The Association between Prognostic Nutritional Index (PNI) and Intraoperative Transfusion in Patients Undergoing Hepatectomy for Hepatocellular Carcinoma: A Retrospective Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. General Anesthesia
2.3. Surgical Technique
2.4. Clinical Data Collection and Outcome Assessments
2.5. Primary and Secondary Outcomes
2.6. Statistical Analysis
3. Results
3.1. Primary Outcomes
3.2. Secondary Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ikeda, K.; Hosaka, T.; Sezaki, H.; Someya, T.; Akuta, N.; Suzuki, F.; Suzuki, Y.; Saitoh, S.; Arase, Y.; et al. Natural history of compensated cirrhosis in the Child-Pugh class a compared between 490 patients with hepatitis C and 167 with B virus infections. J. Med. Virol. 2006, 78, 459–465. [Google Scholar] [CrossRef]
- Yoshida, H.; Taniai, N.; Yoshioka, M.; Hirakata, A.; Kawano, Y.; Shimizu, T.; Ueda, J.; Takata, H.; Nakamura, Y.; Mamada, Y. Current Status of Laparoscopic Hepatectomy. J. Nippon Med. Sch. 2019, 86, 201–206. [Google Scholar] [CrossRef]
- Xiao, L.-K.; Huang, P.; Wu, K.; Xiang, J.-F.; Fu, X.; Zheng, M.-Y.; Song, X.-X.; Xie, W. Effect of infrahepatic inferior vena cava partial clamping on central venous pressure and intraoperative blood loss during laparoscopic hepatectomy. Surg. Endosc. 2021, 35, 2773–2780. [Google Scholar] [CrossRef] [PubMed]
- Spolverato, G.; Ejaz, A.; Kim, Y.; Hall, B.L.; Bilimoria, K.; Cohen, M.; Ko, C.; Pitt, H.; Pawlik, T.M. Patterns of care among patients undergoing hepatic resection: A query of the National Surgical Quality Improvement Program-targeted hepatectomy database. J. Surg. Res. 2015, 196, 221–228. [Google Scholar] [CrossRef]
- Romano, F.; Garancini, M.; Uggeri, F.; Degrate, L.; Nespoli, L.; Gianotti, L.; Nespoli, A.; Uggeri, F. Bleeding in hepatic surgery: Sorting through methods to prevent it. HPB Surg. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- De Boer, M.T.; Molenaar, I.Q.; Porte, R.J. Impact of blood loss on outcome after liver resection. Dig. Surg. 2007, 24, 259–264. [Google Scholar] [CrossRef]
- Onodera, T.; Goseki, N.; Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984, 85, 1001–1005. [Google Scholar]
- Hu, Y.; Shen, J.; Liu, R.; Feng, Z.; Zhang, C.; Ling, L.; Chen, L. Prognostic value of pretreatment prognostic nutritional index in non-small cell lung cancer: A systematic review and meta-analysis. Int. J. Biol. Markers 2018, 33, 372–378. [Google Scholar] [CrossRef]
