The Proportion Cured of Patients with Resected Stage II–III Cutaneous Melanoma in Sweden
Abstract
Simple summary
Abstract
1. Introduction
2. Results
2.1. Clinicopathologic Characteristics
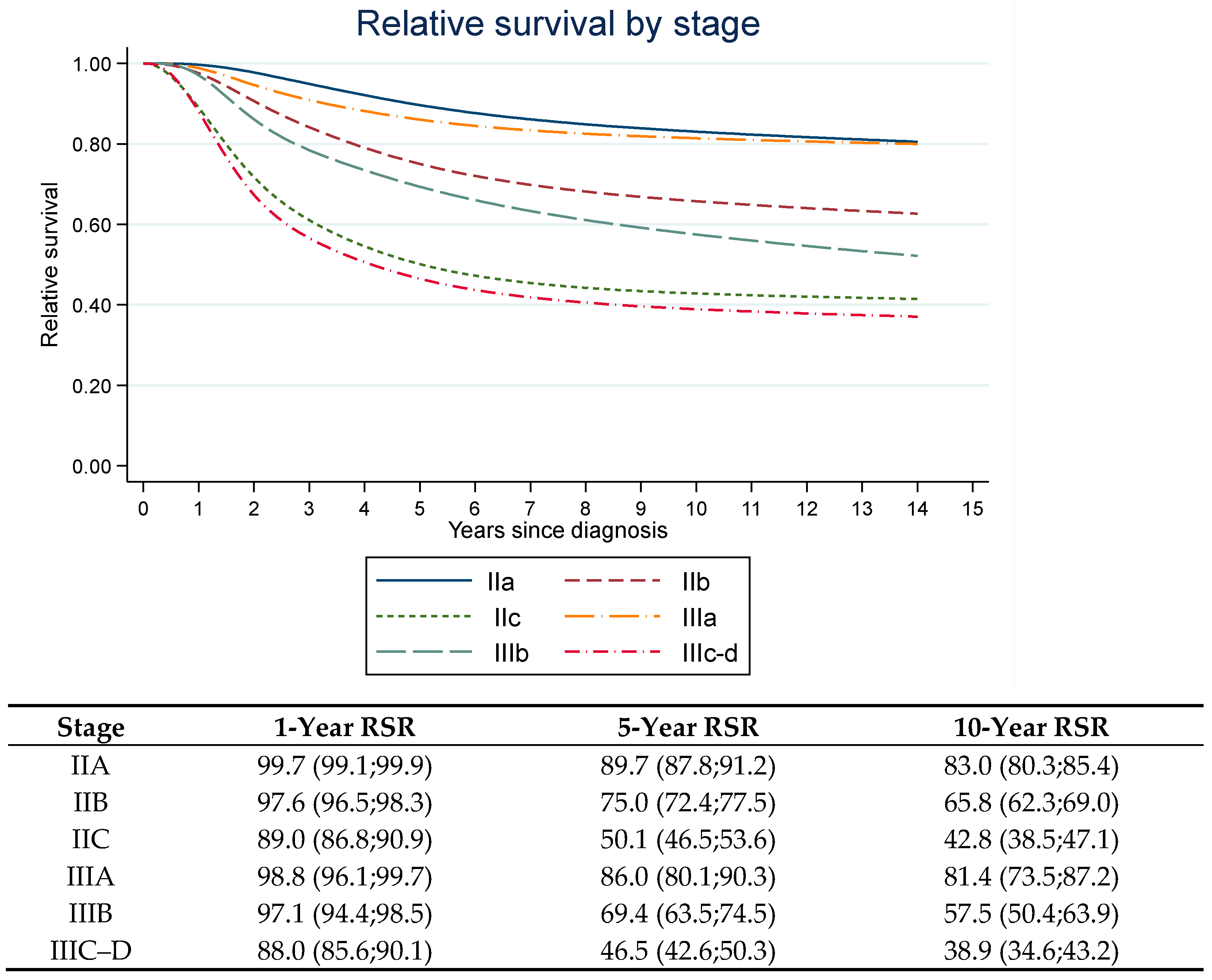
2.2. Relative Survival Ratios, Cure Proportions and Median Survival Times
3. Discussion
4. Materials and Methods
4.1. Patients and Methods
4.2. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
References
- Tas, F. Metastatic behavior in melanoma: Timing, pattern, survival, and influencing factors. J Oncol. 2012, 2012, 647684. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Del Vecchio, M.; Mandala, M.; Gogas, H.; Arance, A.M.; Dalle, S.; Cowey, C.L.; Schenker, M.; Grob, J.J.; Chiarion-Sileni, V.; et al. Adjuvant nivolumab versus ipilimumab in resected stage iiib-c and stage iv melanoma (checkmate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 1465–1477. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Longer follow-up confirms recurrence-free survival benefit of adjuvant pembrolizumab in high-risk stage iii melanoma: Updated results from the eortc 1325-mg/keynote-054 trial. J. Clin. Oncol. 2020, 38, 3925–3936. [Google Scholar] [CrossRef]
- Weber, J.; Mandala, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant nivolumab versus ipilimumab in resected stage iii or iv melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandala, M.; Chiarion-Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; Haydon, A.; et al. Adjuvant dabrafenib plus trametinib in stage iii braf-mutated melanoma. N. Engl. J. Med. 2017, 377, 1813–1823. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Lichinitser, M.; Khattak, A.; Carlino, M.S.; et al. Adjuvant pembrolizumab versus placebo in resected stage iii melanoma. N. Engl. J. Med. 2018, 378, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Amaria, R.N.; Prieto, P.A.; Tetzlaff, M.T.; Reuben, A.; Andrews, M.C.; Ross, M.I.; Glitza, I.C.; Cormier, J.; Hwu, W.J.; Tawbi, H.A.; et al. Neoadjuvant plus adjuvant dabrafenib and trametinib versus standard of care in patients with high-risk, surgically resectable melanoma: A single-centre, open-label, randomised, phase 2 trial. Lancet Oncol. 2018, 19, 181–193. [Google Scholar] [CrossRef]
- Rozeman, E.A.; Hoefsmit, E.P.; Reijers, I.L.M.; Saw, R.P.M.; Versluis, J.M.; Krijgsman, O.; Dimitriadis, P.; Sikorska, K.; van de Wiel, B.A.; Eriksson, H.; et al. Survival and biomarker analyses from the opacin-neo and opacin neoadjuvant immunotherapy trials in stage iii melanoma. Nat. Med. 2021, 27, 256–263. [Google Scholar] [CrossRef]
