Toward a Personalized Therapy in Soft-Tissue Sarcomas: State of the Art and Future Directions
Abstract
Simple Summary
Abstract
1. Introduction
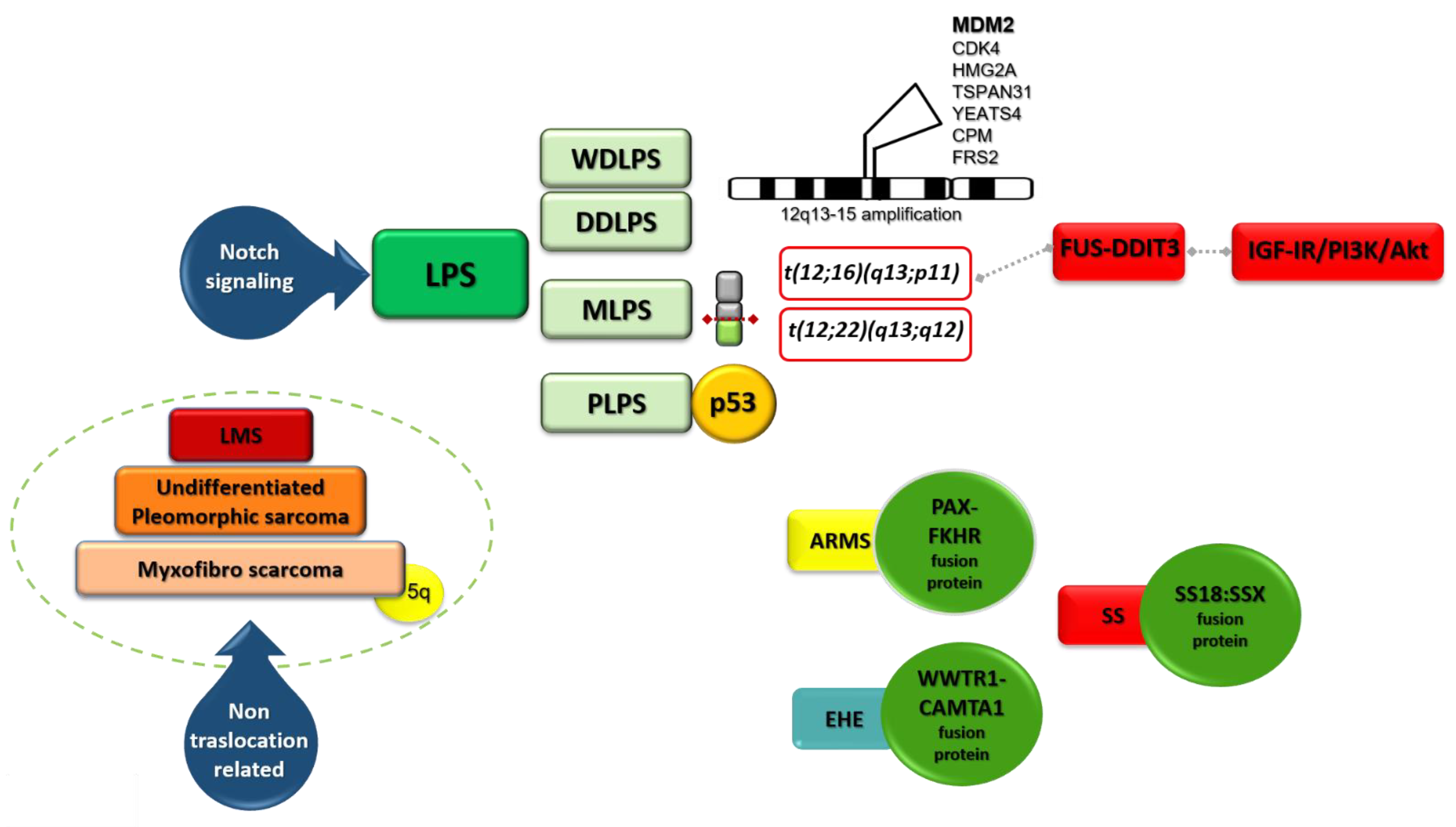
2. Liposarcoma (LPS)
2.1. Genomic Alterations in LPS
2.2. WDLPS and DDLPS
2.3. Aurora Kinases in LPS
2.4. MLPS
2.5. PLPS
2.6. microRNAs in LPS
2.7. Notch Pathway in LPS
2.8. Innovative Therapeutic Approaches in LPS
3. Leiomyosarcoma (LMS)
4. Rhabdomyosarcoma (RMS)
5. Fibroblastic/Myofibroblastic Tumors: Myxofibrosarcoma, Malignant Solitary Fibrous Tumor
6. Malignant Tenosynovial Giant Cell Tumor (TGCT)
7. Vascular Tumors: Angiosarcoma and Epithelioid Hemangioendothelioma
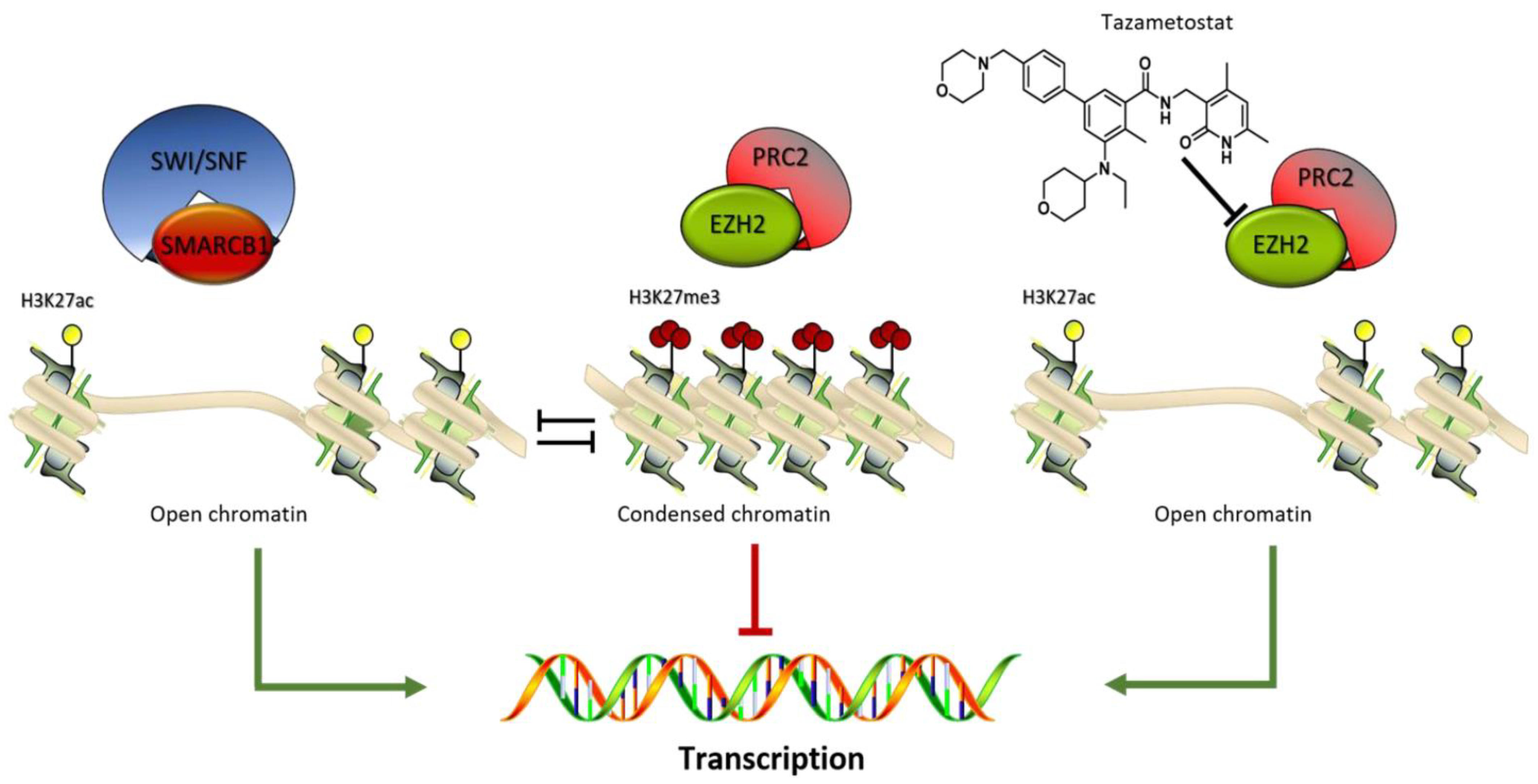
8. Malignant Peripheral Nerve Sheath Tumors (MPNSTs)
9. Tumors of Uncertain Differentiation
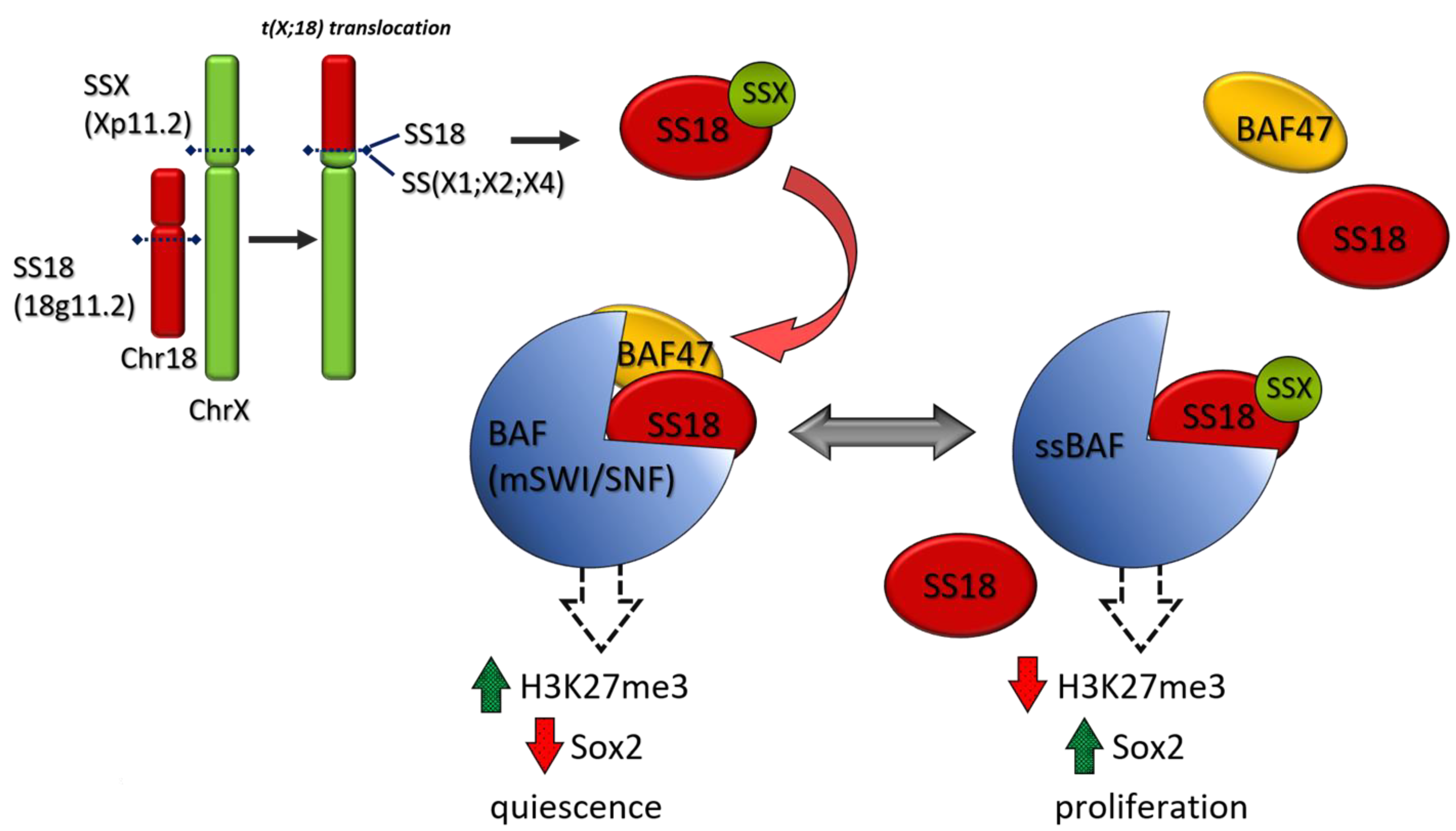
9.1. NTRK-Rearranged Spindle Cell Neoplasm, Synovial Sarcoma, Epithelioid Sarcoma, Undifferentiated Round-Cell Sarcoma
9.1.1. NTRK-Rearranged Spindle Cell Neoplasm
9.1.2. Synovial Sarcoma (SS)
9.1.3. Epithelioid Sarcoma
9.1.4. Undifferentiated Small Round-Cell Sarcoma
10. Immunotherapy
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALT | Alternative Lengthening of Telomeres |
| ARMS | alveolar RMS |
| BCOR | Bcl6 corepressor |
| BMI1 | B cell-specific Moloney murine leukemia virus integration site 1 |
| CAMTA1 | calmodulin-binding transcription activator 1 |
| CAR | Chimeric antigen receptors |
| CIC | capicua transcriptional repressor |
| CPM | Carboxypeptidase M |
| CSF1R | colony stimulating factor-1 receptor |
| CTA | Cancer-testis antigens |
| DDLPS | dedifferentiated liposarcoma |
| EHE | Epithelioid Hemangioendothelioma |
| ERMS | embryonal RMS |
| ES | epithelioid sarcoma |
| EZH2 | Enhancer of zeste homolog 2 |
| EWS | Ewing sarcoma gene |
| FAP | fibroblast activation protein |
| FGF | Fibroblast Growth Factor |
| FKHR | forkhead in rhabdomyosarcoma |
| FLI1 | Friend Leukemia Integration 1 transcription factor |
| GD2 | disialoganglioside |
| GIST | Gastrointestinal Stromal Tumors |
| GLI1 | Glioma-Associated Oncogene Homolog 1 |
| HER2 | human epidermal growth factor receptor 2 |
| HMG2A | (High-mobility group AT-hook 2) |
| IGF-1R | insulin-like growth factor 1 receptor |
| IL-11RA | interleukin 11 Receptor Subunit Alpha |
| LMS | leiomyosarcoma |
| LPS | liposarcoma |
| MDM2 | Mouse Double Minute 2 |
| MLPS | myxoid liposarcoma |
| MPNST | malignant peripheral nerve sheath tumor |
| NF1 | neurofibromatosis type 1 |
| NY-ESO-1 | New York Esophageal Squamous Cell Carcinoma-1 |
| ORR | Overall response Rate |
| OS | Overall Survival |
| PAX | Paired box |
| PDGF | Platelet Derived Growth Factor |
| PDGFR | Platelet Derived Growth Factor Receptor |
| PFS | Progression Free Survival |
| PLPS | pleomorphic liposarcoma |
| PRC2 | polycomb repressive complex 2 |
| PRMS | pleomorphic RMS |
| ROR1 | tyrosine kinase orphan-like receptor 1 |
| SMARCB1 (BAF47)/INI1 | SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1 |
| SPEAR | Specific Peptide Enhanced Affinity Receptor |
| SWI/SNF | SWItch/Sucrose Nonfermentable |
| TGCT | tenosynovial giant cell tumor |
| TAZ | transcriptional coactivator with PDZ binding motif |
| TF | Transcription Factor |
| TKI | Tyrosine Kinase Inhibitor |
| TMB | Tumor Mutational Burden |
| TSPAN31 | Tetraspanin-31 |
| ULMS | uterine leiomyosarcoma |
| VEGF | Vascular Endothelial Growth Factor |
| WDLPS | well-differentiated liposarcoma |
| WWTR1 | WW Domain-containing Transcription Regulator Protein 1 gene |
| YAP | Yes-associated Protein |
| YEATS4 | GAS41 Glioma Amplified Sequence 41 |
References
- Harris, S.J.; Maruzzo, M.; Thway, K.; Al-Muderis, O.; Jones, R.L.; Benson, A.M.C.; Judson, R.I. Metastatic soft tissue sarcoma, an analysis of systemic therapy and impact on survival. J. Clin. Oncol. 2015, 33, 10545. [Google Scholar] [CrossRef]
- Chowdhary, M.; Chowdhary, A.; Sen, N.; Zaorsky, N.G.; Patel, K.R.; Wang, D. Does the addition of chemotherapy to neoadjuvant radiotherapy impact survival in high-risk extremity/trunk soft-tissue sarcoma? Cancer 2019, 125, 3801–3809. [Google Scholar] [CrossRef]
- Tetta, C.; Londero, F.; Micali, L.R.; Parise, G.; Algargoush, A.T.; Algargoosh, M.; Albisinni, U.; Maessen, J.G.; Gelsomino, S. Stereotactic Body Radiotherapy versus Metastasectomy in patients with pulmonary metastases from soft tissue sarcoma. Clin. Oncol. 2020, 32, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Besiroglu, M.; Dane, F.; Ciltas, A.; Benekli, M. Systemic chemotherapy of advanced soft tissue sarcomas. J. Oncol. Sci. 2017, 3, 66–70. [Google Scholar] [CrossRef]
