COVID-19 Risk Factors for Cancer Patients: A First Report with Comparator Data from COVID-19 Negative Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analyses
3. Results
3.1. Cohort Demographics
3.2. Risk of Developing COVID-19
3.3. Risk of COVID-19 Severity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
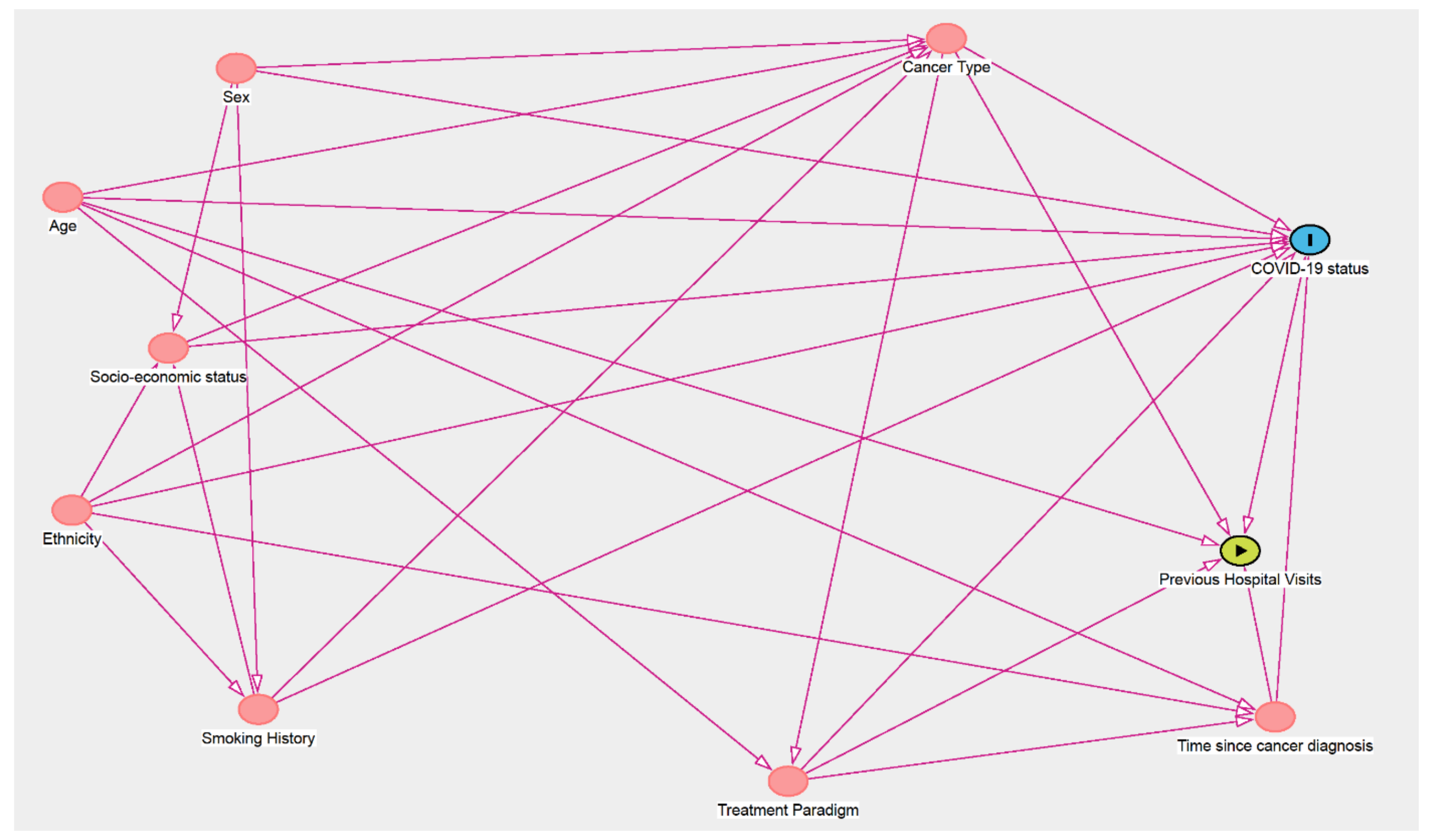
| Main Exposure | Minimal Sufficient Adjustment sets for Estimating the Total Effect of the Exposure Variable on COVID-19 Status/Severity |
|---|---|
| Age | None |
| Sex | None |
| Socio-Economic Status | Ethnicity, Smoking History |
| Ethnicity | None |
| Smoking History | Ethnicity, Sex |
| Cancer Type | Age, Ethnicity, Sex, Smoking History, Socio-economic status |
| Tumour stage | None |
| Treatment paradigm | Age, Cancer Type |
| Time since cancer diagnosis | Age, Ethnicity, Treatment Paradigm |
| Previous Hospital Visits | Age, Cancer type, Time since cancer diagnosis, treatment paradigm |
References
- Pijls, B.G.; Jolani, S.; Atherley, A.; Derckx, R.T.; Dijkstra, J.I.R.; Franssen, G.H.L.; Hendriks, S.; Richters, A.; Venemans-Jellema, A.; Zalpuri, S.; et al. Demographic risk factors for COVID-19 infection, severity, ICU admission and death: A meta-analysis of 59 studies. BMJ Open 2021, 11, e044640. [Google Scholar] [CrossRef]
- Moujaess, E.; Kourie, H.R.; Ghosn, M. Cancer patients and research during COVID-19 pandemic: A systematic review of current evidence. Crit. Rev. Oncol. 2020, 150, 102972. [Google Scholar] [CrossRef]
- Salunke, A.A.; Nandy, K.; Pathak, S.K.; Shah, J.; Kamani, M.; Kottakota, V.; Thivari, P.; Pandey, A.; Patel, K.; Rathod, P.; et al. Impact of COVID -19 in cancer patients on severity of disease and fatal outcomes: A systematic review and meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1431–1437. [Google Scholar] [CrossRef]