- Abe, A.; Hayashi, H.; Ishihama, T.; Furuta, H. Prognostic impact of the prognostic nutritional index in cases of resected oral squamous cell carcinoma: A retrospective study. BMC Oral Health 2021, 21, 1–11. [Google Scholar] [CrossRef]
- Man, Z.; Pang, Q.; Zhou, L.; Wang, Y.; Hu, X.; Yang, S.; Jin, H.; Liu, H. Prognostic significance of preoperative prognostic nutritional index in hepatocellular carcinoma: A meta-analysis. HPB 2018, 20, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ahn, H.J.; Yang, M.; Kim, J.A.; Kim, J.K.; Park, S.J. The prognostic nutritional index and postoperative complications after curative lung cancer resection: A retrospective cohort study. J. Thorac. Cardiovasc. Surg. 2020, 160, 276–285.e1. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, P.; Song, Y.; Sun, J.; Chen, X.; Zhao, J.; Ma, B.; Wang, Z. The prognostic nutritional index is a predictive indicator of prognosis and postoperative complications in gastric cancer: A meta-analysis. Eur. J. Surg. Oncol. (EJSO) 2016, 42, 1176–1182. [Google Scholar] [CrossRef]
- Pham, H.P.; Shaz, B.H. Update on massive transfusion. Br. J. Anaesth. 2013, 111, i71–i82. [Google Scholar] [CrossRef]
- Section 2: AKI Definition. Kidney Int. Suppl. 2012, 2, 19–36. [CrossRef]
- Cook, N.R. Quantifying the added value of new biomarkers: How and how not. Diagn. Progn. Res. 2018, 2, 1–7. [Google Scholar] [CrossRef]
- Girardis, M.; Busani, S.; Marietta, M. Severe Bleeding in Critical Care. In Anaesthesia, Pain, Intensive Care and Emergency A.P.I.C.E.; Springer: Berlin, Germany, 2007; pp. 687–693. [Google Scholar] [CrossRef]
- Janny, S.; Eurin, M.; Dokmak, S.; Toussaint, A.; Farges, O.; Paugam-Burtz, C. Assessment of the external validity of a predictive score for blood transfusion in liver surgery. HPB 2015, 17, 357–361. [Google Scholar] [CrossRef]
- Ghadimi, K.; Levy, J.; Welsby, I. Perioperative management of the bleeding patient. Br. J. Anaesth. 2016, 117, iii18–iii30. [Google Scholar] [CrossRef]
- Glance, L.G.; Dick, A.W.; Mukamel, D.B.; Fleming, F.J.; Zollo, R.A.; Wissler, R.; Salloum, R.; Meredith, U.W.; Osler, T.M. Association between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery. Anesthesiology 2011, 114, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Marietta, M.; Facchini, L.; Pedrazzi, P.; Busani, S.; Torelli, G. Pathophysiology of bleeding in surgery. Transplant. Proc. 2006, 38, 812–814. [Google Scholar] [CrossRef]