- Judson, J.; Verweij, I.; Gelderblom, H.; Hartmann, J.T.; Schöffski, P.; Blay, J.-Y.; Kerst, J.M.; Sufliarsky, J.; Whelan, J.; Hohenberger, P.; et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: A randomised controlled phase 3 trial. Lancet Oncol. 2014, 15, 415–442. [Google Scholar] [CrossRef]
- Demetri, G.D.; von Mehren, M.; Jones, R.L.; Hensley, M.L.; Schuetze, S.M.; Staddon, A.; Milhem, M.; Elias, A.; Ganjoo, K.; Tawbi, H.; et al. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J. Clin. Oncol. 2016, 34, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Grosso, F.; Jones, R.L.; Demetri, G.D.; Judson, I.R.; Blay, J.Y.; Le Cesne, A.; Sanfilippo, R.; Casieri, P.; Collini, P.; Dileo, P.; et al. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: A retrospective study. Lancet Oncol. 2007, 8, 595–602. [Google Scholar] [CrossRef]
- de Sande González, L.M.; Martin-Broto, J.; Kasper, B.; Blay, J.Y.; Le Cesne, A. Real-world evidence of the efficacy and tolerability of trabectedin in patients with advanced soft-tissue sarcoma. Expert Rev. Anticancer Ther. 2020, 20, 957–963. [Google Scholar] [CrossRef]
- Kobayashi, H.; Iwata, S.; Wakamatsu, T.; Hayakawa, K.; Yonemoto, T.; Wasa, J.; Oka, H.; Ueda, T.; Tanaka, S. Efficacy and safety of trabectedin for patients with unresectable and relapsed soft-tissue sarcoma in Japan: A Japanese Musculoskeletal Oncology Group study. Cancer 2020, 126, 1253–1263. [Google Scholar] [CrossRef]
- van der Graaf, W.T.; Blay, J.Y.; Chawla, S.P.; Kim, D.W.; Bui-Nguyen, B.; Casali, P.G.; Schöffski, P.; Aglietta, M.; Staddon, A.P.; Beppu, Y.; et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012, 379, 1879–1886. [Google Scholar] [CrossRef]
- Schöffski, P.; Chawla, S.; Maki, R.G.; Italiano, A.; Gelderblom, H.; Choy, E.; Grignani, G.; Camargo, V.; Bauer, S.; Rha, S.Y.; et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: A randomised, open-label, multicentre, phase 3 trial. Lancet 2016, 387, 1629–1637. [Google Scholar] [CrossRef]
- Antman, K.H. Chemotherapy of advanced sarcomas of bone and soft tissue. Semin. Oncol. 1992, 19 (Suppl. 12), 13–20. [Google Scholar] [PubMed]
- Zucali, P.A.; Bertuzzi, A.; Parra, H.J.S.; Campagnoli, E.; Quagliuolo, V.; Santoro, A. The “old drug” dacarbazine as a second/third line chemotherapy in advanced soft tissue sarcomas. Investig. New Drugs 2008, 26, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Tap, W.D.; Jones, R.L.; Van Tine, B.A.; Chmielowski, B.; Elias, A.D.; Adkins, D.; Agulnik, M.; Cooney, M.M.; Livingston, M.B.; Pennock, G.; et al. Olaratumab and doxorubicin versus doxorubicin alone in soft tissue sarcoma. Lancet 2016, 388, 488–497. [Google Scholar] [CrossRef]
- Tap, W.D.; Wagner, A.J.; Schöffski, P.; Martin-Broto, J.; Krarup-Hansen, A.; Ganjoo, K.N.; Yen, C.C.; Abdul Razak, A.R.; Spira, A.; Kawai, A.; et al. Effect of doxorubicin plus olaratumab vs doxorubicin plus placebo on survival in patients with advanced soft tissue sarcomas: The ANNOUNCE Randomized Clinical Trial. JAMA 2020, 323, 1266–1276. [Google Scholar] [CrossRef]
- Thoenen, E.; Curl, A.; Iwakuma, T. TP53 in bone and soft tissue sarcoma. Pharmacol. Ther. 2019, 202, 149–164. [Google Scholar] [CrossRef]
- Lee, A.T.J.L.; Thway, K.; Huang, P.H.; Jones, R.L. Clinical and molecular spectrum of liposarcoma. J. Clin. Oncol. 2018, 36, 151–159. [Google Scholar] [CrossRef]
- Sbaraglia, M.; Bellan, E.; Dei Tos, A.P. The 2020 WHO Classification of Soft Tissue Tumours: News and perspectives. Pathologica 2020. [Google Scholar] [CrossRef]
- Yang, L.; Chen, S.; Luo, P.; Yan, W.; Wang, C. Liposarcoma: Advances in cellular and molecular genetics alterations and corresponding clinical treatments. J. Cancer 2020, 11, 100–107. [Google Scholar] [CrossRef]
- Jung, J.; Lee, J.S.; Dickson, M.A.; Schwartz, G.K.; Le Cesne, A.; Varga, A.; Bahleda, R.; Wagner, A.J.; Choy, E.; de Jonge, M.J.; et al. TP53 mutations emerge with HDM2 inhibitor SAR405838 treatment in de-differentiated liposarcoma. Nat. Commun. 2016, 7, 12609. [Google Scholar] [CrossRef]
- Zhang, S.; Mo, Q.; Wang, X. Oncological role of HMGA2. Int. J. Oncol. 2019, 55, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Saâda-Bouzid, E.; Burel-Vandenbos, F.; Ranchère-Vince, D.; Birtwisle-Peyrottes, I.; Chetaille, B.; Bouvier, C.; Château, M.-C.; Peoc’h, M.; Battistella, M.; Bazin, A.; et al. Prognostic value of HMGA2, CDK4, and JUN amplification in well-differentiated and dedifferentiated liposarcomas. Mod. Pathol. 2015, 28, 1404–1414. [Google Scholar] [CrossRef]
