Breaching the Blood–Brain Tumor Barrier for Tumor Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. The Blood–Brain Barrier (BBB) and the Blood–Brain Tumor Barrier (BBTB)—Some Fundamentals
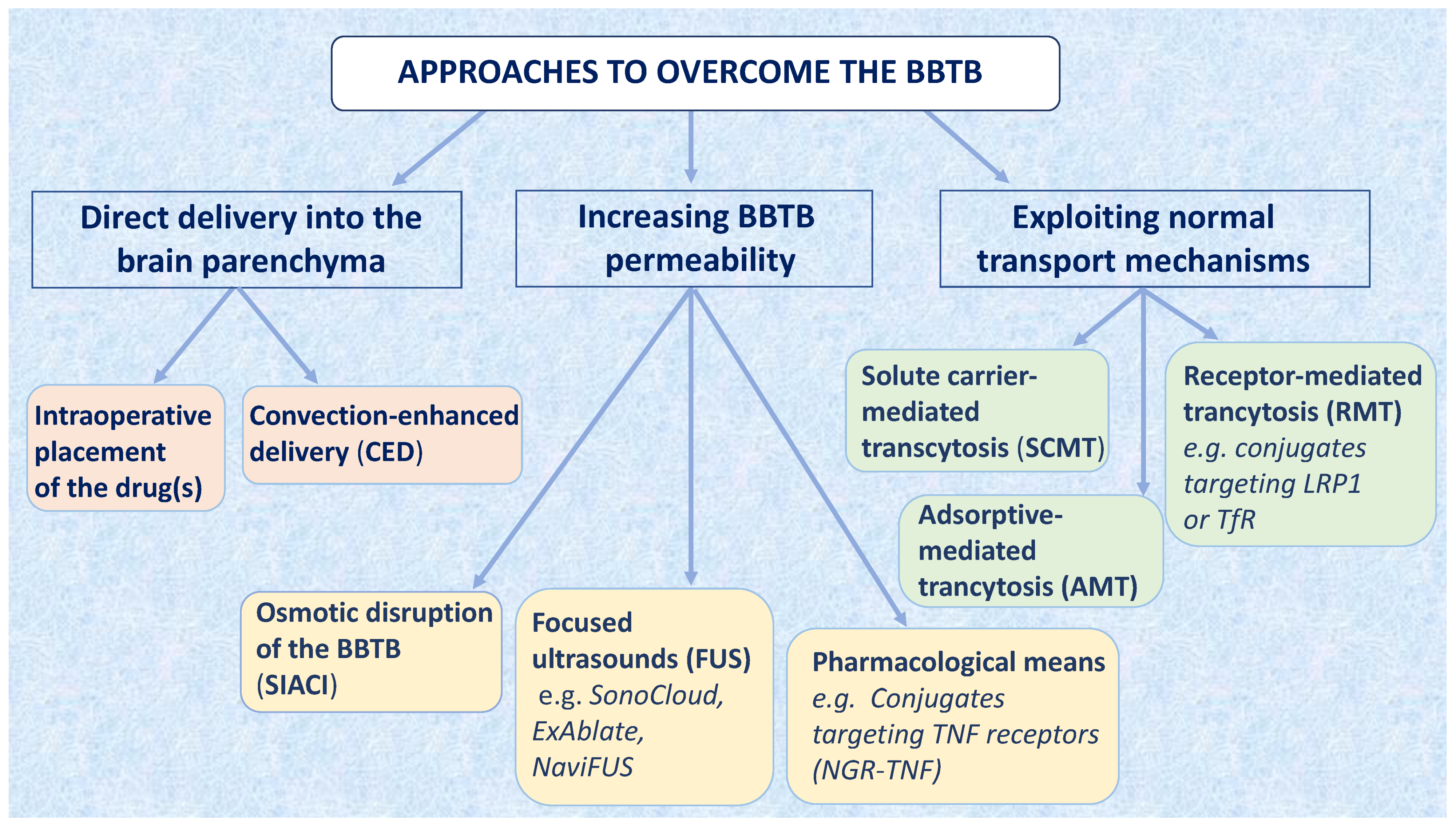
3. Approaches to Overcome the BBTB for Brain Tumor Therapy
3.1. Delivering Drugs Directly into the Brain
3.1.1. Wafers Impregnated with Anticancer Drugs
3.1.2. Convection-Enhanced Delivery (CED)
3.2. Increasing the Permeability of the BBTB
3.2.1. Osmotic Disruption of the BBTB
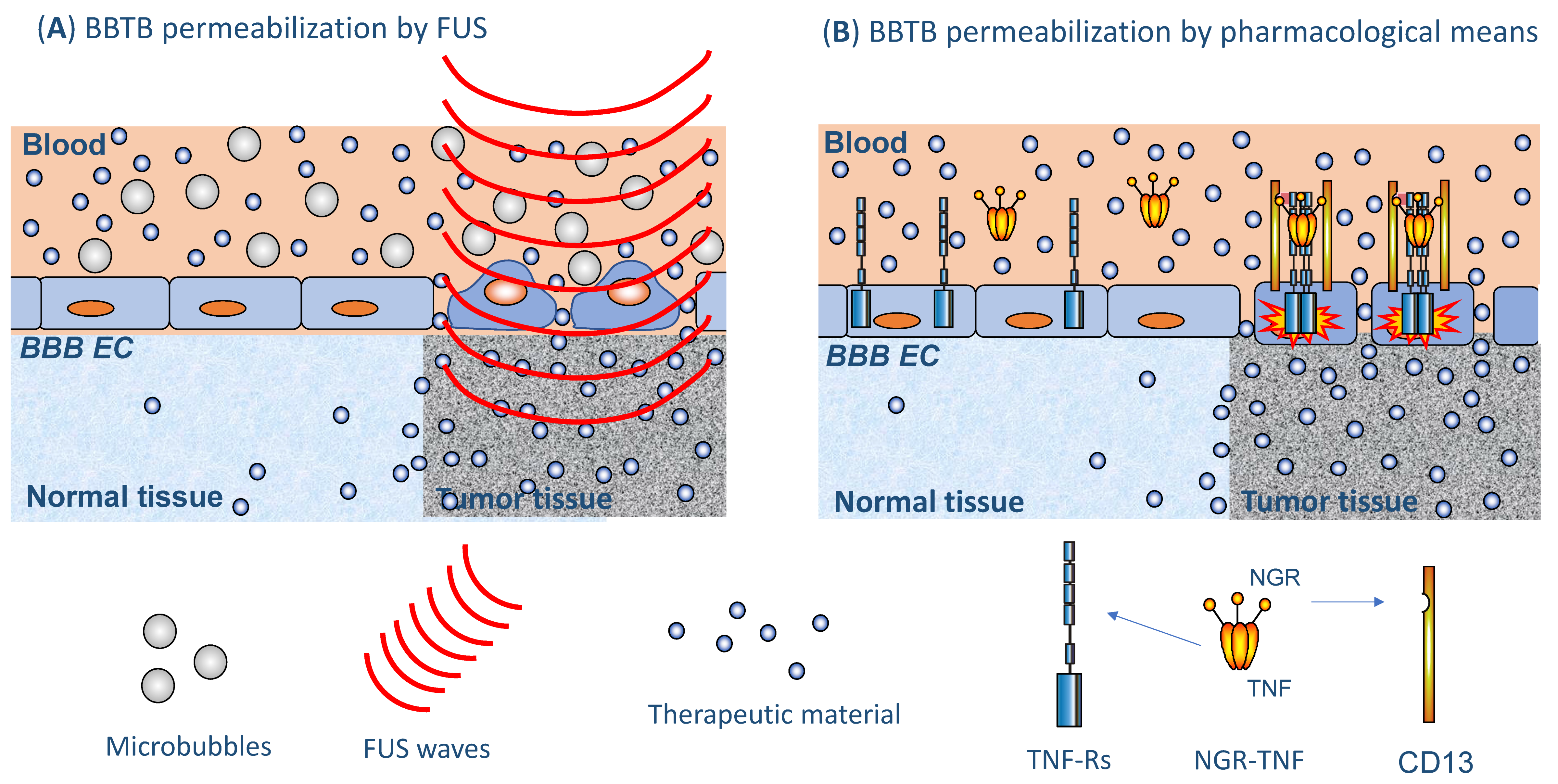
3.2.2. Ultrasound/Focused Ultrasound
3.2.3. Increasing BBTB Permeability by Pharmacological Means
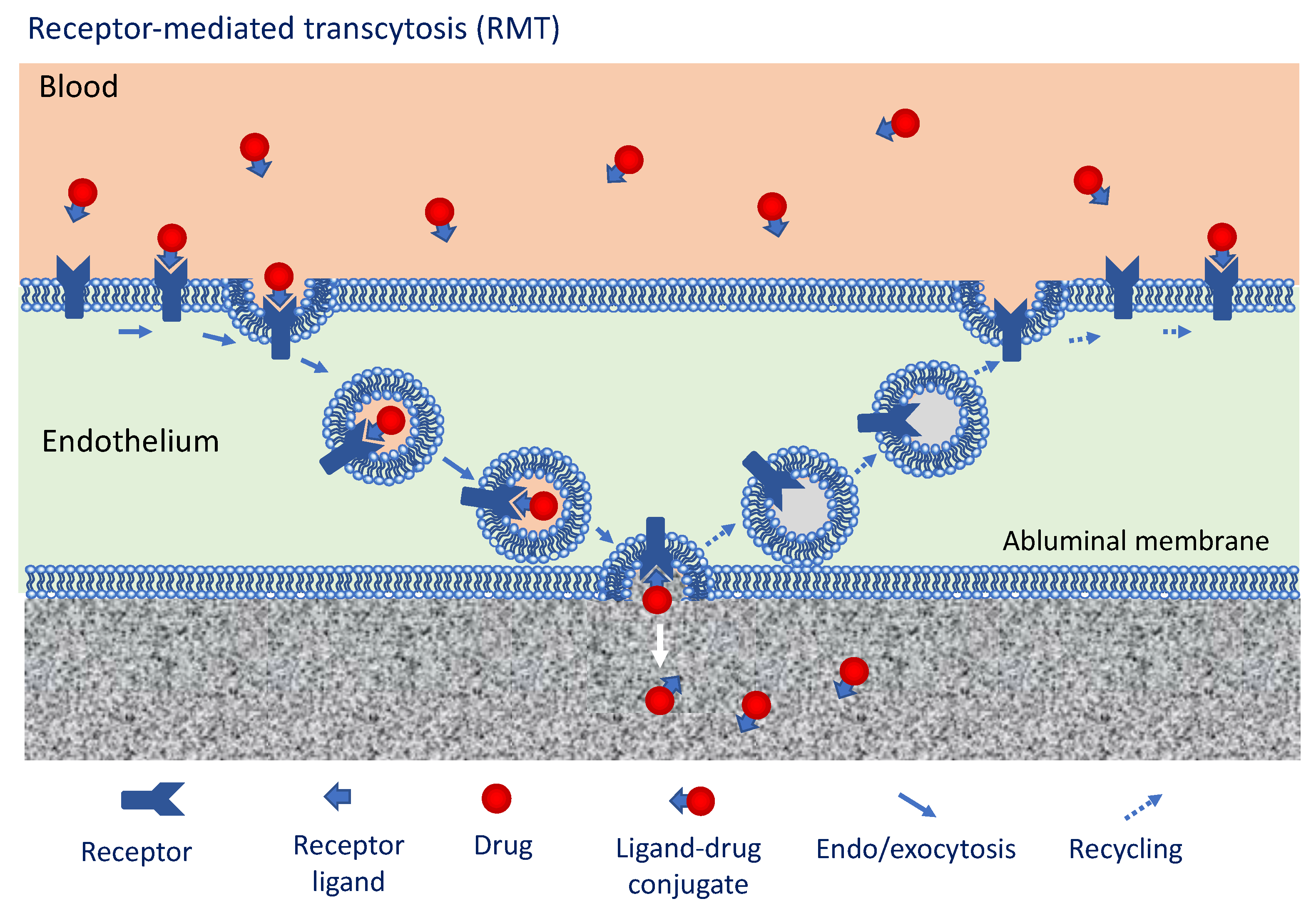
3.3. Approaches to Overcome the BBTB Exploiting Intrinsic Transport Mechanisms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Nelson, A.R.; Betsholtz, C.; Zlokovic, B.V. Establishment and dysfunction of the blood-brain barrier. Cell 2015, 163, 1064–1078. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Vanlandewijck, M.; He, L.; Mäe, M.A.; Andrae, J.; Ando, K.; Del Gaudio, F.; Nahar, K.; Lebouvier, T.; Laviña, B.; Gouveia, L. A molecular atlas of cell types and zonation in the brain vasculature. Nature 2018, 554, 475–480. [Google Scholar] [CrossRef]
- Saunders, A.; Macosko, E.Z.; Wysoker, A.; Goldman, M.; Krienen, F.M.; de Rivera, H.; Bien, E.; Baum, M.; Bortolin, L.; Wang, S. Molecular diversity and specializations among the cells of the adult mouse brain. Cell 2018, 174, 1015–1030. [Google Scholar] [CrossRef]
- Abdul Razzak, R.; Florence, G.J.; Gunn-Moore, F.J. Approaches to CNS drug delivery with a focus on transporter-mediated transcytosis. Int. J. Mol. Sci. 2019, 20, 3108. [Google Scholar] [CrossRef]
- Loscher, W.; Potschka, H. Blood–brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx 2005, 2, 86–98. [Google Scholar] [CrossRef]
- Mittapalli, R.K.; Manda, V.K.; Adkins, C.E.; Geldenhuys, W.J.; Lockman, P.R. Exploiting nutrient transporters at the blood–brain barrier to improve brain distribution of small molecules. Ther. Deliv. 2010, 1, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; di Tomaso, E.; Duda, D.G.; Loeffler, J.S.; Sorensen, A.G.; Batchelor, T.T. Angiogenesis in brain tumours. Nat. Rev. Neurosci. 2007, 8, 610–622. [Google Scholar] [CrossRef]
- Sowers, J.L.; Joohnson, K.M.; Conrad, C.; Patterson, J.T.; Sowers, L.C. The role of inflammation in brain cancer. Adv. Exp. Med. Biol. 2014, 816, 75–105. [Google Scholar]
- Seano, G.; Nia, H.T.; Emblem, K.E.; Datta, M.; Ren, J.; Krishnan, S.; Kloepper, J.; Pinho, M.C.; Ho, W.W.; Ghosh, M.; et al. Solid stress in brain tumours causes neuronal loss and neurological dysfunction and can be reversed by lithium. Nat. Biomed. Eng. 2019, 3, 230. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Lockman, P.R.; Mittapalli, R.K.; Taskar, K.S.; Rudraraju, V.; Gril, B.; Bohn, K.A.; Adkins, C.E.; Roberts, A.; Thorsheim, H.R.; Gaasch, J.A.; et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin. Cancer Res. 2010, 16, 5664–5678. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, F.; Corti, A. How to improve exposure of tumor cells to drugs–Promoter drugs increase tumor uptake and penetration of effector drugs. Adv. Drug. Deliv. Rev. 2012, 64, 53–68. [Google Scholar] [CrossRef]
- Marcucci, F.; Corti, A. Improving drug penetration to curb tumor drug resistance. Drug Discov. Tod. 2012, 17, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Kang, H.J.; Hong, K.T.; Hong, C.R.; Lee, Y.J.; Park, J.D.; Phi, J.H.; Kim, S.K.; Wang, K.C.; Kim, I.H.; et al. Tandem high-dose chemotherapy with topotecan-thiotepa-carboplatin and melphalan-etoposide-carboplatin regimens for pediatric high-risk brain tumors. Int. J. Clin. Oncol. 2019, 24, 1515–1525. [Google Scholar] [CrossRef]
- Ferreri, A.J.; Cwynarski, K.; Pulczynski, E.; Ponzoni, M.; Deckert, M.; Politi, L.S.; Torri, V.; Fox, C.P.; Rosée, P.L.; Schorb, E.; et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: Results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. 2016, 3, e217–e227. [Google Scholar] [CrossRef]
- Xia, Y.; Xu, F.; Xiong, M.; Yang, H.; Lin, W.; Xie, Y.; Xi, H.; Xue, Q.; Ye, T.; Yu, L. Repurposing of antipsychotic trifluoperazine for treating brain metastasis, lung metastasis and bone metastasis of melanoma by disrupting autophagy flux. Pharmacol. Res. 2021, 163, 105295. [Google Scholar] [CrossRef]
