Clearance of Circulating Tumor Cells in Patients with Hepatocellular Carcinoma Undergoing Surgical Resection or Liver Transplantation
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Study Population
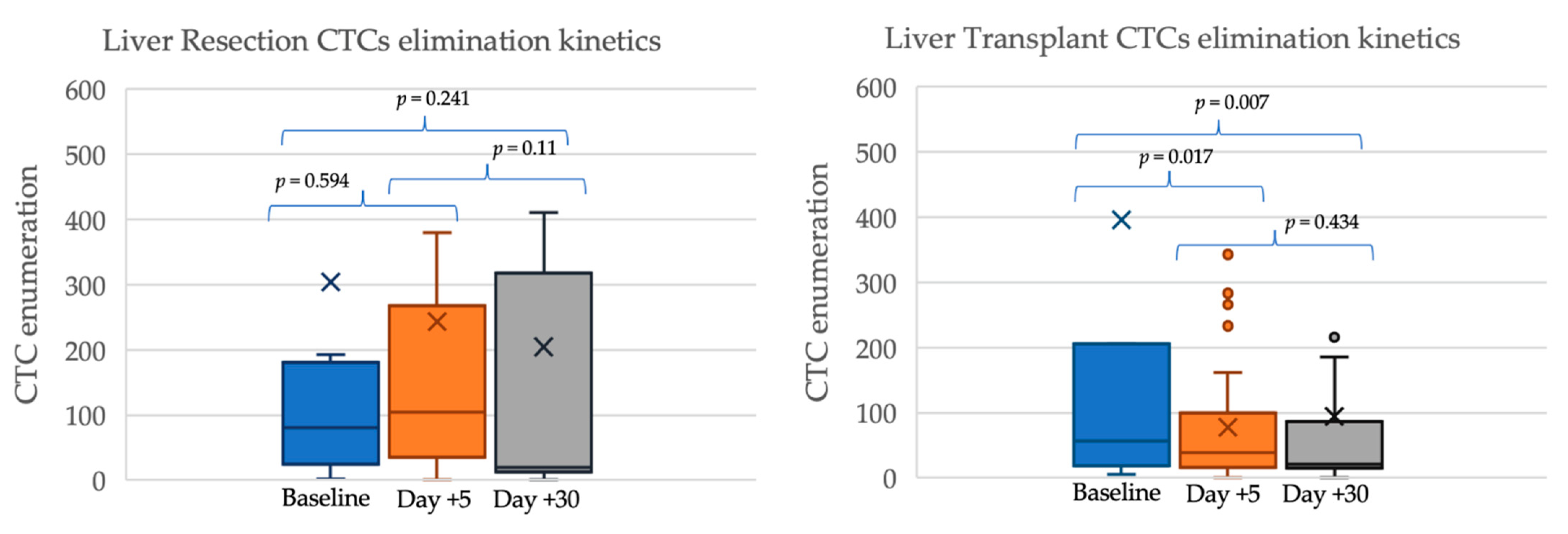
2.2. Circulating Tumor Cells’ Kinetics after Surgery
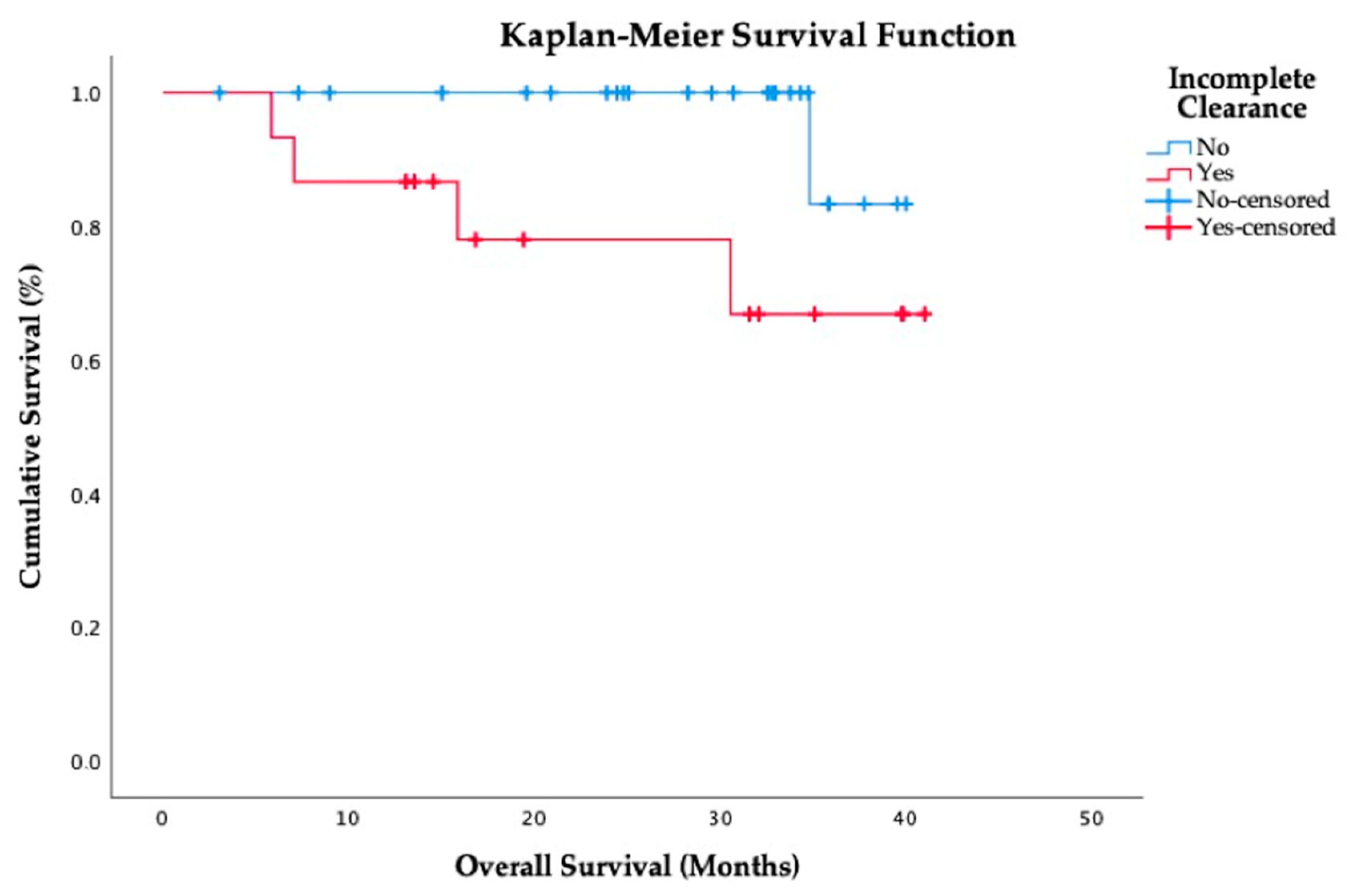
2.3. Prognostic Impact of CTCs
3. Discussion
4. Materials and Methods
4.1. Study Design and Patient Inclusion
4.2. Variables
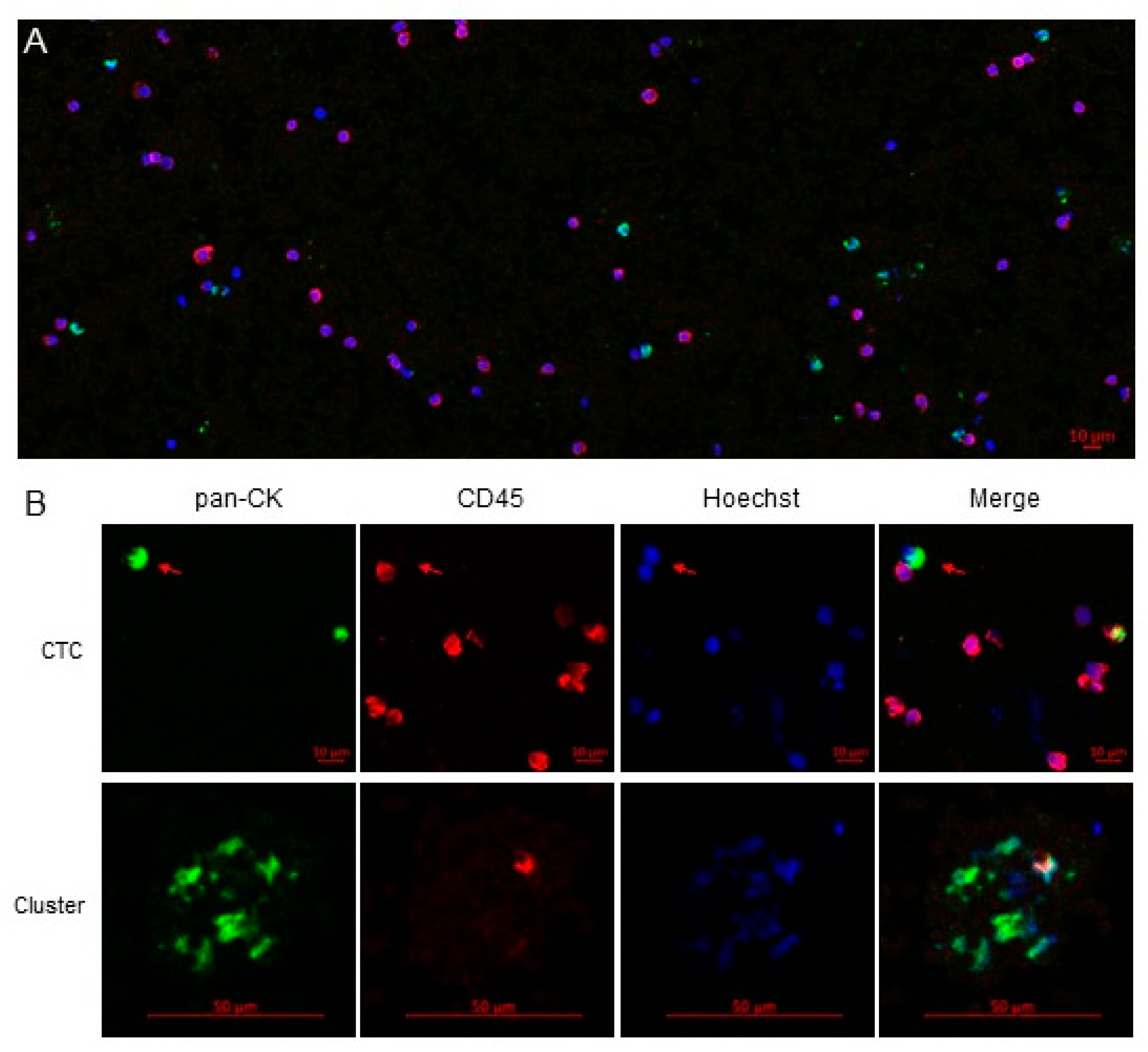
4.3. CTCs Isolation from Peripheral Blood
4.4. CTCs Staining and Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; Artaman, A.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies from 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Bhoori, S.; Sposito, C.; Bongini, M.; Langer, M.; Miceli, R.; Mariani, L. Milan criteria in liver transplantation for hepatocellular carcinoma: An evidence-based analysis of 15 years of experience. Liver Transpl. 2011, 17 (Suppl. S2), S44–S57. [Google Scholar] [CrossRef]
- Rodriguez-Peralvarez, M.; Luong, T.V.; Andreana, L.; Meyer, T.; Dhillon, A.P.; Burroughs, A.K. A systematic review of microvascular invasion in hepatocellular carcinoma: Diagnostic and prognostic variability. Ann. Surg. Oncol. 2013, 20, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Llovet, J.M.; Miceli, R.; Bhoori, S.; Schiavo, M.; Mariani, L.; Camerini, T.; Roayaie, S.; Schwartz, M.E.; Grazi, G.L.; et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: A retrospective, exploratory analysis. Lancet Oncol. 2009, 10, 35–43. [Google Scholar] [CrossRef]
- Yao, F.Y.; Ferrell, L.; Bass, N.M.; Watson, J.J.; Bacchetti, P.; Venook, A.; Ascher, N.L.; Roberts, J.P. Liver transplantation for hepatocellular carcinoma: Expansion of the tumor size limits does not adversely impact survival. Hepatology 2001, 33, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.S.; Xu, X.; Wu, J.; Chen, J.; Wang, W.L.; Zhang, M.; Liang, T.B.; Wu, L.M. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation 2008, 85, 1726–1732. [Google Scholar] [CrossRef]
- Takada, Y.; Uemoto, S. Liver transplantation for hepatocellular carcinoma: The Kyoto experience. J. Hepatobiliary Pancreat. Sci. 2010, 17, 527–532. [Google Scholar] [CrossRef]