- Rozeman, E.A.; Menzies, A.M.; van Akkooi, A.C.J.; Adhikari, C.; Bierman, C.; van de Wiel, B.A.; Scolyer, R.A.; Krijgsman, O.; Sikorska, K.; Eriksson, H.; et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage iii melanoma (opacin-neo): A multicentre, phase 2, randomised, controlled trial. Lancet Oncol. 2019, 20, 948–960. [Google Scholar] [CrossRef]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma staging: Evidence-based changes in the american joint committee on cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef] [PubMed]
- Andersson, T.M.; Eriksson, H.; Hansson, J.; Mansson-Brahme, E.; Dickman, P.W.; Eloranta, S.; Lambe, M.; Lambert, P.C. Estimating the cure proportion of malignant melanoma, an alternative approach to assess long term survival: A population-based study. Cancer Epidemiol. 2014, 38, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, H.; Lyth, J.; Andersson, T.M. The proportion cured of patients diagnosed with stage iii-iv cutaneous malignant melanoma in sweden 1990-2007: A population-based study. Int. J. Cancer 2016, 138, 2829–2836. [Google Scholar] [CrossRef] [PubMed]
- Gershenwald, J.E.; Scolyer, R.A. Melanoma staging: American joint committee on cancer (ajcc) 8th edition and beyond. Ann. Surg. Oncol. 2018, 25, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Isaksson, K.; Katsarelias, D.; Mikiver, R.; Carneiro, A.; Ny, L.; Olofsson Bagge, R. A population-based comparison of the ajcc 7th and ajcc 8th editions for patients diagnosed with stage iii cutaneous malignant melanoma in sweden. Ann. Surg. Oncol. 2019, 26, 2839–2845. [Google Scholar] [CrossRef]
- Garbe, C.; Keim, U.; Suciu, S.; Amaral, T.; Eigentler, T.K.; Gesierich, A.; Hauschild, A.; Heinzerling, L.; Kiecker, F.; Schadendorf, D.; et al. Prognosis of patients with stage iii melanoma according to american joint committee on cancer version 8: A reassessment on the basis of 3 independent stage iii melanoma cohorts. J. Clin. Oncol. 2020, 38, 2543–2551. [Google Scholar] [CrossRef]
- Chan, T.A.; Yarchoan, M.; Jaffee, E.; Swanton, C.; Quezada, S.A.; Stenzinger, A.; Peters, S. Development of tumor mutation burden as an immunotherapy biomarker: Utility for the oncology clinic. Ann. Oncol. 2019, 30, 44–56. [Google Scholar] [CrossRef]
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J.; et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017, 9, 1–14. [Google Scholar] [CrossRef]
- Hauschild, A.; Dummer, R.; Schadendorf, D.; Santinami, M.; Atkinson, V.; Mandala, M.; Chiarion-Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; et al. Longer follow-up confirms relapse-free survival benefit with adjuvant dabrafenib plus trametinib in patients with resected braf v600-mutant stage iii melanoma. J. Clin. Oncol. 2018, 36, 3441–3449. [Google Scholar] [CrossRef]