- Shirabe, K.; Kajiyama, K.; Harimoto, N.; Tsujita, E.; Wakiyama, S.; Maehara, Y. Risk factors for massive bleeding during major hepatectomy. World J. Surg. 2010, 34, 1555–1562. [Google Scholar] [CrossRef]
- Westerkamp, A.C.; Lisman, T.; Porte, R.J. How to minimize blood loss during liver surgery in patients with cirrhosis. HPB 2009, 11, 453–458. [Google Scholar] [CrossRef]
- Alkozai, E.M.; Lisman, T.; Porte, R.J. Bleeding in liver surgery: Prevention and treatment. Clin. Liver Dis. 2009, 13, 145–154. [Google Scholar] [CrossRef]
- Steib, A.; Freys, G.; Lehmann, C.; Meyer, C.; Mahoudeau, G. Intraoperative blood losses and transfusion requirements during adult liver transplantation remain difficult to predict. Can. J. Anesth. J. Can. D’anesthésie 2001, 48, 1075–1079. [Google Scholar] [CrossRef]
- Araújo, T.; Cordeiro, A.; Proença, P.; Perdigoto, R.; Martins, A.; Barroso, E. Predictive variables affecting transfusion requirements in orthotopic liver transplantation. Transplant. Proc. 2010, 42, 1758–1759. [Google Scholar] [CrossRef]
- Massicotte, L.; Beaulieu, D.; Roy, J.-D.; Marleau, D.; Vandenbroucke, F.; Dagenais, M.; Lapointe, R.; Roy, A. MELD Score and blood product requirements during liver transplantation: No link. Transplantation 2009, 87, 1689–1694. [Google Scholar] [CrossRef]
- Caldwell, S.H.; Hoffman, M.; Lisman, T.; Macik, B.G.; Northup, P.G.; Reddy, K.R.; Tripodi, A.; Sanyal, A.J. Coagulation in liver disease group coagulation disorders and hemostasis in liver disease: Pathophysiology and critical assessment of current management. Hepatology 2006, 44, 1039–1046. [Google Scholar] [CrossRef]
- Tripodi, A.; Mannucci, P.M. Abnormalities of hemostasis in chronic liver disease: Reappraisal of their clinical significance and need for clinical and laboratory research. J. Hepatol. 2007, 46, 727–733. [Google Scholar] [CrossRef]
- Choi, S.-S.; Cho, S.-S.; Kim, S.-H.; Jun, I.-G.; Hwang, G.-S.; Kim, Y.-K. Factors associated with blood transfusion in donor hepatectomy. Transplantation 2013, 96, 1000–1007. [Google Scholar] [CrossRef]
- Pandey, C.K. Intraoperative blood loss in orthotopic liver transplantation: The predictive factors. World J. Gastrointest. Surg. 2015, 7, 86–93. [Google Scholar] [CrossRef]
- Ke, M.; Xu, T.; Li, N.; Ren, Y.; Shi, A.; Lv, Y.; He, H. Prognostic nutritional index predicts short-term outcomes after liver resection for hepatocellular carcinoma within the Milan criteria. Oncotarget 2016, 7, 81611–81620. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hong, B.; Park, J.-Y.; Hwang, J.-H.; Kim, Y.-K. Impact of prognostic nutritional index on postoperative pulmonary complications in radical cystectomy: A propensity score-matched analysis. Ann. Surg. Oncol. 2021, 28, 1859–1869. [Google Scholar] [CrossRef]