- Toso, C.; Meeberg, G.; Hernandez-Alejandro, R.; Dufour, J.F.; Marotta, P.; Majno, P.; Kneteman, N.M. Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma: A prospective validation. Hepatology 2015, 62, 158–165. [Google Scholar] [CrossRef]
- Duvoux, C.; Roudot-Thoraval, F.; Decaens, T.; Pessione, F.; Badran, H.; Piardi, T.; Francoz, C.; Compagnon, P.; Vanlemmens, C.; Dumortier, J.; et al. Liver transplantation for hepatocellular carcinoma: A model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012, 143, 986–994. [Google Scholar] [CrossRef]
- Sapisochin, G.; Goldaracena, N.; Laurence, J.M.; Dib, M.; Barbas, A.; Ghanekar, A.; Cleary, S.P.; Lilly, L.; Cattral, M.S.; Marquez, M.; et al. The extended Toronto criteria for liver transplantation in patients with hepatocellular carcinoma: A prospective validation study. Hepatology 2016, 64, 2077–2088. [Google Scholar] [CrossRef]
- Johnson, P.J. The role of serum alpha-fetoprotein estimation in the diagnosis and management of hepatocellular carcinoma. Clin. Liver Dis. 2001, 5, 145–159. [Google Scholar] [CrossRef]
- Ozdemir, F.; Baskiran, A. The Importance of AFP in Liver Transplantation for HCC. J. Gastrointest. Cancer 2020, 51, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Nel, I.; David, P.; Gerken, G.G.; Schlaak, J.F.; Hoffmann, A.C. Role of circulating tumor cells and cancer stem cells in hepatocellular carcinoma. Hepatol. Int. 2014, 8, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Okajima, W.; Komatsu, S.; Ichikawa, D.; Miyamae, M.; Ohashi, T.; Imamura, T.; Kiuchi, J.; Nishibeppu, K.; Arita, T.; Konishi, H.; et al. Liquid biopsy in patients with hepatocellular carcinoma: Circulating tumor cells and cell-free nucleic acids. World J. Gastroenterol. 2017, 23, 5650–5668. [Google Scholar] [CrossRef] [PubMed]
- Bidard, F.C.; Proudhon, C.; Pierga, J.Y. Circulating tumor cells in breast cancer. Mol. Oncol. 2016, 10, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Tong, B.; Wang, M. Circulating tumor cells in patients with lung cancer: Developments and applications for precision medicine. Future Oncol. 2019, 15, 2531–2542. [Google Scholar] [CrossRef]