- von Schuckmann, L.A.; Hughes, M.C.B.; Ghiasvand, R.; Malt, M.; van der Pols, J.C.; Beesley, V.L.; Khosrotehrani, K.; Smithers, B.M.; Green, A.C. Risk of melanoma recurrence after diagnosis of a high-risk primary tumor. JAMA Dermatol. 2019, 155, 688–693. [Google Scholar] [CrossRef]
- Lee, A.Y.; Droppelmann, N.; Panageas, K.S.; Zhou, Q.; Ariyan, C.E.; Brady, M.S.; Chapman, P.B.; Coit, D.G. Patterns and timing of initial relapse in pathologic stage ii melanoma patients. Ann. Surg. Oncol. 2017, 24, 939–946. [Google Scholar] [CrossRef]
- Demierre, M.F.; Chung, C.; Miller, D.R.; Geller, A.C. Early detection of thick melanomas in the united states: Beware of the nodular subtype. Arch. Dermatol. 2005, 141, 745–750. [Google Scholar] [CrossRef]
- Balch, C.M.; Gershenwald, J.E.; Soong, S.J.; Thompson, J.F.; Atkins, M.B.; Byrd, D.R.; Buzaid, A.C.; Cochran, A.J.; Coit, D.G.; Ding, S.; et al. Final version of 2009 ajcc melanoma staging and classification. J. Clin. Oncol. 2009, 27, 6199–6206. [Google Scholar] [CrossRef] [PubMed]
- Joosse, A.; Collette, S.; Suciu, S.; Nijsten, T.; Lejeune, F.; Kleeberg, U.R.; Coebergh, J.W.; Eggermont, A.M.; de Vries, E. Superior outcome of women with stage i/ii cutaneous melanoma: Pooled analysis of four european organisation for research and treatment of cancer phase iii trials. J. Clin. Oncol. 2012, 30, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Joosse, A.; Collette, S.; Suciu, S.; Nijsten, T.; Patel, P.M.; Keilholz, U.; Eggermont, A.M.; Coebergh, J.W.; de Vries, E. Sex is an independent prognostic indicator for survival and relapse/progression-free survival in metastasized stage iii to iv melanoma: A pooled analysis of five european organisation for research and treatment of cancer randomized controlled trials. J. Clin. Oncol. 2013, 31, 2337–2346. [Google Scholar] [CrossRef]
- Scoggins, C.R.; Ross, M.I.; Reintgen, D.S.; Noyes, R.D.; Goydos, J.S.; Beitsch, P.D.; Urist, M.M.; Ariyan, S.; Sussman, J.J.; Edwards, M.J.; et al. Gender-related differences in outcome for melanoma patients. Ann. Surg. 2006, 243, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Andersson, T.M.; Dickman, P.W.; Eloranta, S.; Lambert, P.C. Estimating and modelling cure in population-based cancer studies within the framework of flexible parametric survival models. BMC Med. Res. Methodol. 2011, 11, 1–11. [Google Scholar] [CrossRef]
- Nelson, C.P.; Lambert, P.C.; Squire, I.B.; Jones, D.R. Flexible parametric models for relative survival, with application in coronary heart disease. Stat. Med. 2007, 26, 5486–5498. [Google Scholar] [CrossRef]
- Barbieri, M.; Wilmoth, J.R.; Shkolnikov, V.M.; Glei, D.; Jasilionis, D.; Jdanov, D.; Boe, C.; Riffe, T.; Grigoriev, P.; Winant, C. Data resource profile: The human mortality database (hmd). Int. J. Epidemiol. 2015, 44, 1549–1556. [Google Scholar] [CrossRef] [PubMed]