- Yamashita, K.; Kohashi, K.; Yamada, Y.; Akatsuka, S.; Ikuta, K.; Nishida, Y.; Toyokuni, S.; Oda, Y. Prognostic significance of the MDM2/HMGA2 ratio and histological tumor grade in dedifferentiated liposarcoma. Genes Chromosom. Cancer 2021, 60, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Munnia, A.; Schütz, N.; Romeike, B.F.; Maldener, E.; Glass, B.; Maas, R.; Nastainczyk, W.; Feiden, W.; Fischer, U.; Meese, E. Expression, cellular distribution and protein binding of the glioma amplified sequence (GAS41), a highly conserved putative transcription factor. Oncogene 2001, 20, 4853–4863. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cho, H.J.; Li, H.; Linhares, B.M.; Kim, E.G.; Ndoj, J.; Miao, H.; Grembecka, J.; Cierpicki, T. GAS41 Recognizes Diacetylated Histone H3 through a Bivalent Binding Mode. ACS Chem. Biol. 2018, 13, 2739–2746. [Google Scholar] [CrossRef] [PubMed]
- Erickson-Johnson, M.R.; Seys, A.R.; Roth, C.W.; King, A.A.; Hulshizer, R.L.; Wang, X.; Asmann, Y.W.; Lloyd, R.V.; Jacob, E.K.; Oliveira, A.M. Carboxypeptidase M: A biomarker for the discrimination of well-differentiated liposarcoma from lipoma. Mod. Pathol. 2009, 22, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Perez-Losada, J.; Pintado, B.; Gutierrez-Adan, A.; Flores, T.; Bañares-Gonzalez, B.; Calabia del Campo, J.; Martin-Martin, J.F.; Battaner, E.; Sanchez-Garcia, I. The chimeric FUS/TLS-CHOP fusion protein specifically induces liposarcomas in transgenic mice. Oncogene 2000, 19, 2413–2422. [Google Scholar] [CrossRef]
- Yen, C.-C.; Chen, S.-C.; Hung, G.-Y.; Wu, P.-K.; Chua, W.-Y.; Lin, Y.-C.; Yen, C.-H.; Chen, Y.-C.; Wang, J.-Y.; Yang, M.-H.; et al. Expression profile-driven discovery of AURKA as a treatment target for liposarcoma. Int. J. Oncol. 2019, 55, 938–948. [Google Scholar] [CrossRef]
- Zhang, K.; Chu, K.; Wu, X.; Gao, H.; Wang, J.; Yuan, Y.-C.; Loera, S.; Ho, K.; Wang, Y.; Chow, W.; et al. Amplification of FRS2 and activation of FGFR/FRS2 signaling pathway in high-grade liposarcoma. Cancer Res. 2013, 73, 1298–1307. [Google Scholar] [CrossRef]
- Riggi, N.; Cironi, L.; Provero, P.; Suvà, M.-L.; Stehle, J.-C.; Baumer, K.; Guillou, L.; Stamenkovicet, I. Expression of the FUS-CHOP fusion protein in primary mesenchymal progenitor cells gives rise to a model of myxoid liposarcoma. Cancer Res. 2006, 66, 7016–7023. [Google Scholar] [CrossRef]
- Trautmann, M.; Menzel, J.; Bertling, C.; Cyra, M.; Isfort, I.; Steinestel, K.; Elges, S.; Grünewald, I.; Altvater, B.; Rossig, C.; et al. FUS-DDIT3 Fusion Protein-Driven IGF-IR Signaling is a Therapeutic Target in Myxoid Liposarcoma. Clin. Cancer Res. 2017, 23, 6227–6238. [Google Scholar] [CrossRef] [PubMed]
- Trautmann, M.; Cyra, M.; Isfort, I.; Jeiler, B.; Krüger, A.; Grünewald, I.; Steinestel, K.; Altvater, B.; Rossig, C.; Hafner, S.; et al. Phosphatidylinositol-3-kinase (PI3K)/Akt Signaling is Functionally Essential in Myxoid Liposarcoma. Mol. Cancer Ther. 2019, 18, 834–844. [Google Scholar] [CrossRef]
- Sun, R.; Shen, J.K.; Choy, E.; Yu, Z.; Hornicek, F.J.; Duan, Z. The emerging roles and therapeutic potential of microRNAs (miRs) in liposarcoma. Discov. Med. 2015, 20, 311–324. [Google Scholar] [PubMed]
- Gits, C.M.M.; van Kuijk, P.F.; Jonkers, M.B.E.; Boersma, A.W.M.; Smid, M.; van Ijcken, W.F.; Coindre, J.-M.; Chibon, F.; Verhoef, C.; Mathijssen, R.H.J.; et al. MicroRNA expression profiles distinguish liposarcoma subtypes and implicate miR-145 and miR-451 as tumor suppressors. Int. J. Cancer 2014, 135, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Mazzu, Y.Z.; Hu, Y.; Soni, R.K.; Mojica, K.M.; Qin, L.-X.; Agius, P.; Waxman, Z.M.; Mihailovic, A.; Socci, N.D.; Hendrickson, R.C.; et al. miR-193b-Regulated Signaling Networks Serve as Tumor Suppressors in Liposarcoma and Promote Adipogenesis in Adipose-Derived Stem Cells. Cancer Res. 2017, 77, 5728–5740. [Google Scholar] [CrossRef] [PubMed]
- Ugras, S.; Brill, E.; Jacobsen, A.; Hafner, M.; Socci, N.D.; Decarolis, P.L.; Khanin, R.; O’Connor, R.; Mihailovic, A.; Taylor, B.S.; et al. Small RNA sequencing and functional characterization reveals MicroRNA-143 tumor suppressor activity in liposarcoma. Cancer Res. 2011, 71, 5659–5669. [Google Scholar] [CrossRef] [PubMed]
- Bi, P.; Yue, F.; Karki, A.; Castro, B.; Wirbisky, S.E.; Wang, C.; Durkes, A.; Elzey, B.D.; Andrisani, O.M.; Bidwell, C.A.; et al. Notch activation drives adipocyte dedifferentiation and tumorigenic transformation in mice. J. Exp. Med. 2016, 213, 2019–2037. [Google Scholar] [CrossRef]
- Olsauskas-Kuprys, R.; Zlobin, A.; Osipo, C. Gamma secretase inhibitors of Notch signaling. Oncol. Targets Ther. 2013, 6, 943–955. [Google Scholar]
- U.S. National Library of Medicine Clinicaltrials.Gov. Available online: clinicaltrials.gov (accessed on 17 January 2021).