- Xue, F.; Shi, S.; Zhang, Z.; Xu, C.; Zheng, J.; Qin, T.; Qian, Z.; Zhao, X.; Tong, Y.; Xia, L.; et al. Application of a novel liquid biopsy in patients with hepatocellular carcinoma undergoing liver transplantation. Oncol. Lett. 2018, 15, 5481–5488. [Google Scholar] [CrossRef]
- Sun, Y.F.; Xu, Y.; Yang, X.R.; Guo, W.; Zhang, X.; Qiu, S.J.; Shi, R.Y.; Hu, B.; Zhou, J.; Fan, J. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology 2013, 57, 1458–1468. [Google Scholar] [CrossRef]
- Ou, H.; Huang, Y.; Xiang, L.; Chen, Z.; Fang, Y.; Lin, Y.; Cui, Z.; Yu, S.; Li, X.; Yang, D. Circulating Tumor Cell Phenotype Indicates Poor Survival and Recurrence After Surgery for Hepatocellular Carcinoma. Dig. Dis. Sci. 2018, 63, 2373–2380. [Google Scholar] [CrossRef]
- Schulze, K.; Gasch, C.; Staufer, K.; Nashan, B.; Lohse, A.W.; Pantel, K.; Riethdorf, S.; Wege, H. Presence of EpCAM-positive circulating tumor cells as biomarker for systemic disease strongly correlates to survival in patients with hepatocellular carcinoma. Int. J. Cancer 2013, 133, 2165–2171. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Peralvarez, M.; Tsochatzis, E.; Naveas, M.C.; Pieri, G.; Garcia-Caparros, C.; O’Beirne, J.; Poyato-Gonzalez, A.; Ferrin-Sanchez, G.; Montero-Alvarez, J.L.; Patch, D.; et al. Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma. J. Hepatol. 2013, 59, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Cao, L.; Xu, W.; Yin, Z. Circulating tumor cells in hepatocellular carcinoma: Detection techniques, clinical implications, and future perspectives. Semin. Oncol. 2012, 39, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Sun, Y.F.; Shen, M.N.; Ma, X.L.; Wu, J.; Zhang, C.Y.; Zhou, Y.; Xu, Y.; Hu, B.; Zhang, M.; et al. Circulating Tumor Cells with Stem-Like Phenotypes for Diagnosis, Prognosis, and Therapeutic Response Evaluation in Hepatocellular Carcinoma. Clin. Cancer Res. 2018, 24, 2203–2213. [Google Scholar] [CrossRef] [PubMed]
- Von Felden, J.; Craig, A.J.; Garcia-Lezana, T.; Labgaa, I.; Haber, P.K.; D’Avola, D.; Asgharpour, A.; Dieterich, D.; Bonaccorso, A.; Torres-Martin, M.; et al. Mutations in circulating tumor DNA predict primary resistance to systemic therapies in advanced hepatocellular carcinoma. Oncogene 2021, 40, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Paterlini-Brechot, P.; Vona, G.; Brechot, C. Circulating tumorous cells in patients with hepatocellular carcinoma. Clinical impact and future directions. Semin. Cancer Biol. 2000, 10, 241–249. [Google Scholar] [CrossRef]
