The FIB-4 Index Is a Useful Predictor for the Development of Hepatocellular Carcinoma in Patients with Coexisting Nonalcoholic Fatty Liver Disease and Chronic Hepatitis B
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design
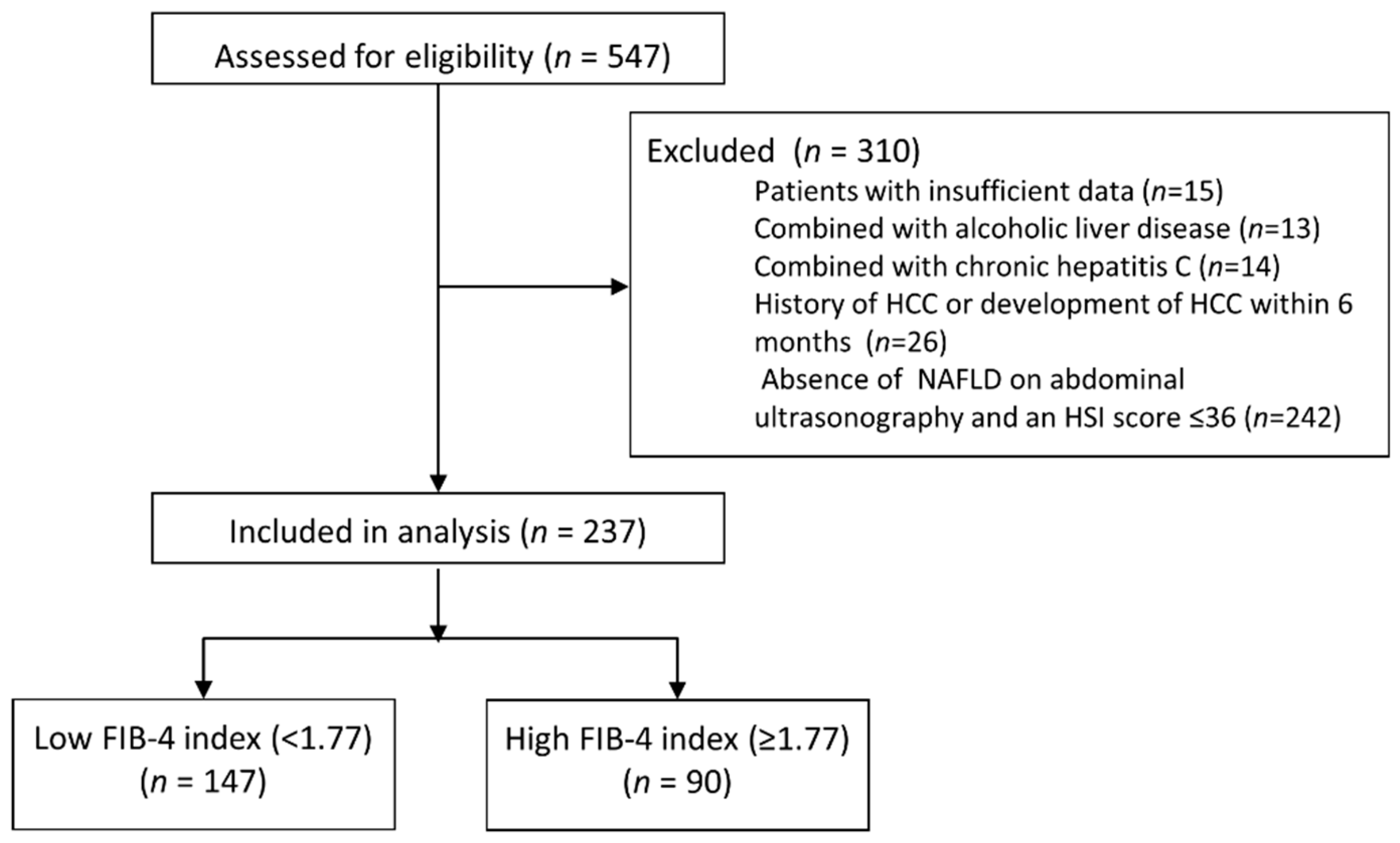
2.2. Patients
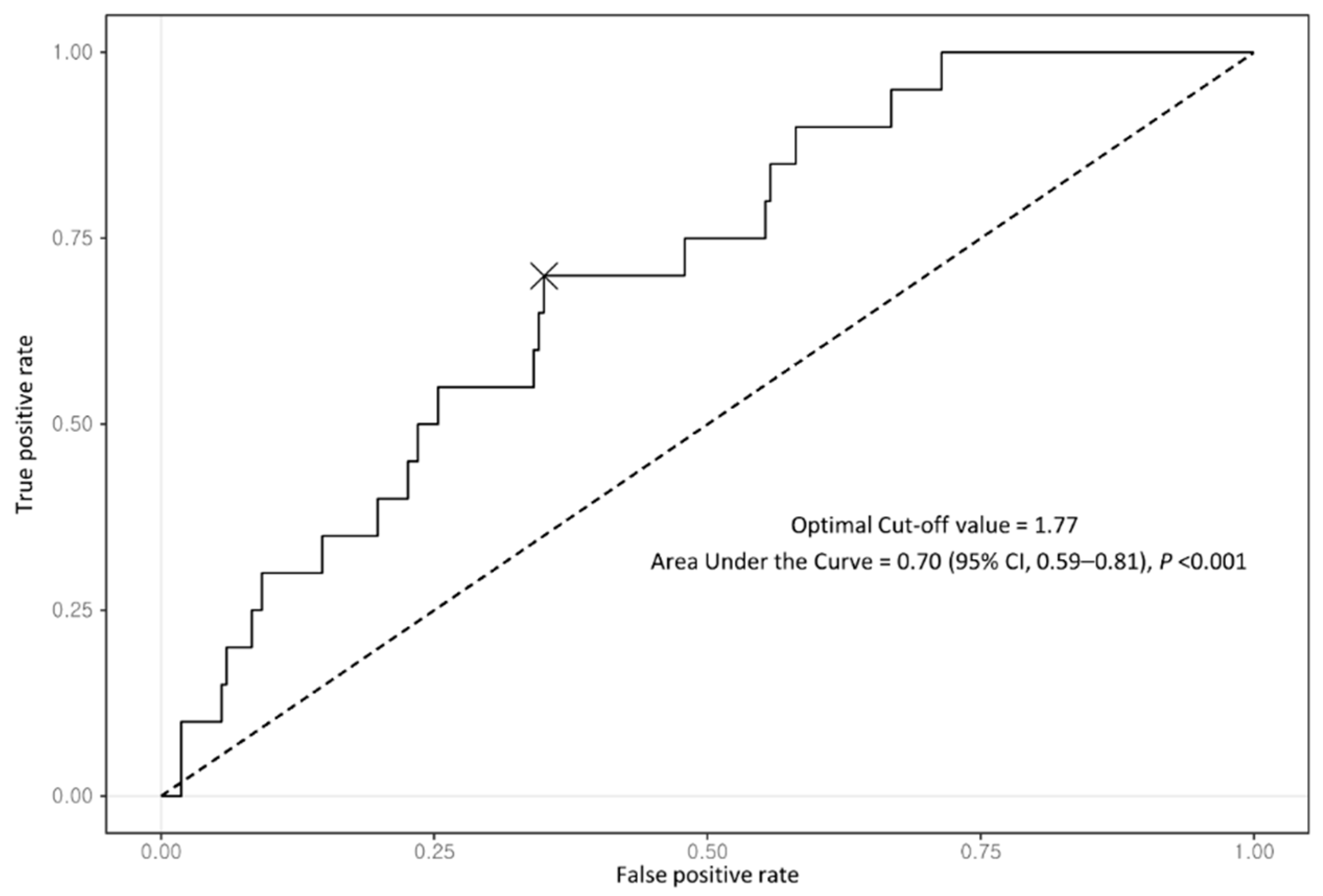
2.3. Statistical Analysis
2.4. Sensitivity Analysis
3. Results
3.1. Baseline Characteristics
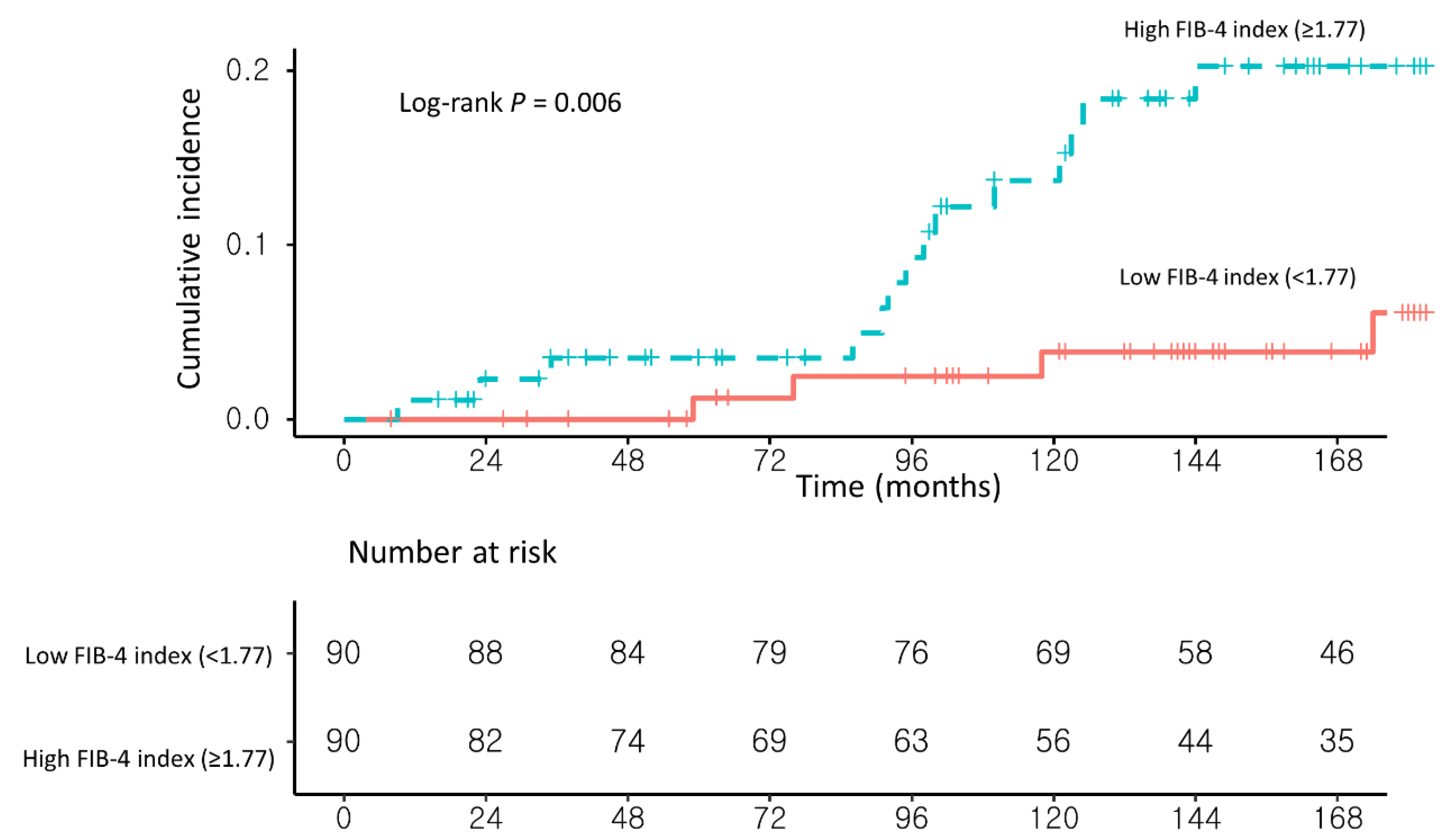
3.2. Risk Factors for Developing HCC in NAFLD–CHB Patients
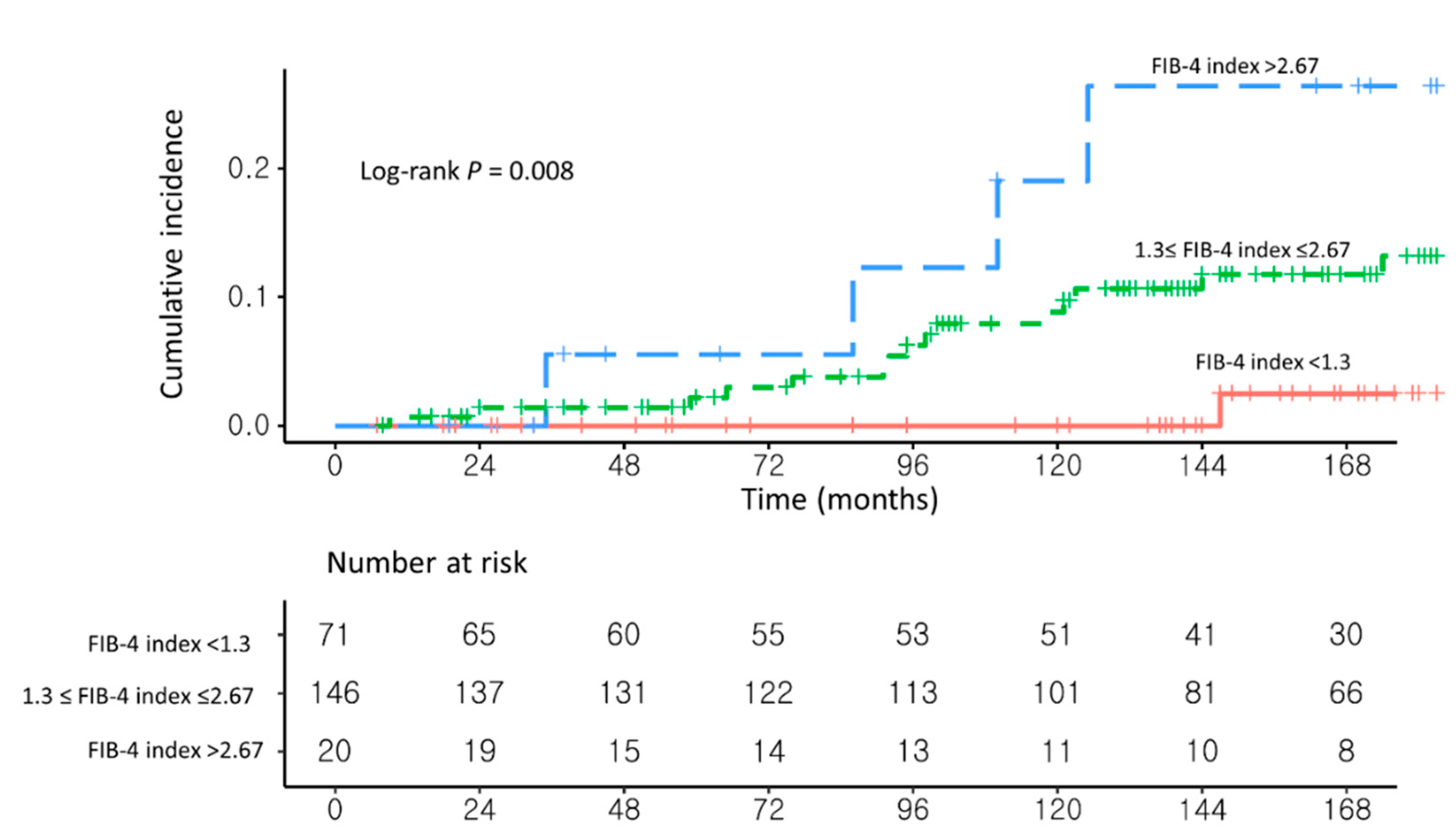
3.3. Sensitivity Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization; Globocan. Liver Fact Sheet. Available online: http://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf. (accessed on 20 February 2021).
- Refolo, M.G.; Messa, C.; Guerra, V.; Carr, B.I.; D’Alessandro, R. Inflammatory Mechanisms of HCC Development. Cancers 2020, 12, 641. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Chan, W.K.; Chitturi, S.; Chawla, Y.; Dan, Y.Y.; Duseja, A.; Fan, J.; Goh, K.L.; Hamaguchi, M.; Hashimoto, E.; et al. Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease guidelines 2017-Part 1: Definition, risk factors and assessment. J. Gastroenterol. Hepatol. 2018, 33, 70–85. [Google Scholar] [CrossRef]
- Younossi, Z.; Tacke, F.; Arrese, M.; Chander Sharma, B.; Mostafa, I.; Bugianesi, E.; Wai-Sun Wong, V.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef]
- Park, S.H.; Plank, L.D.; Suk, K.T.; Park, Y.E.; Lee, J.; Choi, J.H.; Heo, N.Y.; Park, J.; Kim, T.O.; Moon, Y.S.; et al. Trends in the prevalence of chronic liver disease in the Korean adult population, 1998–2017. Clin. Mol. Hepatol. 2020, 26, 209–215. [Google Scholar] [CrossRef] [PubMed]
