Glycolysis on F-18 FDG PET/CT Is Superior to Amino Acid Metabolism on C-11 Methionine PET/CT in Identifying Advanced Renal Cell Carcinoma at Staging
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Logistic Regression Analysis for the Prediction of Advanced-Stage Disease
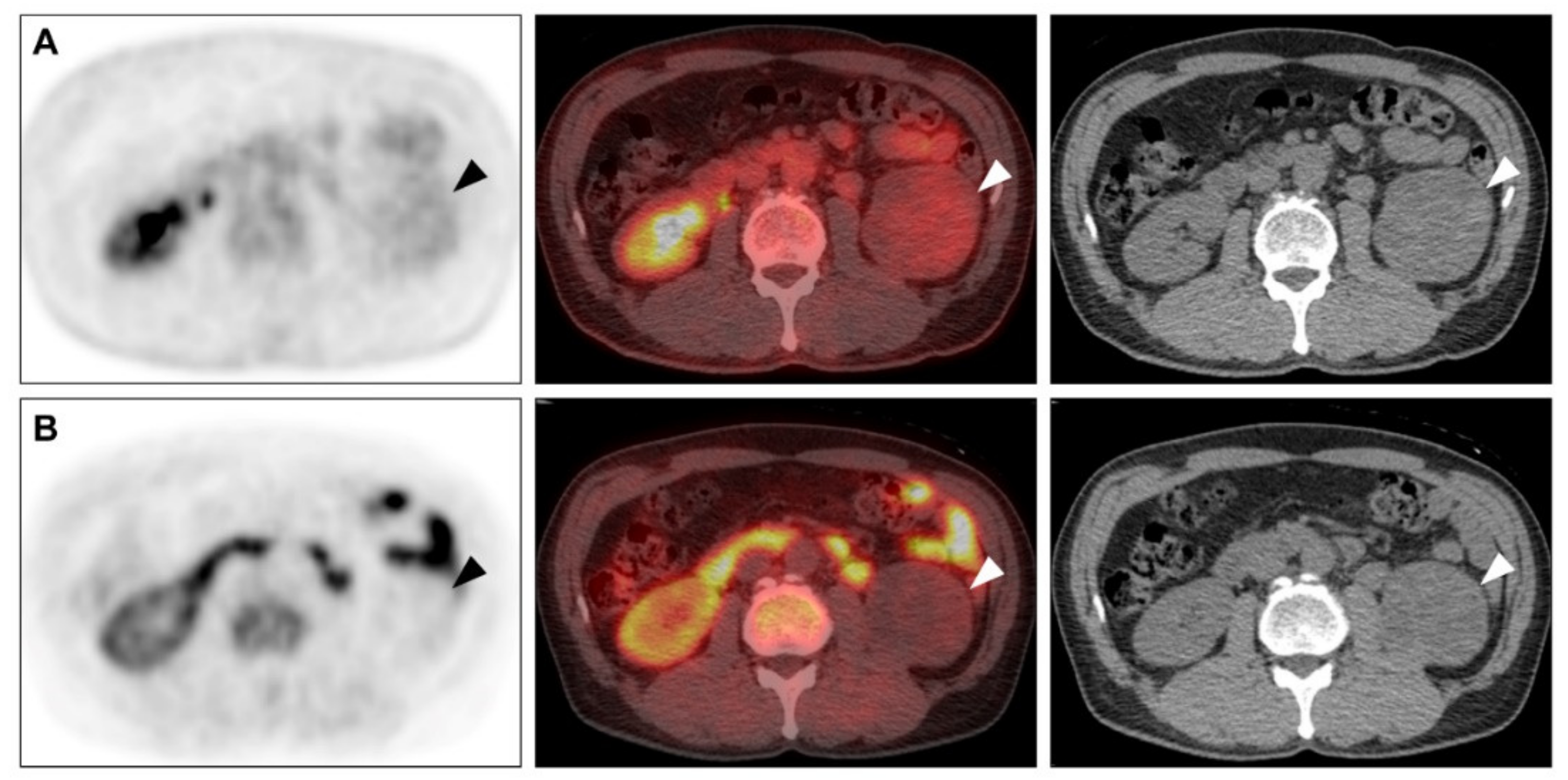
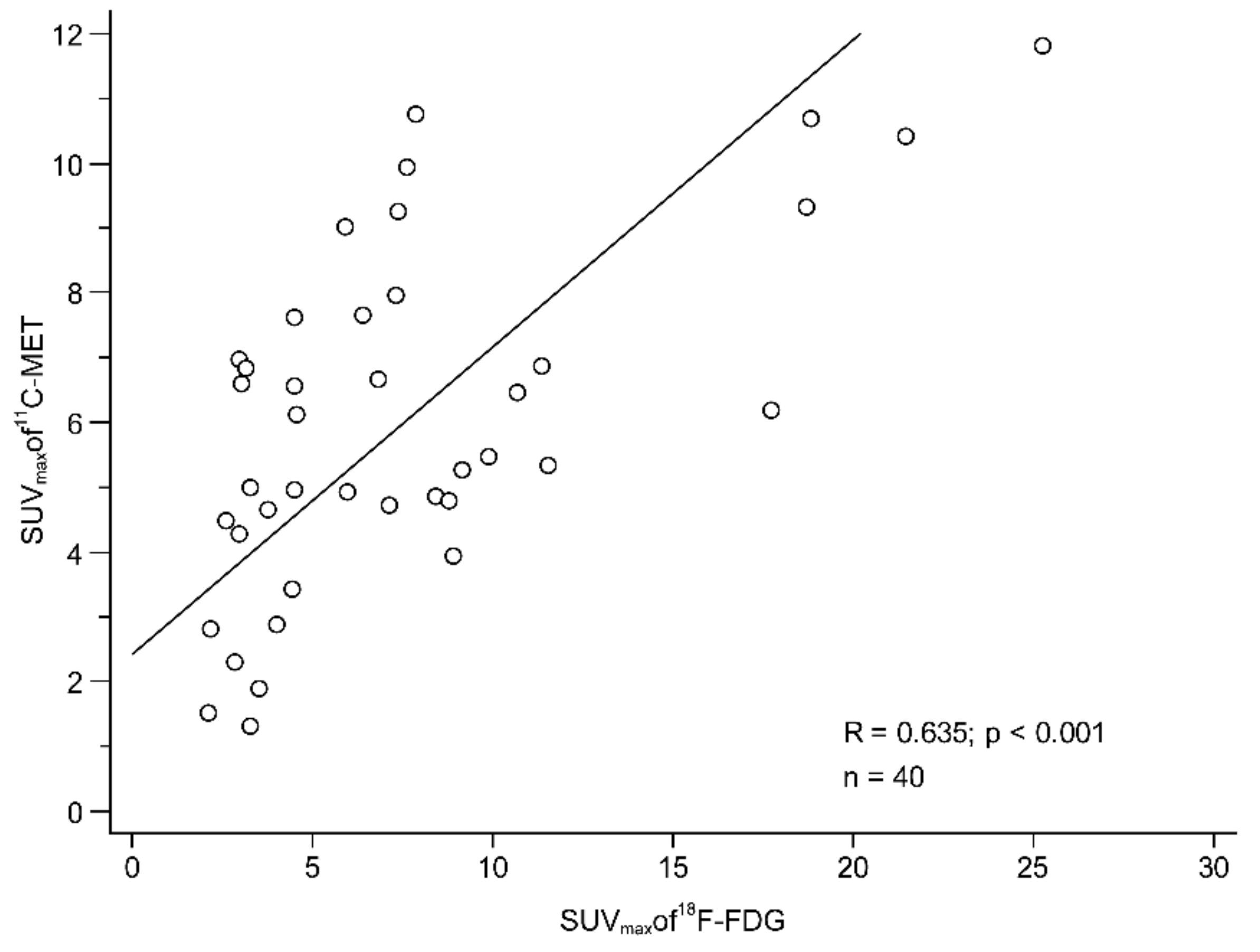
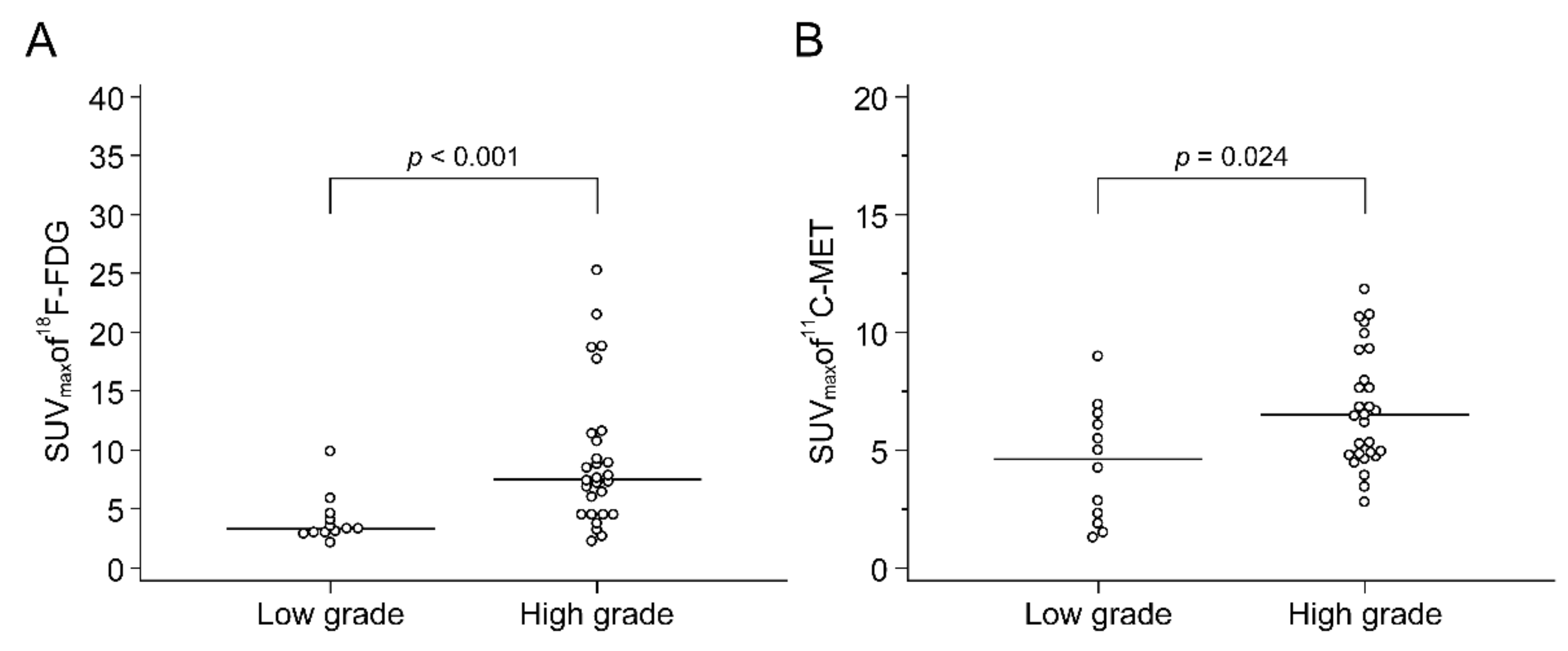
2.3. Comparison of F-18 FDG and C-11 MET Uptake in the Primary Tumour and Histopathological Findings
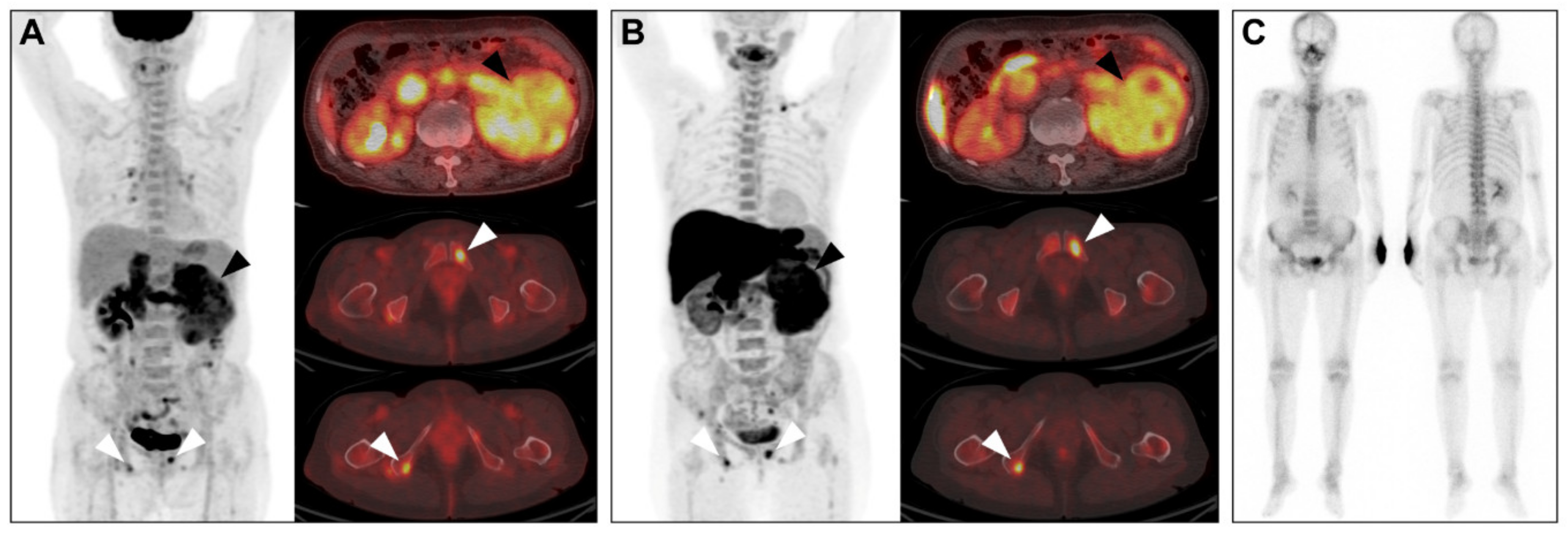
2.4. Comparison of Detection of Extra-Renal Metastases between Conventional Imaging and PET/CT
3. Discussion
4. Materials and Method
4.1. Study Subjects
4.2. PET/CT Imaging Protocol
4.3. PET/CT Image Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef]
- Ozulker, T.; Ozulker, F.; Ozbek, E.; Ozpacaci, T. A prospective diagnostic accuracy study of F-18 fluorodeoxyglucose-positron emission tomography/computed tomography in the evaluation of indeterminate renal masses. Nucl. Med. Commun. 2011, 32, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Lee, H.J.; Cho, N.H.; Kim, J.; Jang, W.S.; Heo, J.E.; Ham, W.S. Risk Prediction Tool for Aggressive Tumors in Clinical T1 Stage Clear Cell Renal Cell Carcinoma Using Molecular Biomarkers. Comput. Struct. Biotechnol. J. 2019, 17, 371–377. [Google Scholar] [CrossRef]
- Wettersten, H.I.; Hakimi, A.A.; Morin, D.; Bianchi, C.; Johnstone, M.E.; Donohoe, D.R.; Trott, J.F.; Aboud, O.A.; Stirdivant, S.; Neri, B.; et al. Grade-Dependent Metabolic Reprogramming in Kidney Cancer Revealed by Combined Proteomics and Metabolomics Analysis. Cancer Res. 2015, 75, 2541–2552. [Google Scholar] [CrossRef] [PubMed]
- Sai, K.K.S.; Zachar, Z.; Bingham, P.M.; Mintz, A. Metabolic PET Imaging in Oncology. AJR Am. J. Roentgenol. 2017, 209, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Fuccio, C.; Ceci, F.; Castellucci, P.; Spinapolice, E.G.; Palumbo, R.; D’Ambrosio, D.; Bernardo, A.; Brunocilla, E.; Schiavina, R.; Maffione, A.M.; et al. Restaging clear cell renal carcinoma with 18F-FDG PET/CT. Clin. Nucl. Med. 2014, 39, e320–e324. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Ding, H.-J.; Chen, J.-H.; Chao, C.-H.; Lu, Y.-Y.; Lin, W.-Y.; Kao, C.-H. Meta-analysis of the diagnostic performance of [18F]FDG-PET and PET/CT in renal cell carcinoma. Cancer Imaging 2012, 12, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Kume, H.; Koyama, K.; Nakagawa, T.; Fujimura, T.; Morikawa, T.; Fukayama, M.; Homma, Y.; Ohtomo, K.; Momose, T. Preoperative evaluation of renal cell carcinoma by using 18F-FDG PET/CT. Clin. Nucl. Med. 2015, 40, 936–940. [Google Scholar] [CrossRef]
- Noda, Y.; Kanematsu, M.; Goshima, S.; Suzui, N.; Hirose, Y.; Matsunaga, K.; Nishibori, H.; Kondo, H.; Watanabe, H.; Kawada, H.; et al. 18-F fluorodeoxyglucose uptake in positron emission tomography as a pathological grade predictor for renal clear cell carcinomas. Eur. Radiol. 2015, 25, 3009–3016. [Google Scholar] [CrossRef] [PubMed]
- Polat, E.C.; Otunctemur, A.; Ozbek, E.; Besiroglu, H.; Dursun, M.; Ozer, K.; Horsanali, M.O. Standardized uptake values highly correlate with tumor size and Fuhrman grade in patients with clear cell renal cell carcinoma. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 7821–7824. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Kamai, T.; Abe, H.; Sakamoto, S.; Kitajima, K.; Nishihara, D.; Yuki, H.; Kambara, T.; Betsunoh, H.; Yashi, M.; et al. Clinically significant association between the maximum standardized uptake value on 18F-FDG PET and expression of phosphorylated Akt and S6 kinase for prediction of the biological characteristics of renal cell cancer. BMC Cancer 2015, 15. [Google Scholar] [CrossRef]
- Kim, D.; Chun, J.H.; Kim, S.H.; Moon, J.H.; Kang, S.G.; Chang, J.H.; Yun, M. Re-evaluation of the diagnostic performance of (11)C-methionine PET/CT according to the 2016 WHO classification of cerebral gliomas. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1678–1684. [Google Scholar] [CrossRef] [PubMed]
- Glaudemans, A.W.; Enting, R.H.; Heesters, M.A.; Dierckx, R.A.; van Rheenen, R.W.; Walenkamp, A.M.; Slart, R.H. Value of 11C-methionine PET in imaging brain tumours and metastases. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 615–635. [Google Scholar] [CrossRef] [PubMed]
