Impact of Advanced Age on the Clinical Presentation and Outcome of Sporadic Medullary Thyroid Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
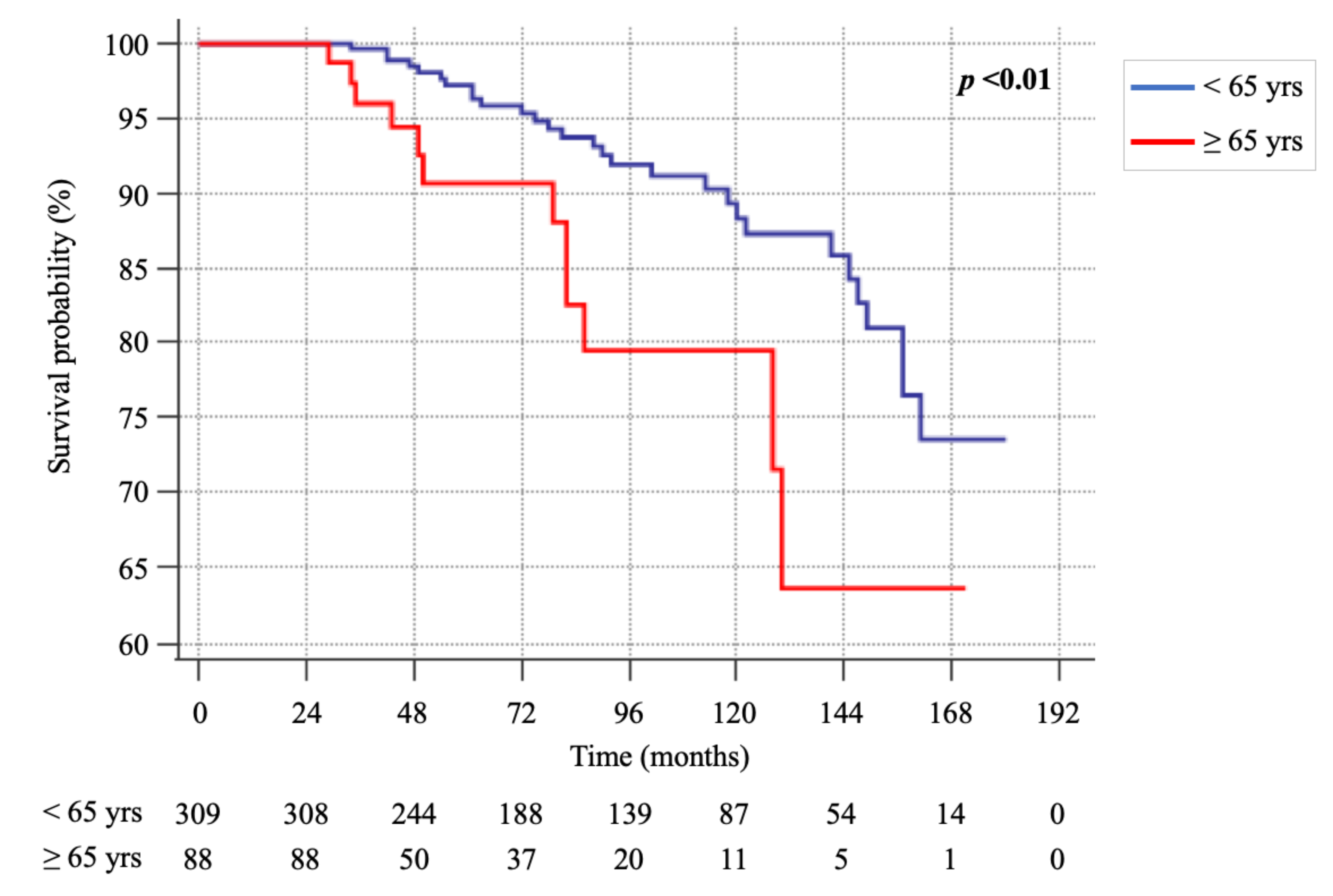
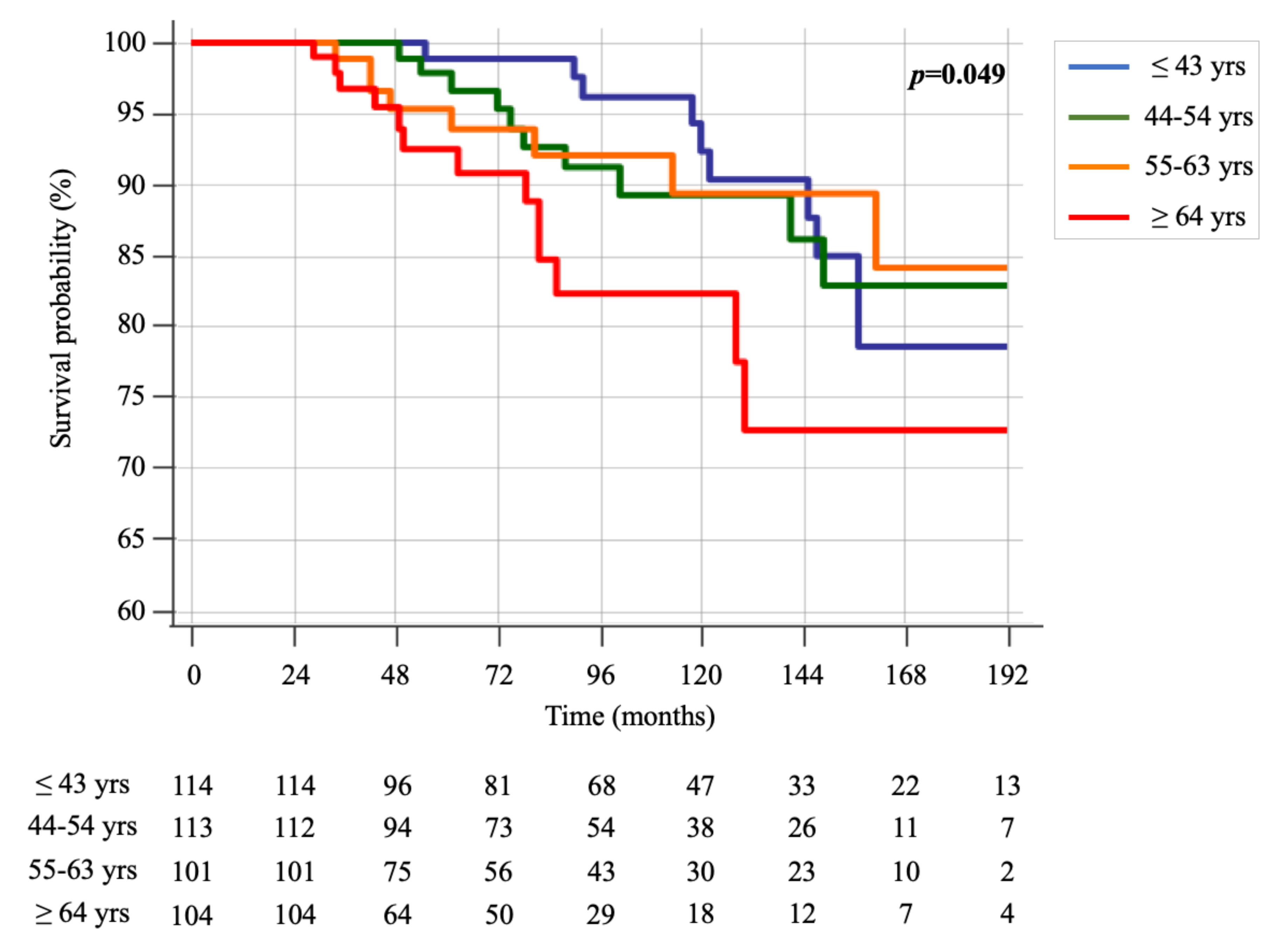
2.1. Differences in Sporadic MTC Patients According to Age < or ≥ 65 Years
2.2. Cancer-Related Death Events
3. Discussion
4. Materials and Methods
4.1. CT Assays
4.2. Histological Analysis
4.3. Molecular Analysis
4.4. Neck US
5. Statistical Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wells, S.A.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.Y.; Machens, A.; Moley, J.F.; Pacini, F.; et al. Revised American Thyroid Association Guidelines for the Management of Medullary Thyroid Carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef] [PubMed]
- Schröder, S.; Böcker, W.; Baisch, H.; Bürk, C.G.; Arps, H.; Meiners, I.; Kastendieck, H.; Heitz, P.U.; Klöppel, G. Prognostic factors in medullary thyroid carcinomas.Survival in relation to age, sex, stage, histology, immunocytochemistry, and DNA content. Cancer 1988, 61, 806–816. [Google Scholar] [CrossRef]
- Modigliani, E.; Cohen, R.; Campos, J.-M.; Conte-Devolx, B.; Maes, B.; Boneu, A.; Schlumberger, M.; Bigorgne, J.-C.; Dumontier, P.; Leclerc, L.; et al. Prognostic factors for survival and for biochemical cure in medullary thyroid carcinoma: Results in 899 patients. Clin. Endocrinol. 1998, 48, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Kotwal, A.; Erickson, D.; Geske, J.; Hay, I.D.; Castro, M.R. Predicting Outcomes in Sporadic and Hereditary Medullary Thyroid Carcinoma over Two Decades. Thyroid 2020. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, O.; Awny, S.; Metwally, I.H. Medullary thyroid cancer: Epidemiological pattern and factors contributing to recurrence and metastasis. Ann. R. Coll. Surg. Engl. 2020, 102, 1–5. [Google Scholar] [CrossRef]
- Twito, O.; Grozinsky-Glasberg, S.; Levy, S.; Bachar, G.; Gross, D.J.; Benbassat, C.; Rozental, A.; Hirsch, D. Clinico-pathologic and dynamic prognostic factors in sporadic and familial medullary thyroid carcinoma: An Israeli multi-center study. Eur. J. Endocrinol. 2019, 181, 13–21. [Google Scholar] [CrossRef]
