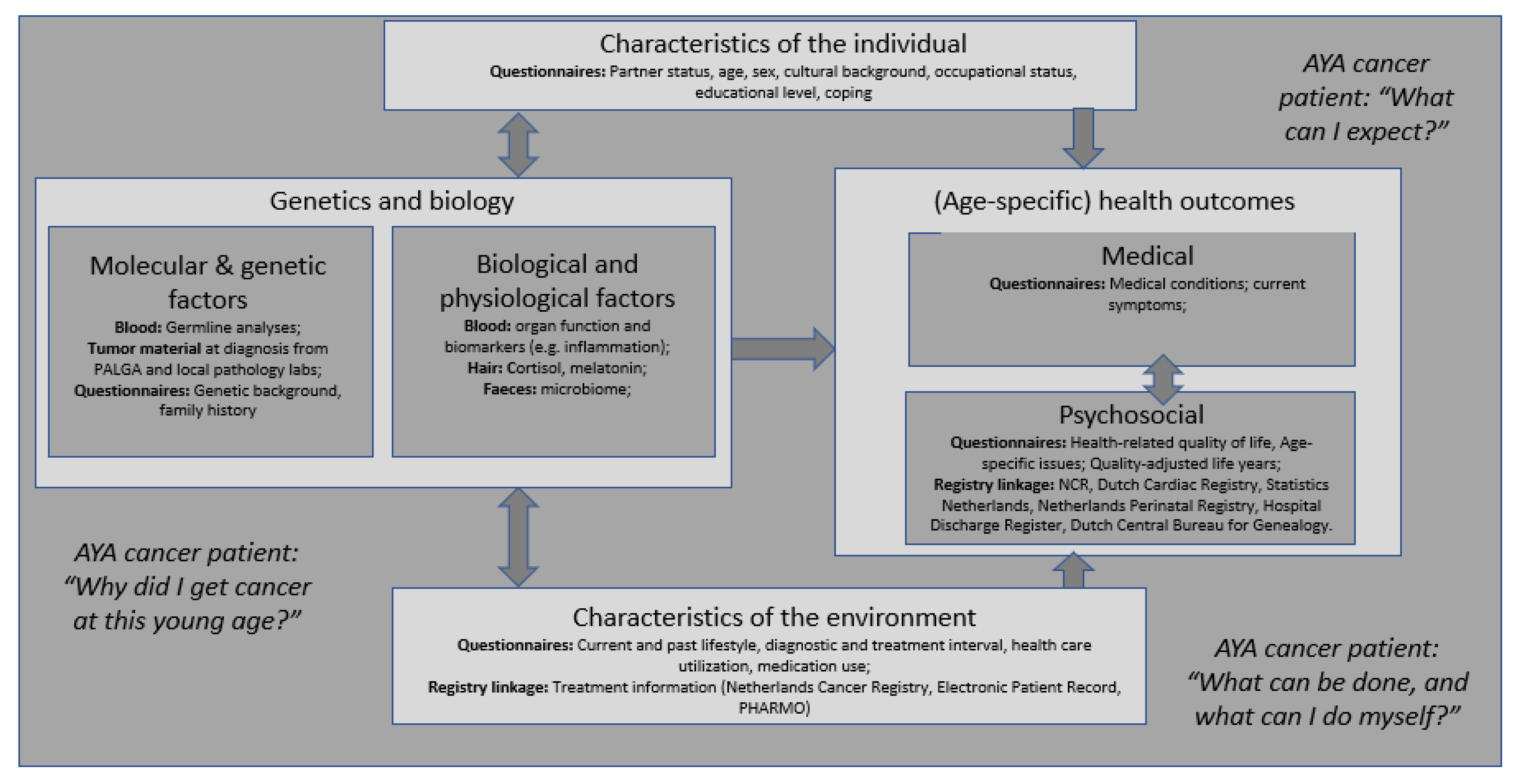
Comprehensive Assessment of Incidence, Risk Factors, and Mechanisms of Impaired Medical and Psychosocial Health Outcomes among Adolescents and Young Adults with Cancer: Protocol of the Prospective Observational COMPRAYA Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
- -
- -
- -
- Distinct age-related physiology, pharmacology, and genomic properties with respect to cancer susceptibility and treatment [9];
- -
- Unequal access to and low participation rates in clinical trials [1];
- -
- -
- Adolescence and emerging and young adulthood are complex phases of life due to the many physical, emotional, cognitive, and social transitions [31]. Important developmental tasks need to be achieved, such as forming one’s own identity and a healthy body image, establishing autonomy, responsibility and independence, finishing education and starting a career, starting a relationship and having children [31]. A cancer diagnosis challenges AYAs’ abilities to achieve these developmental milestones [32,33]. The way in which AYA cancer patients adjust to their cancer experience might have life-long implications for the quality of their survival [34,35,36,37].
