High Serum PD-L1 Levels Are Associated with Poor Survival in Urothelial Cancer Patients Treated with Chemotherapy and Immune Checkpoint Inhibitor Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
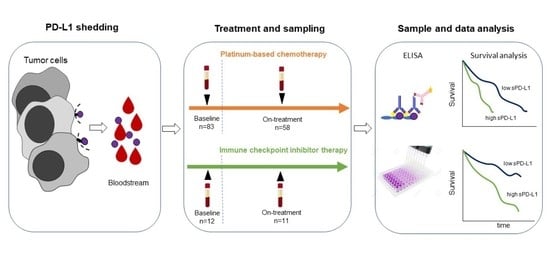
2.1. Patients
2.2. Serum PD-L1 Enzyme-Linked Immunosorbent Assay (ELISA)
2.3. Tissue PD-L1 Immunohistochemical Analysis
2.4. Statistical Analysis
3. Results
3.1. Clinical Background
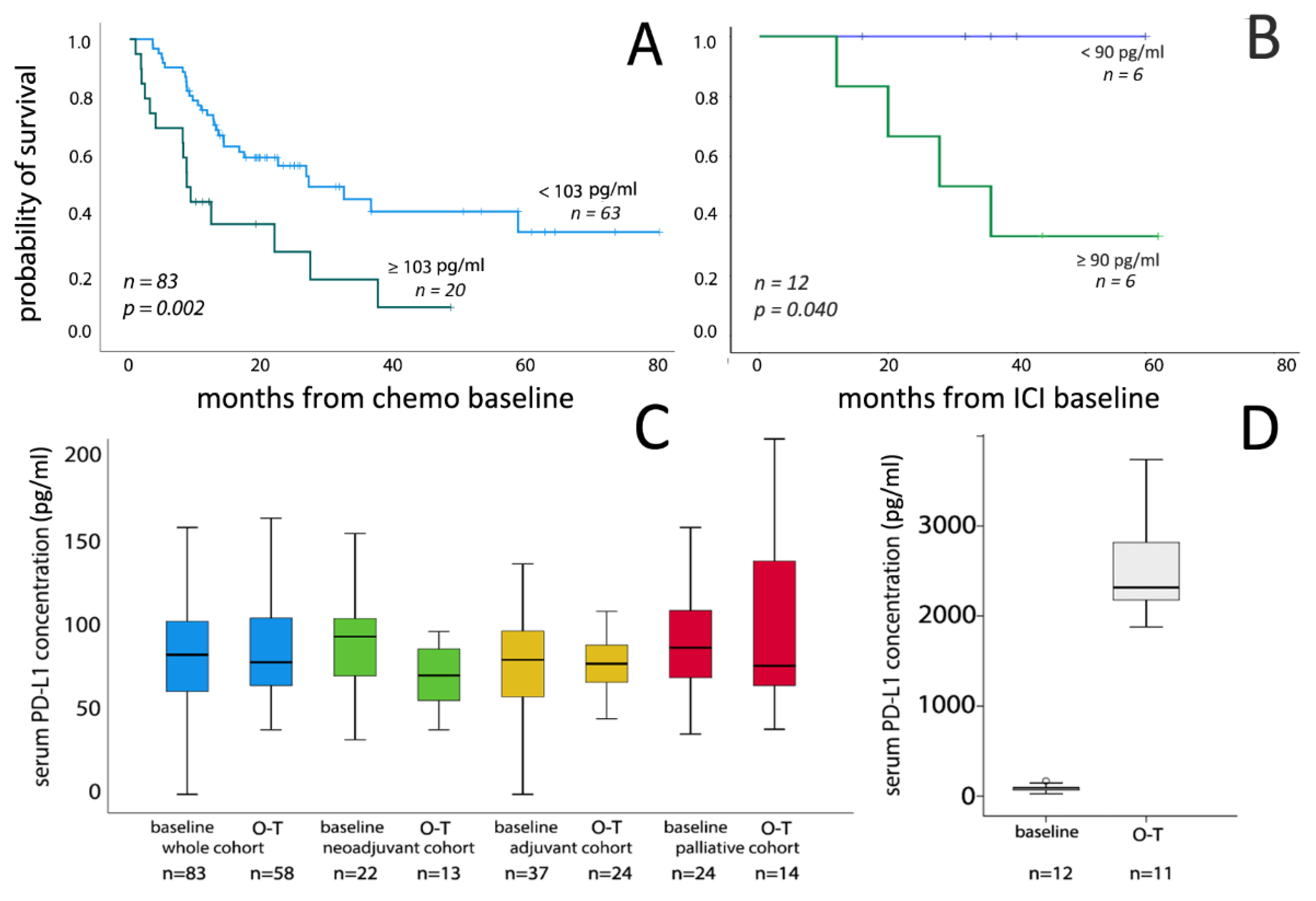
3.2. Correlation of Serum PD-L1 Concentrations with Clinicopathological Parameters and Survival
3.3. Serum PD-L1 Level Changes during Chemotherapy
3.4. Serum PD-L1 Level Changes during ICI Therapy
3.5. Tissue PD-L1 Expression and Its Corrrelation with Serum PD-L1 Levels
3.6. Correlation Between Serum PD-L1 and MMP-7 Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Stein, J.P.; Skinner, D.G. Radical cystectomy for invasive bladder cancer: Long-term results of a standard procedure. World J. Urol. 2006, 24, 296–304. [Google Scholar] [CrossRef]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Espinós, E.L.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Hanna, N.; Trinh, Q.-D.; Seisen, T.; Vetterlein, M.W.; Sammon, J.; Preston, M.A.; Lipsitz, S.R.; Bellmunt, J.; Menon, M.; Choueiri, T.K.; et al. Effectiveness of Neoadjuvant Chemotherapy for Muscle-invasive Bladder Cancer in the Current Real World Setting in the USA. Eur. Urol. Oncol. 2018, 1, 83–90. [Google Scholar] [CrossRef]
- Von Der Maase, H.; Sengelov, L.; Roberts, J.T.; Ricci, S.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Zimmermann, A.; Arning, M. Long-Term Survival Results of a Randomized Trial Comparing Gemcitabine Plus Cisplatin, With Methotrexate, Vinblastine, Doxorubicin, Plus Cisplatin in Patients with Bladder Cancer. J. Clin. Oncol. 2005, 23, 4602–4608. [Google Scholar] [CrossRef] [PubMed]
- E Rosenberg, J.; Hoffman-Censits, J.; Powles, T.; van der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef]
- Bellmunt, J.; De Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef]
- Ning, Y.; Suzman, D.; Maher, V.E.; Zhang, L.; Tang, S.; Ricks, T.; Palmby, T.; Fu, W.; Liu, Q.; Goldberg, K.B.; et al. FDA Approval Summary: Atezolizumab for the Treatment of Patients with Progressive Advanced Urothelial Carcinoma after Platinum-Containing Chemotherapy. Oncologist 2017, 22, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Suzman, D.L.; Agrawal, S.; Ning, Y.; Maher, V.E.; Fernandes, L.L.; Karuri, S.; Tang, S.; Sridhara, R.; Schroeder, J.; Goldberg, K.B.; et al. FDA Approval Summary: Atezolizumab or Pembrolizumab for the Treatment of Patients with Advanced Urothelial Carcinoma Ineligible for Cisplatin-Containing Chemotherapy. Oncologist 2019, 24, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, J.; Wada, Y.; Matsumoto, K.; Azuma, M.; Kikuchi, K.; Ueda, S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol. Immunother. 