- Burgess, A.; Chia, K.M.; Haupt, S.; Thomas, D.; Haupt, Y.; Lim, E. Clinical overview of MDM2/X-targeted therapies. Front. Oncol. 2016, 6, 7. [Google Scholar] [CrossRef]
- Dadone-Montaudié, B.; Laroche-Clary, A.; Mongis, A.; Chamorey, E.; Di Mauro, I.; Chaire, V.; Finetti, P.; Schiappa, R.; Le Loarer, F.; Birtwisle-Peyrottes, I.; et al. Novel Therapeutic Insights in Dedifferentiated Liposarcoma: A Role for FGFR and MDM2 Dual Targeting. Cancers 2020, 12, 3058. [Google Scholar] [CrossRef]
- Vassilev, L.T.; Vu, B.T.; Graves, B.; Carvajal, D.; Podlaski, F.; Filipovic, Z.; Kong, N.; Kammlott, U.; Lukacs, C.; Klein, C.; et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004, 303, 844–848. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Blay, J.Y.; Italiano, A.; Le Cesne, A.; Penel, N.; Zhi, J.; Heil, F.; Rueger, R.; Graves, B.; Ding, M.; et al. Effect of the MDM2 antagonist RG7112 on the P53 pathway in patients with MDM2-amplified, well-differentiated or dedifferentiated liposarcoma: An exploratory proof-of-mechanism study. Lancet Oncol. 2012, 13, 1133–1140. [Google Scholar] [CrossRef]
- de Jonge, M.; de Weger, V.A.; Dickson, M.A.; Langenberg, M.; Le Cesne, A.; Wagner, A.J.; Hsu, K.; Zheng, W.; Macé, S.; Tuffal, G.; et al. A phase I study of SAR405838, a novel human double minute 2 (HDM2) antagonist, in patients with solid tumours. Eur. J. Cancer 2017, 76, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.J.; Banerji, U.; Mahipal, A.; Somaiah, N.; Hirsch, H.; Fancourt, C.; Johnson-Levonas, A.O.; Lam, R.; Meister, A.K.; Russo, G.; et al. Phase I Trial of the Human Double Minute 2 Inhibitor MK-8242 in Patients with advanced solid tumors. J. Clin. Oncol. 2017, 35, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Laroche-Clary, A.; Verbeke, S.; Derieppe, M.-A.; Italiano, A. MDM2 Antagonists Induce a Paradoxical Activation of Erk1/2 through a P53-Dependent Mechanism in Dedifferentiated Liposarcomas: Implications for Combinatorial Strategies. Cancers 2020, 12, 2253. [Google Scholar] [CrossRef] [PubMed]
- Dickson, M.A.; Schwartz, G.K.; Keohan, M.L.; D’Angelo, S.P.; Gounder, M.M.; Chi, P.; Antonescu, C.R.; Landa, J.; Qin, L.-X.; Crago, A.M.; et al. Progression-Free Survival among patients with Well-Differentiated or Dedifferentiated Liposarcoma treated with CDK4 Inhibitor Palbociclib: A Phase 2 Clinical Trial. JAMA Oncol. 2016, 2, 937–940. [Google Scholar] [CrossRef] [PubMed]
- Infante, J.R.; Cassier, P.A.; Gerecitano, J.F.; Witteveen, P.O.; Chugh, R.; Ribrag, V.; Chakraborty, A.; Matano, A.; Dobson, J.R.; Crystal, A.S.; et al. A phase I study of the cyclin-dependent kinase 4/6 inhibitor ribociclib (LEE011) in patients with advanced solid tumors and lymphomas. Clin. Cancer Res. 2016, 22, 5696–5705. [Google Scholar] [CrossRef] [PubMed]
- Laroche-Clary, A.; Chaire, V.; Algeo, M.-P.; Derieppe, M.-A.; Loarer, F.L.; Italiano, A. Combined targeting of MDM2 and CDK4 is synergistic in dedifferentiated liposarcomas. J. Hematol. Oncol. 2017, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Gounder, M.; Razak, A.A.; Somaiah, N.; MartinBroto, J.; Schuetze, S.; Grignani, G.; Chawla, S.P.; Chmielowski, B.; Vincenzi, B.; Silvia Stacchiotti, S.; et al. A phase 2/3, randomized, double blind, cross-over, study of selinexor versus placebo in advanced unresectable dedifferentiated liposarcoma (DDLS). In Proceedings of the Oral presentation at CTOS 2020 Memorial Sloan Kettering Cancer Center, New York, NY, USA, 20 November 2020. [Google Scholar]
- Singh, Z. Leiomyosarcoma: A rare soft tissue cancer arising from multiple organs. J. Cancer Res. Pract. 2018, 1, 1–8. [Google Scholar] [CrossRef]
- Koelsche, C.; Renner, M.; Johann, P.; Leiss, I.; Sahm, F.; Schimmack, S.; Wardelmann, E.; Renker, E.-K.; Schirmacher, P.; Korshunov, A.; et al. Differential nuclear ATRX expression in sarcomas. Histopathology 2016, 68, 738–745. [Google Scholar] [CrossRef]
- Liau, J.-Y.; Tsai, J.-H.; Jeng, Y.-M.; Lee, J.-C.; Hsu, H.-H.; Yang, C.-Y. Leiomyosarcoma with alternative lengthening of telomeres is associated with aggressive histologic features, loss of ATRX expression, and poor clinical outcome. Am. J. Surg. Pathol. 2015, 39, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Seligson, N.D.; Kautto, E.A.; Passen, E.N.; Stets, C.; Toland, A.E.; Millis, S.Z.; Meyer, C.F.; Hays, J.L.; Chen, J.L. BRCA1/2 functional loss defines a targetable subset in leiomyosarcoma. Oncologist 2019, 24, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Weetall, M.; Branstrom, A.; Baird, J.; Cao, L.; Sheedy, J.; O’Keefe, K.; Ingham, M.; Schwartz, G.K.; Spiegel, R.; O’Mara, E. Experimental and Molecular Therapeutics Abstract 292: PTC596 combination therapy for sarcoma. In Proceedings of the AACR Annual Meeting 2019, Atlanta, GA, USA, 29 March–3 April 2019. [Google Scholar] [CrossRef]
- Vikas, P.; Borcherding, N.; Chennamadhavuni, A.; Garje, R. Therapeutic Potential of Combining PARP Inhibitor and Immunotherapy in Solid Tumors. Front. Oncol. 2020, 10, 570. [Google Scholar] [CrossRef] [PubMed]
- Peyraud, F.; Italiano, A. Combined PARP Inhibition and Immune Checkpoint Therapy in Solid Tumors. Cancers 2020, 12, 1502. [Google Scholar] [CrossRef]
- Chi, Y.; Fang, Z.; Hong, X.; Yao, Y.; Sun, P.; Wang, G.; Du, F.; Sun, Y.; Wu, Q.; Qu, G.; et al. Safety and efficacy of Anlotinib, a Multikinase Angiogenesis Inhibitor, in patients with refractory metastatic Soft-Tissue Sarcoma. Clin. Cancer Res. 2018, 24, 5233–5238. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Liu, H.; Zhang, F.; Li, L.; Du, X.; Li, C.; Yang, J.; Wang, J. Retrospective review of the activity and safety of apatinib and anlotinib in patients with advanced osteosarcoma and soft tissue sarcoma. Investig. New Drugs 2020, 38, 1559–1569. [Google Scholar] [CrossRef]
- Wang, H.Y.; Chu, J.F.; Zhang, P.; Wang, J.Q.; Yan, Z.; Yao, S.N.; Yao, Z.H.; Liu, Y.Y. Safety and Efficacy of Chemotherapy Combined with Anlotinib Plus Anlotinib Maintenance in Chinese Patients with Advanced/Metastatic Soft Tissue Sarcoma. Oncol. Targets Ther. 2020, 13, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Shern, J.F.; Chen, L.; Chmielecki, J.; Wei, J.S.; Patidar, R.; Rosenberg, M.; Ambrogio, L.; Auclair, D.; Wang, J.; Song, Y.K.; et al. Comprehensive genomic analysis of rhabdomyosarcoma reveals a landscape of alterations affecting a common genetic axis in fusion-positive and fusion-negative tumors. Cancer Discov. 2014, 4, 216–231. [Google Scholar] [CrossRef]
- Finckenstein, F.G.; Shahbazian, V.; Davicioni, E.; Ren, Y.-X.; Anderson, M.J. PAX-FKHR function as pangenes by simultaneously inducing and inhibiting myogenesis. Oncogene 2008, 27, 2004–2014. [Google Scholar] [CrossRef]