2. Methods and Analysis
2.1. Objectives
2.1.1. Primary Objective
- -
- To identify individual, environmental, biological, and psychological characteristics of AYA cancer patients who are at high risk for impaired medical and psychosocial health outcomes. In other words, to develop a prediction model for impaired medical and psychosocial health outcomes (at baseline and at 2-, 5- and 10-year follow-up).
2.1.2. Secondary Objective(s)
- -
- To assess the incidence of impaired (age-specific) medical (e.g., second tumor) and psychosocial (e.g., social isolation) health outcomes at each time-point (at baseline and at 2-, 5- and 10-year follow-up). We aim to compare some of the patient-reported outcomes and medical registry outcomes of AYA with those of control groups available to get a better idea of the impact of cancer on AYA.
2.1.3. Exploratory Objective(s)
- -
- To analyze the course of medical and psychosocial health outcomes over time (all timepoints needed);
- -
- To analyze mediating mechanisms associated with impaired health outcomes in AYA cancer patients (at baseline and at 2-, 5- and 10-year follow-up).
2.1.4. Other Objective(s)
- -
- To form a prospective observational cohort of patients diagnosed with cancer at AYA age, and follow them over time until death.
2.2. Study Population
2.2.1. Inclusion Criteria
- -
- Pathological-confirmed cancer diagnosis;
- -
- Age 18–39 years at time of first cancer diagnosis;
- -
- Able to understand the informed consent form;
- -
- Willing to provide written informed consent.
2.2.2. Exclusion Criteria
- -
- Mentally incompetent patients based on the opinion of treating physician;
- -
- Inability to understand the Dutch language;
- -
- Life expectancy less than 6 months based on the opinion of treating physician. Patients living with an uncertain or poor cancer prognosis have the option to participate in the INVAYA study. This qualitative interview study primarily aims to get a better understanding of the experiences and needs of AYA with life-limiting cancer in daily life and in the healthcare system, through the lens of the AYA themselves, the informal caregiver, and the healthcare professional [16].
2.3. Data Collection
2.4. Measures
2.4.1. Medical and Psychosocial Health Outcomes (Questionnaires and Registries)
AYA Impact of Cancer
Health-Related Quality of Life
Psychological Distress
Medical History/Conditions
Costs Related to Productivity and Medical Consumption
Registry Linkage
2.4.2. Characteristics of the Individual and Environment
Clinical and Health Care Characteristics (Registries, Medical Records, and Questionnaires)
2.4.3. Psychosocial Characteristics (Questionnaire)
Coping Style
Resilience
Illness Perceptions
Autonomy
Spirituality
2.4.4. Lifestyle and Other Environmental Exposures (Questionnaire)
2.4.5. Genetics and Biology
Genetic Background and Family History (Questionnaire)
Special Phenotypic Features of the Patient (Questionnaire)
Tumor Material (PALGA Linkage)
Blood (Hospital Visit)
- (1)
- Germline DNA for research on cancer susceptibility genes, SNP-array, methylation profile, and telomere length (separate informed consent from patient for this part);
- (2)
- Biomarkers of impaired health outcomes (metabolic syndrome, markers of inflammation, fertility hormones, methylation profile, markers of the senescence-associated secretory phenotype (SASP), biochemical markers for cardiovascular damage, and telomere length assessed in white blood cell DNA as a measure of ageing).