- MacLachlan, J.H.; Cowie, B.C. Hepatitis B virus epidemiology. Cold Spring Harb. Perspect. Med. 2015, 5, a021410. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.J.; Brouwer, W.P.; Zanjir, W.M.R.; de Man, R.A.; Feld, J.J.; Hansen, B.E.; Janssen, H.L.A.; Patel, K. Nonalcoholic Steatohepatitis Is Associated With Liver-Related Outcomes and All-Cause Mortality in Chronic Hepatitis B. Hepatology 2020, 71, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.W.; Wong, G.L.; Chan, H.Y.; Tong, J.H.; Yu, Y.H.; Choi, P.C.; Chan, H.L.; To, K.F.; Wong, V.W. Concurrent fatty liver increases risk of hepatocellular carcinoma among patients with chronic hepatitis B. J. Gastroenterol. Hepatol. 2017, 32, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.B.; Ha, Y.; Chon, Y.E.; Kim, M.N.; Lee, J.H.; Park, H.; Kim, K.I.; Kim, S.H.; Rim, K.S.; Hwang, S.G. Association between hepatic steatosis and the development of hepatocellular carcinoma in patients with chronic hepatitis B. Clin. Mol. Hepatol. 2019, 25, 52–64. [Google Scholar] [CrossRef]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar]
- Imajo, K.; Kessoku, T.; Honda, Y.; Tomeno, W.; Ogawa, Y.; Mawatari, H.; Fujita, K.; Yoneda, M.; Taguri, M.; Hyogo, H.; et al. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology 2016, 150, 626–637.e627. [Google Scholar] [CrossRef]
- Suh, B.; Park, S.; Shin, D.W.; Yun, J.M.; Yang, H.K.; Yu, S.J.; Shin, C.I.; Kim, J.S.; Ahn, E.; Lee, H.; et al. High liver fibrosis index FIB-4 is highly predictive of hepatocellular carcinoma in chronic hepatitis B carriers. Hepatology 2015, 61, 1261–1268. [Google Scholar] [CrossRef]
- Tseng, T.-C.; Liu, C.-J.; Su, T.-H.; Yang, W.-T.; Chen, C.-L.; Yang, H.-C.; Wang, C.-C.; Kuo, S.F.-T.; Liu, C.-H.; Chen, P.-J.; et al. Fibrosis-4 index helps identify HBV carriers with the lowest risk of hepatocellular carcinoma. Am. J. Gasteroenterol. 2017, 112, 1564–1574. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Lai, H.C.; Hu, T.H.; Su, W.P.; Lu, S.N.; Lin, C.H.; Hung, C.H.; Chuang, P.H.; Wang, J.H.; Lee, M.H.; et al. Stratification of hepatocellular carcinoma risk through modified FIB-4 index in chronic hepatitis B patients on entecavir therapy. J. Gastroenterol. Hepatol. 2019, 34, 442–449. [Google Scholar] [PubMed]