- Orthmann, A.; Peiker, L.; Fichtner, I.; Hoffmann, A.; Hilger, R.A.; Zeisig, R. Improved treatment of MT-3 breast cancer and brain metastases in a mouse xenograft by LRP-targeted oxaliplatin liposomes. J. Biomed. Nanotechnol. 2016, 12, 56–68. [Google Scholar] [CrossRef]
- Kobus, T.; Zervantonakis, I.K.; Zhang, Y.; McDannold, N.J. Growth inhibition in a brain metastasis model by antibody delivery using focused ultrasound-mediated blood-brain barrier disruption. J. Control. Release 2016, 238, 281–288. [Google Scholar] [CrossRef]
- Perry, J.; Chambers, A.; Spithoff, K.; Laperriere, N. Gliadel wafers in the treatment of malignant glioma: A systematic review. Curr. Oncol. 2007, 14, 189–194. [Google Scholar] [CrossRef]
- Brem, H.; Piantadosi, S.; Burger, P.C.; Walker, M.; Selker, R.; Vick, N.A.; Black, K.; Sisti, M.; Brem, S.; Mohr, G.; et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet 1995, 345, 1008–1012. [Google Scholar] [CrossRef]
- Westphal, M.; Hilt, D.C.; Bortey, E.; Delavault, P.; Olivares, R.; Warnke, P.C.; Whittle, I.R.; Jääskeläinen, J.; Ram, Z. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003, 5, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Pallud, J.; Audureau, E.; Noel, G.; Corns, R.; Lechapt-Zalcman, E.; Duntze, J.; Pavlov, V.; Guyotat, J.; Hieu, P.D.; Le Reste, P.J.; et al. Long-term results of carmustine wafer implantation for newly diagnosed glioblastomas: A controlled propensity-matched analysis of a French multicenter cohort. Neuro Oncol. 2015, 17, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Kadota, T.; Saito, R.; Kumabe, T.; Mizusawa, J.; Katayama, H.; Sumi, M.; Igaki, H.; Kinoshita, M.; Komori, T.; Ichimura, K. A multicenter randomized phase III study for newly diagnosed maximally resected glioblastoma comparing carmustine wafer implantation followed by chemoradiotherapy with temozolomide with chemoradiotherapy alone; Japan Clinical Oncology Group Study JCOG1703 (MACS study). Jpn. J. Clin. Oncol. 2019, 49, 1172–1175. [Google Scholar] [PubMed]
- Lee, J.; Cho, H.R.; Cha, G.D.; Seo, H.; Lee, S.; Park, C.K.; Kim, J.W.; Qiao, S.; Wang, L.; Kang, D. Flexible, sticky, and biodegradable wireless device for drug delivery to brain tumors. Nat. Commun. 2019, 10, 5205. [Google Scholar] [CrossRef]
- Bobo, R.H.; Laske, D.W.; Akbasak, A.; Morrison, P.F.; Dedrick, R.L.; Oldfield, E.H. Convection-enhanced delivery of macromolecules in the brain. Proc. Natl. Acad. Sci. USA 1994, 91, 2076–2080. [Google Scholar] [CrossRef]
- Souweidane, M.M.; Kramer, K.; Pandit-Taskar, N.; Zhou, Z.; Haque, S.; Zanzonico, P.; Carrasquillo, J.A.; Lyashchenko, S.K.; Thakur, S.B.; Donzelli, M.; et al. Convection-enhanced delivery for diffuse intrinsic pontine glioma: A single-centre, dose-escalation, phase 1 trial. Lancet Oncol. 2018, 19, 1040–1050. [Google Scholar] [CrossRef]
- Saito, R.; Kanamori, M.; Sonoda, Y.; Yamashita, Y.; Nagamatsu, K.; Murata, T.; Mugikura, S.; Kumabe, T.; Wembacher-Schröder, E.; Thomson, R.; et al. Phase I trial of convection-enhanced delivery of nimustine hydrochloride (ACNU) for brainstem recurrent glioma. Neurooncol. Adv. 2020, 2, vdaa033. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.H.; Akabani, G.; Archer, G.E.; Bigner, D.D.; Berger, M.S.; Friedman, A.H.; Friedman, H.S.; Herndon, J.E., 2nd; Kunwar, S.; Marcus, S.; et al. Progress report of a Phase I study of the intracerebral microinfusion of a recombinant chimeric protein composed of transforming growth factor (TGF)-alpha and a mutated form of the Pseudomonas exotoxin termed PE-38 (TP-38) for the treatment of malignant brain tumors. J. Neurooncol. 2003, 65, 27–35. [Google Scholar]
- Weber, F.W.; Floeth, F.; Asher, A.; Bucholz, R.; Berger, M.; Prados, M.; Chang, S.; Bruce, J.; Hall, W.; Rainov, N.G.; et al. Local convection enhanced delivery of IL4-Pseudomonas exotoxin (NBI-3001) for treatment of patients with recurrent malignant glioma. Acta Neurochir. Suppl. 2003, 88, 93–103. [Google Scholar]
- Heiss, J.D.; Jamshidi, A.; Shah, S.; Martin, S.; Wolters, P.L.; Argersinger, D.P.; Warren, K.E.; Lonser, R.R. Phase I trial of convection-enhanced delivery of IL13-Pseudomonas toxin in children with diffuse intrinsic pontine glioma. J. Neurosurg. Pediatr. 2018, 23, 333–342. [Google Scholar] [CrossRef]
- Kunwar, S.; Chang, S.; Westphal, M.; Vogelbaum, M.; Sampson, J.; Barnett, G.; Shaffrey, M.; Ram, Z.; Piepmeier, J.; Prados, M.; et al. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010, 12, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, A.; Gromeier, M.; Herndon, J.E., 2nd; Beaubier, N.; Bolognesi, D.P.; Friedman, A.H.; Friedman, H.S.; McSherry, F.; Muscat, A.M.; Nair, S.; et al. Recurrent glioblastoma treated with recombinant poliovirus. N. Engl. J. Med. 2018, 379, 150–161. [Google Scholar] [CrossRef]