- Li, R.; Pei, S.; Chen, B.; Song, Y.; Zhang, T.; Yang, W.; Shaman, J. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science 2020, 368, 489–493. [Google Scholar] [CrossRef]
- Moss, C.; Dolly, S.; Russell, B.; Lei, M.; Papa, S.; Sullivan, R.; Hemelrijck, M.V.; Rigg, A. One Piece of the Jigsaw for the Cancer Recovery Strategy: Prevalence of COVID-19 in patients with cancer. Cancer Control 2020, 27, 1073274820950844. [Google Scholar] [CrossRef]
- World Health Organization Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. Interim guidance. Pediatr. Med. Rodz. 2020, 16, 9–26. [Google Scholar] [CrossRef]
- Russell, B.; Moss, C.; Papa, S.; Irshad, S.; Ross, P.; Spicer, J.; Kordasti, S.; Crawley, D.; Wylie, H.; Cahill, F.; et al. Factors Affecting COVID-19 Outcomes in Cancer Patients: A First Report from Guy’s Cancer Center in London. Front. Oncol. 2020, 10, 1279. [Google Scholar] [CrossRef] [PubMed]
- Palmer, K.; Sylva, R.; Russell, B.; Moss, C.; Shah, V.; Ko, T.K.; George, G.; Kordasti, S.; Crawley, D.; Wylie, H.; et al. Factors affecting COVID-19 outcomes in cancer patients from Guy’s Cancer Center and King’s College Hospital. In AACR Virtual Meeting: COVID-19 and Cancer. AACR 2021. [Google Scholar]
- Moss, C.; Haire, A.; Cahill, F.; Enting, D.; Hughes, S.; Smith, D.; Sawyer, E.; Davies, A.; Zylstra, J.; Haire, K.; et al. Guy’s cancer cohort-real world evidence for cancer pathways. BMC Cancer 2020, 20, 1–6. [Google Scholar] [CrossRef]
- Office of National Statistics. English Indices of Deprivation 2020. 2011. Available online: https://www.gov.uk/government/statistics/english-indices-of-deprivatio-2010 (accessed on 13 January 2021).