- Kanwal, F.; Kramer, J.R.; Mapakshi, S.; Natarajan, Y.; Chayanupatkul, M.; Richardson, P.A.; Li, L.; Desiderio, R.; Thrift, A.P.; Asch, S.M.; et al. Risk of Hepatocellular Cancer in Patients With Non-Alcoholic Fatty Liver Disease. Gastroenterology 2018, 155, 1828–1837.e1822. [Google Scholar] [CrossRef]
- Alexander, M.; Loomis, A.K.; van der Lei, J.; Duarte-Salles, T.; Prieto-Alhambra, D.; Ansell, D.; Pasqua, A.; Lapi, F.; Rijnbeek, P.; Mosseveld, M.; et al. Risks and clinical predictors of cirrhosis and hepatocellular carcinoma diagnoses in adults with diagnosed NAFLD: Real-world study of 18 million patients in four European cohorts. BMC Med. 2019, 17, 95. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, D.; Kim, H.J.; Lee, C.-H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.-H.; Cho, S.-H.; Sung, M.-W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Korean Association for the Study of the Liver. Clinical practice guidelines for management of chronic hepatitis B. Clin. Mol. Hepatol. 2019, 25, 93. [Google Scholar] [CrossRef]
- Terrault, N.A.; Lok, A.S.; McMahon, B.J.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar]
- European Association for the Study of the Liver. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. J. Hepatol. 2018, 69, 154–181. [Google Scholar]
- Haukoos, J.S.; Lewis, R.J. The Propensity Score. JAMA 2015, 314, 1637–1638. [Google Scholar] [CrossRef]
- Yang, J.D.; Kim, W.R.; Coelho, R.; Mettler, T.A.; Benson, J.T.; Sanderson, S.O.; Therneau, T.M.; Kim, B.; Roberts, L.R. Cirrhosis is present in most patients with hepatitis B and hepatocellular carcinoma. Clin. Hepatol. Gastroenterol. 2011, 9, 64–70. [Google Scholar] [CrossRef]