- Wedman, J.; Pruim, J.; Langendijk, J.A.; van der Laan, B.F. Visualization of small glottic laryngeal cancer using methyl-labeled 11C-methionine positron emission tomography. Oral. Oncol. 2009, 45, 703–705. [Google Scholar] [CrossRef]
- Chesnay, E.; Babin, E.; Constans, J.M.; Agostini, D.; Bequignon, A.; Regeasse, A.; Sobrio, F.; Moreau, S. Early response to chemotherapy in hypopharyngeal cancer: Assessment with (11)C-methionine PET, correlation with morphologic response, and clinical outcome. J. Nucl. Med. 2003, 44, 526–532. [Google Scholar] [PubMed]
- Nunez, R.; Macapinlac, H.A.; Yeung, H.W.; Akhurst, T.; Cai, S.; Osman, I.; Gonen, M.; Riedel, E.; Scher, H.I.; Larson, S.M. Combined 18F-FDG and 11C-methionine PET scans in patients with newly progressive metastatic prostate cancer. J. Nucl. Med. 2002, 43, 46–55. [Google Scholar] [PubMed]
- Lindholm, P.; Leskinen, S.; Lapela, M. Carbon-11-methionine uptake in squamous cell head and neck cancer. J. Nucl. Med. 1998, 39, 1393–1397. [Google Scholar] [PubMed]
- Lindholm, P.; Leskinen-Kallio, S.; Minn, H.; Bergman, J.; Haaparanta, M.; Lehikoinen, P.; Nagren, K.; Ruotsalainen, U.; Teras, M.; Joensuu, H. Comparison of fluorine-18-fluorodeoxyglucose and carbon-11-methionine in head and neck cancer. J. Nucl. Med. 1993, 34, 1711–1716. [Google Scholar] [PubMed]
- Leskinen-Kallio, S.; Nagren, K.; Lehikoinen, P.; Ruotsalainen, U.; Teras, M.; Joensuu, H. Carbon-11-methionine and PET is an effective method to image head and neck cancer. J. Nucl. Med. 1992, 33, 691–695. [Google Scholar]
- Walvekar, A.S.; Srinivasan, R.; Gupta, R.; Laxman, S. Methionine coordinates a hierarchically organized anabolic program enabling proliferation. Mol. Biol. Cell 2018, 29, 3183–3200. [Google Scholar] [CrossRef] [PubMed]
- Bénard, F.; Romsa, J.; Hustinx, R. Imaging gliomas with positron emission tomography and single-photon emission computed tomography. Semin. Nucl. Med. 2003, 33, 148–162. [Google Scholar] [CrossRef]