- Van Heerden, J.A.; Grant, C.S.; Gharib, H.; Hay, I.D.; Ilstrup, D.M. Long-term Course of Patients with Persistent Hypercalcitoninemia After Apparent Curative Primary Surgery for Medullary Thyroid Carcinoma. Ann. Surg. 1990, 212, 395–401. [Google Scholar] [CrossRef]
- Prabhu, M.; Shakya, S.; Ballal, S.; Shamim, S.A.; Bal, C. RET gene mutation analysis and long-term clinical outcomes of medullary thyroid cancer patients. Nucl. Med. Commun. 2020, 41, 1136–1142. [Google Scholar] [CrossRef]
- Kebebew, E.; Ituarte, P.H.; Siperstein, A.E.; Duh, Q.Y.; Clark, O.H. Medullary thyroid carcinoma: Clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer 2000, 88, 1139–1148. [Google Scholar] [CrossRef]
- Sahli, Z.T.; Canner, J.K.; Zeiger, M.A.; Mathur, A. Association between age and disease specific mortality in medullary thyroid cancer. Am. J. Surg. 2020. [Google Scholar] [CrossRef]
- Orimo, H. Reviewing the definition of elderly. Nippon. Ronen Igakkai Zasshi Jpn. J. Geriatr. 2006, 43, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Donis-Keller, H.; Dou, S.; Chi, D.; Carlson, K.M.; Toshima, K.; Lairmore, T.C.; Howe, J.R.; Moley, J.F.; Goodfellow, P.; Wells, J.S.A. Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Hum. Mol. Genet. 1993, 2, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Marsh, D.J.; Learoyd, D.L.; Andrew, S.D.; Krishnan, L.; Pojer, R.; Richardson, A.-L.; Delbridge, L.; Eng, C.; Robinson, B.G. Somatic mutations in the RET proto-oncogene in sporadic medullary thyroid carcinoma. Clin. Endocrinol. 1996, 44, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Ganly, I.; Nixon, I.J.; Wang, L.Y.; Palmer, F.L.; Migliacci, J.C.; Aniss, A.; Sywak, M.S.; Eskander, A.E.; Freeman, J.L.; Campbell, M.J.; et al. Survival from Differentiated Thyroid Cancer: What Has Age Got to Do with It? Thyroid 2015, 25, 1106–1114. [Google Scholar] [CrossRef]
- Gülben, K.; Berberoğlu, U.; Boyabatlı, M. Prognostic Factors for Sporadic Medullary Thyroid Carcinoma. World J. Surg. 2005, 30, 84–90. [Google Scholar] [CrossRef]
- Massimo, E.D.; Assi, A.; Sironi, M.; Sangalli, G.; Spreafico, G.; Colombo, L. Multivariate analysis of patients with medullary thyroid carcinoma: Prognostic significance and impact on treatment of clinical and pathologic variables. Cancer 1996, 77, 1556–1565. [Google Scholar] [CrossRef]