Feces
Hair
2.4.6. Physiological Characteristics (Hospital)
Bioimpedence
Grip Strength (GS)
2.5. Statistical Analysis and Power Calculation
2.5.1. Sample Size
2.5.2. Statistical Analyses
Primary Endpoint
Secondary
Exploratory
3. Discussion
3.1. Limitations
3.1.1. Anticipated Uptake and Representativeness Study Sample
3.1.2. Logistical Challenges
3.2. Strengths and Opportunities
3.2.1. Future Innovative Cohort Multiple-Randomized Controlled Trial
3.2.2. Accessibility Data, (Inter)national Collaboration, Continuity Research
3.2.3. COMPRAYA Patient Platform
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meeneghan, M.R.; Wood, W.A. Challenges for cancer care delivery to adolescents and young adults: Present and future. Acta Haematol. 2014, 132, 414–422. [Google Scholar] [CrossRef]
- Adolescent and Young Adult Oncology Review Group. Closing the Gap: Research and Care Imperatives for Adolescents and Young Adults with Cancer; National Institute of Health, National Cancer Institute, and Livestrong Young Ault Alliance: Bethesda, MD, USA, 2006.
- Lewis, D.R.; Seibel, N.L.; Smith, A.W.; Stedman, M.R. Adolescent and young adult cancer survival. J. Natl. Cancer Inst. Monogr. 2014, 49, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.W.; Seibel, N.L.; Lewis, D.R.; Albritton, K.H.; Blair, D.F.; Blanke, C.D.; Bleyer, W.A.; Freyer, D.R.; Geiger, A.M.; Hayes-Lattin, B.; et al. Next steps for adolescent and young adult oncology workshop: An update on progress and recommendations for the future. Cancer Am. Cancer Soc. 2016, 122, 988–999. [Google Scholar] [CrossRef]
- Marshall, S.; Grinyer, A.; Limmer, M. The ‘lost tribe’ reconsidered: Teenagers and young adults treated for cancer in adult settings in the UK. Eur. J. Oncol. Nurs. 2018, 33, 85–90. [Google Scholar] [CrossRef]
- Michelagnoli, M.P.; Pritchard, J.; Phillips, M.B. Adolescent oncology—A homeland for the “lost tribe”. Eur. J. Cancer 2003, 39, 2571–2572. [Google Scholar] [CrossRef] [PubMed]
- Netherlands Cancer Registry. 2021. Available online: www.cijfersoverkanker.nl (accessed on 12 April 2021).
- Aben, K.K.; van Gaal, C.; van Gils, N.A.; van der Graaf, W.T.; Zielhuis, G.A. Cancer in adolescents and young adults (15–29 years): A population-based study in the Netherlands 1989–2009. Acta Oncol. 2012, 51, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Bleyer, A.; Barr, R.; Hayes-Lattin, B.; Thomas, D.; Ellis, C.; Anderson, B.; on behalf of the Biology and Clinical Trials Subgroups of the US National Cancer Institute Progress Review Group in Adolescent and Young Adult Oncology. The distinctive biology of cancer in adolescents and young adults. Nat. Rev. Cancer 2008, 8, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Trama, A.; Botta, L.; Foschi, R.; Ferrari, A.; Stiller, C.; Desandes, E.; Maule, M.M.; Merletti, F.; Gatta, G.; Group, E.-W. Survival of European adolescents and young adults diagnosed with cancer in 2000–2007: Population-based data from EUROCARE-5. Lancet Oncol. 2016, 17, 896–906. [Google Scholar] [CrossRef]
- Keegan, T.H.; Ries, L.A.; Barr, R.D.; Geiger, A.M.; Dahlke, D.V.; Pollock, B.H.; Bleyer, W.A.; for the National Cancer Institute Next Steps for Adolescent and Young Adult Oncology Epidemiology Working Group. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer 2016, 122, 1009–1016. [Google Scholar] [CrossRef]
- Bleyer, A.; Budd, T.; Montello, M. Adolescents and young adults with cancer: The scope of the problem and criticality of clinical trials. Cancer 2006, 107, 1645–1655. [Google Scholar] [CrossRef]