- Jager, P.L.; Vaalburg, W.; Pruim, J.; de Vries, E.G.; Langen, K.J.; Piers, D.A. Radiolabeled amino acids: Basic aspects and clinical applications in oncology. J. Nucl. Med. 2001, 42, 432–445. [Google Scholar]
- Edgren, M.; Westlin, J.; Ahlstrom, H.; Nilsson, S. A comparison of positron emission tomography (pet) utilizing C-11 methionine and computerized-tomography in the diagnosis of renal-cell adenocarcinoma. Oncol. Rep. 1995, 2, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.-M.; Zhang, W.; Zhang, B.; Chen, Y.-Y.; Li, J.-H.; Wu, Y.-W. Correlation between the Uptake of 18F-Fluorodeoxyglucose (18F-FDG) and the Expression of Proliferation-Associated Antigen Ki-67 in Cancer Patients: A Meta-Analysis. PLoS ONE 2015, 10, e0129028. [Google Scholar] [CrossRef]
- Kato, T.; Shinoda, J.; Oka, N.; Miwa, K.; Nakayama, N.; Yano, H.; Maruyama, T.; Muragaki, Y.; Iwama, T. Analysis of 11C-methionine Uptake in Low-Grade Gliomas and Correlation with Proliferative Activity. Am. J. Neuroradiol. 2008, 29, 1867–1871. [Google Scholar] [CrossRef] [PubMed]
- Mitamura, K.; Yamamoto, Y.; Norikane, T.; Hatakeyama, T.; Okada, M.; Nishiyama, Y. Correlation of (18)F-FDG and (11)C-methionine uptake on PET/CT with Ki-67 immunohistochemistry in newly diagnosed intracranial meningiomas. Ann. Nucl. Med. 2018, 32, 627–633. [Google Scholar] [CrossRef]
- Narayanan, T.K.; Said, S.; Mukherjee, J.; Christian, B.; Satter, M.; Dunigan, K.; Shi, B.; Jacobs, M.; Bernstein, T.; Padma, M.; et al. A comparative study on the uptake and incorporation of radiolabeled methionine, choline and fluorodeoxyglucose in human astrocytoma. Mol. Imaging Biol. 2002, 4, 147–156. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Gatto, F.; Nookaew, I.; Nielsen, J. Chromosome 3p loss of heterozygosity is associated with a unique metabolic network in clear cell renal carcinoma. Proc. Natl. Acad. Sci. USA 2014, 111, E866–E875. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013, 499, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Seino, Y.; Fukumoto, H.; Koh, G.; Yano, H.; Inagaki, N.; Yamada, Y.; Inoue, K.; Manabe, T.; Imura, H. Over-expression of facilitative glucose transporter genes in human cancer. Biochem. Biophys. Res. Commun. 1990, 170, 223–230. [Google Scholar] [CrossRef]