2007, 56, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Dezutter-Dambuyant, C.; Durand, I.; Alberti, L.; Bendriss-Vermare, N.; Valladeau-Guilemond, J.; Duc, A.; Magron, A.; Morel, A.-P.; Sisirak, V.; Rodriguez, C.; et al. A novel regulation of PD-1 ligands on mesenchymal stromal cells through MMP-mediated proteolytic cleavage. OncoImmunology 2016, 5, e1091146. [Google Scholar] [CrossRef] [PubMed]
- Costantini, A.; Julie, C.; Dumenil, C.; Hélias-Rodzewicz, Z.; Tisserand, J.; Dumoulin, J.; Giraud, V.; Labrune, S.; Chinet, T.; Emile, J.-F.; et al. Predictive role of plasmatic biomarkers in advanced non-small cell lung cancer treated by nivolumab. OncoImmunology 2018, 7, e1452581. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Kamai, T.; Masuda, A.; Nukui, A.; Abe, H.; Arai, K.; Yoshida, K. Higher preoperative serum levels of PD -L1 and B7-H4 are associated with invasive and metastatic potential and predictable for poor response to VEGF -targeted therapy and unfavorable prognosis of renal cell carcinoma. Cancer Med. 2016, 5, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Kushlinskii, N.; Gershtein, E.S.; Morozov, A.A.; Goryacheva, I.O.; Filipenko, M.L.; Alferov, A.A.; Bezhanova, S.D.; Bazaev, V.V.; Kazantseva, I.A. Soluble Ligand of the Immune Checkpoint Receptor (sPD-L1) in Blood Serum of Patients with Renal Cell Carcinoma. Bull. Exp. Biol. Med. 2019, 166, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Szarvas, T.; Becker, M.; Dorp, F.V.; Gethmann, C.; Tötsch, M.; Bánkfalvi, Á.; Schmid, K.W.; Romics, I.; Rübben, H.; Ergün, S. Matrix metalloproteinase-7 as a marker of metastasis and predictor of poor survival in bladder cancer. Cancer Sci. 2010, 101, 1300–1308. [Google Scholar] [CrossRef]
- Szarvas, T.; Singer, B.B.; Becker, M.; Dorp, F.V.; Jäger, T.; Szendrői, A.; Riesz, P.; Romics, I.; Rübben, H.; Ergün, S. Urinary matrix metalloproteinase-7 level is associated with the presence of metastasis in bladder cancer. BJU Int. 2010, 107, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Szarvas, T.; Hoffmann, M.J.; Olah, C.; Szekely, E.; Kiss, A.; Hess, J.; Tschirdewahn, S.; Hadaschik, B.; Grotheer, V.; Nyirady, P.; et al. MMP-7 Serum and Tissue Levels Are Associated with Poor Survival in Platinum-Treated Bladder Cancer Patients. Diagnostics 2020, 11, 48. [Google Scholar] [CrossRef]