- Satheesha, S.; Manzella, G.; Bovay, A.; Casanova, E.A.; Bode, P.K.; Belle, R.; Feuchtgruber, S.; Jaaks, P.; Dogan, N.; Koscielniak, E.; et al. Targeting hedgehog signaling reduces self-renewal in embryonal rhabdomyosarcoma. Oncogene 2016, 35, 2020–2030. [Google Scholar] [CrossRef]
- Stewart, E.; McEvoy, J.; Wang, H.; Chen, X.; Honnell, V.; Ocarz, M.; Gordon, B.; Dapper, J.; Blankenship, K.; Yang, Y.; et al. St. Jude Children’s Research Hospital—Washington University Pediatric Cancer Genome Project. Identification of Therapeutic Targets in Rhabdomyosarcoma through Integrated Genomic, Epigenomic, and Proteomic Analyses. Cancer Cell 2018, 34, 411–426.e19. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Barr, F.G. Therapeutic Approaches Targeting PAX3-FOXO1 and Its Regulatory and Transcriptional Pathways in Rhabdomyosarcoma. Molecules 2018, 23, 2798. [Google Scholar] [CrossRef]
- Heitzer, E.; Sunitsch, S.; Gilg, M.M.; Lohberger, B.; Rinner, B.; Kashofer, K.; Stündl, N.; Ulz, P.; Szkandera, J.; Leithner, A.; et al. Expanded molecular profiling of myxofibrosarcoma reveals potentially actionable targets. Mod. Pathol. 2017, 30, 1698–1709. [Google Scholar] [CrossRef]
- Ogura, K.; Hosoda, F.; Arai, Y.; Nakamura, H.; Hama, N.; Totoki, Y.; Yoshida, A.; Nagai, M.; Kato, M.; Arakawa, E.; et al. Integrated genetic and epigenetic analysis of myxofibrosarcoma. Nat. Commun. 2018, 9, 2765. [Google Scholar] [CrossRef] [PubMed]
- Idbaih, A.; Coindre, J.M.; Derre, J.; Mariani, O.; Terrier, P.; Ranchère, D.; Mairal, A.; Aurias, A. Myxoid malignant fibrous histiocytoma and pleomorphic liposarcoma share very similar genomic imbalances. Lab. Investig. 2005, 85, 176–181. [Google Scholar] [CrossRef]
- Okada, T.; Lee, A.Y.; Qin, L.X.; Agaram, N.; Mimae, T.; Shen, Y.; O’Connor, R.; López-Lago, M.A.; Craig, A.; Miller, M.L.; et al. Integrin-alpha10 Dependency Identifies RAC and RICTOR as Therapeutic Targets in High-Grade Myxofibrosarcoma. Cancer Discov. 2016, 6, 1148–1165. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Yu, D.B.; Sung, M.; Oh, E.; Kim, M.; Song, J.-Y.; Lee, M.-S.; Jung, K.; Noh, K.-W.; An, S.; et al. Molecular changes in solitary fibrous tumor progression. J. Mol. Med. 2019, 97, 1413–1425. [Google Scholar] [CrossRef]
- Park, Y.S.; Kim, H.S.; Kim, J.H.; Choi, S.H.; Kim, D.S.; Ryoo, Z.Y.; Kim, J.Y.; Lee, S. NAB2-STAT6 fusion protein mediates cell proliferation and oncogenic progression via EGR-1 regulation. Biochem. Biophys. Res. Commun. 2020, 526, 287–292. [Google Scholar] [CrossRef]
- West, R.B.; Rubin, B.P.; Miller, M.A.; Subramanian, S.; Kaygusuz, G.; Montgomery, K.; Zhu, S.; Marinelli, R.J.; De Luca, A.; Downs-Kelly, E.; et al. A landscape effect in tenosynovial giant-cell tumor from activation of CSF1 expression by a translocation in a minority of tumor cells. Proc. Natl. Acad. Sci. USA 2006, 103, 690–695. [Google Scholar] [CrossRef]
- Tap, W.D.; Gelderblom, H.; Palmerini, E.; Desai, J.; Bauer, S.; Blay, J.Y.; Alcindor, T.; Ganjoo, K.; Martín-Broto, J.; Ryan, C.W.; et al. Pexidartinib for advanced tenosynovial giant cell tumor: Results of the randomized phase 3 ENLIVEN study. Lancet 2019, 394, 478–487. [Google Scholar] [CrossRef]
- Cao, J.; Wang, J.; He, C.; Fang, M. Angiosarcoma: A review of diagnosis and current treatment. Am. J. Cancer Res. 2019, 9, 2303–2313. [Google Scholar]
- Murali, R.; Chandramohan, R.; Möller, I.; Scholz, S.L.; Berger, M.; Huberman, K.; Viale, A.; Pirun, M.; Socci, N.D.; Bouvier, N.; et al. Targeted massively parallel sequencing of angiosarcomas reveals frequent activation of the mitogen activated protein kinase pathway. Oncotarget 2015, 6, 36041–36052. [Google Scholar] [CrossRef] [PubMed]
- Kollár, A.; Jones, R.L.; Stacchiotti, S.; Gelderblom, H.; Guida, M.; Grignani, G.; Steeghs, N.; Safwat, A.; Katz, D.; Duffaud, F.; et al. Pazopanib in advanced vascular sarcomas: An EORTC Soft Tissue and Bone Sarcoma Group (STBSG) retrospective analysis. Acta Oncol. 2017, 56, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Italiano, A.; Bompas, E.; Le Cesne, A.; Robin, Y.M.; Chevreau, C.; Bay, J.O.; Bousquet, G.; Piperno-Neumann, S.; French Sarcoma Group (GSF/GETO); et al. Sorafenib for patients with advanced angiosarcoma: A phase II Trial from the French Sarcoma Group (GSF/GETO). Oncologist 2012, 17, 260–266. [Google Scholar] [CrossRef]
- Lee, K.W.; Lee, K.H.; Zang, D.Y.; Park, Y.I.; Shin, D.B.; Kim, J.W.; Im, S.A.; Koh, S.A.; Yu, K.S.; Cho, J.Y.; et al. Phase I/II Study of Weekly Oraxol for the Second-Line Treatment of Patients with Metastatic or Recurrent Gastric Cancer. Oncologist 2015, 20, 896–897. [Google Scholar] [CrossRef] [PubMed]
- Mehta, C.R.; Liu, L.; Theuer, C. An adaptive population enrichment phase III trial of TRC105 and pazopanib versus pazopanib alone in patients with advanced angiosarcoma (TAPPAS trial). Ann. Oncol. 2019, 30, 103–108. [Google Scholar] [CrossRef]
- Florou, V.; Rosenberg, A.E.; Wieder, E.; Komanduri, K.V.; Kolonias, D.; Uduman, M.; Castle, J.C.; Buell, J.S.; Trent, J.C.; Wilky, B.A. Angiosarcoma patients treated with immune checkpoint inhibitors: A case series of seven patients from a single institution. J. Immunother. Cancer 2019, 7, 213. [Google Scholar] [CrossRef]
- Kaplon, H.; Muralidharan, M.; Schneider, Z.; Reichert, J.M. Antibodies to watch in 2020. MAbs 2020, 12, 1703531. [Google Scholar] [CrossRef]
- Hay, C.M.; Sult, E.; Huang, Q.; Mulgrew, K.; Fuhrmann, S.R.; McGlinchey, K.A.; Hammond, S.A.; Rothstein, R.; Rios-Doria, J.; Poon, E.; et al. Targeting CD73 in the tumor microenvironment with MEDI9447. Oncoimmunology 2016, 5, e1208875. [Google Scholar] [CrossRef]
- Martin-Broto, J.; Hindi, N.; Grignani, G.; Martinez-Trufero, J.; Redondo, A.; Valverde, C.; Stacchiotti, S.; Lopez-Pousa, A.; D’Ambrosio, L.; Gutierrez, A.; et al. Nivolumab and sunitinib combination in advanced soft tissue sarcomas: A multicenter, single-arm, phase Ib/II trial. J. Immunother. Cancer 2020, 8, e001561. [Google Scholar] [CrossRef]
- Rosenbaum, E.; Jadeja, B.; Xu, B.; Zhang, L.; Agaram, N.P.; Travis, W.; Singer, S.; Tap, W.D.; Antonescu, C.R. Prognostic stratification of clinical and molecular epithelioid hemangioendothelioma subsets. Mod. Pathol. 2020, 33, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Tanas, M.R.; Ma, S.; Jadaan, F.O.; Ng, C.K.Y.; Weigelt, B.; Reis-Filho, J.S.; Rubin, B.P. Mechanism of action of a WWTR1(TAZ)-CAMTA1 fusion oncoprotein. Oncogene 2016, 35, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Antonescu, C.R.; Le Loarer, F.; Mosquera, J.M.; Sboner, A.; Zhang, L.; Chen, C.L.; Chen, H.W.; Pathan, N.; Krausz, T.; Dickson, B.C.; et al. Novel YAP1-TFE3 fusion defines a distinct subset of epithelioid hemangioendothelioma. Genes Chromosom. Cancer 2013, 52, 775–784. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Simeone, N.; Lo Vullo, S.; Baldi, G.G.; Brunello, A.; Vincenzi, B.; Palassini, E.; Dagrada, G.; Collini, P.; Morosi, C.; et al. Activity of sirolimus in patients with progressive epithelioid hemangioendothelioma: A case-series analysis within the Italian rare cancer network. Cancer 2021, 127, 569–576. [Google Scholar] [CrossRef]