- Ramirez, P.; Saenz, L.; Cascales-Campos, P.A.; Gonzalez Sanchez, M.R.; Llacer-Millan, E.; Sanchez-Lorencio, M.I.; Diaz-Rubio, E.; De La Orden, V.; Mediero-Valeros, B.; Navarro, J.L.; et al. Oncological Evaluation by Positron-emission Tomography, Circulating Tumor Cells and Alpha Fetoprotein in Patients With Hepatocellular Carcinoma on the Waiting List for Liver Transplantation. Transplant. Proc. 2016, 48, 2962–2965. [Google Scholar] [CrossRef]
- Ye, X.; Li, G.; Han, C.; Han, Q.; Shang, L.; Su, H.; Han, B.; Gong, Y.; Lu, G.; Peng, T. Circulating tumor cells as a potential biomarker for postoperative clinical outcome in HBV-related hepatocellular carcinoma. Cancer Manag. Res. 2018, 10, 5639–5647. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, Y.; Liu, J.; Huo, F.; Zhou, J. Analysis of circulating tumor cells in patients with hepatocellular carcinoma recurrence following liver transplantation. J. Investig. Med. 2018. [Google Scholar] [CrossRef]
- Yu, J.J.; Xiao, W.; Dong, S.L.; Liang, H.F.; Zhang, Z.W.; Zhang, B.X.; Huang, Z.Y.; Chen, Y.F.; Zhang, W.G.; Luo, H.P.; et al. Effect of surgical liver resection on circulating tumor cells in patients with hepatocellular carcinoma. BMC Cancer 2018, 18, 835. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Li, W.; Yang, R.; Zhang, X.; Ye, Y.; Yu, J.; Ye, L.; Tang, W. Circulating tumor cells undergoing EMT are poorly correlated with clinical stages or predictive of recurrence in hepatocellular carcinoma. Sci. Rep. 2019, 9, 7084. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lin, X.; Chen, C.; Chen, Y.; Zhao, Q.; Wu, L.; Wang, D.; Ma, Y.; Ju, W.; Chen, M.; et al. Analysis of preoperative circulating tumor cells for recurrence in patients with hepatocellular carcinoma after liver transplantation. Ann. Transl. Med. 2020, 8, 1067. [Google Scholar] [CrossRef]