- Fattovich, G.; Bortolotti, F.; Donato, F. Natural history of chronic hepatitis B: Special emphasis on disease progression and prognostic factors. J. Hepatol. 2008, 48, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Corey, K.E.; Alkhouri, N.; Noureddin, M.; Jacobson, I.; Lam, B.; Clement, S.; Basu, R.; Gordon, S.C.; Ravendhra, N.; et al. Clinical assessment for high-risk patients with non-alcoholic fatty liver disease in primary care and diabetology practices. Aliment. Pharmacol. Ther. 2020, 52, 513–526. [Google Scholar] [CrossRef]
- Shah, A.G.; Lydecker, A.; Murray, K.; Tetri, B.N.; Contos, M.J.; Sanyal, A.J.; Nash Clinical Research, N. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2009, 7, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Seko, Y.; Yano, K.; Takahashi, A.; Okishio, S.; Kataoka, S.; Okuda, K.; Umemura, A.; Yamaguchi, K.; Moriguchi, M.; Tanaka, S.; et al. The Appropriate Opportunity for Evaluating Liver Fibrosis by Using the FIB-4 Index in Patients with Nonalcoholic Fatty Liver Disease in Japan. Diagnostics 2020, 10, 842. [Google Scholar] [CrossRef] [PubMed]
- Macías, J.; Girón-González, J.A.; González-Serrano, M.; Merino, D.; Cano, P.; Mira, J.A.; Arizcorreta-Yarza, A.; Ruíz-Morales, J.; Lomas-Cabeza, J.M.; García-García, J.A.; et al. Prediction of liver fibrosis in human immunodeficiency virus/hepatitis C virus coinfected patients by simple non-invasive indexes. Gut 2006, 55, 409–414. [Google Scholar] [CrossRef]
- Fontana, R.J.; Lok, A.S.F. Noninvasive monitoring of patients with chronic hepatitis C. Hepatology 2002, 36, S57–S64. [Google Scholar]
- Calès, P.; Oberti, F.; Michalak, S.; Hubert-Fouchard, I.; Rousselet, M.C.; Konaté, A.; Gallois, Y.; Ternisien, C.; Chevailler, A.; Lunel, F. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology 2005, 42, 1373–1381. [Google Scholar] [CrossRef]