- Ad, N.; Massimiano, P.S.; Burton, N.A.; Halpin, L.; Pritchard, G.; Shuman, D.J.; Holmes, S.D. Effect of patient age on blood product transfusion after cardiac surgery. J. Thorac. Cardiovasc. Surg. 2015, 150, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Cleland, S.; Corredor, C.; Ye, J.J.; Srinivas, C.; A McCluskey, S. Massive haemorrhage in liver transplantation: Consequences, prediction and management. World J. Transplant. 2016, 6, 291–305. [Google Scholar] [CrossRef]
- Eghbal, M.H.; Samadi, K.; Khosravi, M.B.; Sahmeddini, M.A.; Ghaffaripoor, S.; Ghorbani, M.; Shokrizadeh, S. The impact of preoperative variables on intraoperative blood loss and transfusion requirements during orthotopic liver transplant. Exp. Clin. Transplant. 2019, 17, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Takahara, T.; Wakabayashi, G.; Beppu, T.; Aihara, A.; Hasegawa, K.; Gotohda, N.; Hatano, E.; Tanahashi, Y.; Mizuguchi, T.; Kamiyama, T.; et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: A multi-institutional Japanese study. J. Hepato-Biliary-Pancreatic Sci. 2015, 22, 721–727. [Google Scholar] [CrossRef]
- Kozek-Langenecker, S.A. Effects of Hydroxyethyl starch solutions on hemostasis. Anesthesiology 2005, 103, 654–660. [Google Scholar] [CrossRef]
- Hilbert-Carius, P.; Schwarzkopf, D.; Reinhart, K.; Hartog, C.S.; Lefering, R.; Bernhard, M.; Struck, M.F. Synthetic colloid resuscitation in severely injured patients: Analysis of a nationwide trauma registry (TraumaRegister DGU). Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Carson, J.L.; Noveck, H.; Berlin, J.A.; Gould, S.A. Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion 2002, 42, 812–818. [Google Scholar] [CrossRef]
- Finch, C.A.; Lenfant, C. Oxygen transport in man. N. Engl. J. Med. 1972, 286, 407–415. [Google Scholar] [CrossRef]
- Goodnough, L.T.; Levy, J.H.; Murphy, M.F. Concepts of blood transfusion in adults. Lancet 2013, 381, 1845–1854. [Google Scholar] [CrossRef]
- Yang, Z.-L.; Guo, T.; Zhu, D.-L.; Zheng, S.; Han, D.-D.; Chen, Y. Risk factors of portal vein thrombosis after splenectomy in patients with liver cirrhosis. Hepatoma Res. 2020, 2020, 37. [Google Scholar] [CrossRef]