- Sun, Y.F.; Wang, P.X.; Cheng, J.W.; Gong, Z.J.; Huang, A.; Zhou, K.Q.; Hu, B.; Gao, P.T.; Cao, Y.; Qiu, S.J.; et al. Postoperative circulating tumor cells: An early predictor of extrahepatic metastases in patients with hepatocellular carcinoma undergoing curative surgical resection. Cancer Cytopathol. 2020, 128, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Nel, I.; Baba, H.A.; Ertle, J.; Weber, F.; Sitek, B.; Eisenacher, M.; Meyer, H.E.; Schlaak, J.F.; Hoffmann, A.C. Individual profiling of circulating tumor cell composition and therapeutic outcome in patients with hepatocellular carcinoma. Transl. Oncol. 2013, 6, 420–428. [Google Scholar] [CrossRef]
- Von Felden, J.; Schulze, K.; Krech, T.; Ewald, F.; Nashan, B.; Pantel, K.; Lohse, A.W.; Riethdorf, S.; Wege, H. Circulating tumor cells as liquid biomarker for high HCC recurrence risk after curative liver resection. Oncotarget 2017, 8, 89978–89987. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.C.; Teng, P.C.; Chen, P.J.; Posadas, E.; Tseng, H.R.; Lu, S.C.; Yang, J.D. Detection of Circulating Tumor Cells and Their Implications as a Biomarker for Diagnosis, Prognostication, and Therapeutic Monitoring in Hepatocellular Carcinoma. Hepatology 2021, 73, 422–436. [Google Scholar] [CrossRef]
- Sanchez-Lorencio, M.I.; Ramirez, P.; Saenz, L.; Martinez Sanchez, M.V.; De La Orden, V.; Mediero-Valeros, B.; Veganzones-De-Castro, S.; Baroja-Mazo, A.; Revilla Nuin, B.; Gonzalez, M.R.; et al. Comparison of Two Types of Liquid Biopsies in Patients with Hepatocellular Carcinoma Awaiting Orthotopic Liver Transplantation. Transplant. Proc. 2015, 47, 2639–2642. [Google Scholar] [CrossRef] [PubMed]
- Polyak, K.; Weinberg, R.A. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat. Rev. Cancer 2009, 9, 265–273. [Google Scholar] [CrossRef]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef]
- Satelli, A.; Li, S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol. Life Sci. 2011, 68, 3033–3046. [Google Scholar] [CrossRef]
- Jolly, M.K.; Boareto, M.; Huang, B.; Jia, D.; Lu, M.; Ben-Jacob, E.; Onuchic, J.N.; Levine, H. Implications of the Hybrid Epithelial/Mesenchymal Phenotype in Metastasis. Front. Oncol. 2015, 5, 155. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Forgues, M.; Wang, W.; Kim, J.W.; Ye, Q.; Jia, H.; Budhu, A.; Zanetti, K.A.; Chen, Y.; Qin, L.X.; et al. EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer Res. 2008, 68, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Ding, T.; Guo, Z.W.; Yu, X.J.; Hu, Y.Z.; Zheng, L.; Xu, J. Expression pattern of tumour-associated antigens in hepatocellular carcinoma: Association with immune infiltration and disease progression. Br. J. Cancer 2013, 109, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, H.A.; Steiner, P.E. Primary carcinoma of the liver: A study of 100 cases among 48,900 necropsies. Cancer 1954, 7, 462–503. [Google Scholar] [CrossRef]
| Variable | Resection (n = 10) | Transplantation (n = 31) | p |
|---|---|---|---|
| Age (years) | 63.2 ± 8.5 | 57.2 ± 4.7 | 0.058 |
| Sex (men), n (%) | 8 (80%) | 26 (83.9%) | 0.556 |
| Hepatitis B, n (%) | 2 (20%) | 4 (12.9%) | 0.458 |
| Hepatitis C, n (%) | 4 (40%) | 19 (61.3%) | 0.208 |
| Alcoholic liver disease, n (%) | 4 (40%) | 18 (58.1%) | 0.264 |
| Portal Hypertension, n (%) | 1 (10%) | 24 (77.4%) | 0.001 |
| Child-Pugh score | 5 (5–5) | 6 (5–7) | 0.003 |
| MELD score | 6 (6–7) | 9 (7–12) | 0.002 |
| Number of nodules, n (%) | |||
| Single nodule | 9 (90%) | 19 (61.3%) | 0.092 |
| Multinodular | 1 (10%) | 12 (38.7%) | |
| Main nodule diameter (mm) | 34.4 ± 22.8 | 22.1 ± 14.4 | 0.051 |
| Histological differentiation (moderate–poor), n (%) | 5 (50%) | 17 (54.8%) | 0.537 |
| Microvascular invasion, n (%) | 1 (10%) | 6 (19.5%) | 0.444 |
| Baseline AFP (ng/mL) | 8.33 | 4.55 | 0.421 |
| (IQR 1.9–186.7) | (IQR 2–16.2) | ||
| Bridging locoregional therapy, n (%) | 2 (20%) | 28 (90.3%) | 0.001 |
| Primary immunosuppression | |||
| Tacrolimus, n (%) | - | 31 (100%) | - |
| Mycophenolate, n (%) | - | 31 (100%) | - |
| Everolimus, n (%) | - | 1 (3.2%) | - |
| Basiliximab induction, n (%) | - | 3 (9.7%) | - |
| Tacrolimus trough levels in the first month (ng/mL) | - | 6.9 ± 1.7 | - |
| Median follow-up after surgery (months) | 33 (IQR 30–37) | 25 (IQR 15–34) | 0.075 |
| HCC recurrence after surgery, n (%) | 4 (40%) | 5 (16.1%) | 0.127 |
| Mortality, n (%) | 1 (10%) | 4 (12.9%) | 0.647 |
| Variable | Resection (n = 10) | Transplant (n = 31) | p |
|---|---|---|---|
| Baseline CTC enumeration | 81 (24–178) | 56 (18–204) | 0.990 |