- Lidar, Z.; Mardor, Y.; Jonas, T.; Pfeffer, R.; Faibel, M.; Nass, D.; Hadani, M.; Ram, Z. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: A phase I/II clinical study. J. Neurosurg. 2004, 100, 472–479. [Google Scholar] [CrossRef]
- Tanner, P.G.; Holtmannspötter, M.; Tonn, J.C.; Goldbrunner, R. Effects of drug efflux on convection-enhanced paclitaxel delivery to malignant gliomas: Technical note. Neurosurgery 2007, 61, E880–E882. [Google Scholar] [CrossRef]
- Sampson, J.H.; Akabani, G.; Archer, G.E.; Berger, M.S.; Coleman, R.E.; Friedman, A.H.; Friedman, H.S.; Greer, K.; Herndon, J.E., 2nd; Kunwar, S.; et al. Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant tumors. Neuro Oncol. 2008, 10, 320–329. [Google Scholar] [CrossRef]
- Choi, C.; Kim, H.M.; Shon, J.; Park, J.; Kim, H.T.; Oh, S.H.; Kim, N.K.; Kim, O.J. Additional increased effects of mannitol-temozolomide combined treatment on blood-brain barrier permeability. Biochem. Biophys. Res. Commun. 2018, 497, 769–775. [Google Scholar] [CrossRef]
- Bhattacharjee, A.K.; Nagashima, T.; Kondoh, T.; Tamaki, N. The effects of the Na(+)/Ca(++) exchange blocker on osmotic blood-brain barrier disruption. Brain Res. 2001, 900, 157–162. [Google Scholar] [CrossRef]
- Rodriguez, A.; Tatter, S.B.; Debinski, W. Neurosurgical techniques for disruption of the blood–brain barrier for glioblastoma treatment. Pharmaceutics 2015, 7, 175–187. [Google Scholar] [CrossRef]
- Chakraborty, S.; Filippi, C.G.; Wong, T.; Ray, A.; Fralin, S.; Tsiouris, A.J.; Praminick, B.; Demopoulos, A.; McCrea, H.J.; Bodhinayake, I.; et al. Superselective intraarterial cerebral infusion of cetuximab after osmotic blood/brain barrier disruption for recurrent malignant glioma: Phase I study. J. Neurooncol. 2016, 128, 405–415. [Google Scholar] [CrossRef]
- Chakraborty, S.; Filippi, C.G.; Wong, T.; Ray, A.; Fralin, S.; Tsiouris, A.J.; Praminick, B.; Demopoulos, A.; McCrea, H.J.; Bodhinayake, I.; et al. Safety and maximum tolerated dose of superselective intraarterial cerebral infusion of bevacizumab after osmotic blood-brain barrier disruption for recurrent malignant glioma. J. Neurosurg. 2011, 114, 624–632. [Google Scholar]
- Chakraborty, S.; Filippi, C.G.; Burkhardt, J.K.; Fralin, S.; Ray, A.; Wong, T.; Ortiz, R.; Langer, D.J.; Boockvar, J.A. Durability of single dose intra-arterial bevacizumab after blood/brain barrier disruption for recurrent glioblastoma. J. Exp. Ther. Oncol. 2016, 11, 261–267. [Google Scholar]
- Angelov, L.; Doolittle, N.D.; Kraemer, D.F.; Siegal, T.; Barnett, G.H.; Peereboom, D.M.; Stevens, G.; McGregor, J.; Jahnke, K.; Lacy, C.A.; et al. Blood-brain barrier disruption and intra-arterial methotrexate-based therapy for newly diagnosed primary CNS lymphoma: A multi-institutional experience. J. Clin. Oncol. 2009, 27, 3503–3509. [Google Scholar] [CrossRef] [PubMed]
- Beccaria, K.; Canney, M.; Bouchoux, G.; Desseaux, C.; Grill, J.; Heimberger, A.B.; Carpentier, A. Ultrasound-induced blood-brain barrier disruption for the treatment of gliomas and other primary CNS tumors. Cancer Lett. 2020, 479, 13–22. [Google Scholar] [CrossRef]
- Parodi, A.; Rudzińska, M.; Deviatkin, A.A.; Soond, S.M.; Baldin, A.V.; Zamyatnin, A.A., Jr. Established and emerging strategies for drug delivery across the blood-brain barrier in brain cancer. Pharmaceutics 2019, 11, E245. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.K.; Chu, P.C.; Chai, W.Y.; Kang, S.T.; Tsai, C.H.; Fan, C.H.; Yeh, C.K.; Liu, H.L. Characterization of different microbubbles in assisting focused ultrasound-induced blood-brain barrier opening. Sci. Rep. 2017, 7, 46689. [Google Scholar] [CrossRef] [PubMed]
- Horodyckid, C.; Canney, M.; Vignot, A.; Boisgard, R.; Drier, A.; Huberfeld, G.; François, C.; Prigent, A.; Santin, M.D.; Adam, C.; et al. Safe long-term repeated disruption of the blood-brain barrier using an implantable ultrasound device: A multiparametric study in primates. J. Neurosurg. 2017, 126, 1351–1361. [Google Scholar] [CrossRef]
- Goldwirt, L.; Canney, M.; Horodyckid, C.; Poupon, J.; Mourah, S.; Vignot, A.; Chapelon, J.Y.; Carpentier, A. Enhanced brain distribution of carboplatin in a primate model after blood–brain barrier disruption using an implantable ultrasound device. Cancer Chemother. Pharmacol. 2016, 77, 211–216. [Google Scholar] [CrossRef]