- Hira-Miyazawa, M.; Nakamura, H.; Hirai, M.; Kobayashi, Y.; Kitahara, H.; Bou-Gharios, G.; Kawashiri, S. Regulation of programmed-death ligand in the human head and neck squamous cell carcinoma microenvironment is mediated through matrix metalloproteinase-mediated proteolytic cleavage. Int. J. Oncol. 2017, 52, 379–388. [Google Scholar] [CrossRef]
- Chen, Y.; Li, M.; Liu, J.; Pan, T.; Zhou, T.; Liu, Z.; Tan, R.; Wang, X.; Tian, L.; Chen, E.; et al. sPD-L1 Expression is Associated with Immunosuppression and Infectious Complications in Patients with Acute Pancreatitis. Scand. J. Immunol. 2017, 86, 100–106. [Google Scholar] [CrossRef]
- Szarvas, T.; Oláh, C.; Riesz, P.; Géczi, L.; Nyirády, P. A húgyhólyag urothelialis daganatainak molekuláris alcsoportbeosztása és annak klinikai vonatkozásai. Orvosi Hetil. 2019, 160, 1647–1654. [Google Scholar] [CrossRef]
- Als, A.B.; Dyrskjøt, L.; Von Der Maase, H.; Koed, K.; Mansilla, F.; Toldbod, H.E.; Jensen, J.L.; Ulhøi, B.P.; Sengeløv, L.; Jensen, K.M.; et al. Emmprin and Survivin Predict Response and Survival following Cisplatin-Containing Chemotherapy in Patients with Advanced Bladder Cancer. Clin. Cancer Res. 2007, 13, 4407–4414. [Google Scholar] [CrossRef] [PubMed]
- Groenendijk, F.H.; de Jong, J.; van de Putte, E.E.F.; Michaut, M.; Schlicker, A.; Peters, D.; Velds, A.; Nieuwland, M.; Heuvel, M.M.V.D.; Kerkhoven, R.M.; et al. ERBB2 Mutations Characterize a Subgroup of Muscle-invasive Bladder Cancers with Excellent Response to Neoadjuvant Chemotherapy. Eur. Urol. 2016, 69, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76. [Google Scholar] [CrossRef]
- Pichler, R.; Heidegger, I.; Fritz, J.; Danzl, M.; Sprung, S.; Zelger, B.; Brunner, A.; Pircher, A. PD-L1 expression in bladder cancer and metastasis and its influence on oncologic outcome after cystectomy. Oncotarget 2017, 8, 66849–66864. [Google Scholar] [CrossRef]
- Shigemori, T.; Toiyama, Y.; Okugawa, Y.; Yamamoto, A.; Yin, C.; Narumi, A.; Ichikawa, T.; Ide, S.; Shimura, T.; Fujikawa, H.; et al. Soluble PD-L1 Expression in Circulation as a Predictive Marker for Recurrence and Prognosis in Gastric Cancer: Direct Comparison of the Clinical Burden Between Tissue and Serum PD-L1 Expression. Ann. Surg. Oncol. 2018, 26, 876–883. [Google Scholar] [CrossRef]
