |
| Total dose | 0.0016 | −0.021, 0.024 | 0.89 | 0.00062 | −0.025, 0.026 | 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komatsu, S.; Okamoto, M.; Shiba, S.; Kaminuma, T.; Okazaki, S.; Kiyohara, H.; Yanagawa, T.; Nakano, T.; Ohno, T. Prospective Evaluation of Quality of Life and Functional Outcomes after Carbon Ion Radiotherapy for Inoperable Bone and Soft Tissue Sarcomas. Cancers 2021, 13, 2591. https://doi.org/10.3390/cancers13112591
Komatsu S, Okamoto M, Shiba S, Kaminuma T, Okazaki S, Kiyohara H, Yanagawa T, Nakano T, Ohno T. Prospective Evaluation of Quality of Life and Functional Outcomes after Carbon Ion Radiotherapy for Inoperable Bone and Soft Tissue Sarcomas. Cancers. 2021; 13(11):2591. https://doi.org/10.3390/cancers13112591
Chicago/Turabian StyleKomatsu, Shuichiro, Masahiko Okamoto, Shintaro Shiba, Takuya Kaminuma, Shohei Okazaki, Hiroki Kiyohara, Takashi Yanagawa, Takashi Nakano, and Tatsuya Ohno. 2021. "Prospective Evaluation of Quality of Life and Functional Outcomes after Carbon Ion Radiotherapy for Inoperable Bone and Soft Tissue Sarcomas" Cancers 13, no. 11: 2591. https://doi.org/10.3390/cancers13112591
APA StyleKomatsu, S., Okamoto, M., Shiba, S., Kaminuma, T., Okazaki, S., Kiyohara, H., Yanagawa, T., Nakano, T., & Ohno, T. (2021). Prospective Evaluation of Quality of Life and Functional Outcomes after Carbon Ion Radiotherapy for Inoperable Bone and Soft Tissue Sarcomas. Cancers, 13(11), 2591. https://doi.org/10.3390/cancers13112591