- Carpentier, A.; Canney, M.; Vignot, A.; Reina, V.; Beccaria, K.; Horodyckid, C.; Karachi, C.; Leclercq, D.; Lafon, C.; Chapelon, J.Y.; et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl. Med. 2016, 8, 343re2. [Google Scholar] [CrossRef] [PubMed]
- Idbaih, A.; Canney, M.; Belin, L.; Desseaux, C.; Vignot, A.; Bouchoux, G.; Asquier, N.; Law-Ye, B.; Leclercq, D.; Bissery, A.; et al. Safety and feasibility of repeated and transient blood-brain barrier disruption by pulsed ultrasound in patients with recurrent glioblastoma. Clin. Cancer Res. 2019, 25, 3793–3801. [Google Scholar] [CrossRef]
- Dick, E.A.; Gedroyc, W.M. ExAblate magnetic resonance-guided focused ultrasound system in multiple body applications. Expert Rev. Med. Devices 2010, 7, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Z.R.; Zaubermann, J.; Harnof, S.; Mardor, Y.; Nass, D.; Zadicario, E.; Hananel, A.; Castel, D.; Faibel, M.; Ram, Z. Magnetic resonance imaging-guided focused ultrasound for thermal ablation in the brain: A feasibility study in a swine model. Neurosurgery 2007, 60, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Lipsman, N.; Meng, Y.; Bethune, A.J.; Huang, Y.; Lam, B.; Masellis, M.; Herrmann, N.; Heyn, C.; Aubert, I.; Boutet, A.; et al. Blood-brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat. Commun. 2018, 9, 2336. [Google Scholar] [CrossRef] [PubMed]
- Rezai, A.R.; Ranjan, M.; D’Haese, P.F.; Haut, M.W.; Carpenter, J.; Najib, U.; Mehta, R.I.; Chazen, J.L.; Zibly, Z.; Yates, J.R.; et al. Noninvasive hippocampal blood-brain barrier opening in Alzheimer’s disease with focused ultrasound. Proc. Natl. Acad. Sci. USA 2020, 117, 9180–9182. [Google Scholar] [CrossRef]
- Stewart, E.A.; Rabinovici, J.; Tempany, C.M.; Inbar, Y.; Regan, L.; Gostout, B.; Hesley, G.; Kim, H.S.; Hengst, S.; Gedroyc, W.M. Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil. Steril. 2006, 85, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.Y.; Chu, P.C.; Tsai, C.H.; Lin, C.Y.; Yang, H.W.; Lai, H.Y.; Liu, H.L. Image-guided focused-ultrasound CNS molecular delivery: An implementation via dynamic contrast-enhanced magnetic-resonance imaging. Sci. Rep. 2018, 8, 4151. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.T.; Lin, Y.J.; Chai, W.Y.; Lin, C.J.; Chen, P.Y.; Huang, C.Y.; Kuo, J.S.; Liu, H.L.; Wei, K.C. Neuronavigation-guided focused ultrasound (NaviFUS) for transcranial blood-brain barrier opening in recurrent glioblastoma patients: Clinical trial protocol. Ann. Transl. Med. 2020, 8, 673. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Ye, D.; Laforest, R.; Williamson, J.; Liu, Y.; Chen, H. Cavitation dose painting for focused ultrasound-induced blood-brain barrier disruption. Sci. Rep. 2019, 9, 2840. [Google Scholar] [CrossRef]
- Gasca-Salas, C.; Fernández-Rodríguez, B.; Pineda-Pardo, J.A.; Rodríguez-Rojas, R.; Obeso, I.; Hernández-Fernández, F.; Del Álamo, M.; Mata, D.; Guida, P.; Ordás-Bandera, C.; et al. Blood-brain barrier opening with focused ultrasound in Parkinson’s disease dementia. Nat. Commun. 2021, 12, 779. [Google Scholar] [CrossRef]
- Conti, A.; Mériaux, S.; Larrat, B. About the Marty model of blood-brain barrier closure after its disruption using focused ultrasound. Phys. Med. Biol. 2019, 64, 14NT02. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.; Poon, C.; Hynynen, K. Evaluating the safety profile of focused ultrasound and microbubble-mediated treatments to increase blood-brain barrier permeability. Expert Opin. Drug Deliv. 2019, 16, 129–142. [Google Scholar] [CrossRef]
- Doctrow, S.R.; Abelleira, S.M.; Curry, L.A.; Heller-Harrison, R.; Kozarich, J.W.; Malfroy, B.; McCarroll, L.A.; Morgan, K.G.; Morrow, A.R.; Musso, G.F.; et al. The bradykinin analog RMP-7 increases extracellular free calcium levels in rat brain microvascular endothelial cells. J. Pharmacol. Exp. Ther. 1994, 271, 229–237. [Google Scholar] [PubMed]
- Gregor, A.; Lind, M.; Newman, H.; Grant, R.; Hadley, D.M.; Barton, T.; Osborn, C. Phase II studies of RMP-7 and carboplatin in the treatment of recurrent high grade glioma. RMP-7 European Study Group. J. Neurooncol. 1999, 44, 137–145. [Google Scholar] [CrossRef]
- Warren, K.E.; Patel, M.C.; Aikin, A.A.; Widemann, B.; Libucha, M.; Adamson, P.C.; Neuwirth, R.; Benziger, D.; O’Toole, T.; Ford, K.; et al. Phase I trial of lobradimil (RMP-7) and carboplatin in children with brain tumors. Cancer Chemother. Pharmacol. 2001, 48, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Prados, M.D.; Schold, S.C., Jr.; Fine, H.A.; Jaeckle, K.; Hochberg, F.; Mechtler, L.; Fetell, M.R.; Phuphanich, S.; Feun, L.; Janus, T.J.; et al. A randomized, double-blind, placebo-controlled, phase 2 study of RMP-7 in combination with carboplatin administered intravenously for the treatment of recurrent malignant glioma. Neuro Oncol. 2003, 5, 96–103. [Google Scholar] [CrossRef]