- Barr, R.D.; Ries, L.A.; Lewis, D.R.; Harlan, L.C.; Keegan, T.H.; Pollock, B.H.; Bleyer, W.A.; for the US National Cancer Institute Science of Adolescent and Young Adult Oncology Epidemiology Working Group. Incidence and incidence trends of the most frequent cancers in adolescent and young adult Americans, including “nonmalignant/noninvasive” tumors. Cancer 2016, 122, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Trama, A.; Bernasconi, A.; McCabe, M.G.; Guevara, M.; Gatta, G.; Botta, L.; Group, R.A.W.; Ries, L.; Bleyer, A. Is the cancer survival improvement in European and American adolescent and young adults still lagging behind that in children? Pediatr. Blood Cancer 2019, 66, e27407. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, D.J.; Karim-Kos, H.E.; van der Mark, M.; Aben, K.K.H.; Bijlsma, R.M.; Rijneveld, A.W.; van der Graaf, W.T.A.; Husson, O. Incidence, Survival, and Mortality Trends of Cancers Diagnosed in Adolescents and Young Adults (15–39 Years): A Population-Based Study in The Netherlands 1990–2016. Cancers 2020, 12, 3421. [Google Scholar] [CrossRef] [PubMed]
- Burgers, V.W.G.; van der Graaf, W.T.A.; van der Meer, D.J.; McCabe, M.G.; Rijneveld, A.W.; van den Bent, M.J.; Husson, O. Adolescents and Young Adults Living with an Uncertain or Poor Cancer Prognosis: The “New” Lost Tribe. J. Natl. Compr. Cancer Netw. 2021, 19, 240–246. [Google Scholar] [CrossRef]
- De, R.; Sutradhar, R.; Kurdyak, P.; Aktar, S.; Pole, J.D.; Baxter, N.; Nathan, P.C.; Gupta, S. Incidence and Predictors of Mental Health Outcomes Among Survivors of Adolescent and Young Adult Cancer: A Population-Based Study Using the IMPACT Cohort. J. Clin. Oncol. 2021, 39, 1010–1019. [Google Scholar] [CrossRef]
- Fidler, M.M.; Reulen, R.C.; Bright, C.J.; Henson, K.E.; Kelly, J.S.; Jenney, M.; Ng, A.; Whelan, J.; Winter, D.L.; Frobisher, C.; et al. Respiratory mortality of childhood, adolescent and young adult cancer survivors. Thorax 2018, 73, 959–968. [Google Scholar] [CrossRef]
- Keegan, T.H.M.; Bleyer, A.; Rosenberg, A.S.; Li, Q.; Goldfarb, M. Second Primary Malignant Neoplasms and Survival in Adolescent and Young Adult Cancer Survivors. JAMA Oncol. 2017, 3, 1554–1557. [Google Scholar] [CrossRef]
- Keegan, T.H.M.; Li, Q.; Steele, A.; Alvarez, E.M.; Brunson, A.; Flowers, C.R.; Glaser, S.L.; Wun, T. Sociodemographic disparities in the occurrence of medical conditions among adolescent and young adult Hodgkin lymphoma survivors. Cancer Causes Control 2018, 29, 551–561. [Google Scholar] [CrossRef]
- John, T.D.; Sender, L.S.; Bota, D.A. Cognitive Impairment in Survivors of Adolescent and Early Young Adult Onset Non-CNS Cancers: Does Chemotherapy Play a Role? J. Adolesc. Young Adult Oncol. 2016, 5, 226–231. [Google Scholar] [CrossRef]
- Hayashi, R.J. Adolescent and young adult cancer survivorship: The new frontier for investigation. Cancer 2019, 125, 1976–1978. [Google Scholar] [CrossRef]
- Woodward, E.; Jessop, M.; Glaser, A.; Stark, D. Late effects in survivors of teenage and young adult cancer: Does age matter? Ann. Oncol. 2011, 22, 2561–2568. [Google Scholar] [CrossRef]
- Dommett, R.M.; Redaniel, M.T.; Stevens, M.C.; Hamilton, W.; Martin, R.M. Features of cancer in teenagers and young adults in primary care: A population-based nested case-control study. Br. J. Cancer 2013, 108, 2329–2333. [Google Scholar] [CrossRef]
- Fern, L.A.; Birch, R.; Whelan, J.; Cooke, M.; Sutton, S.; Neal, R.D.; Gerrand, C.; Hubbard, G.; Smith, S.; Lethaby, C.; et al. Why can’t we improve the timeliness of cancer diagnosis in children, teenagers, and young adults? BMJ 2013, 347, f6493. [Google Scholar] [CrossRef]