- Yoneda, M.; Imajo, K.; Takahashi, H.; Ogawa, Y.; Eguchi, Y.; Sumida, Y.; Yoneda, M.; Kawanaka, M.; Saito, S.; Tokushige, K.; et al. Clinical strategy of diagnosing and following patients with nonalcoholic fatty liver disease based on invasive and noninvasive methods. J. Gastroenterol. 2018, 53, 181–196. [Google Scholar] [CrossRef]
- Davyduke, T.; Tandon, P.; Al-Karaghouli, M.; Abraldes, J.G.; Ma, M.M. Impact of Implementing a “FIB-4 First” Strategy on a Pathway for Patients With NAFLD Referred From Primary Care. Hepatol. Commun. 2019, 3, 1322–1333. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.R.; Berg, T.; Asselah, T.; Flisiak, R.; Fung, S.; Gordon, S.C.; Janssen, H.L.; Lampertico, P.; Lau, D.; Bornstein, J.D.; et al. Evaluation of APRI and FIB-4 scoring systems for non-invasive assessment of hepatic fibrosis in chronic hepatitis B patients. J. Hepatol. 2016, 64, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [PubMed]
- Fattovich, G.; Stroffolini, T.; Zagni, I.; Donato, F. Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology 2004, 127, S35–S50. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Friedman, S.L.; Goossens, N.; Hoshida, Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J. Hepatol. 2018, 68, 526–549. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, L.; Leung, L.; Yen, T.S.; Lee, C.; Chan, J.Y. Liver-specific inactivation of the Nrf1 gene in adult mouse leads to nonalcoholic steatohepatitis and hepatic neoplasia. Proc. Natl. Acad Sci. USA 2005, 102, 4120–4125. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2017, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Kamada, Y.; Matsumoto, H.; Tamura, S.; Fukushima, J.; Kiso, S.; Fukui, K.; Igura, T.; Maeda, N.; Kihara, S.; Funahashi, T.; et al. Hypoadiponectinemia accelerates hepatic tumor formation in a nonalcoholic steatohepatitis mouse model. J. Hepatol. 