- Tominaga, T.; Akiyoshi, T.; Yamamoto, N.; Taguchi, S.; Mori, S.; Nagasaki, T.; Fukunaga, Y.; Ueno, M. Clinical significance of soluble programmed cell death-1 and soluble programmed cell death-ligand 1 in patients with locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. PLoS ONE 2019, 14, e0212978. [Google Scholar] [CrossRef]
- Rossille, D.; Gressier, M.; Damotte, D.; Maucort-Boulch, D.; Pangault, C.; Semana, G.; Le Gouill, S.; Haioun, C.; Tarte, K.; Lamy, T.; et al. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: Results from a French multicenter clinical trial. Leukemia 2014, 28, 2367–2375. [Google Scholar] [CrossRef]
- Ruf, M.; Moch, H.; Schraml, P. PD-L1 expression is regulated by hypoxia inducible factor in clear cell renal cell carcinoma. Int. J. Cancer 2016, 139, 396–403. [Google Scholar] [CrossRef]
- E Aguirre, J.; Beswick, E.J.; Grim, C.; Uribe, G.; Tafoya, M.; Palma, G.C.; Samedi, V.; McKee, R.; Villeger, R.; Fofanov, Y.; et al. Matrix metalloproteinases cleave membrane-bound PD-L1 on CD90+ (myo-)fibroblasts in Crohn’s disease and regulate Th1/Th17 cell responses. Int. Immunol. 2020, 32, 57–68. [Google Scholar] [CrossRef]
- Zhu, X.; Lang, J. Soluble PD-1 and PD-L1: Predictive and prognostic significance in cancer. Oncotarget 2017, 8, 97671–97682. [Google Scholar] [CrossRef]
- Sarfaty, M.; Rosenberg, J.E. Antibody-Drug Conjugates in Urothelial Carcinomas. Curr. Oncol. Rep. 2020, 22, 13. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, E.; Fujita, K.; Pena, M.R.; Taheri, D.; Banno, E.; Kato, T.; Hatano, K.; Kawashima, A.; Ujike, T.; Uemura, M.; et al. Expression of Nectin-4 and PD-L1 in Upper Tract Urothelial Carcinoma. Int. J. Mol. Sci. 2020, 21, 5390. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Kockx, M.; Rodriguez-Vida, A.; Duran, I.; Crabb, S.J.; Van Der Heijden, M.S.; Szabados, B.; Pous, A.F.; Gravis, G.; Herranz, U.A.; et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat. Med. 2019, 25, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
| Variables | Whole Chemo Cohort | ICI Cohort |
|---|---|---|
| n (%) | n (%) | |
| Total number of patients | 83 | 12 |
| Age at baseline median (range) | 67 (41–81) | 69 (63–77) |
| Gender | ||
| male | 64 (77) | 10 (83) |
| female | 19 (33) | 2 (17) |
| ECOG PS at enrollment | ||
| 0 | 48 (58) | 10 (84) |
| 1 | 32 (37) | 1 (8) |
| 2 | 3 (3) | 1 (8) |
| Cystectomy data | ||
| Cystectomy performed | 59 (71) | 8 (67) |
| pT0 | 3 (5) | 1 (8) |
| pT1 | 2 (3) | 0 (0) |
| pT2 | 11 (19) | 1 (8) |
| pT3 | 30 (51) | 5 (42) |
| pT4 | 12 (20) | 1 (8) |
| n.a. | 1 | 4 (33) |
| R | 10 (17) | 0 (0) |