- Creighton, C.J.; Morgan, M.; Gunaratne, P.H.; Wheeler, D.A.; Gibbs, R.A.; Gordon Robertson, A.; Chu, A.; Beroukhim, R.; Cibulskis, K.; Signoretti, S.; et al. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013, 499, 43–49. [Google Scholar] [CrossRef]
- Wise, D.R.; Thompson, C.B. Glutamine addiction: A new therapeutic target in cancer. Trends Biochem. Sci. 2010, 35, 427–433. [Google Scholar] [CrossRef]
- Qiu, B.; Ackerman, D.; Sanchez, D.J.; Li, B.; Ochocki, J.D.; Grazioli, A.; Bobrovnikova-Marjon, E.; Diehl, J.A.; Keith, B.; Simon, M.C. HIF2α-Dependent Lipid Storage Promotes Endoplasmic Reticulum Homeostasis in Clear-Cell Renal Cell Carcinoma. Cancer Discov. 2015, 5, 652–667. [Google Scholar] [CrossRef] [PubMed]
- Metallo, C.M.; Gameiro, P.A.; Bell, E.L.; Mattaini, K.R.; Yang, J.; Hiller, K.; Jewell, C.M.; Johnson, Z.R.; Irvine, D.J.; Guarente, L.; et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 2011, 481, 380–384. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Mancuso, A.; Daikhin, E.; Nissim, I.; Yudkoff, M.; Wehrli, S.; Thompson, C.B. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 19345–19350. [Google Scholar] [CrossRef]
- Palanichamy, K.; Chakravarti, A. Diagnostic and Prognostic Significance of Methionine Uptake and Methionine Positron Emission Tomography Imaging in Gliomas. Frontiers in Oncology 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Betsunoh, H.; Fukuda, T.; Anzai, N.; Nishihara, D.; Mizuno, T.; Yuki, H.; Masuda, A.; Yamaguchi, Y.; Abe, H.; Yashi, M.; et al. Increased expression of system large amino acid transporter (LAT)-1 mRNA is associated with invasive potential and unfavorable prognosis of human clear cell renal cell carcinoma. BMC Cancer 2013, 13, 509. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Chen, J.; Gao, H.; Kong, S.N.; Deivasigamani, A.; Shi, M.; Xie, T.; Hui, K.M. Hypoxia-induced modulation of glucose transporter expression impacts (18)F-fluorodeoxyglucose PET-CT imaging in hepatocellular carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 787–797. [Google Scholar] [CrossRef]