- Ki, D.H.; He, S.; Rodig, S.; Look, A.T. Overexpression of PDGFRA cooperates with loss of NF1 and p53 to accelerate the molecular pathogenesis of malignant peripheral nerve sheath tumors. Oncogene 2017, 36, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Stewart, D.R.; Reilly, K.M.; Viskochil, D.; Miettinen, M.M.; Widemann, B.C. Malignant Peripheral Nerve Sheath Tumors State of the Science: Leveraging Clinical and Biological Insights into Effective Therapies. Sarcoma 2017, 2017, 7429697. [Google Scholar] [CrossRef]
- Schaefer, I.-M.; Dong, F.; Garcia, E.P.; Fletcher, C.D.M.; Jo, V.Y. Recurrent SMARCB1 Inactivation in Epithelioid Malignant Peripheral Nerve Sheath Tumors. Am. J. Surg. Pathol. 2019, 43, 835–843. [Google Scholar] [CrossRef]
- Kawano, S.; Grassian, A.R.; Tsuda, M.; Knutson, S.K.; Warholic, N.M.; Kuznetsov, G.; Xu, S.; Xiao, Y.; Pollock, R.M.; Smith, J.S.; et al. Preclinical Evidence of Anti-Tumor Activity Induced by EZH2 Inhibition in Human Models of Synovial Sarcoma. PLoS ONE 2016, 11, e0158888. [Google Scholar] [CrossRef]
- Pemov, A.; Li, H.; Presley, W.; Wallace, M.R.; Miller, D.T. Genetics of human malignant peripheral nerve sheath tumors. Neurooncol. Adv. 2020, 2 (Suppl. 1), i50–i61. [Google Scholar] [CrossRef]
- Kim, K.H.; Roberts, C.W.M. Mechanisms by which SMARCB1 loss drives rhabdoid tumor growth. Cancer Genet. 2014, 207, 365–372. [Google Scholar] [CrossRef]
- Morel, D.; Almouzni, G.; Soria, J.C.; Postel-Vinay, S. Targeting chromatin defects in selected solid tumors based on oncogene addiction, synthetic lethality and epigenetic antagonism. Ann. Oncol. 2017, 28, 254–269. [Google Scholar] [CrossRef] [PubMed]
- Kohashi, K.; Oda, Y. Oncogenic roles of SMARCB1/INI1 and its deficient tumors. Cancer Sci. 2017, 108, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Agaimy, A.; Foulkes, W.D. Hereditary SWI/SNF complex deficiency syndromes. Semin. Diagn. Pathol. 2018, 35, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Knutson, S.K.; Warholic, N.M.; Wigle, T.J.; Klaus, C.R.; Allain, C.J.; Raimondi, A.; Scott, M.P.; Chesworth, R.; Moyer, M.P.; Copeland, R.A.; et al. Durable tumor regression in genetically altered malignant rhabdoid tumors by inhibition of methyltransferase EZH2. Proc. Natl. Acad. Sci. USA 2013, 110, 7922–7927. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Murray, B.; Mo, G.; Shern, J.F. The Role of Polycomb Repressive Complex in Malignant Peripheral Nerve Sheath Tumor. Genes 2020, 11, 287. [Google Scholar] [CrossRef]
- Wojcik, J.B.; Marchione, D.M.; Sidoli, S.; Djedid, A.; Lisby, A.; Majewski, J.; Garcia, B.A. Epigenomic Reordering Induced by Polycomb Loss Drives Oncogenesis but Leads to Therapeutic Vulnerabilities in Malignant Peripheral Nerve Sheath Tumors. Cancer Res. 2019, 79, 3205–3219. [Google Scholar] [CrossRef]
- Nishida, Y.; Urakawa, H.; Nakayama, R.; Kobayashi, E.; Ozaki, T.; Ae, K.; Matsumoto, Y.; Tsuchiya, H.; Goto, T.; Hiraga, H.; et al. Phase II clinical trial of pazopanib for patients with unresectable or metastatic malignant peripheral nerve sheath tumors. Int. J. Cancer 2021, 148, 140–149. [Google Scholar] [CrossRef]
- Lee, P.; Malik, D.; Perkons, N.; Huangyang, P.; Khare, S.; Rhoades, S.; Gong, Y.Y.; Burrows, M.; Finan, J.M.; Nissim, I.; et al. Targeting glutamine metabolism slows soft tissue sarcoma growth. Nat. Commun. 2020, 11, 498. [Google Scholar] [CrossRef]
- Demetri, G.D.; Antonescu, C.R.; Bjerkehagen, B.; Bovée, J.V.M.G.; Boye, K.; Chacón, M.; Dei Tos, A.P.; Desai, J.; Fletcher, J.A.; Gelderblom, H.; et al. Diagnosis and management of tropomyosin receptor kinase (TRK) fusion sarcomas: Expert recommendations from the World Sarcoma Network. Ann. Oncol. 2020, 31, 1506–1517. [Google Scholar] [CrossRef]
- Kadoch, C.; Crabtree, G.R. Reversible Disruption of mSWI/SNF (BAF) Complexes by the SS18-SSX Oncogenic Fusion in Synovial Sarcoma. Cell 2013, 153, 71–85. [Google Scholar] [CrossRef]
- Kohashi, K.; Oda, Y.; Yamamoto, H.; Tamiya, S.; Matono, H.; Iwamoto, Y.; Taguchi, T.; Tsuneyoshi, M. Reduced expression of SMARCB1/INI1 protein in synovial sarcoma. Mod. Pathol. 2010, 23, 981–990. [Google Scholar] [CrossRef]
- Briski, L.M.; Thomas, D.G.; Patel, R.M.; Lawlor, E.R.; Chugh, R.; McHugh, J.B.; Lucas, D.R. Canonical Wnt/beta-catenin signaling activation in soft-tissue sarcomas: A comparative study of synovial sarcoma and leiomyosarcoma. Rare Tumors 2018, 10, 2036361318813431. [Google Scholar] [CrossRef]
- He, M.; Abro, B.; Kaushal, M.; Chen, L.; Chen, T.; Gondim, M.; Yan, W.; Neidich, J.; Dehner, L.P.; Pfeifer, J.D. Tumor mutation burden and checkpoint immunotherapy markers in primary and metastatic synovial sarcoma. Hum. Pathol. 2020, 100, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Iura, K.; Maekawa, A.; Kohashi, K.; Ishii, T.; Bekki, H.; Otsuka, H.; Yamada, Y.; Yamamoto, H.; Harimaya, K.; Iwamoto, Y.; et al. Cancer-testis antigen expression in synovial sarcoma: NY-ESO-1, PRAME, MAGEA4, and MAGEA1. Hum. Pathol. 2017, 61, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Keung, E.Z.; Tawbi, H.A. Engineered T Cells in Synovial Sarcoma: Persistence Pays Off! Cancer Discov. 2018, 8, 914–917. [Google Scholar] [CrossRef] [PubMed]
- Somaiah, N.; Block, M.S.; Kim, J.W.; Shapiro, G.I.; Do, K.T.; Hwu, P.; Eder, J.P.; Jones, R.L.; Lu, H.; ter Meulen, J.; et al. First-in-Class, First-in-Human Study Evaluating LV305, a Dendritic-Cell Tropic Lentiviral Vector, in Sarcoma and Other Solid Tumors Expressing NY-ESO-1. Clin. Cancer Res. 2019, 25, 5808–5817. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, S.N.; Chetty, R. Gene of the month: SMARCB1. J. Clin. Pathol. 2016, 69, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.E. Epigenetic therapies for cancer. N. Engl. J. Med. 2020, 383, 650–663. [Google Scholar] [CrossRef]
- Groisberg, R.; Subbiah, V. EZH2 inhibition for epithelioid sarcoma and follicular lymphoma. Lancet Oncol. 2020, 21, 1388–1390. [Google Scholar] [CrossRef]
- Gounder, M.; Schöffski, P.; Jones, R.L.; Agulnik, M.; Cote, G.M.; Villalobos, V.M.; Attia, S.; Chugh, R.; Chen, T.W.; Jahan, T.; et al. Tazemetostat in advanced epithelioid sarcoma with loss of INI1/SMARCB1: An international, open-label, phase 2 basket study. Lancet Oncol. 2020, 21, 1423–1432. [Google Scholar] [CrossRef]