- Tricoli, J.V.; Blair, D.G.; Anders, C.K.; Bleyer, W.A.; Boardman, L.A.; Khan, J.; Kummar, S.; Hayes-Lattin, B.; Hunger, S.P.; Merchant, M.; et al. Biologic and clinical characteristics of adolescent and young adult cancers: Acute lymphoblastic leukemia, colorectal cancer, breast cancer, melanoma, and sarcoma. Cancer 2016, 122, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Tricoli, J.V.; Seibel, N.L.; Blair, D.G.; Albritton, K.; Hayes-Lattin, B. Unique characteristics of adolescent and young adult acute lymphoblastic leukemia, breast cancer, and colon cancer. J. Natl. Cancer Inst. 2011, 103, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Potosky, A.L.; Harlan, L.C.; Albritton, K.; Cress, R.D.; Friedman, D.L.; Hamilton, A.S.; Kato, I.; Keegan, T.H.; Keel, G.; Schwartz, S.M.; et al. Use of appropriate initial treatment among adolescents and young adults with cancer. J. Natl. Cancer Inst. 2014, 106, dju300. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.D.; Ferrari, A.; Ries, L.; Whelan, J.; Bleyer, W.A. Cancer in Adolescents and Young Adults: A Narrative Review of the Current Status and a View of the Future. JAMA Pediatr. 2016, 170, 495–501. [Google Scholar] [CrossRef]
- Ferrari, A.; Stark, D.; Peccatori, F.A.; Fern, L.; Laurence, V.; Gaspar, N.; Bozovic-Spasojevic, I.; Smith, O.; de Munter, J.; Derwich, K.; et al. Adolescents and young adults (AYA) with cancer: A position paper from the AYA Working Group of the European Society for Medical Oncology (ESMO) and the European Society for Paediatric Oncology (SIOPE). ESMO Open 2021, 6, 100096. [Google Scholar] [CrossRef]
- Zebrack, B.J. Psychological, social, and behavioral issues for young adults with cancer. Cancer-Am. Cancer Soc. 2011, 117, 2289–2294. [Google Scholar] [CrossRef]
- Zebrack, B.; Isaacson, S. Psychosocial care of adolescent and young adult patients with cancer and survivors. J. Clin. Oncol. 2012, 30, 1221–1226. [Google Scholar] [CrossRef]
- Sodergren, S.C.; Husson, O.; Rohde, G.E.; Tomasewska, I.M.; Vivat, B.; Yarom, N.; Griffiths, H.; Darlington, A.S. A Life Put on Pause: An Exploration of the Health-Related Quality of Life Issues Relevant to Adolescents and Young Adults with Cancer. J. Adolesc. Young Adult Oncol. 2018, 7, 453–464. [Google Scholar] [CrossRef]
- Husson, O.; Huijgens, P.C.; van der Graaf, W.T.A. Psychosocial challenges and health-related quality of life of adolescents and young adults with hematologic malignancies. Blood 2018, 132, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Husson, O.; Zebrack, B.J.; Block, R.; Embry, L.; Aguilar, C.; Hayes-Lattin, B.; Cole, S. Health-Related Quality of Life in Adolescent and Young Adult Patients with Cancer: A Longitudinal Study. J. Clin. Oncol. 2017, 35, 652–659. [Google Scholar] [CrossRef]
- Greup, S.R.; Kaal, S.E.J.; Jansen, R.; Manten-Horst, E.; Thong, M.S.Y.; van der Graaf, W.T.A.; Prins, J.B.; Husson, O. Post-Traumatic Growth and Resilience in Adolescent and Young Adult Cancer Patients: An Overview. J. Adolesc. Young Adult Oncol. 2018, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Harju, E.; Roser, K.; Dehler, S.; Michel, G. Health-related quality of life in adolescent and young adult cancer survivors. Support. Care Cancer 2018, 26, 3099–3110. [Google Scholar] [CrossRef]