2007, 47, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Ponziani, F.R.; Bhoori, S.; Castelli, C.; Putignani, L.; Rivoltini, L.; Del Chierico, F.; Sanguinetti, M.; Morelli, D.; Paroni Sterbini, F.; Petito, V.; et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology 2019, 69, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Malehmir, M.; Pfister, D.; Gallage, S.; Szydlowska, M.; Inverso, D.; Kotsiliti, E.; Leone, V.; Peiseler, M.; Surewaard, B.G.; Rath, D.; et al. Platelet GPIbα is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat. Med. 2019, 25, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Makary, M.S.; Khandpur, U.; Cloyd, J.M.; Mumtaz, K.; Dowell, J.D. Locoregional therapy approaches for hepatocellular carcinoma: Recent advances and management strategies. Cancers 2020, 12, 1914. [Google Scholar] [CrossRef] [PubMed]
| Variables | Before Propensity Score Matching | After Propensity Score Matching * | |||||
|---|---|---|---|---|---|---|---|
| Total Population (n = 237) | Low FIB-4 Index | High FIB-4 Index | p Value | Low FIB-4 Index | High FIB-4 Index | p Value | |
| (n = 147) | (n = 90) | (n = 90) | (n = 90) | ||||
| Male (vs. female) | 214 (90.3%) | 137 (93.2%) | 77 (85.6%) | 0.09 | 83 (92.2%) | 77 (85.6%) | 0.24 |
| Diabetes mellitus (yes vs. no) | 55 (23.2%) | 36 (24.5%) | 19 (21.1%) | 0.66 | 27 (30.0%) | 19 (21.1%) | 0.13 |
| Hypertension (yes vs. no) | 52(21.9%) | 28 (19.0%) | 24 (26.7%) | 0.22 | 23 (25.6%) | 24 (26.7%) | 1.00 |
| USG-LC (yes vs. no) | 37 (15.6%) | 18 (12.2%) | 19 (21.1%) | 0.1 | 16 (17.8%) | 19 (21.1%) | 0.85 |
| Body mass index (kg/m2) | 25.0 (25.0–26.8) | 25.0 (25.0–26.6) | 25.0 (25.0–27.2) | 0.56 | 25.0 (24.7–26.3) | 25.0 (25.0–27.2) | 0.37 |
| Antiviral treatment at baseline (yes vs. no) | 84 (35.4%) | 42 (28.6%) | 42 (46.7%) | 0.007 | 33 (36.7%) | 42 (46.7%) | 0.23 |