- Krzanicki, D.; Sugavanam, A.; Mallett, S. Intraoperative hypercoagulability during liver transplantation as demonstrated by thromboelastography. Liver Transplant. 2013, 19, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.F.; Lo, J.W. The development of hypercoagulability state, as measured by thrombelastography, associated with in-traoperative surgical blood loss. Anaesth. Intensive Care 1996, 24, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Bothe, A.; Steele, G. Is there a role for perioperative nutritional support in liver resection? HPB Surg. 1997, 10, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Ponziani, F.R. Portal vein thrombosis: Insight into physiopathology, diagnosis, and treatment. World J. Gastroenterol. 2010, 16, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Campillo, B.; Richardet, J.-P.; Bories, P.-N. Validation of body mass index for the diagnosis of malnutrition in patients with liver cirrhosis. Gastroentérologie Clinique Biologique 2006, 30, 1137–1143. [Google Scholar] [CrossRef]
- Lee, K.R.; Park, S.O.; Kim, S.Y.; Hong, D.Y.; Kim, J.W.; Baek, K.J.; Shin, D.H.; Lee, Y.H. Red cell distribution width as a novel marker for predicting high-risk from upper gastro-intestinal bleeding patients. PLoS ONE 2017, 12, e0187158. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, J.R.; Wolberg, A.S. Red blood cells in thrombosis. Blood 2017, 130, 1795–1799. [Google Scholar] [CrossRef]
- Semba, R.D.; Patel, K.V.; Ferrucci, L.; Sun, K.; Roy, C.N.; Guralnik, J.M.; Fried, L.P. Serum antioxidants and inflammation predict red cell distribution width in older women: The women’s health and aging study I. Clin. Nutr. 2010, 29, 600–604. [Google Scholar] [CrossRef]
- Moon, D.-B.; Lee, S.-G.; Hwang, S.; Kim, K.-H.; Ahn, C.-S.; Ha, T.-Y.; Song, G.-W.; Jung, D.-H.; Park, G.-C.; Namkoong, J.-M.; et al. More than 300 consecutive living donor liver transplants a year at a single center. Transplant. Proc. 2013, 45, 1942–1947. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Chen, Y.-S.; Lee, P.-H.; Lo, G.-H.; Hsu, C.-C.; Hsieh, P.-M.; Koh, K.W.; Bair, M.-J.; Dai, C.-Y.; Huang, J.-F.; et al. Significant predictors of overall survival in patients with hepatocellular carcinoma after surgical resection. PLoS ONE 2018, 13, e0202650. [Google Scholar] [CrossRef] [PubMed]
| Demographic, Laboratory, and Intraoperative Variables | |||
|---|---|---|---|
| Prognostic Nutritional Index | |||
| <44 (n = 333) | ≥44 (n = 732) | p-Value | |
| Demographic variables | |||
| Age; years | 57.14 ± 10.67 | 55.06 ± 10.10 | 0.026 |
| Sex; female | 85 (25.53) | 124 (16.94) | 0.002 |