- Assaad, S.; Avrillon, V.; Fournier, M.-L.; Mastroianni, B.; Russias, B.; Swalduz, A.; Cassier, P.; Eberst, L.; Steineur, M.-P.; Kazes, M.; et al. High mortality rate in cancer patients with symptoms of COVID-19 with or without detectable SARS-COV-2 on RT-PCR. Eur. J. Cancer 2020, 135, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Haitao, T., Vermunt; Ghamrawi, R.; Gunaratne, M., Jayachandran; Narang, K.; Parashuram, S.; Suvakov, S.; Garovic, V.D. COVID-19 and Sex Differences: Mechanisms and Biomarkers. Mayo Clin. Proc. 2020, 95, 2189–2203. [Google Scholar]
- Park, R.; Chidharla, A.; Mehta, K.; Sun, W.; Wulff-Burchfield, E.; Kasi, A. Sex-bias in COVID-19-associated illness severity and mortality in cancer patients: A systematic review and meta-analysis. EClinicalMedicine 2020, 26, 100519. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.S.; Moulin, T.C.; Schiöth, H.B. Sex differences in COVID-19: The role of androgens in disease severity and progression. Endocrine 2021, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Raisi-Estabragh, Z.; McCracken, C.; Bethell, M.S.; Cooper, J.; Cooper, C.; Caulfield, M.J.; Munroe, P.B.; Harvey, N.C.; Petersen, S.E. Greater risk of severe COVID-19 in Black, Asian and Minority Ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25(OH)-vitamin D status: Study of 1326 cases from the UK Biobank. J. Public Health 2020, 42, 451–460. [Google Scholar] [CrossRef]
- Niedzwiedz, C.L.; O’Donnell, C.A.; Jani, B.D.; Demou, E.; Ho, F.K.; Celis-Morales, C.; Nicholl, B.I.; Mair, F.S.; Welsh, P.; Sattar, N.; et al. Ethnic and socioeconomic differences in SARS-CoV-2 infection: Prospective cohort study using UK Biobank. BMC Med. 2020, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Tai, D.B.G.; Shah, A.; A Doubeni, C.; Sia, I.G.; Wieland, M.L. The Disproportionate Impact of COVID-19 on Racial and Ethnic Minorities in the United States. Clin. Infect. Dis. 2021, 72, 703–706. [Google Scholar] [CrossRef]
- Webb Hooper, M.; Nápoles, A.M.; Pérez-Stable, E.J. COVID-19 and Racial/Ethnic Disparities. JAMA 2020, 323, 2466–2467. [Google Scholar] [CrossRef] [PubMed]
- Sorouri, M.; Kasaeian, A.; Mojtabavi, H.; Radmard, A.R.; Kolahdoozan, S.; Anushiravani, A.; Khosravi, B.; Pourabbas, S.M.; Eslahi, M.; Sirusbakht, A.; et al. Clinical characteristics, outcomes, and risk factors for mortality in hospitalized patients with COVID-19 and cancer history: A propensity score-matched study. Infect. Agents Cancer 2020, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Thachil, J. What do monitoring platelet counts in COVID-19 teach us? J. Thromb. Haemost. 2020, 18, 2071–2072. [Google Scholar] [CrossRef]
- Bange, E.M.; Han, N.A.; Wileyto, P.; Kim, J.Y.; Gouma, S. CD8 T cells compensate for impaired humoral immunity in COVID-19 patients with hematologic cancer. Res. Sq. 2021, preprint. [Google Scholar]