- Bretheau, D.; Lechevallier, E.; de Fromont, M.; Sault, M.C.; Rampal, M.; Coulange, C. Prognostic value of nuclear grade of renal cell carcinoma. Cancer 1995, 76, 2543–2549. [Google Scholar] [CrossRef]
- Cheville, J.C.; Lohse, C.M.; Zincke, H.; Weaver, A.L.; Blute, M.L. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am. J. Surg. Pathol. 2003, 27, 612–624. [Google Scholar] [CrossRef] [PubMed]
- Patard, J.J.; Leray, E.; Rioux-Leclercq, N.; Cindolo, L.; Ficarra, V.; Zisman, A.; De La Taille, A.; Tostain, J.; Artibani, W.; Abbou, C.C.; et al. Prognostic value of histologic subtypes in renal cell carcinoma: A multicenter experience. J. Clin. Oncol. 2005, 23, 2763–2771. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.E.; White, R.L., Jr.; Zuger, J.H.; Sasser, H.C.; Teigland, C.M. Clinical use of fluorodeoxyglucose F 18 positron emission tomography for detection of renal cell carcinoma. J. Urol. 2004, 171, 1806–1809. [Google Scholar] [CrossRef] [PubMed]
- Aide, N.; Cappele, O.; Bottet, P.; Bensadoun, H.; Regeasse, A.; Comoz, F.; Sobrio, F.; Bouvard, G.; Agostini, D. Efficiency of [(18)F]FDG PET in characterising renal cancer and detecting distant metastases: A comparison with CT. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1236–1245. [Google Scholar] [CrossRef]
- Ramdave, S.; Thomas, G.W.; Berlangieri, S.U.; Bolton, D.M.; Davis, I.; Danguy, H.T.; Macgregor, D.; Scott, A.M. Clinical role of F-18 fluorodeoxyglucose positron emission tomography for detection and management of renal cell carcinoma. J. Urol. 2001, 166, 825–830. [Google Scholar] [CrossRef]
- Sohaib, S.A.; Cook, G.; Allen, S.D.; Hughes, M.; Eisen, T.; Gore, M. Comparison of whole-body MRI and bone scintigraphy in the detection of bone metastases in renal cancer. Br. J. Radiol. 2009, 82, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Staudenherz, A.; Steiner, B.; Puig, S.; Kainberger, F.; Leitha, T. Is there a diagnostic role for bone scanning of patients with a high pretest probability for metastatic renal cell carcinoma? Cancer 1999, 85, 153–155. [Google Scholar] [CrossRef]