- Pecora, A.; Halpern, S.; Weber, M.; Paleoudis, E.G.; Panush, D.; Patterson, F.; Toretsky, J. Rapid and Complete Response to Combination Anti-CTLA-4 and Anti-PD-1 Checkpoint Inhibitor Therapy in a Patient with Stage IV Refractory End-stage Epithelioid Sarcoma: A Case Report. J. Immunother. 2020, 43, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Casey, D.L.; Lin, T.-Y.; Cheung, N.-K.V. Exploiting Signaling Pathways and Immune Targets beyond the Standard of Care for Ewing Sarcoma. Front. Oncol. 2019, 9, 537. [Google Scholar] [CrossRef] [PubMed]
- Brčić, I.; Brodowicz, T.; Cerroni, L.; Kashofer, K.; Serbanescu, G.L.; Kasseroler, M.T.; Amann, G.; Scheipl, S.; Szkandera, J.; Leithner, A.; et al. Undifferentiated round cell sarcomas with CIC-DUX4 gene fusion: Expanding the clinical spectrum. Pathology 2020, 52, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; Felisiak-Golabek, A.; Luiña Contreras, A.; Glod, J.; Kaplan, R.N.; Killian, J.K.; Lasota, J. New fusion sarcomas: Histopathology and clinical significance of selected entities. Hum. Pathol. 2019, 86, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Antonescu, C.R.; Owosho, A.A.; Zhang, L.; Chen, S.; Deniz, K.; Huryn, J.M.; Kao, Y.C.; Huang, S.C.; Singer, S.; Tap, W.; et al. Sarcomas With CIC-rearrangements Are a Distinct Pathologic Entity with Aggressive Outcome: A Clinicopathologic and Molecular Study of 115 Cases. Am. J. Surg. Pathol. 2017, 41, 941–949. [Google Scholar] [CrossRef]
- Watson, S.; Kendall, G.C.; Rakheja, D.; McFaul, M.E.; Draper, B.W.; Tirode, F.; Delattre, O.; Amatruda, J.F. CIC-DUX4 expression drives the development of small round cell sarcoma in transgenic zebrafish: A new model revealing a role for ETV4 in CIC-mediated sarcomagenesis. bioRxiv 2019, 517722. [Google Scholar] [CrossRef]
- Orth, M.F.; Buecklein, V.L.; Kampmann, E.; Subklewe, M.; Noessner, E.; Cidre-Aranaz, F.; Romero-Pérez, L.; Wehweck, F.S.; Lindner, L.; Issels, R.; et al. A comparative view on the expression patterns of PD-L1 and PD-1 in soft tissue sarcomas. Cancer Immunol. Immunother. 2020, 69, 1353–1362. [Google Scholar] [CrossRef]
- Vargas, A.C.; Maclean, F.M.; Sioson, L.; Tran, D.; Bonar, F.; Mahar, A.; Cheah, A.L.; Russell, P.; Grimison, P.; Richardson, L.; et al. Prevalence of PD-L1 expression in matched recurrent and/or metastatic sarcoma samples and in a range of selected sarcomas subtypes. PLoS ONE 2020, 15, e0222551. [Google Scholar] [CrossRef]
- Zheng, C.; You, W.; Wan, P.; Jiang, X.; Chen, J.; Zheng, Y.; Li, W.; Tan, J.; Zhang, S. Clinicopathological and prognostic significance of PD-L1 expression in sarcoma: A systematic review and meta-analysis. Medicine 2018, 97, e11004. [Google Scholar] [CrossRef]
- Wang, F.; Yu, T.; Ma, C.; Yuan, H.; Zhang, H.; Zhang, Z. Prognostic value of Programmed Cell Death 1 Ligand-1 in patients with bone and soft tissue sarcomas: A Systemic and Comprehensive Meta-Analysis Based on 3680 Patients. Front. Oncol. 2020, 10, 749. [Google Scholar] [CrossRef]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef]
- Samstein, R.M.; Lee, C.H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.B.; Light, N.; Fabrizio, D.; Zatzman, M.; Fuligni, F.; de Borja, R.; Davidson, S.; Edwards, M.; Elvin, J.A.; Hodel, K.P.; et al. Comprehensive Analysis of Hypermutation in Human Cancer. Cell 2017, 171, 1042–1056.e10. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.A.; Nowak, J.A.; Nathenson, M.J.; Thornton, K.; Wagner, A.J.; Johnson, J.M.; Albrayak, A.; George, S.; Sholl, L.M. Characteristics of mismatch repair deficiency in sarcomas. Mod. Pathol. 2019, 32, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Steele, C.D.; Tarabichi, M.; Oukrif, D.; Webster, A.P.; Ye, H.; Fittall, M.; Lombard, P.; Martincorena, I.; Tarpey, P.S.; Collord, G.; et al. Undifferentiated Sarcomas Develop through Distinct Evolutionary Pathways. Cancer Cell. 2019, 35, 441–456.e8. [Google Scholar] [CrossRef]
- Gu, H.Y.; Lin, L.L.; Zhang, C.; Yang, M.; Zhong, H.C.; Wei, R.X. The Potential of Five Immune-Related Prognostic Genes to Predict Survival and Response to Immune Checkpoint Inhibitors for Soft Tissue Sarcomas Based on Multi-Omic Study. Front. Oncol. 2020, 10, 1317. [Google Scholar] [CrossRef]
- Petitprez, F.; de Reyniès, A.; Keung, E.Z.; Chen, T.W.; Sun, C.M.; Calderaro, J.; Jeng, Y.M.; Hsiao, L.P.; Lacroix, L.; Bougoüin, A.; et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 2020, 577, 556–560. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
- Keung, E.Z.; Burgess, M.; Salazar, R.; Parra, E.R.; Rodrigues-Canales, J.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Attia, S.; Riedel, R.F.; et al. Correlative Analyses of the SARC028 Trial Reveal an Association Between Sarcoma-Associated Immune Infiltrate and Response to Pembrolizumab. Clin. Cancer Res. 2020, 26, 1258–1266. [Google Scholar] [CrossRef]
- Blay, J.-Y.; Chevret, S.; Penel, N.; Bertucci, F.; Bompas, E.; Saada-Bouzid, E.; Eymard, J.; Lotz, J.; Coquan, E.E.; Schott, R.; et al. High clinical benefit rates of single agent pembrolizumab in selected rare sarcoma histotypes: First results of the AcSé Pembrolizumab study. Ann. Oncol. 2020, 31 (Suppl. 4), S914–S933. [Google Scholar] [CrossRef]
- Monga, V.; Skubitz, K.M.; Maliske, S.; Mott, S.L.; Dietz, H.; Hirbe, A.C.; Van Tine, B.A.; Oppelt, P.; Okuno, S.; Robinson, S.; et al. A Retrospective Analysis of the Efficacy of Immunotherapy in Metastatic Soft-Tissue Sarcomas. Cancers 2020, 12, 1873. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Mahoney, M.R.; Van Tine, B.A.; Atkins, J.; Milhem, M.M.; Jahagirdar, B.N.; Antonescu, C.R.; Horvath, E.; Tap, W.D.; Schwartz, G.K.; et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): Two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018, 19, 416–426. [Google Scholar] [CrossRef]
- Wilky, B.A.; Trucco, M.M.; Subhawong, T.K.; Florou, V.; Park, W.; Kwon, D.; Wieder, E.D.; Kolonias, D.; Rosenberg, A.E.; Kerr, D.A.; et al. Axitinib plus pembrolizumab in patients with advanced sarcomas including alveolar soft-part sarcoma: A single-centre, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 837–848. [Google Scholar] [CrossRef]
- Scheinberg, T.; Lomax, A.; Tattersall, M.; Thomas, D.; McCowage, G.; Sullivan, M.; Karim, R.; Luk, P.P.; Mahar, A.; Bonar, F.; et al. PD-1 blockade using pembrolizumab in adolescent and young adult patients with advanced bone and soft tissue sarcoma. Cancer Rep. 2020, e1327. [Google Scholar] [CrossRef]
- Thanindratarn, P.; Dean, D.C.; Nelson, S.D.; Hornicek, F.J.; Duan, Z. Chimeric antigen receptor T (CAR-T) cell immunotherapy for sarcomas: From mechanisms to potential clinical applications. Cancer Treat. Rev. 2020, 82, 101934. [Google Scholar] [CrossRef]