| Clinico-Pathological Characteristics | Stage II n (%) | Stage III n (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All | A | B | C | All | A | B | C | D | |
| Number of patients | 5156 | 2114 (41.0) | 1810 (35.1) | 1232 (23.9) | 1310 | 210 (16.0) | 321 (24.5) | 733 (56.0) | 46 (3.5) |
| Median age at diagnosis (years) | 72 | 64 | |||||||
| Age groups (years) | |||||||||
| 18–50 | 625 (12.1) | 371 (59.4) | 186 (29.8) | 68 (10.9) | 324 (24.7) | 90 (27.8) | 84 (25.9) | 147 (45.4) | 3 (0.9) |
| >50–70 | 1735 (33.7) | 826 (47.6) | 609 (35.1) | 300 (17.3) | 560 (42.7) | 95 (17.0) | 151 (27.0) | 293 (52.3) | 21 (3.8) |
| >70 | 2796 (54.2) | 917 (32.8) | 1015 (36.3) | 864 (30.9) | 426 (32.5) | 25 (5.9) | 86 (20.2) | 293 (68.8) | 22 (5.2) |
| Sex | |||||||||
| Men | 2773 (53.8) | 1101 (39.7) | 1003 (36.2) | 669 (24.1) | 783 (60.0) | 113 (14.4) | 172 (22.0) | 461 (58.9) | 37 (4.7) |
| Women | 2383 (46.2) | 1013 (42.5) | 807 (33.9) | 563 (23.6) | 527 (40.2) | 97 (18.4) | 149 (28.3) | 272 (51.6) | 9 (1.7) |
| Tumor site | |||||||||
| Upper/lower extremity, acral sites | 2240 (43.4) | 960 (42.9) | 745 (33.3) | 535 (23.9) | 583 (44.5) | 98 (16.8) | 140 (24.0) | 332 (56.9) | 13 (2.2) |
| Trunk | 1958 (38.0) | 792 (40.4) | 725 (37.0) | 441 (22.5) | 626 (47.8) | 106 (16.9) | 156 (24.9) | 338 (54.0) | 26 (4.2) |
| Head/neck | 932 (18.1) | 349 (37.4) | 333 (35.7) | 250 (26.8) | 95 (7.3) | 5 (5.3) | 23 (24.2) | 60 (63.2) | 7 (7.4) |
| Unknown information | 26 (0.5) | 13 (50.0) | 7 (26.9) | 6 (23.1) | 6 (0.5) | 1 (16.7) | 2 (33.3) | 3 (50.0) | 0 (0) |
| Median tumor thickness (mm) | 3.3 | 3.1 | |||||||
| T-stage | |||||||||
| T1–T2a | - | - | - | - | 302 (23.1) | 210 (69.5) | 56 (18.5) | 36 (11.9) | - |
| T2b | 713 (13.8) | 713 (100) | - | - | 77 (5.9) | - | 65 (84.4) | 12 (15.6) | - |
| T3a | 1401 (27.1) | 1401 (100) | - | - | 217 (16.6) | - | 200 (92.2) | 17 (7.8) | - |
| T3b | 1161 (22.5) | - | 1161 (100) | - | 238 (18.2) | - | - | 238 (100) | - |
| T4a | 649 (12.6) | - | 649 (100) | - | 133 (10.2) | - | - | 133 (100) | - |
| T4b | 1232 (23.9) | - | - | 1232 | 343 (26.2) | - | - | 297 (86.9) | 46 (13.4) |
| Histologic subtype | |||||||||
| NM | 2486 (48.2) | 700 (28.2) | 968 (38.9) | 818 (32.9) | 563 (43.0) | 33 (5.9) | 104 (18.5) | 396 (70.3) | 30 (5.3) |
| SSM | 1714 (33.2) | 977 (57) | 526 (30.7) | 211 (12.3) | 530 (40.5) | 140 (26.4) | 155 (29.2) | 224 (42.3) | 11 (0.6) |
| LMM | 237 (4.6) | 111 (46.8) | 75 (31.6) | 51 (21.5) | 27 (2.1) | 7 (18.9) | 9 (24.3) | 11 (29.7) | 0 (0) |
| ALM | 96 (1.9) | 33 (34.4) | 37 (38.5) | 26 (27.1) | 31 (2.4) | 1 (2.7) | 9 (29.0) | 21 (67.7) | 0 (0) |