- Yan, H.; Winchester, D.J.; Prinz, R.A.; Wang, C.-H.; Nakazato, Y.; Moo-Young, T.A. Differences in the Impact of Age on Mortality in Well-Differentiated Thyroid Cancer. Ann. Surg. Oncol. 2018, 25, 3193–3199. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.B.; American Joint Committee on Cancer. AJCC Cancer Staging Manual, 8th ed.; Springer: Cham, Switzerland, 2017; 1024p. [Google Scholar]
- Elisei, R.; Romei, C.; Renzini, G.; Bottici, V.; Cosci, B.; Molinaro, E.; Agate, L.; Cappagli, V.; Miccoli, P.; Berti, P.; et al. The Timing of Total Thyroidectomy inRETGene Mutation Carriers Could Be Personalized and Safely Planned on the Basis of Serum Calcitonin: 18 Years Experience at One Single Center. J. Clin. Endocrinol. Metab. 2012, 97, 426–435. [Google Scholar] [CrossRef]
- Elisei, R.; Cosci, B.; Romei, C.; Bottici, V.; Renzini, G.; Molinaro, E.; Agate, L.; Vivaldi, A.; Faviana, P.; Basolo, F.; et al. Prognostic Significance of SomaticRETOncogene Mutations in Sporadic Medullary Thyroid Cancer: A 10-Year Follow-Up Study. J. Clin. Endocrinol. Metab. 2008, 93, 682–687. [Google Scholar] [CrossRef]
- DeLellis, R.A.; Al Ghuzlan, A.; Albores Saavedra, J.; Baloch, Z.W.; Basolo, F.; Elisei, R.; Kaserer, K.; LiVolsi, V.; Matias-Guiu, X.; Mete, O.; et al. Medullary thyroid carcinoma. In WHO Classification of Tumors of Endocrine Organs In Tumors of the Thyroid Gland, 4th ed.; Lloyd, R., Osamura, R., Kloppel, G., Rosai, J., Eds.; International Agency for Research on Cancer: Lyon, France, 2017; pp. 108–113. [Google Scholar]
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer Statistics, 2010. CA A Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef]
- Owonikoko, T.K.; Ragin, C.C.; Belani, C.P.; Oton, A.B.; Gooding, W.E.; Taioli, E.; Ramalingam, S.S. Lung Cancer in Elderly Patients: An Analysis of the Surveillance, Epidemiology, and End Results Database. J. Clin. Oncol. 2007, 25, 5570–5577. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.C.; Gruber, S.B.; Hollenbeck, B.K.; Montie, J.E.; Wei, J.T. Incidence of Initial Local Therapy Among Men With Lower-Risk Prostate Cancer in the United States. J. Natl. Cancer Inst. 2006, 98, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, M.; Rowland, J.H.; Yancik, R. Cancer Survivors in the United States: Age, Health, and Disability. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2003, 58, M82–M91. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.W.; Reeve, B.B.; Bellizzi, K.M.; Harlan, L.C.; Klabunde, C.N.; Amsellem, M.; Bierman, A.S.; Hays, R.D. Cancer, Comorbidities, and Health-Related Quality of Life of Older Adults. Health Care Financ. Rev. 2008, 29, 41–56. [Google Scholar]
- Louwman, W.; Janssen-Heijnen, M.; Houterman, S.; Voogd, A.; Van Der Sangen, M.; Nieuwenhuijzen, G.; Coebergh, J.; Van Der Sangen, M.J.C. Less extensive treatment and inferior prognosis for breast cancer patient with comorbidity: A population-based study. Eur. J. Cancer 2005, 41, 779–785. [Google Scholar] [CrossRef]
- Romei, C.; Cosci, B.; Renzini, G.; Bottici, V.; Molinaro, E.; Agate, L.; Passannanti, P.; Viola, D.; Biagini, A.; Basolo, F.; et al. RET genetic screening of sporadic medullary thyroid cancer (MTC) allows the preclinical diagnosis of unsuspected gene carriers and the identification of a relevant percentage of hidden familial MTC (FMTC). Clin. Endocrinol. 2011, 74, 241–247. [Google Scholar] [CrossRef]
- Ciampi, R.; Romei, C.; Ramone, T.; Prete, A.; Tacito, A.; Cappagli, V.; Bottici, V.; Viola, D.; Torregrossa, L.; Ugolini, C.; et al. Genetic Landscape of Somatic Mutations in a Large Cohort of Sporadic Medullary Thyroid Carcinomas Studied by Next-Generation Targeted Sequencing. iScience 2019, 20, 324–336. [Google Scholar] [CrossRef]
| Features | n (%) | |
|---|---|---|
| Sex | M | 188 (43.5) |
| F | 244 (56.5) | |
| Age (years) | Median (IQR) | 54 (43–63) |
| Min–Max | 16–83 | |
| Pre-operative calcitonin a | ≤100 pg/mL | 147 (41.1) |
| >100 pg/mL | 211 (58.9) | |
| Tumor size b (cm) | Median (IQR) | 1.5 (0.8–2.7) |
| Min–Max | 0.1–9 | |
| Tumor size category | ≤1 cm | 156 (36.1) |
| 1.1–4 cm | 239 (55.3) | |
| >4 cm | 28 (6.5) | |
| Multifocality | Yes | 69 (16) |
| No | 363 (84) | |
| mETE | Yes | 86 (19.9) |
| No | 346 (80.1) | |
| Histologic pattern c | Conventional | 157 (59.5) |
| Spindle Cell | 57 (21.6) | |
| Others d | 50 (18.9) | |
| T stage | T1a | 147 (34) |
| T1b | 94 (21.8) | |
| T2 | 89 (20.6) | |