- Yakoub-Agha, I.; Chabannon, C.; Bader, P.; Basak, G.W.; Bonig, H.; Ciceri, F.; Corbacioglu, S.; Duarte, R.F.; Einsele, H.; Hudecek, M.; et al. Management of adults and children undergoing chimeric antigen receptor T-cell therapy: Best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE). Haematologica 2020, 105, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Lucchesi, C.; Khalifa, E.; Laizet, Y.; Soubeyran, I.; Mathoulin-Pelissier, S.; Chomienne, C.; Italiano, A. Targetable alterations in adult patients with soft-tissue sarcomas: Insights for personalized therapy. JAMA Oncol. 2018, 4, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Gounder, M.M.; Ali, S.M.; Robinson, V.; Bailey, M.; Ferraro, R.; Patel, N.M.; Krishnan, A.; Millis, S.Z.; Dickson, M.A.; D’Angelo, S.P.; et al. Impact of next-generation sequencing (NGS) on diagnostic and therapeutic options in soft-tissue and bone sarcoma. J. Clin. Oncol. 2017, 35, 11001. [Google Scholar] [CrossRef]

| Malignant adipocytic tumors (15–25%) |
|
| LMS (5–10%) | |
| RMS (<3%) |
|
| Fibroblastic/myofibroblastic tumors |
|
| TGCT | |
| Vascular tumors |
|
| MPNST | |
| Tumors of uncertain differentiation |
|
| Undifferentiated small round-cell sarcoma |
|
| Tumor Type | Translocations/Fusion Protein (Reported Incidence) | Genetic Aberration | Receptor Overexpression | Pathways |
|---|---|---|---|---|
| Malignant adipocytic tumors | p53 (10–20%) | Notch signaling | ||
|
amplification 12q13-15 amplification of MDM2, CDK4, HMG2A, TSPAN31, YEATS4, CPM | MET, IGFR, AXL, EGFR | ||
|
amplification 12q13-15 amplification of MDM2, CDK4, HMG2A, TSPAN31, YEATS4, CPM aurora kinase | MET, IGFR, AXL, EGFR | ||
|
t(12;16)(q13;p11) FUS-DDIT3 (90%) t(12;22)(q13;q12) EWSR1-DDIT3 (5%) | PI3K/Akt (26%) | ||
| p53 (60%) | |||
|
LMS ULMS |
P53 Rb1 ATRX loss BRCA | |||
| RMS |
P53 MDM2 |
IGF/RAS/MEK/ERK PI3K/AKT/mTOR MET, FGFR, PDGFR | ||
|
T(2;13)(q35;q14), t(1;13)(p36;q14) PAX-FKHR fusion protein | |||
| Hedgehog signaling | |||
| ||||
| Fibroblastic/myofibroblastic tumors | ||||
|
P53 (>40%) Amplification of 5q region | |||
|
inv12(q13q13) NAB2-STAT6 fusion protein |
P53 TERT promoter | ||
| Malignant tenosynovial giant cell tumor | 1p13 (CSF) | |||
| Vascular tumor | ||||
|
P53 (50%) MDM2 (>60%) Amplification 8q24 Amplification 5q35 | MYC FLT MAPK (RAS, BRAF, MAPK1, NF1) | ||
|
t(1;3)(p36.3;q25)
WWTR1-CAMPTA1 fusion protein YAP-TFE3 | PI3KCa/Akt/mTOR | ||
| MPNST |
Loss of SMARCB1/INI1 Mutations EED/SUZ12 |
Sonic Hedgehog pathway Wnt/β-catenin pathway | ||
| Tumors of uncertain differentiation | ||||
| t(X;18) (p 11.2; q11.2) → SS18:SSX fusion proteins | Wnt/β-catenin pathway | ||
| Loss of SMARCB1/INI1 | |||
| Undifferentiated small round-cell sarcoma | ||||
| t(11;22)(q24;q12) → EWS-FLI1 fusion protein | |||
| ||||
| t(4;19) or t(10;19) translocation → CIC-DUX4 fusion protein | |||
|
| Trial Identifier | STS Histotype | Drugs | Phase | Study Hallmarks | Treatment Arms | Estimated Enrollment | Status |
|---|---|---|---|---|---|---|---|
| NCT01636479 | LPS | SAR405838 | I | Completed | |||
| NCT01463696 | MK 8242 | I | Completed | ||||
| NCT01209598 | Palbociclib | II | Completed | ||||
| NCT03114527 | LPS-LMS | Ribociclib everolimus | II | Active | |||
| NCT04438824 | LPS | Palbociclib Anti-PD-1 | II | ||||
| NCT02606461 (SEAL) | LPS | Selixenor | II/III |
| 342 | Active not recruiting | |
| NCT02978859 | LPS | Sitravanib | II | Active | |||
| NCT03761095 | LMS | PTC596 Dacarbazine | I | Active | |||
| NCT04242238 | STS | Avelumab DCC-3014 | I | Active | |||
| NCT03526679 (LEADER) | LMS LPS Adult STS Advanced cancer | Lenvatinib Eribulin | I/II | Active | |||
| NCT03123276 (GEMMK) | LMS UPS | Pembrolizumab Gemcitabine | I/II | ||||
| NCT04624178 | LMS | Rucaparib Nivolumab | II | Active | |||
| NCT03536780 (EAGLES) | LMS | Avelumab Gemcitabine | II | Active | |||
| NCT03810976 | LMS | Eribulin Gemcitabine | II | Active | |||
| NCT04200443 | LMS | Cabozantinib Temozolamide | II | Active | |||
| NCT02203760 (PazoDoble) | ULMS | Pazopanib Gemcitabine | II randomized |
| 107 | Active | |
| NCT03114527 | DDLPS LMS | Ribociclib Everolimus | II | Pretreated | Active | ||
| NCT03851614 (DAPPER) | LMS MMRp-CRC PA | Durvalumab Olaparib Cediranib | II randomized | Basket study |
| 90 | Active |
| NCT03718091 | LMS Osteosarcoma Solid tumors | MSS20 | II | 223 | Active not recruiting | ||
| NCT03899805 | LMS LPS UPS | Eribulin Pembrolizumab | II | Active | |||
| NCT02406781 (PEMBROSARC) | LMS STS | Metronomic CP Pembrolizumab | II | Active | |||
| NCT03016819 (APROMISS) | LMS SS ASPS | AL3818 Dacarbazine (DTIC) | III | Pretreated LMS/SS |
| 325 | Active |
| NCT04480502 (ENVASARC) | UPS Myxofibrosarcoma | Envafolimab Ipilimumab | II randomized |
| 160 | Active | |
| NCT03512834 (ASAP) | Angiosarcoma | Paclitaxel Avelumab | II | Active | |||
| NCT04607200 | Angiosarcoma | AGEN2034 AGEN1884 | II | Active | |||
| NCT03277924 (ImmunoSarc) | STS Bone tumors | I/II | 270 | Active | |||
| NCT02834013 | Rare tumors | II | |||||
| NCT02601950 | INI-1 negative tumors SS | Tazemetostat | II | 250 | Active | ||
| NCT02584647 | Sarcoma MPNST | PLX3397 Sirolimus | I/II | Active | |||
| NCT03433183 (SARC031) | MPNST | Selumetinib (AZD6244) Sirolimus | II | Active | |||
| NCT03872427 (BeGIN) | NF1 Aberrations, NF1 Mutant MPNST, KEAP1/NRF2, and LKB1 Aberrant Tumors | Telaglenastat Hydrochloride | II | basket | 108 | Active | |
| NCT04204941 | STS ES | Tazemetostat Doxorubicin | Ib/III |
| 164 | Active | |
| NCT04416568 | INI1-negative tumors | Nivolumab Ipilimumab | II | Active |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montella, L.; Altucci, L.; Sarno, F.; Buonerba, C.; De Simone, S.; Facchini, B.A.; Franzese, E.; De Vita, F.; Tafuto, S.; Berretta, M.; et al. Toward a Personalized Therapy in Soft-Tissue Sarcomas: State of the Art and Future Directions. Cancers 2021, 13, 2359. https://doi.org/10.3390/cancers13102359
Montella L, Altucci L, Sarno F, Buonerba C, De Simone S, Facchini BA, Franzese E, De Vita F, Tafuto S, Berretta M, et al. Toward a Personalized Therapy in Soft-Tissue Sarcomas: State of the Art and Future Directions. Cancers. 2021; 13(10):2359. https://doi.org/10.3390/cancers13102359
Chicago/Turabian StyleMontella, Liliana, Lucia Altucci, Federica Sarno, Carlo Buonerba, Stefano De Simone, Bianca Arianna Facchini, Elisena Franzese, Ferdinando De Vita, Salvatore Tafuto, Massimiliano Berretta, and et al. 2021. "Toward a Personalized Therapy in Soft-Tissue Sarcomas: State of the Art and Future Directions" Cancers 13, no. 10: 2359. https://doi.org/10.3390/cancers13102359
APA StyleMontella, L., Altucci, L., Sarno, F., Buonerba, C., De Simone, S., Facchini, B. A., Franzese, E., De Vita, F., Tafuto, S., Berretta, M., & Facchini, G. (2021). Toward a Personalized Therapy in Soft-Tissue Sarcomas: State of the Art and Future Directions. Cancers, 13(10), 2359. https://doi.org/10.3390/cancers13102359