- Robison, L.L.; Mertens, A.C.; Boice, J.D.; Breslow, N.E.; Donaldson, S.S.; Green, D.M.; Li, F.P.; Meadows, A.T.; Mulvihill, J.J.; Neglia, J.P.; et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: A multi-institutional collaborative project. Med. Pediatr. Oncol. 2002, 38, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Teepen, J.C.; van Leeuwen, F.E.; Tissing, W.J.; van Dulmen-den Broeder, E.; van den Heuvel-Eibrink, M.M.; van der Pal, H.J.; Loonen, J.J.; Bresters, D.; Versluys, B.; Neggers, S.; et al. Long-Term Risk of Subsequent Malignant Neoplasms After Treatment of Childhood Cancer in the DCOG LATER Study Cohort: Role of Chemotherapy. J. Clin. Oncol. 2017, 35, 2288–2298. [Google Scholar] [CrossRef]
- van de Poll-Franse, L.V.; Horevoorts, N.; van Eenbergen, M.; Denollet, J.; Roukema, J.A.; Aaronson, N.K.; Vingerhoets, A.; Coebergh, J.W.; de Vries, J.; Essink-Bot, M.L.; et al. The Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship registry: Scope, rationale and design of an infrastructure for the study of physical and psychosocial outcomes in cancer survivorship cohorts. Eur. J. Cancer 2011, 47, 2188–2194. [Google Scholar] [CrossRef]
- Wilson, I.B.; Cleary, P.D. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA 1995, 273, 59–65. [Google Scholar] [CrossRef]
- Ferrans, C.E.; Zerwic, J.J.; Wilbur, J.E.; Larson, J.L. Conceptual model of health-related quality of life. J. Nurs. Sch. 2005, 37, 336–342. [Google Scholar] [CrossRef]
- Sprangers, M.A.; Sloan, J.A.; Barsevick, A.; Chauhan, C.; Dueck, A.C.; Raat, H.; Shi, Q.; Van Noorden, C.J.; Consortium, G. Scientific imperatives, clinical implications, and theoretical underpinnings for the investigation of the relationship between genetic variables and patient-reported quality-of-life outcomes. Qual. Life Res. 2010, 19, 1395–1403. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bellizzi, K.M.; Smith, A.; Schmidt, S.; Keegan, T.H.; Zebrack, B.; Lynch, C.F.; Deapen, D.; Shnorhavorian, M.; Tompkins, B.J.; Simon, M.; et al. Positive and negative psychosocial impact of being diagnosed with cancer as an adolescent or young adult. Cancer 2012, 118, 5155–5162. [Google Scholar] [CrossRef]
- Ganz, P.A.; Desmond, K.A.; Leedham, B.; Rowland, J.H.; Meyerowitz, B.E.; Belin, T.R. Quality of life in long-term, disease-free survivors of breast cancer: A follow-up study. J. Natl. Cancer Inst. 2002, 94, 39–49. [Google Scholar] [CrossRef]
- Zebrack, B.J.; Mills, J.; Weitzman, T.S. Health and supportive care needs of young adult cancer patients and survivors. J. Cancer Surviv. Res. Pract. 2007, 1, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Zebrack, B. Developing a new instrument to assess the impact of cancer in young adult survivors of childhood cancer. J. Cancer Surviv. Res. Pract. 2009, 3, 174–180. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Bouwmans, C.; Krol, M.; Brouwer, W.; Severens, J.L.; Koopmanschap, M.A.; Hakkaart, L. IMTA Productivity Cost Questionnaire (IPCQ). Value Health 2014, 17, A550. [Google Scholar] [CrossRef] [PubMed]
- Koopmanschap, M.A. PRODISQ: A modular questionnaire on productivity and disease for economic evaluation studies. Expert Rev. Pharm. Outcomes Res. 2005, 5, 23–28. [Google Scholar] [CrossRef]
- van Herk-Sukel, M.P.; van de Poll-Franse, L.V.; Lemmens, V.E.; Vreugdenhil, G.; Pruijt, J.F.; Coebergh, J.W.; Herings, R.M. New opportunities for drug outcomes research in cancer patients: The linkage of the Eindhoven Cancer Registry and the PHARMO Record Linkage System. Eur. J. Cancer 2010, 46, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Sperling, C.D.; Petersen, G.S.; Holge-Hazelton, B.; Graugaard, C.; Winther, J.F.; Gudmundsdottir, T.; Ahrensberg, J.; Schmiegelow, K.; Boisen, K.A.; Olsen, P.R.; et al. Being Young and Getting Cancer: Development of a Questionnaire Reflecting the Needs and Experiences of Adolescents and Young Adults with Cancer. J. Adolesc. Young Adult Oncol. 2016, 6, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.M.; Fern, L.A.; Solanki, A.; Hooker, L.; Carluccio, A.; Pye, J.; Jeans, D.; Frere-Smith, T.; Gibson, F.; Barber, J.; et al. Development and validation of the BRIGHTLIGHT Survey, a patient-reported experience measure for young people with cancer. Health Qual. Life Outcomes 2015, 13, 107. [Google Scholar] [CrossRef]