| T3 | 77 (17.8) | |
| T4 | 21 (4.9) | |
| Tx | 4 (0.9) | |
| Central compartment lymph node dissection | Yes | 378 (87.5) |
| No | 54 (12.5) | |
| Latero-cervical lymph node dissection | Yes | 158 (36.6) |
| No | 274 (63.4) | |
| N stage | N0 | 204 (47.2) |
| N1a | 66 (15.3) | |
| N1b | 36 (8.3) | |
| N1a + N1b | 93 (21.5) | |
| Nx | 33 (7.6) | |
| M stage | M0 | 22 (5.1) |
| M1 | 48 (11.1) | |
| Mx | 362 (83.8) | |
| Somatic mutation investigation e | Positive | 94 (58.8) |
| Negative | 66 (41.3) | |
| Somatic mutation result | RET M918T | 63 (67) |
| Others | 31 (33) | |
| Follow-up time (months) | Median (IQR) | 88.5 (50–138.8) |
| Min–Max | 25–247 | |
| Second neck surgery | Yes | 48 (11.1) |
| No | 384 (88.9) | |
| Local treatments f | Yes | 44 (10.2) |
| No | 388 (89.8) | |
| Systemic treatments | Yes | 59 (13.7) |
| No | 373 (86.3) | |
| Type of systemic treatment | Chemotherapy | 5 (8.5) |
| TKI | 47 (79.7) | |
| Chemotherapy + TKI | 5 (8.5) | |
| Somatostatin analogs | 2 (3.4) | |
| Clinical outcome | Excellent Response | 229 (53) |
| Biochemical Incomplete Response | 67 (15.5) | |
| Local Metastatic disease | 29 (6.7) | |
| Distant Metastatic disease | 107 (24.8) | |
| Site of surgery | Pisa | 323 (74.8) |
| No Pisa | 109 (25.2) |
| Features | Group A (<65) (338—78.2%) | Group B (≥65) (94—21.8%) | p | |
|---|---|---|---|---|
| Sex | M | 145 (42.9) | 43 (45.7) | 0.62 |
| F | 193 (57.1) | 51 (54.3) | ||
| Age (years) | Median (IQR) | 49 (40–57) | 70 (67–76) | <0.01 |
| Min–Max | (16–64) | (65–83) | ||
| Pre-operative calcitonin a | ≤100 pg/mL | 112 (40.4) | 35 (43.2) | 0.66 |
| >100 pg/mL | 165 (59.6) | 46 (56.8) | ||
| Tumor size b (cm) | Median (IQR) | 1.5 (0.8–2.5) | 1.6 (0.8–2.8) | 0.7 |
| Min–Max | 0.1–9 | 0.2–8 | ||
| Tumor size category | ≤1 cm | 121 (36.4) | 35 (38.5) | 0.81 |
| 1.1–4 cm | 190 (57.2) | 49 (53.8) | ||
| >4 cm | 21 (6.3) | 7 (7.7) | ||
| Multifocality | Yes | 53 (15.7) | 16 (17) | 0.75 |
| No | 285 (84.3 | 78 (83) | ||
| mETE | Yes | 67 (19.8) | 19 (20.2) | 0.93 |
| No | 271 (80.2) | 75 (79.8) | ||
| Histologic pattern c | Conventional | 117 (57.4) | 40 (66.7) | 0.2 |
| Spindle Cell | 49 (24) | 8 (13.3) | ||
| Others d | 38 (18.6) | 12 (20) | ||
| T stage | T1a | 115 (34) | 32 (34) | 0.92 |