| Antiviral treatment after baseline (yes vs. no) | 132 (55.7%) | 74 (50.3%) | 58 (64.4%) | 0.047 | 51 (56.7%) | 58 (64.4%) | 0.29 |
| Aspirin use (yes vs. no) | 42 (17.7%) | 25 (32.9%) | 17 (18.9%) | 0.85 | 20 (22.2%) | 17 (18.9%) | 0.71 |
| Statin use (yes vs. no) | 49 (20.7%) | 32 (21.8%) | 17 (18.9%)) | 0.71 | 20 (22.2%) | 17 (18.9%)) | 0.47 |
| HBeAg positivity (yes vs. no) | 121 (51.1%) | 74 (50.3%) | 47 (52.2%) | 0.88 | 45 (50.0%) | 47 (52.2%) | 1 |
| HBV DNA > 2000 IU/mL (yes vs. no) | 96 (40.5%) | 61 (41.5%) | 140 (22.2%) | <0.001 | 38 (42.2%) | 35 (38.9%) | 0.65 |
| Total bilirubin (IU/L) | 0.8 (0.6–1.0) | 0.8 (0.6–1.0) | 0.8 (0.6–1.1) | 0.26 | 0.8 (0.6–1.1) | 0.8 (0.6–1.1) | 0.23 |
| Cholesterol (mg/dL) | 171.0 (151.0–194.5) | 172.0 (150.0–194.5) | 168.0 (151.0–193.0) | 0.77 | 168.0 (150.0–193.0) | 168.0 (151.0–193.0) | 0.82 |
| FIB-4 index | 1.6 (1.2–2.0) | 1.3 (1.1–1.5) | 2.2 (1.9–2.6) | <0.001 | 1.4 (1.2–1.6) | 2.2 (1.9–2.6) | <0.001 |
| Age (years) | 46.0 (39.0–52.0) | 43.0 (36.5–49.0) | 51.5 (47.0–55.0) | <0.001 | 46.5 (42.0–52.0) | 51.5 (47.0–55.0) | <0.001 |
| AST (IU/L) | 30.0 (23.0–46.0) | 25.0 (20.5–31.0) | 49.0 (36.0–67.0) | <0.001 | 24.0 (20.0–30.0) | 49.0 (36.0–67.0) | <0.001 |
| ALT (IU/L) | 41.0 (28.0–70.0) | 35.0 (26.0–51.0) | 63.5 (35.0–94.0) | <0.001 | 34.5 (25.0–48.0) | 63.5 (35.0–94.0) | <0.001 |
| Platelet (109/L) | 182.0 (150.0–220.0) | 197.0 (161.5–231.5) | 156.5 (121.0–197.0) | <0.001 | 193.0 (155.0–224.0) | 156.5 (121.0–197.0) | 0.001 |
| Variables | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | aHR (95% CI) | p Value | |
| Male (vs. female) | 1.32 (0.17–9.99) | 0.78 | – | – |
| Age (years) | 1.00 (0.95–1.07) | 0.88 | – | – |
| Diabetes mellitus (yes vs. no) | 1.65 (0.62–4.41) | 0.31 | – | – |
| USG-LC (yes vs. no) | 7.59 (2.94–19.59) | <0.001 | 7.84 (3.03–20.28) | <0.001 |
| Body mass index ≥25 (vs. <25) | 1.55 (0.61–3.93) | 0.35 | – | – |