- Warren, K.; Jakacki, R.; Widemann, B.; Aikin, A.; Libucha, M.; Packer, R.; Vezina, G.; Reaman, G.; Shaw, D.; Krailo, M.; et al. Phase II trial of intravenous lobradimil and carboplatin in childhood brain tumors: A report from the Children’s Oncology Group. Cancer Chemother. Pharmacol. 2006, 5, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Carman, A.J.; Mills, J.H.; Krenz, A.; Kim, D.G.; Bynoe, M.S. Adenosine receptor signaling modulates permeability of the blood-brain barrier. J. Neurosci. 2011, 31, 13272–13280. [Google Scholar] [CrossRef]
- Jackson, S.; George, R.T.; Lodge, M.A.; Piotrowski, A.; Wahl, R.L.; Gujar, S.K.; Grossman, S.A. The effect of regadenoson on the integrity of the human blood-brain barrier, a pilot study. J. Neurooncol. 2017, 132, 513–519. [Google Scholar] [CrossRef]
- Jackson, S.; Weingart, J.; Nduom, E.K.; Harfi, T.T.; George, R.T.; McAreavey, D.; Ye, X.; Anders, N.M.; Peer, C.; Figg, W.D.; et al. The effect of an adenosine A2A agonist on intra-tumoral concentrations of temozolomide in patients with recurrent glioblastoma. Fluids Barriers CNS 2018, 15, 2. [Google Scholar] [CrossRef]
- Connell, J.J.; Chatain, G.; Cornelissen, B.; Vallis, K.A.; Hamilton, A.; Seymour, L.; Anthony, D.C.; Sibson, N.R. Selective permeabilization of the blood-brain barrier at sites of metastasis. J. Natl. Cancer Inst. 2013, 105, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Corti, A.; Curnis, F.; Rossoni, G.; Marcucci, F.; Gregorc, V. Peptide-mediated targeting of cytokines to tumor vasculature: The NGR-hTNF example. BioDrugs 2013, 27, 591–603. [Google Scholar] [CrossRef]
- Curnis, F.; Sacchi, A.; Borgna, L.; Magni, F.; Gasparri, A.; Corti, A. Enhancement of tumor necrosis factor alpha antitumor immunotherapeutic properties by targeted delivery to aminopeptidase N (CD13). Nat. Biotechnol. 2000, 18, 1185–1190. [Google Scholar] [CrossRef]
- Di Matteo, P.; Arrigoni, G.L.; Alberici, L.; Corti, A.; Gallo-Stampino, C.; Traversari, C.; Doglioni, C.; Rizzardi, G.P. Enhanced expression of CD13 in vessels of inflammatory and neoplastic tissues. J. Histochem. Cytochem. 2011, 59, 47–59. [Google Scholar] [CrossRef]
- Ferreri, A.J.M.; Calimeri, T.; Conte, G.M.; Cattaneo, D.; Fallanca, F.; Ponzoni, M.; Scarano, E.; Curnis, F.; Nonis, A.; Lopedote, P.; et al. R-CHOP preceded by blood-brain barrier permeabilization with engineered tumor necrosis factor-alpha in primary CNS lymphoma. Blood 2019, 134, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, A.J.M.; Calimeri, T.; Ponzoni, M.; Curnis, F.; Conte, G.M.; Scarano, E.; Rrapaj, E.; De Lorenzo, D.; Cattaneo, D.; Fallanca, F.; et al. Blood-brain barrier permeabilization with engineered tumor necrosis factor-α followed by R-CHOP is an active and safe salvage therapy in primary CNS lymphoma. Blood Adv. 2020, 4, 3648–3658. [Google Scholar] [CrossRef] [PubMed]
- Curnis, F.; Sacchi, A.; Corti, A. Improving chemotherapeutic drug penetration in tumors by vascular targeting and barrier alteration. J. Clin. Investig. 2002, 110, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, A.; Gasparri, A.; Gallo-Stampino, C.; Toma, S.; Curnis, F.; Corti, A. Synergistic antitumor activity of cisplatin, paclitaxel, and gemcitabine with tumor vasculature-targeted tumor necrosis factor-alpha. Clin. Cancer Res. 2006, 12, 175–182. [Google Scholar] [CrossRef]
- Gregorc, V.; Gaafar, R.M.; Favaretto, A.; Grossi, F.; Jassem, J.; Polychronis, A.; Bidoli, P.; Tiseo, M.; Shah, R.; Taylor, P.; et al. NGR-hTNF in combination with best investigator choice in previously treated malignant pleural mesothelioma (NGR015): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2018, 19, 799–811. [Google Scholar] [CrossRef]