| T1b | 77 (22.8) | 17 (18.1) | ||
| T2 | 67 (19.8) | 22 (23.4) | ||
| T3 | 59 (17.5) | 18 (19.1) | ||
| T4 | 17 (5) | 4 (4.3) | ||
| Tx | 3 (0.9) | 1 (1.1) | ||
| Central compartment lymph node dissection | Yes | 296 (87.6) | 82 (87.2) | 0.93 |
| No | 42 (12.4) | 12 (12.8) | ||
| Latero-cervical lymph node dissection | Yes | 126 (37.3) | 32 (34) | 0.57 |
| No | 212 (62.7) | 62 (66) | ||
| N stage | N0 | 157 (46.4) | 47 (50) | 0.91 |
| N1a | 54 (16) | 12 (12.8) | ||
| N1b | 28 (8.3) | 8 (8.5) | ||
| N1a + N1b | 72 (21.3) | 21 (22.3) | ||
| Nx | 27 (8) | 6 (6.4) | ||
| M stage | M0 | 21 (6.2) | 1 (1.1) | 0.13 |
| M1 | 38 (11.2) | 10 (10.6) | ||
| Mx | 279 (82.5) | 83 (88.3) | ||
| Somatic mutation investigation e | Positive | 81 (60.9) | 13 (48.1) | 0.22 |
| Negative | 52 (39.1) | 14 (51.9) | ||
| Somatic mutation result | RET M918T | 56 (69.1) | 7 (53.8) | 0.28 |
| Others | 25 (30.9) | 6 (46.2) | ||
| Follow-up time (months) | Median (IQR) | 96 (56–144.3) | 61 (38–106) | <0.01 |
| Min–Max | 25–247 | 25–242 | ||
| Second neck surgery | Yes | 40 (11.8) | 8 (85) | 0.36 |
| No | 298 (88.2) | 86 (91.5) | ||
| Local treatments f | Yes | 38 (11.2) | 6 (6.4) | 0.17 |
| No | 300 (88.8) | 88 (93.6) | ||
| Systemic treatments | Yes | 47 (13.9) | 12 (12.8) | 0.78 |
| No | 291 (86.1) | 82 (87.2) | ||
| Type of systemic treatment | Chemotherapy | 3 (6.4) | 2 (16.7) | 0.46 |
| TKI | 39 (83) | 8 (66.7) | ||
| Chemotherapy + TKI | 4 (8.5) | 1 (8.3) | ||
| Somatostatin analogs | 1 (2.1) | 1 (8.3) | ||
| Clinical outcome | Excellent Response | 175 (51.8) | 54 (57.4) | 0.51 |
| Biochemical Incomplete Response | 57 (16.9) | 10 (10.6) | ||
| Local Metastatic disease | 23 (6.8) | 6 (6.4) | ||
| Distant Metastatic disease | 83 (24.6) | 24 (25.5) | ||
| Site of surgery | Pisa | 250 (74) | 73 (77.7) | 0.47 |
| No Pisa | 88 (26) | 21 (22.3) |
| Features | Group A Dead 29/338 (8.6%) | Group B Dead 12/94 (12.8%) | p | |
|---|---|---|---|---|
| Sex | Male | 21 (72.4) | 10 (83.3) | 0.46 |
| Female | 8 (27.6) | 2 (16.7) | ||
| Age (years) | Median (IQR) | 47 (41.5–58.5) | 70.5 (67.3–74.3) | <0.01 |
| Min–Max | (26–64) | (65–79) | ||
| Pre-operative calcitonin a | ≤100 pg/mL | 2 (10) | - | 0.28 |