- Schwyzer, M.; Ferraro, D.A.; Muehlematter, U.J.; Curioni-Fontecedro, A.; Huellner, M.W.; von Schulthess, G.K.; Kaufmann, P.A.; Burger, I.A.; Messerli, M. Automated detection of lung cancer at ultralow dose PET/CT by deep neural networks - Initial results. Lung Cancer 2018, 126, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, S.A.; Lasky, L.C.; Limas, C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am. J. Surg. Pathol. 1982, 6, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.B. AJCC Cancer Staging Manual; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
| Characteristics | AJCC Prognostic Groups | |||
|---|---|---|---|---|
| Stage I (n = 5) | Stage II (n = 10) | Stage III (n = 16) | Stage IV (n = 9) | |
| Sex | ||||
| Male, n (%) | 3 (60.0%) | 4 (40.0%) | 11 (68.8%) | 3 (33.3%) |
| Female, n (%) | 2 (40.0%) | 6 (60.0%) | 5 (31.3%) | 6 (66.7%) |
| Age, years, median (Q1–Q3) | 61 (58–64) | 50 (39–76) | 61 (58–67) | 62 (56–69) |
| Tumour size, cm, median (Q1–Q3) | 5.4 (4.1–5.8) | 8.0 (7.3–8.5) | 8.4 (7.1–9.8) | 9.0 (8.1–11.5) |
| Histopathology | ||||
| Clear cell type, n (%) | 3 (60.0%) | 8 (80.0%) | 14 (87.5%) | 9 (100.0%) |
| Chromophobe type, n (%) | 1 (20.0%) | 2 (20.0%) | 1 (6.3%) | 0 (0.0%) |
| Papillary type, n (%) | 1 (20.0%) | 0 (0.0%) | 1 (6.3%) | 0 (0.0%) |
| F-18 FDG PET/CT | ||||
| SUVmax, median (Q1–Q3) | 3.8 (2.9–4.9) | 3.4 (2.9–4.5) | 8.0 (5.3–11.5) | 7.9 (7.0–12.2) |
| MTV, median (Q1–Q3) | 2.3 (1.1–11.6) | 0.6 (0.2–5.5) | 109.1 (45.9–194.1) | 112.8 (53.9–295.5) |
| C-11 MET PET/CT | ||||
| SUVmax, median (Q1–Q3) | 4.5 (3.9–5.5) | 4.9 (2.3–6.1) | 6.5 (5.1–7.2) | 9.2 (5.1–10.7) |
| MTV, median (Q1–Q3) | 0.0 (0.0–1.4) | 3.8 (0.0–9.8) | 7.5 (1.4–47.4) | 25.8 (9.0–41.4) |
| Fuhrman nuclear grade | ||||
| Grade 1, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Grade 2, n (%) | 2 (40.0%) | 6 (60.0%) | 1 (6.3%) | 3 (33.3%) |