| Other | 617 (12.0) | 291 (47.2) | 201 (32.6) | 125 (20.3) | 156 (11.9) | 29 (18.9) | 44 (28.2) | 79 (50.6) | 4 (2.6) |
| Unknown information | 2 (33.3) | 3 (50.0) | 1 (16.7) | 3 (0.2) | 0 (0) | 0 (0) | 2 (66.7) | 1 (33.3) | |
| Stage | Standardized 1-Year RSR (95% CI) | Difference in Standardized 1-Year RSR | Standardized 5-Year RSR (95% CI) | Difference in Standardized 5-Year RSR | Standardized Cure Proportion (95% CI) | Difference Standardized Cure Proportion | Standardized MST (Years) of Uncured (95% CI) | Difference in MST |
|---|---|---|---|---|---|---|---|---|
| IIA | 1.00 (0.99;1.00) | - | 0.88 (0.86;0.89) | - | 0.80 (0.77;0.83) | - | 4.2 (3.8;4.7) | - |
| IIB | 0.97 (0.96;0.98) | 0.03 (0.02;0.04) | 0.74 (0.71;0.76) | 0.14 (0.11;0.17) | 0.62 (0.59;0.66) | 0.17 (0.13;0.22) | 3.4 (3.1;3.7) | 0.8 (0.3;1.4) |
| IIC | 0.90 (0.88;0.92) | 0.09 (0.08;0.11) | 0.52 (0.49;0.56) | 0.35 (0.31;0.39) | 0.42 (0.37;0.46) | 0.38 (0.33;0.43) | 2.3 (2.1;2.5) | 2.0 (1.5;2.4) |
| IIIA | 0.98 (0.96;1.00) | - | 0.82 (0.75;0.88) | - | 0.76 (0.68;0.84) | - | 2.7 (1.8;3.5) | - |
| IIIB | 0.95 (0.93;0.98) | 0.03 (−0.006; 0.06) | 0.64 (0.58;0.69) | 0.18 (0.10;0.26) | 0.52 (0.45;0.59) | 0.25 (0.14;0,35) | 2.8 (2.2;3.4) | −0.18 (−1.23;0.87) |
| IIIC–D | 0.85 (0.82;0.88) | 0.13 (0.10;0.16) | 0.45 (0.41;0.49) | 0.36 (0.29;0.43) | 0.35 (0.30;0.39) | 0.42 (0.33;0.51) | 1.9 (1.7;2.1) | 0.74 (−0.14;1.63) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eriksson, H.; Utjés, D.; Olofsson Bagge, R.; Gillgren, P.; Isaksson, K.; Lapins, J.; Schultz, I.L.; Lyth, J.; Andersson, T.M.-L. The Proportion Cured of Patients with Resected Stage II–III Cutaneous Melanoma in Sweden. Cancers 2021, 13, 2456. https://doi.org/10.3390/cancers13102456
Eriksson H, Utjés D, Olofsson Bagge R, Gillgren P, Isaksson K, Lapins J, Schultz IL, Lyth J, Andersson TM-L. The Proportion Cured of Patients with Resected Stage II–III Cutaneous Melanoma in Sweden. Cancers. 2021; 13(10):2456. https://doi.org/10.3390/cancers13102456
Chicago/Turabian StyleEriksson, Hanna, Deborah Utjés, Roger Olofsson Bagge, Peter Gillgren, Karolin Isaksson, Jan Lapins, Inkeri Leonardsson Schultz, Johan Lyth, and Therese M.-L. Andersson. 2021. "The Proportion Cured of Patients with Resected Stage II–III Cutaneous Melanoma in Sweden" Cancers 13, no. 10: 2456. https://doi.org/10.3390/cancers13102456
APA StyleEriksson, H., Utjés, D., Olofsson Bagge, R., Gillgren, P., Isaksson, K., Lapins, J., Schultz, I. L., Lyth, J., & Andersson, T. M.-L. (2021). The Proportion Cured of Patients with Resected Stage II–III Cutaneous Melanoma in Sweden. Cancers, 13(10), 2456. https://doi.org/10.3390/cancers13102456