| Baseline CTC clusters | 1 (0–2) | 1 (0–5) | 0.764 |
| CTC enumeration day +5 | 103 (34.5–258) | 38 (16–99) | 0.138 |
| CTC clusters day +5 | 1 (0–13) | 0 (0–1) | 0.114 |
| CTC enumeration day +30 | 20 (13–311) | 21 (15–85) | 0.777 |
| CTC clusters day +30 | 0 (0–5) | 0 (0–1) | 0.846 |
| Incomplete clearance | 3 (30%) | 12 (38.7%) | 0.460 |
| Variable | Relative Change of CTCs | p | Increase in CTC Clusters | p | |
|---|---|---|---|---|---|
| Sex | Male (n = 34) | −71.34% (−91.79–−6.25%) | 0.058 | 11.8% | 0.458 |
| Female (n = 7) | +33.3% (−70.9–382.85%) | 0% | |||
| Age | <60 (n = 26) | −65.3% (−89.9–60.76%) | 0.799 | 15.4% | 0.148 |
| ≥60 (n = 15) | −74.36% (−91.25–33.33%) | 0% | |||
| Type of surgery | Resection (n = 10) | −71.34% (−84.9–−20.2%) | 0.846 | 20% | 0.245 |
| Transplant (n = 31) | −62.8% (−91.25–60%) | 6.5% | |||
| AFP (ng/mL) | <400 (n = 34) | −69.6% (−91.8–40.76%) | 0.349 | 8.8% | 0.838 |
| ≥400 (n = 2) | +21.5% (−48–21.5%) | 0% | |||
| Number of nodules | Single nodule (n = 28) | −72.6% (−93.19–68.2%) | 0.552 | 10.7% | 0.623 |
| Multinodular (n = 13) | −62.8% (−83.9–−2.3%) | 7.7% | |||
| Dominant nodule size (mm) | <30 (n = 30) | −60.6% (−86.07–75.16%) | 0.065 | 10% | 0.712 |
| ≥30 (n=11) | −89.4% (−93.26–−48%) | 9.1% | |||
| Histological Differentiation | Well (n = 22) | −81.46% (−91.8–62.5%) | 0.261 | 9.1% | 0.639 |
| Moderate-poor (n = 19) | −48% (−85.9–33.3%) | 10.5% | |||
| Microvascular invasion | Absent (n = 34) | −68.1% (−88–12.5%) | 0.799 | 5.9% | 0.128 |
| Present (n = 7) | −62.85% (−94.11–90.9%) | 28.6% | |||
| Reference | n | Therapy | CTCs Technique | CTCs Threshold | Time-Point Analysis | Prognostic Impact |
|---|---|---|---|---|---|---|
| Sun et al. [19] | 123 | LR | CellSearch® | ≥2/7.5 mL | Baseline and at 1 month | Increased tumor recurrence |
| Ramirez et al. [27] | 24 | LT | Isoflux® | Not defined | Baseline, at 1 month and at 6 months | No association with outcomes |
| Ou et al. [20] | 165 | LR | CanPatrol® | ≥2/5 mL | Baseline | Shorter overall survival |
| Xue et al. [18] | 30 | LT | iFISH + CellSearch® | ≥5/7.5 mL | Baseline and at 3 months | Reduced recurrence-free survival |
| Ye et al. [28] | 42 | LR | CanPatrol® | ≥5/5 mL | Baseline and at 1 month | Reduced recurrence-free survival |