- Garnefski, N.; Kraaij, V. Cognitive emotion regulation questionnaire–development of a short 18-item version (CERQ-short). Personal. Individ. Differ. 2006, 41, 1045–1053. [Google Scholar] [CrossRef]
- Smith, B.; Dalen, J.; Wiggins, K.; Tooley, E.; Christopher, P.; Bernard, J. The Brief Resilience Scale: Assessing the Ability to Bounce Back. Int. J. Behav. Med. 2008, 15, 194–200. [Google Scholar] [CrossRef]
- Broadbent, E.; Petrie, K.J.; Main, J.; Weinman, J. The brief illness perception questionnaire. J. Psychosom. Res. 2006, 60, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Vernooij-Dassen, M.J.; Osse, B.H.; Schade, E.; Grol, R.P. Patient autonomy problems in palliative care: Systematic development and evaluation of a questionnaire. J. Pain Symptom Manag. 2005, 30, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Smeets, W. Het spirituele aspect in het detecteren van psychosociale behoeften in de oncologische praktijk. Psyche Geloof 2010, 21, 178–195. [Google Scholar]
- Wendel-Vos, G.C.; Schuit, A.J.; Saris, W.H.; Kromhout, D. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J. Clin. Epidemiol. 2003, 56, 1163–1169. [Google Scholar] [CrossRef]
- Wright, K.P., Jr.; Drake, A.L.; Frey, D.J.; Fleshner, M.; Desouza, C.A.; Gronfier, C.; Czeisler, C.A. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav. Immun. 2015, 47, 24–34. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using SAS; SAGE Publications Ltd: New York City, NY, USA, 2010; p. 197. [Google Scholar]
- Hayes, A.F.; Rockwood, N.J. Regression-based statistical mediation and moderation analysis in clinical research: Observations, recommendations, and implementation. Behav. Res. Ther. 2017, 98, 39–57. [Google Scholar] [CrossRef]
- Husson, O.; Zebrack, B.J. Perceived impact of cancer among adolescents and young adults: Relationship with health-related quality of life and distress. Psychooncology 2017, 26, 1307–1315. [Google Scholar] [CrossRef]
- Murnane, A.; Gough, K.; Thompson, K.; Holland, L.; Conyers, R. Adolescents and young adult cancer survivors: Exercise habits, quality of life and physical activity preferences. Support. Care Cancer 2015, 23, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Kaal, S.E.J.; Husson, O.; van Duivenboden, S.; Jansen, R.; Manten-Horst, E.; Servaes, P.; Prins, J.B.; van den Berg, S.W.; van der Graaf, W.T.A. Empowerment in adolescents and young adults with cancer: Relationship with health-related quality of life. Cancer 2017, 123, 4039–4047. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.R.; Bona, K.; Wharton, C.M.; Bradford, M.; Shaffer, M.L.; Wolfe, J.; Baker, K.S. Adolescent and Young Adult Patient Engagement and Participation in Survey-Based Research: A Report From the “Resilience in Adolescents and Young Adults with Cancer” Study. Pediatr. Blood Cancer 2016, 63, 734–736. [Google Scholar] [CrossRef] [PubMed]
- Husson, O.; Sodergren, S.C.; Darlington, A.S. The importance of a collaborative health-related quality of life measurement strategy for adolescents and young adults with cancer. Cancer 2021, 127, 1714–1715. [Google Scholar] [CrossRef] [PubMed]