| Antiviral treatment at baseline (yes vs. no) | 0.65 (0.24–1.73) | 0.39 | – | – |
| Antiviral treatment after baseline (yes vs. no) | 2.02 (0.67–6.15) | 0.21 | – | – |
| Aspirin use (yes vs. no) | 0.21 (0.03–1.57) | 0.13 | – | – |
| Statin use (yes vs. no) | 0.20 (0.02–1.54) | 0.12 | – | – |
| HBeAg positivity (yes vs. no) | 0.93 (0.37–2.33) | 0.87 | – | – |
| HBV DNA >2000 IU/mL (yes vs. no) | 0.63 (0.24–1.67) | 0.35 | – | – |
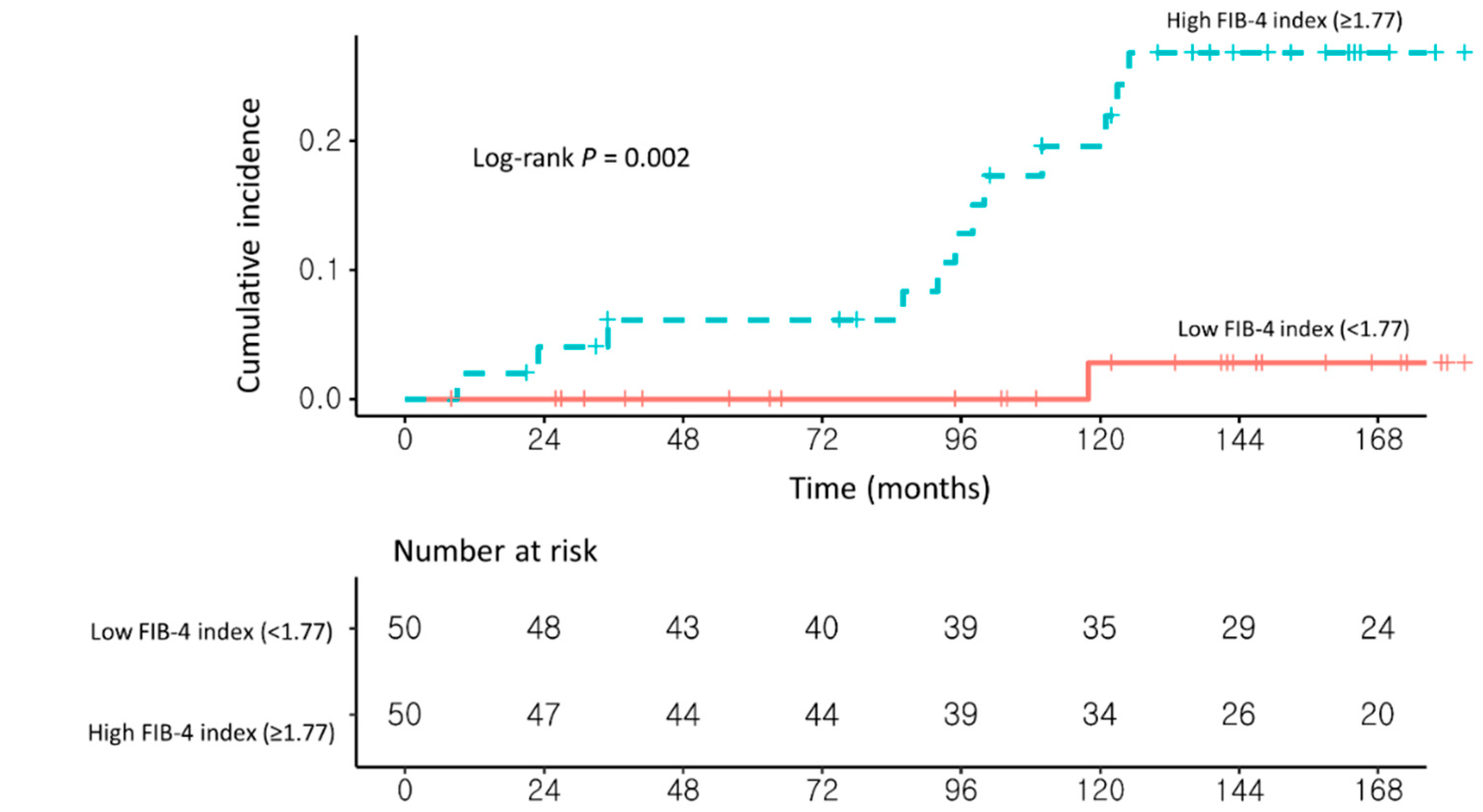
| High FIB-4 index (≥1.77) (vs. <1.77) | 4.16 (1.37–12.65) | 0.01 | 4.35 (1.42–13.24) | 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Lee, Y.; Yoon, J.S.; Lee, M.; Kye, S.S.; Kim, S.W.; Cho, Y. The FIB-4 Index Is a Useful Predictor for the Development of Hepatocellular Carcinoma in Patients with Coexisting Nonalcoholic Fatty Liver Disease and Chronic Hepatitis B. Cancers 2021, 13, 2301. https://doi.org/10.3390/cancers13102301
Kim M, Lee Y, Yoon JS, Lee M, Kye SS, Kim SW, Cho Y. The FIB-4 Index Is a Useful Predictor for the Development of Hepatocellular Carcinoma in Patients with Coexisting Nonalcoholic Fatty Liver Disease and Chronic Hepatitis B. Cancers. 2021; 13(10):2301. https://doi.org/10.3390/cancers13102301
Chicago/Turabian StyleKim, Minah, Yeonju Lee, Jun Sik Yoon, Minjong Lee, So Shin Kye, Sun Woong Kim, and Yuri Cho. 2021. "The FIB-4 Index Is a Useful Predictor for the Development of Hepatocellular Carcinoma in Patients with Coexisting Nonalcoholic Fatty Liver Disease and Chronic Hepatitis B" Cancers 13, no. 10: 2301. https://doi.org/10.3390/cancers13102301
APA StyleKim, M., Lee, Y., Yoon, J. S., Lee, M., Kye, S. S., Kim, S. W., & Cho, Y. (2021). The FIB-4 Index Is a Useful Predictor for the Development of Hepatocellular Carcinoma in Patients with Coexisting Nonalcoholic Fatty Liver Disease and Chronic Hepatitis B. Cancers, 13(10), 2301. https://doi.org/10.3390/cancers13102301