- Guarnieri, G.; Sarchielli, E.; Comeglio, P.; Herrera-Puerta, E.; Piaceri, I.; Nacmias, B.; Benelli, M.; Kelsey, G.; Maggi, M.; Gallina, P.; et al. Tumor necrosis factor α influences phenotypic plasticity and promotes epigenetic changes in human basal forebrain cholinergic neuroblasts. Int. J. Mol. Sci. 2020, 21, 6128. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, Y.; Naito, T.; Yuge, T.; Furukawa, A.; Yamada, S.; Pardridge, W.M.; Kimura, R. Blood-brain barrier transport of 125I-labeled basic fibroblast growth factor. Pharm. Res. 2000, 17, 63–69. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, Y.; Meng, F.; Deng, C.; Cheng, R.; Zhang, J.; Feijen, J.; Zhong, Z. Highly efficacious and specific anti-glioma chemotherapy by tandem nanomicelles co-functionalized with brain tumor-targeting and cell-penetrating peptides. J. Control. Release 2018, 278, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Valiante, S.; Falanga, A.; Cigliano, L.; Iachetta, G.; Busiello, R.A.; La Marca, V.; Galdiero, M.; Lombardi, A.; Galdiero, S. Peptide gH625 enters into neuron and astrocyte cell lines and crosses the blood-brain barrier in rats. Int. J. Nanomed. 2015, 10, 1885–1898. [Google Scholar]
- Bai, X.; Moraes, T.F.; Reithmeier, R.A.F. Structural biology of solute carrier (SLC) membrane transport proteins. Mol. Membr. Biol. 2017, 34, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Patching, S.G. Glucose transporters at the blood-brain barrier: Function, regulation and gateways for drug delivery. Mol. Neurobiol. 2017, 54, 1046–1077. [Google Scholar] [CrossRef]
- Geier, E.G.; Schlessinger, A.; Fan, H.; Gable, J.E.; Irwin, J.J.; Sali, A.; Giacomini, K.M. Structure-based ligand discovery for the large-neutral amino acid transporter 1, LAT-1. Proc. Natl. Acad. Sci. USA 2013, 110, 5480–5485. [Google Scholar] [CrossRef]
- Gao, B.; Hagenbuch, B.; Kullak-Ublick, G.A.; Benke, D.; Aguzzi, A.; Meier, P.J. Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J. Pharmacol. Exp. Ther. 2000, 294, 73–79. [Google Scholar] [PubMed]
- Tuma, P.; Hubbard, A.L. Transcytosis: Crossing cellular barriers. Physiol. Rev. 2003, 83, 871–932. [Google Scholar] [CrossRef]
- Begg, D.P. Insulin transport into the brain and cerebrospinal fluid. Vitam. Horm. 2015, 98, 229–248. [Google Scholar] [PubMed]
- Johnsen, K.B.; Burkhart, A.; Melander, F.; Kempen, P.J.; Vejlebo, J.B.; Siupka, P.; Nielsen, M.S.; Andresen, T.L.; Moos, T. Targeting transferrin receptors at the blood-brain barrier improves the uptake of immunoliposomes and subsequent cargo transport into the brain parenchyma. Sci. Rep. 2017, 7, 10396. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Qian, M.; Wang, Y.; Huang, J.; Chen, J.; Ni, L.; Huang, Q.; Liu, Q.; Gong, P.; Hou, S.; et al. Combination glioma therapy mediated by a dual-targeted delivery system constructed using OMCN-PEG-Pep22/DOX. Small 2018, 14, e1801905. [Google Scholar] [CrossRef]
- Tosi, G.; Vilella, A.; Veratti, P.; Belletti, D.; Pederzoli, F.; Ruozi, B.; Vandelli, M.A.; Zoli, M.; Forni, F. Exploiting bacterial pathways for BBB crossing with PLGA nanoparticles modified with a mutated form of diphtheria toxin (CRM197): In vivo experiments. Mol. Pharm. 2015, 12, 3672–3684. [Google Scholar] [CrossRef]
- Chai, Z.; Hu, X.; Wei, X.; Zhan, C.; Lu, L.; Jiang, K.; Su, B.; Ruan, H.; Ran, D.; Fang, R.H.; et al. A facile approach to functionalizing cell membrane-coated nanoparticles with neurotoxin-derived peptide for brain-targeted drug delivery. J. Control. Release 2017, 264, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Soe, Z.C.; Kwon, J.B.; Thapa, R.K.; Ou, W.; Nguyen, H.T.; Gautam, M.; Oh, K.T.; Choi, H.G.; Ku, S.K.; Yong, C.S.; et al. Transferrin-conjugated polymeric nanoparticle for receptor-mediated delivery of doxorubicin in doxorubicin-resistant breast cancer cells. Pharmaceutics 2019, 11, 63. [Google Scholar] [CrossRef]
- Demeule, M.; Currie, J.C.; Bertrand, Y.; Ché, C.; Nguyen, T.; Régina, A.; Gabathule, R.; Castaigne, J.P.; Béliveau, R. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector angiopep-2. J. Neurochem. 2008, 106, 1534–1544. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, Y.; Currie, J.C.; Poirier, J.; Demeule, M.; Abulrob, A.; Fatehi, D.; Stanimirovic, D.; Sartelet, H.; Castaigne, J.P.; Béliveau, R. Influence of glioma tumour microenvironment on the transport of ANG1005 via low-density lipoprotein receptor-related protein 1. Br. J. Cancer 2011, 105, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Li, Y.; Lee, J.; Schwartz, A.L.; Bu, G. Low-density lipoprotein receptor-related protein 1 promotes cancer cell migration and invasion by inducing the expression of matrix metalloproteinases 2 and 9. Cancer Res. 2009, 69, 879–886. [Google Scholar] [CrossRef]