| Grade 3, n (%) | 2 (40.0%) | 4 (40.0%) | 11 (68.8%) | 5 (55.6%) |
| Grade 4, n (%) | 1 (20.0%) | 0 (0.0%) | 4 (25.0%) | 1 (11.1%) |
| Characteristics | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Sex (male vs. female) | 1.46 (0.40–5.26) | 0.568 | ||
| Age (years) | 1.05 (0.99–1.11) | 0.121 | ||
| Tumour size (cm) | 1.47 (1.03–2.09) | 0.033 * | ||
| F-18 FDG PET/CT | ||||
| SUVmax (≤4.6 vs. >4.6) | 26.00 (4.38–154.53) | <0.001 * | ||
| MTV (≤21.3 vs. >21.3) | 102.67 (9.69–1087.65) | <0.001 * | 102.67 (9.69–1087.65) | <0.001 * |
| C-11 MET PET/CT | ||||
| SUVmax (≤5.0 vs. >5.0) | 8.71 (2.01–37.76) | 0.004 * | ||
| MTV (≤4.2 vs. >4.2) | 7.07 (1.68–29.83) | 0.008 * | ||
| Fuhrman nuclear grade (1–2 vs. 3–4) | 6.00 (1.37–26.20) | 0.017 * | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-H.; Park, J.-S.; Kim, H.; Kim, D.; Lee, S.-H.; Ham, W.-S.; Han, W.-K.; Choi, Y.-D.; Yun, M. Glycolysis on F-18 FDG PET/CT Is Superior to Amino Acid Metabolism on C-11 Methionine PET/CT in Identifying Advanced Renal Cell Carcinoma at Staging. Cancers 2021, 13, 2381. https://doi.org/10.3390/cancers13102381
Lee S-H, Park J-S, Kim H, Kim D, Lee S-H, Ham W-S, Han W-K, Choi Y-D, Yun M. Glycolysis on F-18 FDG PET/CT Is Superior to Amino Acid Metabolism on C-11 Methionine PET/CT in Identifying Advanced Renal Cell Carcinoma at Staging. Cancers. 2021; 13(10):2381. https://doi.org/10.3390/cancers13102381
Chicago/Turabian StyleLee, Suk-Hyun, Jee-Soo Park, Hyunjeong Kim, Dongwoo Kim, Seung-Hwan Lee, Won-Sik Ham, Woong-Kyu Han, Young-Deuk Choi, and Mijin Yun. 2021. "Glycolysis on F-18 FDG PET/CT Is Superior to Amino Acid Metabolism on C-11 Methionine PET/CT in Identifying Advanced Renal Cell Carcinoma at Staging" Cancers 13, no. 10: 2381. https://doi.org/10.3390/cancers13102381
APA StyleLee, S.-H., Park, J.-S., Kim, H., Kim, D., Lee, S.-H., Ham, W.-S., Han, W.-K., Choi, Y.-D., & Yun, M. (2021). Glycolysis on F-18 FDG PET/CT Is Superior to Amino Acid Metabolism on C-11 Methionine PET/CT in Identifying Advanced Renal Cell Carcinoma at Staging. Cancers, 13(10), 2381. https://doi.org/10.3390/cancers13102381