| Weight; kg | 64.21 ± 10.16 | 67.56 ± 10.17 | 0.185 |
| BMI; kg.m−2 | 23.83 ± 3.00 | 24.33 ± 2.82 | 0.038 |
| Liver cirrhosis | 124 (37.24) | 264 (36.7) | 0.764 |
| Etiology | 0.623 | ||
| HBV | 258 (77.48) | 540 (73.77) | |
| HCV | 26 (7.81) | 70 (9.56) | |
| Alcoholic | 30 (9.01) | 76 (10.38) | |
| NAFLD | 19 (5.71) | 46 (6.28) | |
| Antiviral therapy | 143 (42.94) | 320 (43.72) | 0.866 |
| TACE | 66 (19.82) | 132 (18.03) | 0.542 |
| TNM staging | 0.046 | ||
| 1 | 194 (58.38) | 491 (67.03) | |
| 2 | 20 (5.99) | 41 (5.61) | |
| 3A | 8 (2.4) | 8 (1.09) | |
| 3B | 2 (0.6) | 3 (0.41) | |
| 4A | 95 (28.44) | 174 (23.8) | |
| 4B | 14 (4.19) | 15 (2.05) | |
| Number of tumors | |||
| Solitary | 290 (87.09) | 658 (89.89) | 0.211 |
| ≥2 | 43 (12.91) | 74 (10.11) | |
| Tumor size | 5.61 ± 4.83 | 4.00 ± 2.97 | <0.001 |
| Lymph node invasion | 103 (30.93) | 183 (0.25) | 0.025 |
| Metastasis | 14 (4.20) | 16 (2.19) | 0.100 |
| DM | 24 (7.21) | 43 (5.87) | 0.487 |
| HTN | 29 (8.71) | 45 (6.15) | 0.163 |
| CAD | 2 (0.60) | 7 (0.96) | 0.728 |
| MELD scores | 7.70 ± 1.42 | 7.01 ± 1.00 | <0.001 |
| CTP scores | 5.72 ± 0.57 | 5.08 ± 0.27 | <0.001 |
| Laboratory Variables | |||
| WBC | 4.65 ± 1.84 | 5.74 ± 1.65 | <0.001 |
| Hemoglobin | 12.98 ± 1.66 | 14.33 ± 1.40 | <0.001 |
| Platelets | 152.20 ± 85.86 | 168.57 ± 55.21 | 0.163 |
| Prothrombin time | 1.07 ± 0.09 | 1.02 ± 0.06 | <0.001 |
| Creatinine; mg.dL−1 | 0.79 ± 0.18 | 0.84 ± 0.17 | <0.001 |
| eGFR; mL/min/1.73m2 | 68.70 ± 14.66 | 74.28 ± 13.09 | 0.125 |
| Total bilirubin | 0.84 ± 0.47 | 0.77 ± 0.34 | 0.001 |
| AST | 49.17 ± 45.70 | 35.66 ± 20.68 | <0.001 |
| ALT | 37.38 ± 32.29 | 36.39 ± 25.55 | 0.049 |
| Sodium | 139.27 ± 2.96 | 140.10 ± 2.30 | <0.001 |
| RDW | 13.52 ± 1.55 | 12.91 ± 1.03 | <0.001 |
| PNI | 40.38 ± 3.18 | 49.77 ± 4.01 | <0.001 |
| Intraoperative Variables | |||
| Operation time; min | 273.83 ± 85.49 | 266.20 ± 76.29 | 0.028 |
| Laparoscopic surgery | 67 (20.12) | 166 (22.68) | 0.392 |
| Extensive surgery (≥3 segments) | 13 (3.90) | 29 (3.96) | 0.901 |
| Total fluids; mL/kg | 42.01 ± 22.02 | 38.06 ± 18.06 | 0.729 |
| Colloid (mL/kg) | 4.80 ± 7.05 | 4.61 ± 5.20 | 0.002 |
| Synthetic colloid use | 142 (42.64) | 388 (53.01) | 0.002 |
| Urine output; mL/kg/hr | 1.89 ± 1.38 | 1.67 ± 1.05 | 0.469 |
| Surgical outcomes of the study population | |||
| Prognostic Nutritional Index | |||
| <44 (n = 333) | ≥44 (n = 732) | p-Value | |
| Transfusions | |||
| RBC transfusion | 42 (12.61) | 21 (2.87) | <0.001 |