- Regina, A.; Demeule, M.; Che, C.; Lavallée, I.; Poirier, J.; Gabathuler, R.; Béliveau, R.; Castaigne, J.P. Antitumour activity of ANG1005, a conjugate between paclitaxel and the new brain delivery vector Angiopep-2. Br. J. Pharmacol. 2008, 155, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Drappatz, J.; Brenner, A.; Wong, E.T.; Eichler, A.; Schiff, D.; Groves, M.D.; Mikkelsen, T.; Rosenfeld, S.; Sarantopoulos, J.; Meyers, C.A.; et al. Phase I study of GRN1005 in recurrent malignant glioma. Clin. Cancer Res. 2013, 19, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Kumthekar, P.; Tang, S.C.; Brenner, A.J.; Kesari, S.; Piccioni, D.E.; Anders, C.; Carrillo, J.; Chalasani, P.; Kabos, P.; Puhalla, S.; et al. ANG1005, a brain-penetrating peptide–drug conjugate, shows activity in patients with breast cancer with leptomeningeal carcinomatosis and recurrent brain metastases. Clin. Cancer Res. 2020, 26, 2789–2799. [Google Scholar] [CrossRef]
- Ché, C.; Yang, G.; Thiot, C.; Lacoste, M.C.; Currie, J.C.; Demeule, M.; Régina, A.; Béliveau, R.; Castaigne, J.P. New Angiopep-modified doxorubicin (ANG1007) and etoposide (ANG1009) chemotherapeutics with increased brain penetration. J. Med. Chem. 2010, 53, 2814–2824. [Google Scholar] [CrossRef]
- Regina, A.; Demeule, M.; Tripathy, S.; Lord-Dufour, S.; Currie, J.C.; Iddir, M.; Annabi, B.; Castaigne, J.P.; Lachowicz, J.E. ANG4043, a novel brain-penetrant peptide-mAb conjugate, is efficacious against HER2-positive intracranial tumors in mice. Mol. Cancer Ther. 2015, 14, 129–140. [Google Scholar] [CrossRef]
- Camp, E.R.; Wang, C.; Little, E.C.; Watson, P.M.; Pirollo, K.F.; Rait, A.; Cole, D.J.; Chang, E.H.; Watson, D.K. Transferrin receptor targeting nanomedicine delivering wild-type p53 gene sensitizes pancreatic cancer to gemcitabine therapy. Cancer Gene Ther. 2013, 20, 222–228. [Google Scholar] [CrossRef]
- Kim, S.S.; Rait, A.; Kim, E.; Pirollo, K.F.; Nishida, M.; Farkas, N.; Dagata, J.A.; Chang, E.H. A nanoparticle carrying the p53 gene targets tumors including cancer stem cells, sensitizes glioblastoma to chemotherapy and improves survival. ACS Nano 2014, 8, 5494–5514. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Rait, A.; Kim, E.; Pirollo, K.F.; Chang, E.H. A tumor-targeting p53 nanodelivery system limits chemoresistance to temozolomide prolonging survival in a mouse model of glioblastoma multiforme. Nanomedicine 2015, 11, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Harford, J.B.; Moghe, M.; Slaughter, T.; Doherty, C.; Chang, E.H. A tumor-targeting nanomedicine carrying the p53 gene crosses the blood-brain barrier and enhances anti-PD-1 immunotherapy in mouse models of glioblastoma. Int. J. Cancer 2019, 145, 2535–2546. [Google Scholar] [CrossRef]
- Kim, S.S.; Harford, J.B.; Moghe, M.; Rait, A.; Chang, E.H. Combination with SGT-53 overcomes tumor resistance to a checkpoint inhibitor. Oncoimmunology 2018, 7, e1484982. [Google Scholar] [CrossRef] [PubMed]
- Kariolis, M.S.; Wells, R.C.; Getz, J.A.; Kwan, W.; Mahon, C.S.; Tong, R.; Kim, D.J.; Srivastava, A.; Bedard, C.; Henne, K.R.; et al. Brain delivery of therapeutic proteins using an Fc fragment blood-brain barrier transport vehicle in mice and monkeys. Sci. Transl. Med. 2020, 12, eaay1359. [Google Scholar] [CrossRef] [PubMed]
- Ullman, J.C.; Arguello, A.; Getz, J.A.; Bhalla, A.; Mahon, C.S.; Wang, J.; Giese, T.; Bedard, C.; Kim, D.J.; Blumenfeld, J.R.; et al. Brain delivery and activity of a lysosomal enzyme using a blood-brain barrier transport vehicle in mice. Sci. Transl. Med. 2020, 12, eaay1163. [Google Scholar] [CrossRef]
| Drug | Clinical Indication | Phase | Clinicaltrials.Gov Number |
|---|---|---|---|
| Placing drug during surgery | |||
| Surgery + Gliadel Wafer (carmustine) vs. Surgery + radiation therapy after surgery. | Metastatic brain disease | II | NCT04222062 |
| Surgery with 5-ALA given together with Gliadel Wafer, followed by radiation therapy and temozolomide. | Glioblastoma | II | NCT01310868 |
| Surgery + Gliadel Wafer | Metastatic brain cancer | II | NCT00525590 |
| Convection-enhanced delivery | |||
| Nanoparticle formulation of panobinostat (MTX110) | HGG (pontine) | I/II | NCT03566199 NCT04264143 |
| Topotecan | HGG | I | NCT03154996 NCT03927274 NCT02278510 |
| Carboplatin | HGG | I | NCT01644955 |
| Liposomal formulation of irinotecan | HGG | I | NCT03086616 NCT02022644 |
| Liposomal formulation of rhenium (186RNL) | Glioma | I/II | NCT01906385 |
| 124I-labeled anti-B7-H3 mAb 8H9 | HGG (pontine) treated with radiation therapy | I | NCT01502917 |
| D2C7 immunotoxin (scFv from the anti-EGFR mAb D2C7 linked to the Pseudomonas exotoxin PE38KDEL) | HGG | I | NCT02303678 |