- Abdul-Jawad, S.; Baù, L.; Alaguthurai, T.; Barrio, I.D.M.D.; Laing, A.G.; Hayday, T.S.; Monin, L.; Muñoz-Ruiz, M.; McDonald, L.; Quijorna, I.F.; et al. Acute Immune Signatures and Their Legacies in Severe Acute Respiratory Syndrome Coronavirus-2 Infected Cancer Patients. Cancer Cell 2021, 39, 257–275.e6. [Google Scholar] [CrossRef]
- Lee, L.Y.W.; Cazier, J.-B.; Angelis, V.; Arnold, R.; Bisht, V.; Campton, N.A.; Chackathayil, J.; Cheng, V.W.; Curley, H.M.; Fittall, M.W.; et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study. Lancet 2020, 395, 1919–1926. [Google Scholar] [CrossRef]
- Lunski, M.J.; Burton, J.; Tawagi, K.; Maslov, D.; Simenson, V.; Barr, D.; Yuan, H.; Johnson, D.; Matrana, M.; Cole, J.; et al. Multivariate mortality analyses in COVID-19: Comparing patients with cancer and patients without cancer in Louisiana. Cancer 2021, 127, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Höllein, A.; Bojko, P.; Schulz, S.; Neitz, J.; Stötzer, O.; Pihusch, R.; Abedinpour, F.; Schmidt, B.; Hentrich, M. Characteristics and outcomes of patients with cancer and COVID-19: Results from a cohort study. Acta Oncol. 2021, 60, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Brar, G.; Pinheiro, L.C.; Shusterman, M.; Swed, B.; Reshetnyak, E.; Soroka, O.; Chen, F.; Yamshon, S.; Vaughn, J.; Martin, P.; et al. COVID-19 Severity and Outcomes in Patients with Cancer: A Matched Cohort Study. J. Clin. Oncol. 2020, 38, 3914–3924. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Qiu, X.; Wang, C.; Zhao, J.; Jiang, X.; Niu, W.; Huang, J.-C.; Zhang, F. Cancer Associates with Risk and Severe Events of COVID-19: A Systematic Review and Meta-Analysis. SSRN Electron. J. 2020, 148, 363–374. [Google Scholar] [CrossRef]
- Monin-Aldama, L.; Laing, A.G.; Muñoz-Ruiz, M.; McKenzie, D.R.; Barrio, I.d.M.d.; Alaguthurai, Y.; Domingo-Vila, C.; Hayday, T.S.; Graham, G. Interim results of the safety and immune-efficacy of 1 versus 2 doses of COVID-19 vaccine BNT162b2 for cancer patients in the context of the UK vaccine priority guidelines. medRxiv 2021, preprint. [Google Scholar]
- Yusuf, A.; Sarfati, D.; Booth, C.L.M.; Pramesh, C.S.; Lombe, D.; Aggarwal, A.; Bhoo-Pathy, N.; Tsunoda, A.; Vanderpuye, V.; Kutluk, T.; et al. Cancer and COVID-19 vaccines: A complex global picture. Lancet Oncol. 2021, 2045. [Google Scholar] [CrossRef]
| Variable | Total (n = 2152) | COVID-19 Status | ||||
|---|---|---|---|---|---|---|
| Positive (n = 190) | Negative (n = 1962) | |||||
| n | % | n | % | n | % | |
| Sex | ||||||
| Male | 969 | 45.00 | 112 | 58.90 | 857 | 43.70 |
| Female | 1183 | 55.00 | 78 | 41.10 | 1105 | 56.30 |
| Age | ||||||
| <50 | 362 | 16.80 | 30 | 15.80 | 332 | 16.90 |
| 50–59 | 503 | 23.40 | 36 | 18.90 | 467 | 23.80 |