- Dodd, S.; Harman, N.; Taske, N.; Minchin, M.; Tan, T.; Williamson, P.R. Core outcome sets through the healthcare ecosystem: The case of type 2 diabetes mellitus. Trials 2020, 21, 570. [Google Scholar] [CrossRef]
| Screening/ Baseline | Follow-Up 1 Year | Follow-Up 2 Years | Follow-Up 3,4,6,7,8,9 Years | Follow-Up 5 Years | Follow-Up 10 Years | |
|---|---|---|---|---|---|---|
| (profiles only) | (profiles only) | |||||
| Informed consent | X | |||||
| Background characteristics 1 | X | X | X | X | X | X |
| Clinical and treatment characteristics | X | X | X | X | ||
| Genetic background and family history/special phenotypic features | X | |||||
| Lifestyle and other environmental exposures 2 | X | X | X | X | ||
| Medical history/conditions | X | X | X | X | ||
| Physical examination/vital parameters 3 | X | X | X | |||
| Blood sample + standard questionnaire | X | X | X | |||
| Feces sample + standard questionnaire 4 | X | X | X | |||
| Hair sample + standard questionnaire | X | X | X | |||
| Questionnaires | ||||||
| Impact cancer 5 | X | X | X | X | X | X |
| Health-related quality of life 6 | X | X | X | X | X | X |
| Psychological distress 7 | X | X | X | X | X | X |
| Psychosocial characteristics 8 | X | X | X | X | X | |
| Costs related to productivity and medical consumption 9 | X | X | X | X | X | X |
| Food-intake diaries | X | X | X | X | X | |
| Survival and registry linkage | ----------------------------------------------------------------------------------> | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Husson, O.; Ligtenberg, M.J.L.; van de Poll-Franse, L.V.; Prins, J.B.; van den Bent, M.J.; van Eenbergen, M.C.; Fles, R.; Manten-Horst, E.; Gietema, J.A.; van der Graaf, W.T.A. Comprehensive Assessment of Incidence, Risk Factors, and Mechanisms of Impaired Medical and Psychosocial Health Outcomes among Adolescents and Young Adults with Cancer: Protocol of the Prospective Observational COMPRAYA Cohort Study. Cancers 2021, 13, 2348. https://doi.org/10.3390/cancers13102348
Husson O, Ligtenberg MJL, van de Poll-Franse LV, Prins JB, van den Bent MJ, van Eenbergen MC, Fles R, Manten-Horst E, Gietema JA, van der Graaf WTA. Comprehensive Assessment of Incidence, Risk Factors, and Mechanisms of Impaired Medical and Psychosocial Health Outcomes among Adolescents and Young Adults with Cancer: Protocol of the Prospective Observational COMPRAYA Cohort Study. Cancers. 2021; 13(10):2348. https://doi.org/10.3390/cancers13102348
Chicago/Turabian StyleHusson, Olga, Marjolijn J. L. Ligtenberg, Lonneke V. van de Poll-Franse, Judith B. Prins, Martin J. van den Bent, Mies C. van Eenbergen, Renske Fles, Eveliene Manten-Horst, Jourik A. Gietema, and Winette T. A. van der Graaf. 2021. "Comprehensive Assessment of Incidence, Risk Factors, and Mechanisms of Impaired Medical and Psychosocial Health Outcomes among Adolescents and Young Adults with Cancer: Protocol of the Prospective Observational COMPRAYA Cohort Study" Cancers 13, no. 10: 2348. https://doi.org/10.3390/cancers13102348
APA StyleHusson, O., Ligtenberg, M. J. L., van de Poll-Franse, L. V., Prins, J. B., van den Bent, M. J., van Eenbergen, M. C., Fles, R., Manten-Horst, E., Gietema, J. A., & van der Graaf, W. T. A. (2021). Comprehensive Assessment of Incidence, Risk Factors, and Mechanisms of Impaired Medical and Psychosocial Health Outcomes among Adolescents and Young Adults with Cancer: Protocol of the Prospective Observational COMPRAYA Cohort Study. Cancers, 13(10), 2348. https://doi.org/10.3390/cancers13102348