| D2C7-immunotoxin in combination with anti-PD-L1 mAb atezolizumab | HGG (recurrent) | I | NCT04160494 |
| Anti-CD40 mAb (2141-V11) with D2C7-immunotoxin | Grade III/IV malignant glioma | I | NCT04547777 |
| IL4 linked to a modified version of Pseudomonas exotoxin A (MDNA55) | HGG (recurrent or progressive) | I | NCT02858895 |
| Bone morphogenetic protein (BMP) 4 | HGG (progressive and/or recurrent) | I | NCT02869243 |
| Safety study of replication-competent Adenovirus (Delta-24-rgd) | Recurrent glioblastoma | I/IIC | NCT01582516 |
| Oncolytic poliovirus therapy with PVSRIPO | HGG (recurrent) | I | NCT03043391 NCT01491893 |
| Oncolytic poliovirus therapy with PVSRIPO with anti-PD-1 mAb pembrolizumab | Glioblastoma | I | NCT04479241 |
| GRm13Z40-2, an allogeneic CD8+ cytolitic T-cell line expressing IL13-Zetakine with IL-2. | Glioma and other brain tumors | IC | NCT01082926 |
| Osmotic Disruption/SIACI | |||
|---|---|---|---|
| Drug(s) | Clinical Indication | Phase | Clinicaltrials.gov number |
| Cetuximab and Bevacizumab | Relapsed/refractory glioma in patients under 22. | I/II | NCT01884740 |
| Repeated infusion of bevacizumab | Newly diagnosed glioblastoma Relapsed glioblastoma and anaplastic astrocytoma | I/II I/II | NCT01811498 NCT01269853 |
| Repeated infusion of cetuximab | Newly diagnosed glioblastoma | I/II | NCT02861898 |
| Temozolomide | Newly diagnosed and anaplastic astrocytoma | I | NCT01180816 |
| Cetuximab | Relapsed glioblastoma and anaplastic astrocytoma | I | NCT01238237 |
| Bevacizumab | Relapsed/refractory glioblastoma and anaplastic astrocytoma Recurrent glioblastoma | I I | NCT00968240 NCT02285959 |
| FUS | |||
| US-emitting device | Drug(s) and clinical indication | Phase | Clinicaltrials.gov number |
| ExAblate 4000 Type 2 | With carboplatin in recurrent glioblastoma. | I/II I/II | NCT04440358 NCT04417088 |
| Safety and feasibility in opening the BBTB in malignant gliomas before or during standard of care therapy or surgery | NA NA NA NA NA NA | NCT03322813 NCT03551249 NCT03712293 NCT01473485 NCT03616860 NCT00147056 | |
| Safety and feasibility in opening BBTB in brain tumors other than glioblastoma (e.g., metastases) | NA NA NA | NCT03714243 NCT03714243 NCT02343991 * | |
| SonoCloud | Safety of opening BBTB in patients with recurrent glioblastoma before systemic carboplatin chemotherapy. | I/II | NCT02253212 |
| BBTB opening and administration of albumin-bound paclitaxel in recurrent GBM. | I/II | NCT04528680 | |
| DLT of escalating numbers of ultrasound beams (Phase 1); safety and efficacy (Phase 2a expansion) in HGG | I/II | NCT03744026 | |
| Safety and efficacy of BBTB opening with nivolumab ± ipilimumab in brain melanoma metastases | I/II | NCT04021420 | |
| NaviFUS system | Efficacy and safety with bevacizumab in recurrent glioblastoma | NA | NCT04446416 |
| Safety and feasibility of transient opening of the BBTB in recurrent glioblastoma | NA | NCT03626896 |
| Carrier–Drug Combination | Clinical Indication | Phase | Clinicaltrials.Gov Number |
|---|---|---|---|
| ANG1005 (Angiopep-2 conjugated to paclitaxel) | HER2- breast cancer patients with newly diagnosed leptomeningeal disease and previously treated brain metastases | III | NCT03613181 |
| Breast cancer patients with recurrent brain metastases. | II | NCT02048059 | |
| Patients with recurrent high-grade glioma with or without bevacizumab. | II | NCT01967810 | |
| Breast cancer patients with recurrent brain metastases with or without trastuzumab. | II | NCT01480583 | |
| SGT-53 (cationic liposomes encapsulating plasmid for human tumor suppressor gene TP53) | In combination with irradiation and/or chemotherapy in pediatric patients with recurrent or progressive CNS malignancies | I | NCT03554707 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcucci, F.; Corti, A.; Ferreri, A.J.M. Breaching the Blood–Brain Tumor Barrier for Tumor Therapy. Cancers 2021, 13, 2391. https://doi.org/10.3390/cancers13102391
Marcucci F, Corti A, Ferreri AJM. Breaching the Blood–Brain Tumor Barrier for Tumor Therapy. Cancers. 2021; 13(10):2391. https://doi.org/10.3390/cancers13102391
Chicago/Turabian StyleMarcucci, Fabrizio, Angelo Corti, and Andrés J. M. Ferreri. 2021. "Breaching the Blood–Brain Tumor Barrier for Tumor Therapy" Cancers 13, no. 10: 2391. https://doi.org/10.3390/cancers13102391
APA StyleMarcucci, F., Corti, A., & Ferreri, A. J. M. (2021). Breaching the Blood–Brain Tumor Barrier for Tumor Therapy. Cancers, 13(10), 2391. https://doi.org/10.3390/cancers13102391