| 60–69 | 613 | 28.50 | 55 | 28.90 | 558 | 28.40 |
| 70–79 | 476 | 22.10 | 40 | 21.10 | 436 | 22.20 |
| ≥80 | 198 | 9.20 | 29 | 15.30 | 169 | 8.60 |
| Mean (SD) | 62.60 (13.30) | 63.80 (14.80) | 62.50 (13.20) | |||
| SES | ||||||
| Low | 1848 | 85.90 | 157 | 82.60 | 1691 | 86.20 |
| Medium | 36 | 1.70 | 0 | 0.00 | 36 | 1.80 |
| High | 150 | 7.00 | 19 | 10.00 | 131 | 6.70 |
| Missing | 118 | 5.50 | 14 | 7.40 | 104 | 5.30 |
| Ethnicity | ||||||
| White British | 923 | 42.90 | 84 | 44.20 | 839 | 42.80 |
| White Other | 196 | 9.10 | 14 | 7.40 | 182 | 9.30 |
| Black Caribbean | 89 | 4.10 | 10 | 5.30 | 79 | 4.00 |
| Black African | 102 | 4.70 | 17 | 8.90 | 85 | 4.30 |
| Black Other | 78 | 3.60 | 15 | 7.90 | 63 | 3.20 |
| Asian | 62 | 2.90 | 7 | 3.70 | 55 | 2.80 |
| Mixed | 25 | 1.20 | 2 | 1.10 | 23 | 1.20 |
| Other | 43 | 2.00 | 4 | 2.10 | 39 | 2.00 |
| Unknown | 634 | 29.50 | 37 | 19.50 | 597 | 30.40 |
| Smoking history | ||||||
| Never | 563 | 26.20 | 66 | 34.70 | 497 | 25.30 |
| Current | 206 | 9.60 | 19 | 10.00 | 187 | 9.50 |
| Ex-smoker | 432 | 20.10 | 48 | 25.30 | 384 | 19.60 |
| Unknown | 951 | 44.20 | 57 | 30.00 | 894 | 45.60 |
| Cancer type | ||||||
| Urological | 320 | 14.90 | 41 | 21.50 | 279 | 14.20 |
| Gynaecological | 226 | 10.50 | 10 | 5.30 | 216 | 11.00 |
| Gastro-intestinal | 395 | 18.40 | 29 | 15.30 | 366 | 18.70 |
| Skin/Head & neck | 199 | 9.20 | 16 | 8.40 | 183 | 9.30 |
| Central Nervous System | 39 | 1.80 | 12 | 6.30 | 27 | 1.40 |
| Breast | 456 | 21.20 | 27 | 14.20 | 429 | 21.90 |
| Lung | 300 | 13.90 | 22 | 11.60 | 278 | 14.20 |
| Haematological | 195 | 9.10 | 33 | 17.40 | 162 | 8.30 |
| Other | 22 | 1.00 | 0 | 0.00 | 22 | 1.10 |
| Cancer stage | ||||||
| I | 233 | 10.80 | 21 | 11.10 | 212 | 10.80 |
| II | 304 | 14.10 | 34 | 17.90 | 270 | 13.80 |
| III | 450 | 20.90 | 34 | 17.90 | 416 | 21.20 |
| IV | 889 | 41.30 | 76 | 40.00 | 813 | 41.40 |
| Missing | 276 | 12.80 | 25 | 13.20 | 251 | 12.80 |
| Treatment Paradigm | ||||||
| Treatment naive | 56 | 2.60 | 18 | 9.50 | 38 | 1.90 |
| Neoadjuvant | 117 | 5.40 | 10 | 5.30 | 107 | 5.50 |
| Adjuvant | 419 | 19.50 | 12 | 6.30 | 407 | 20.70 |
| Radical | 388 | 18.00 | 49 | 25.80 | 339 | 17.30 |
| Palliative | 970 | 45.10 | 78 | 41.10 | 892 | 45.50 |
| Watch and wait | 96 | 4.50 | 18 | 9.50 | 78 | 4.00 |
| Missing | 106 | 4.90 | 5 | 2.60 | 101 | 5.10 |
| Line of Palliative Treatment (n = 970) | ||||||
| 1 | 370 | 38.60 | 38 | 50.70 | 332 | 37.60 |
| 2 | 243 | 25.40 | 22 | 29.30 | 221 | 25.00 |
| 3 | 108 | 11.30 | 7 | 9.30 | 101 | 11.40 |
| >4 | 53 | 5.60 | 1 | 1.30 | 52 | 5.90 |
| Missing | 196 | 20.20 | 10 | 12.80 | 186 | 20.90 |
| Systemic Treatment (n = 1448) | ||||||
| Chemotherapy | 708 | 48.90 | 65 | 61.90 | 643 | 47.90 |
| Immunotherapy | 110 | 7.00 | 9 | 8.60 | 101 | 7.50 |
| Biological | 163 | 11.30 | 14 | 13.30 | 149 | 11.10 |