| Wang et al. [29] | 47 | LT | CanPatrol® | Not defined | Baseline and at 1 month | No association with outcomes |
| Yu et al. [30] | 139 | LR | CellSearch® | ≥2/7.5 mL | Baseline and at 3 days | Reduced overall survival |
| Chen et al. [31] | 143 | LR | CanPatrol® | Not defined | Baseline and after therapy | No association with outcomes |
| Chen et al. [32] | 50 | LT | Negative enrichment + imFISH | ≥2/3.2 mL | Baseline | Increased recurrence and reduced survival |
| Sun et al. [33] | 197 | LR | CellSearch® | ≥3/7.5 mL | Baseline and at 1 month | Reduced survival |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amado, V.; González-Rubio, S.; Zamora, J.; Alejandre, R.; Espejo-Cruz, M.L.; Linares, C.; Sánchez-Frías, M.; García-Jurado, G.; Montero, J.L.; Ciria, R.; et al. Clearance of Circulating Tumor Cells in Patients with Hepatocellular Carcinoma Undergoing Surgical Resection or Liver Transplantation. Cancers 2021, 13, 2476. https://doi.org/10.3390/cancers13102476
Amado V, González-Rubio S, Zamora J, Alejandre R, Espejo-Cruz ML, Linares C, Sánchez-Frías M, García-Jurado G, Montero JL, Ciria R, et al. Clearance of Circulating Tumor Cells in Patients with Hepatocellular Carcinoma Undergoing Surgical Resection or Liver Transplantation. Cancers. 2021; 13(10):2476. https://doi.org/10.3390/cancers13102476
Chicago/Turabian StyleAmado, Víctor, Sandra González-Rubio, Javier Zamora, Rafael Alejandre, María Lola Espejo-Cruz, Clara Linares, Marina Sánchez-Frías, Gema García-Jurado, José Luis Montero, Rubén Ciria, and et al. 2021. "Clearance of Circulating Tumor Cells in Patients with Hepatocellular Carcinoma Undergoing Surgical Resection or Liver Transplantation" Cancers 13, no. 10: 2476. https://doi.org/10.3390/cancers13102476
APA StyleAmado, V., González-Rubio, S., Zamora, J., Alejandre, R., Espejo-Cruz, M. L., Linares, C., Sánchez-Frías, M., García-Jurado, G., Montero, J. L., Ciria, R., Rodríguez-Perálvarez, M., Ferrín, G., & De la Mata, M. (2021). Clearance of Circulating Tumor Cells in Patients with Hepatocellular Carcinoma Undergoing Surgical Resection or Liver Transplantation. Cancers, 13(10), 2476. https://doi.org/10.3390/cancers13102476