| FFP transfusion | 5 (1.50) | 2 (0.27) | 0.034 |
| Platelet transfusion | 3 (0.90) | 0 (0.00) | 0.030 |
| Massive transfusion (≥4 units) | 17 (5.11) | 7 (0.96) | <0.001 |
| Postoperative transfusion | 11 (3.30) | 22 (3.00) | 0.945 |
| Surgical outcomes | |||
| Hospital days | 21.18 ± 14.14 | 20.79 ± 12.65 | 0.851 |
| AKI | 24 (7.21) | 41 (5.60) | 0.334 |
| PHLF | 61 (18.32) | 34 (4.64) | <0.001 |
| ICU admission | 25 (7.51) | 53 (7.24) | 0.899 |
| ICU stay (≥2 days) | 14 (4.20) | 18 (2.46) | 0.125 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| PNI (<44) | 5.25 | 3.04–9.08 | <0.001 | 2.20 | 1.06–4.60 | 0.035 |
| Age | 1.04 | 1.02–1.07 | 0.002 | 1.04 | 1.01–1.08 | 0.010 |
| Sex (male) | 0.94 | 0.50–1.76 | 0.835 | |||
| BMI | 0.87 | 0.79–0.96 | 0.004 | |||
| DM | 1.62 | 0.67–3.92 | 0.280 | |||
| HTN | 1.74 | 0.77–3.98 | 0.186 | |||
| MELD scores | 1.37 | 1.15–1.63 | <0.001 | 1.26 | 1.00–1.61 | 0.054 |
| CTP scores | 12.40 | 4.55–33.80 | <0.001 | |||
| TNM staging | 0.008 | |||||
| 1 | 1.00(Ref.) | |||||
| 2 | 2.10 | 0.78–5.64 | 0.143 | |||
| 3 | 1.17 | 0.15–9.06 | 0.878 | |||
| 4 | 2.53 | 1.48–4.33 | 0.001 | |||
| Hemoglobin | 0.57 | 0.49–0.67 | <0.001 | 0.59 | 0.47–0.74 | <0.001 |
| RDW | 1.34 | 1.16–1.53 | <0.001 | 1.07 | 0.83–1.38 | 0.610 |
| Operation time; min | 1.01 | 1.01–1.02 | <0.001 | 1.02 | 1.01–1.02 | <0.001 |
| Synthetic colloid use | 3.45 | 1.91–6.25 | <0.001 | 2.30 | 1.12–4.73 | 0.024 |
| Extensive surgery (≥3 segments) | 6.63 | 3.15–13.92 | <0.001 | 1.36 | 0.48–3.85 | 0.561 |
| Laparoscopic surgery | 0.17 | 0.05–0.54 | 0.003 | 0.16 | 0.04–0.57 | 0.005 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| PNI (<44) | 2.20 | 1.71–2.83 | <0.001 | 1.68 | 1.27–2.24 | <0.001 |
| Age | 1.00 | 0.99–1.01 | 0.642 | 0.99 | 0.98–1.01 | 0.301 |
| Sex (male) | 1.25 | 0.90–1.75 | 0.186 | 1.14 | 0.81–1.61 | 0.461 |
| BMI | 0.94 | 0.90–0.98 | 0.041 | |||
| DM | 1.53 | 0.99–2.37 | 0.057 | 1.49 | 0.94–2.35 | 0.087 |
| HTN | 0.83 | 0.49–1.40 | 0.488 | |||
| CAD | 0.94 | 0.23–3.75 | 0.924 | |||
| MELD scores | 1.24 | 1.14–1.35 | <0.001 | 1.17 | 1.06–1.29 | 0.002 |
| CTP scores | 2.66 | 1.32–5.39 | 0.006 | |||
| TNM staging | <0.001 | <0.001 | ||||
| 1 | 1.00(Ref.) | 1.00(Ref.) | ||||
| 2 | 1.65 | 0.94–2.88 | 0.081 | 1.57 | 0.90–2.76 | 0.013 |
| 3 | 3.06 | 1.49–6.29 | 0.002 | 2.61 | 1.26–5.39 | 0.010 |
| 4 | 3.72 | 2.86–4.85 | <0.001 | 3.72 | 2.84–4.88 | <0.001 |
| Hemoglobin | 0.85 | 0.79–0.92 | <0.001 | |||
| RDW | 1.16 | 1.09–1.22 | <0.001 | |||