| Targeted Therapy | 202 | 14.00 | 6 | 5.70 | 196 | 14.60 |
| Combination Therapy | 265 | 18.30 | 11 | 10.50 | 254 | 18.90 |
| Time since cancer diagnosis | ||||||
| <1 year | 974 | 45.30 | 96 | 50.50 | 878 | 44.80 |
| 1–2 years | 340 | 15.80 | 23 | 12.10 | 317 | 16.20 |
| 2–5 years | 399 | 18.50 | 29 | 15.30 | 370 | 18.90 |
| ≥5 years | 323 | 15.00 | 36 | 18.90 | 287 | 14.60 |
| Missing | 116 | 5.40 | 6 | 3.20 | 110 | 5.60 |
| Variable | COVID-19 Positive n (%) | COVID-19 Negative n (%) | OR * | 95% CI |
|---|---|---|---|---|
| Sex | ||||
| Female | 78 (41) | 1105 (56) | 1.00 | Ref. |
| Male | 112 (59) | 857 (44) | 1.85 | (1.37–2.51) |
| Age | ||||
| ≤60 | 66 (35) | 799 (41) | 1.00 | Ref. |
| >60 | 124 (65) | 1163 (59) | 1.29 | (0.94–1.76) |
| SES | ||||
| Low | 157 (83) | 1691 (86) | 1.00 | Ref. |
| Middle/High | 19 (0) | 167 (9) | 1.58 | (0.94–2.66) |
| Ethnicity | ||||
| White | 98 (52) | 1021 (52) | 1.00 | Ref. |
| Black | 42 (22) | 227 (12) | 1.93 | (1.31–2.84) |
| Asian | 7 (4) | 55 (3) | 1.33 | (0.59–2.99) |
| Other | 6 (3) | 62 (3) | 1.01 | (0.43–2.39) |
| Smoking History | ||||
| Never | 66 (35) | 497 (25) | 1.00 | Ref. |
| Ever | 67 (35) | 571 (29) | 0.91 | (0.61–1.37) |
| Cancer Type | ||||
| Solid | 157 (83) | 1778 (91) | 1.00 | Ref. |
| Haematological | 33 (17) | 162 (8) | 2.29 | (1.45–3.62) |
| Tumour Stage | ||||
| I-III | 43 (23) | 1103 (56) | 1.00 | Ref. |
| IV | 17 (9) | 302 (15) | 1.44 | (0.81–2.57) |
| Treatment Paradigm | ||||
| No active treatment | 18 (0) | 78 (4) | 1.00 | Ref. |
| Radical/Curative | 71 (37) | 853 (43) | 0.37 | (0.20–0.66) |
| Palliative | 78 (41) | 892 (45) | 0.39 | (0.22–0.70) |
| Time since cancer diagnosis | ||||
| <1 year | 96 (51) | 878 (45) | 1.00 | Ref. |
| 1–2 years | 23 (12) | 317 (16) | 0.70 | (0.42–1.16) |
| 2–5 years | 29 (15) | 370 (19) | 0.73 | (0.45–1.18) |
| ≥5 years | 36 (19) | 287 (15) | 1.11 | (0.70–1.76) |
| Variable | COVID-19 Negative | Mild/Moderate COVID-19 | Risk of Mild/Moderate | Severe COVID-19 | Risk of Severe COVID-19 | ||
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | OR * | 95% CI | n (%) | OR * | 95% CI | |
| Sex | |||||||
| Female | 1105 (56) | 66 (44) | 1.00 | Ref. | 12 (29) | 1.00 | Ref. |
| Male | 857 (44) | 83 (56) | 1.62 | (1.16–2.27) | 29 (71) | 3.12 | (1.58–6.14) |
| Age | |||||||
| ≤60 | 799 (41) | 54 (36) | 1.00 | Ref. | 12 (29) | 1.00 | Ref. |
| >60 | 1163 (59) | 95 (64) | 1.21 | (0.86–1.71) | 29 (71) | 1.66 | (0.84–3.27) |
| SES | |||||||
| Low | 1691 (86) | 124 (83) | 1.00 | Ref. | 33 (80) | 1.00 | Ref. |
| Middle/High | 167 (9) | 16 (11) | 1.64 | (0.93–2.88) | 3 (7) | 1.38 | (0.40–4.76) |
| Ethnicity | |||||||
| White | 1021 (52) | 73 (49) | 1.00 | Ref. | 25 (61) | 1.00 | Ref. |
| Black | 227 (12) | 35 (23) | 2.16 | (1.41–3.31) | 7 (17) | 1.26 | (0.54–2.95) |