| LN metastasis at RC | 30 (51) | 5 (42) |
| Chemo baseline data | ||
| LN at baseline | 36 (43) | 4 (33) |
| Distant metastasis at baseline | 12 (15) | 7 (58) |
| Soft tissue lesions (lung/liver) | 9 (11) | 5 (42) |
| Bone metastasis | 3 (3) | 2 (17) |
| Setting of chemotherapy | ||
| neoadjuvant | 22 (27) | - |
| adjuvant | 33 (40) | - |
| palliative | 28 (33) | - |
| Chemotherapy regimen | ||
| Gem/Cis | 61 (73) | - |
| Gem/Carbo | 22 (27) | - |
| Atezolizumab | - | 11 (92) |
| Pembrolizumab | - | 1 (8) |
| Number of cycles median (range) | 3 (1–9) | 5 (2–17) |
| single (only one series) | 9 | 0 |
| Number of patients died (%) | 45 (54) | 4 (33) |
| Follow-up time in months median (range) | 14 (1–80) | 17 (6–31) |
| PD-L1 serum levels | ||
| PD-L1 median (range) baseline [pg/mL] | 83.0 (0.0–781) | 90.0 (25.3–169.0) |
| PD-L1 median (range) 2–3 cycle [pg/mL] | 78.5 (0.0–273.1) | 2316 (42.5–3818) |
| Variables | All Patients | Whole Chemo Cohort | ICI Cohort | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Median (Range) | p | n | Median (Range) | p | n | Median (Range) | p | ||
| Age | ≤65 | 38 | 84.4 (0.0–780.9) | 0.566 | 35 | 82.2 (0.0–780.9) | 0.430 | 3 | 86.6 (59.0–169.0) | - |
| >65 | 57 | 84.9 (25.3–243.1) | 48 | 84.0 (35.8–243.1) | 9 | 93.3 (25.3–145.0) | ||||
| Gender | Male | 74 | 86.1 (0.0–780.9) | 0.950 | 64 | 84.0 (0.0–780.9) | 0.435 | 10 | 94.2 (59.0–169.0) | - |
| Female | 10 | 82.2 (25.3–194.9) | 19 | 82.2 (40.5–194.9) | 2 | 26.1 (25.3–26.89) | ||||
| ECOG | 0 | 58 | 80.8 (0.0–169.0) | 0.022 | 48 | 80.4 (0.0–158.5) | 0.007 | 10 | 91.9 (26.9–169.0) | - |
| 1–2 | 37 | 95.9 (25.3–780.9) | 35 | 96.2 (35.8–780.9) | 2 | 59.3 (25.3–93.3) | ||||
| Stage at cystectomy | Not performed | 28 | 24 | 4 | ||||||
| n.a. | 1 | 1 | 0 | |||||||
| pT0 | 4 | 92.2 (46.0–111.4) | 0.792 | 3 | 97.7 (46.0–111.0) | 0.858 | 1 | 86.6 | - | |
| pT1 | 2 | 135.2 (75.6–194.9) | 2 | 135.2 (75.6–194.9) | 0 | - | ||||
| pT2 | 12 | 78.4 (26.9–154.9) | 11 | 80.7 (32.4–154.9) | 1 | 26.9 | ||||
| pT3 | 35 | 81.5 (25.8–324.0) | 30 | 80.4 (25.8–324.0) | 5 | 99.1 (59.0–169.0) | ||||
| pT4 | 13 | 88.7 (0.0–158.5) | 12 | 84.4 (0.0–158.5) | 1 | 93.3 | ||||
| pT1–pT2 | 18 | 83.7 (26.9–194.9) | 0.259 | 16 | 84.7 (32.4–194.9) | 0.741 | 2 | 56.8 (26.9–86.6) | - | |
| pT3–pT4 | 48 | 85.1 (0.0–324.0) | 42 | 80.4 (0.0–324.0) | 6 | 97.2 (59.0–169.0) | ||||
| LN/M status | N0/M0 | 40 | 88.6 (25.8–324.0) | 0.277 | 38 | 87.0 (38.5–324.0) | 0.209 | 2 | 87.1 (80.9–93.3) | - |