| Operation time; min | 1.00 | 1.00–1.00 | <0.001 | |||
| Synthetic Colloid use | 1.49 | 1.16–1.92 | 0.002 | 1.70 | 1.31–2.22 | <0.001 |
| Laparoscopic surgery | 0.50 | 0.35–0.72 | <0.001 | |||
| Transfusion | 4.00 | 2.83–5.66 | <0.001 | 2.21 | 1.51–3.23 | <0.001 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI * | p-Value | |
| RBC transfusion | 5.25 | 3.04–9.08 | <0.001 | 2.20 | 1.06–4.60 | 0.035 |
| PHLF | 4.60 | 2.96–7.16 | <0.001 | 3.02 | 1.87–4.87 | <0.001 |
| HR | 95% CI | p-value | HR | 95% CI † | p-value | |
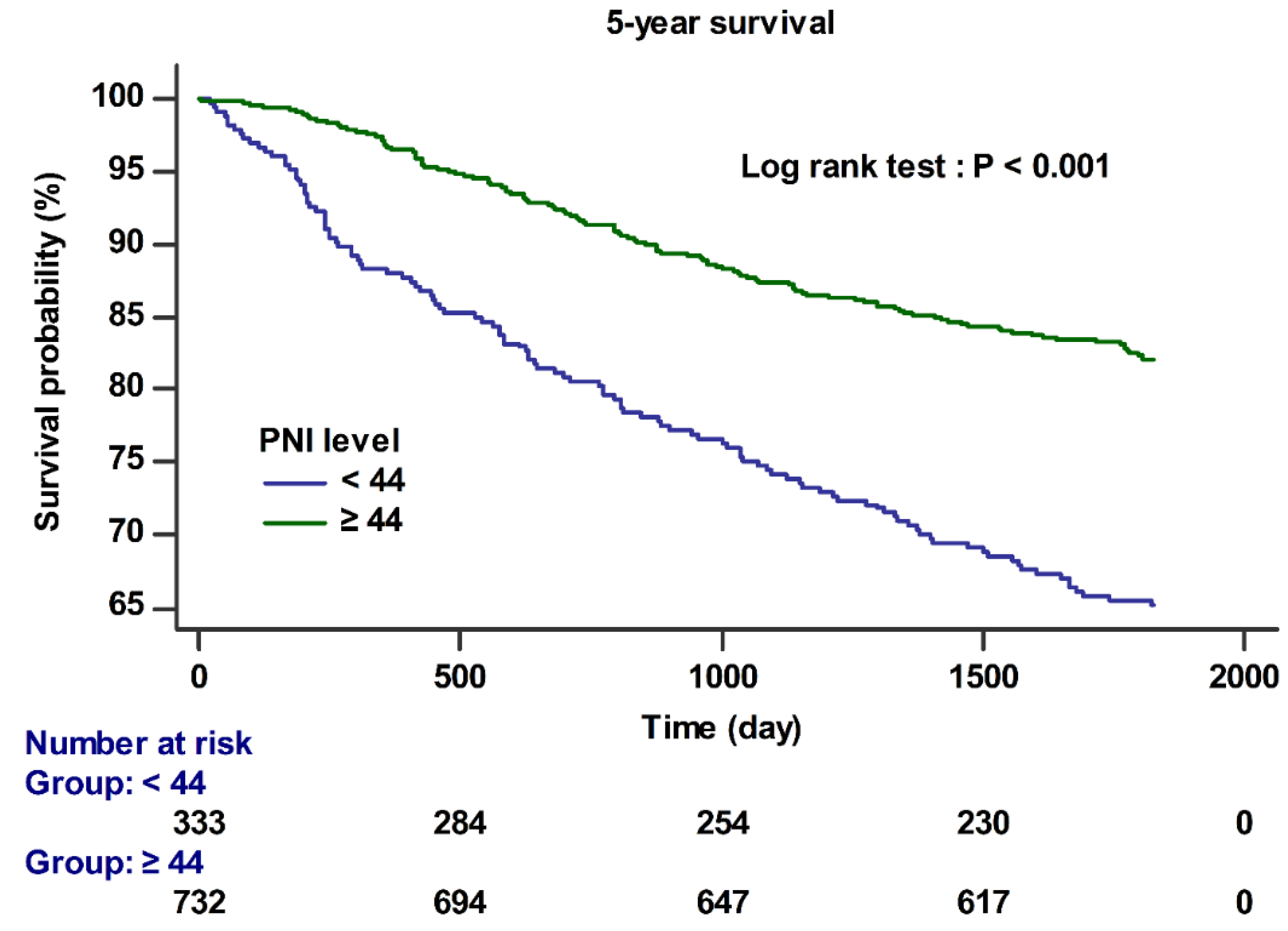
| 1-year survival | 3.86 | 2.30–6.48 | <0.001 | 2.98 | 1.66–5.38 | <0.001 |
| 5-year survival | 2.45 | 1.83–3.29 | <0.001 | 1.68 | 1.27–2.24 | <0.001 |
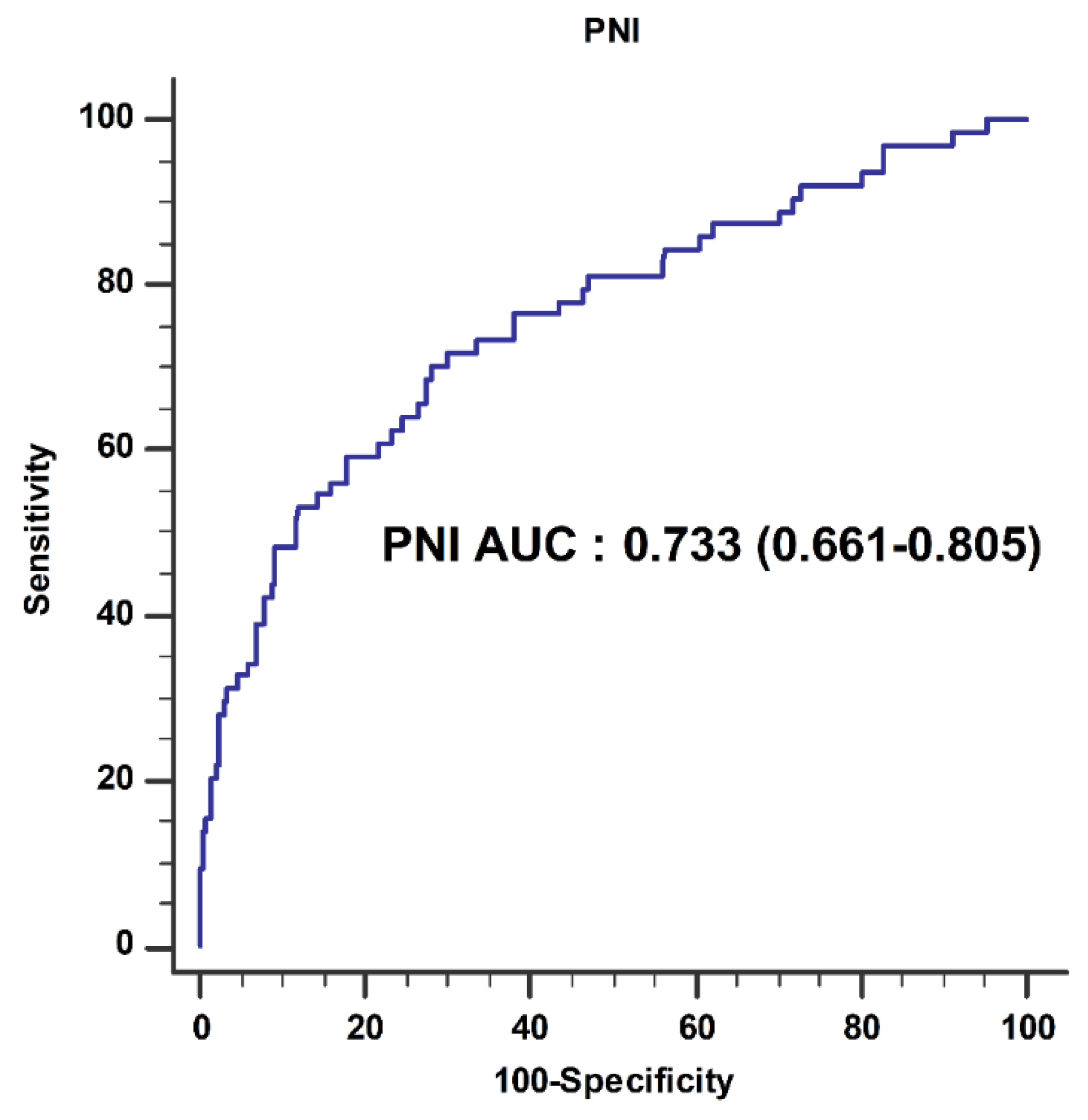
| AUC (95% CI) | p-Value | NRI (95% CI) | p-Value | ||
|---|---|---|---|---|---|
| Transfusion | Model 1 * | 0.757 (0.694–0.820) | |||
| Model 1 * + PNI | 0.768 (0.702–0.834) | 0.506 | 0.392 (0.143–0.641) | 0.002 | |
| 5-year survival | Model 2 † | 0.725 (0.692–0.758) | |||
| Model 2 † + PNI | 0.742 (0.710–0.773) | 0.021 | 0.136 (0.041–0.213) | 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, J.H.; Kim, S.-H.; Jun, I.-G.; Kang, S.-J.; Kim, B.; Kim, S.; Song, J.-G. The Association between Prognostic Nutritional Index (PNI) and Intraoperative Transfusion in Patients Undergoing Hepatectomy for Hepatocellular Carcinoma: A Retrospective Cohort Study. Cancers 2021, 13, 2508. https://doi.org/10.3390/cancers13112508
Sim JH, Kim S-H, Jun I-G, Kang S-J, Kim B, Kim S, Song J-G. The Association between Prognostic Nutritional Index (PNI) and Intraoperative Transfusion in Patients Undergoing Hepatectomy for Hepatocellular Carcinoma: A Retrospective Cohort Study. Cancers. 2021; 13(11):2508. https://doi.org/10.3390/cancers13112508
Chicago/Turabian StyleSim, Ji Hoon, Sung-Hoon Kim, In-Gu Jun, Sa-Jin Kang, Bomi Kim, Seonok Kim, and Jun-Gol Song. 2021. "The Association between Prognostic Nutritional Index (PNI) and Intraoperative Transfusion in Patients Undergoing Hepatectomy for Hepatocellular Carcinoma: A Retrospective Cohort Study" Cancers 13, no. 11: 2508. https://doi.org/10.3390/cancers13112508
APA StyleSim, J. H., Kim, S.-H., Jun, I.-G., Kang, S.-J., Kim, B., Kim, S., & Song, J.-G. (2021). The Association between Prognostic Nutritional Index (PNI) and Intraoperative Transfusion in Patients Undergoing Hepatectomy for Hepatocellular Carcinoma: A Retrospective Cohort Study. Cancers, 13(11), 2508. https://doi.org/10.3390/cancers13112508