| Asian | 55 (3) | 3 (2) | 0.76 | (0.23–2.50) | 4 (10) | 2.97 | (1.00–8.83) |
| Other | 62 (3) | 4 (3) | 0.90 | (0.32–2.55) | 2 (5) | 1.32 | (0.31–5.69) |
| Smoking History | |||||||
| Never | 497 (25) | 53 (36) | 1.00 | Ref. | 13 (32) | 1.00 | Ref. |
| Ever | 571 (29) | 51 (34) | 0.91 | (0.58–1.42) | 16 (39) | 0.90 | (0.38–2.10) |
| Cancer Type | |||||||
| Solid | 1778 (91) | 126 (85) | 1.00 | Ref. | 31 (76) | 1.00 | Ref. |
| Haematological | 162 (8) | 23 (15) | 2.30 | (1.38–3.83) | 10 (24) | 2.43 | (1.00–5.90) |
| Tumour Stage | |||||||
| I-III | 1103 (56) | 37 (25) | 1.00 | Ref. | 6 (15) | 1.00 | Ref. |
| IV | 302 (15) | 15 (10) | 1.48 | (0.80–2.73) | 2 (5) | 1.22 | (0.24–6.06) |
| Treatment Paradigm | |||||||
| No active treatment | 78 (4) | 15 (10) | 1.00 | Ref. | 3 (7) | 1.00 | Ref. |
| Radical/Curative | 853 (43) | 58 (39) | 0.37 | (0.20–0.69) | 13 (32) | 0.37 | (0.10–1.38) |
| Palliative | 892 (45) | 59 (40) | 0.36 | (0.19–0.66) | 19 (46) | 0.56 | (0.16–1.95) |
| Time since cancer diagnosis | |||||||
| <1 year | 878 (45) | 83 (56) | 1.00 | Ref. | 13 (32) | 1.00 | Ref. |
| 1–2 years | 317 (16) | 18 (12) | 0.63 | (0.36–1.11) | 5 (12) | 1.27 | (0.41–3.90) |
| 2–5 years | 370 (19) | 20 (13) | 0.59 | (0.34–1.02) | 9 (22) | 1.69 | (0.62–4.59) |
| ≥5 years | 287 (15) | 24 (16) | 0.87 | (0.51–1.46) | 12 (29) | 2.89 | (1.12–7.50) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russell, B.; Moss, C.L.; Palmer, K.; Sylva, R.; D’Souza, A.; Wylie, H.; Haire, A.; Cahill, F.; Steel, R.; Hoyes, A.; et al. COVID-19 Risk Factors for Cancer Patients: A First Report with Comparator Data from COVID-19 Negative Cancer Patients. Cancers 2021, 13, 2479. https://doi.org/10.3390/cancers13102479
Russell B, Moss CL, Palmer K, Sylva R, D’Souza A, Wylie H, Haire A, Cahill F, Steel R, Hoyes A, et al. COVID-19 Risk Factors for Cancer Patients: A First Report with Comparator Data from COVID-19 Negative Cancer Patients. Cancers. 2021; 13(10):2479. https://doi.org/10.3390/cancers13102479
Chicago/Turabian StyleRussell, Beth, Charlotte L. Moss, Kieran Palmer, Rushan Sylva, Andrea D’Souza, Harriet Wylie, Anna Haire, Fidelma Cahill, Renee Steel, Angela Hoyes, and et al. 2021. "COVID-19 Risk Factors for Cancer Patients: A First Report with Comparator Data from COVID-19 Negative Cancer Patients" Cancers 13, no. 10: 2479. https://doi.org/10.3390/cancers13102479
APA StyleRussell, B., Moss, C. L., Palmer, K., Sylva, R., D’Souza, A., Wylie, H., Haire, A., Cahill, F., Steel, R., Hoyes, A., Wilson, I., Macneil, A., Shifa, B., Monroy-Iglesias, M. J., Papa, S., Irshad, S., Ross, P., Spicer, J., Kordasti, S., ... Dolly, S. (2021). COVID-19 Risk Factors for Cancer Patients: A First Report with Comparator Data from COVID-19 Negative Cancer Patients. Cancers, 13(10), 2479. https://doi.org/10.3390/cancers13102479