| N+ or M+ | 55 | 85.1 (0.0–158.8) | 45 | 80.7 (0.0–780.9) | 10 | 90.9 (25.3–169.0) | ||||
| Variables | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| n | HR | 95% CI | p | HR | 95% CI | p | ||
| Ag | ≤65 | 35 | ref. | - | - | - | ||
| >65 | 48 | 1.297 | 0.716–2.351 | 0.391 | - | - | - | |
| Gender | Male | 64 | ref. | - | - | - | ||
| Female | 19 | 0.815 | 0.392–1.694 | 0.584 | - | - | - | |
| ECOG | 0 | 48 | ref. | ref. | ||||
| 1–2 | 35 | 2.615 | 1.381–4.950 | 0.003 | 3.692 | 1.870–7.287 | <0.001 | |
| Stage at cystectomy * | T0–T2 | 16 | ref. | - | - | - | ||
| T3–T4 | 42 | 1.135 | 0.483–2.664 | 0.772 | - | - | - | |
| LN/M status * | N0/M0 | 38 | ref. | ref. | ||||
| N+ or M+ | 45 | 1.914 | 1.017–3.601 | 0.044 | 2.904 | 1.502–5.615 | 0.002 | |
| PD-L1 serum cc./upper 25% | <103 pg/mL | 63 | ref. | ref. | ||||
| ≥103 pg/mL | 20 | 1.381 | 1.120–1.703 | 0.002 | 1.41 | 1.135–1.752 | 0.002 | |
| PD-L1 serum cc. continuous | - | 83 | 1.002 | 0.999–1.004 | 0.126 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krafft, U.; Olah, C.; Reis, H.; Kesch, C.; Darr, C.; Grünwald, V.; Tschirdewahn, S.; Hadaschik, B.; Horvath, O.; Kenessey, I.; et al. High Serum PD-L1 Levels Are Associated with Poor Survival in Urothelial Cancer Patients Treated with Chemotherapy and Immune Checkpoint Inhibitor Therapy. Cancers 2021, 13, 2548. https://doi.org/10.3390/cancers13112548
Krafft U, Olah C, Reis H, Kesch C, Darr C, Grünwald V, Tschirdewahn S, Hadaschik B, Horvath O, Kenessey I, et al. High Serum PD-L1 Levels Are Associated with Poor Survival in Urothelial Cancer Patients Treated with Chemotherapy and Immune Checkpoint Inhibitor Therapy. Cancers. 2021; 13(11):2548. https://doi.org/10.3390/cancers13112548
Chicago/Turabian StyleKrafft, Ulrich, Csilla Olah, Henning Reis, Claudia Kesch, Christopher Darr, Viktor Grünwald, Stephan Tschirdewahn, Boris Hadaschik, Orsolya Horvath, Istvan Kenessey, and et al. 2021. "High Serum PD-L1 Levels Are Associated with Poor Survival in Urothelial Cancer Patients Treated with Chemotherapy and Immune Checkpoint Inhibitor Therapy" Cancers 13, no. 11: 2548. https://doi.org/10.3390/cancers13112548
APA StyleKrafft, U., Olah, C., Reis, H., Kesch, C., Darr, C., Grünwald, V., Tschirdewahn, S., Hadaschik, B., Horvath, O., Kenessey, I., Nyirady, P., Varadi, M., Modos, O., Csizmarik, A., & Szarvas, T. (2021). High Serum PD-L1 Levels Are Associated with Poor Survival in Urothelial Cancer Patients Treated with Chemotherapy and Immune Checkpoint Inhibitor Therapy. Cancers, 13(11), 2548. https://doi.org/10.3390/cancers13112548