| >100 pg/mL | 18 (90) | 11 (100) | ||
| Tumor size b (cm) | Median (IQR) | 2 (1.2–3.8) | 3 (2.2–4.5) | 0.17 |
| Min–Max | 0.2–9 | 0.3–8 | ||
| Tumor size category | ≤1 cm | 5 (17.9) | 1 (9.1) | 0.69 |
| 1.1–4 cm | 18 (64.3) | 7 (63.6) | ||
| >4 cm | 5 (17.9) | 3 (27.3) | ||
| Multifocality | Yes | 8 (27.6) | 5 (41.7) | 0.38 |
| No | 21 (72.4) | 7 (58.3) | ||
| mETE | Yes | 15 (51.7) | 6 (50) | 0.92 |
| No | 14 (48.3) | 6 (50) | ||
| Histologic pattern c | Conventional | 3 (37.5) | 3 (75) | 0.07 |
| Spindle Cell | 5 (62.5) | - | ||
| Others d | - | 1 (25) | ||
| T stage | T1a | 4 (13.8) | 2 (16.7) | 0.36 |
| T1b | 4 (13.8) | - | ||
| T2 | 4 (13.8) | 3 (25) | ||
| T3 | 14 (48.3) | 4 (33.3) | ||
| T4 | 3 (10.3) | 2 (16.7) | ||
| Tx | - | 1 (8.3) | ||
| Central compartment lymph node dissection | Yes | 23 (79.3) | 10 (83.3) | 0.68 |
| No | 6 (20.7) | 2 (16.7) | ||
| Latero-cervical lymph node dissection | Yes | 24 (82.8) | 11(91.7) | 0.46 |
| No | 5 (17.2) | 1 (8.3) | ||
| N stage | N0 | 3 (10.3) | 1 (8.3) | 0.92 |
| N1a | 1 (3.4) | 1 (8.3) | ||
| N1b | 11 (37.9) | 5 (41.7) | ||
| N1a + N1b | 13 (44.8) | 5 (41.7) | ||
| Nx | 1 (3.4) | - | ||
| M stage | M0 | 3 (10.3) | 1 (8.3) | 0.97 |
| M1 | 15 (51.7) | 6 (50) | ||
| Mx | 11 (37.9) | 5 (41.7) | ||
| Somatic mutation investigation e | Positive | 21 (87.5) | 5 (71.4) | 0.31 |
| Negative | 3 (12.5) | 2 (28.6) | ||
| Somatic mutation result | RET M918 | 16 (76.2) | 4 (80) | 0.86 |
| Others | 5 (23.8) | 1 (20) | ||
| Follow-up time (months) | Median (IQR) | 84 (53.5–131.5) | 61.5 (36–81.8) | 0.08 |
| Min–Max | 31–167 | 28–160 | ||
| Second neck surgery | Yes | 12 (41.4) | 4 (33.3) | 0.63 |
| No | 17 (58.6) | 8 (66.7) | ||
| Local treatments f | Yes | 14 (48.3) | 6 (50) | 0.92 |
| No | 15 (51.7) | 6 (50) | ||
| Systemic treatments | Yes | 21 (72.4) | 9 (75) | 0.87 |
| No | 8 (27.6) | 3 (25) | ||
| Type of systemic treatment | Chemotherapy | 2 (9.5) | 1 (11.1) | 0.91 |
| TKI | 15 (71.4) | 7 (77.8) | ||
| Chemotherapy + TKI | 3 (14.3) | 1 (11.1) | ||
| Somatostatin analogs | 1 (4.8) | - | ||
| Site of surgery | Pisa | 11 (37.9) | 7 (58.3) | 0.23 |
| No Pisa | 18 (62.1) | 5 (41.7) |
| Follow Up Time Interval (Years) | Group A n° (%) | Group B n° (%) | p | |
|---|---|---|---|---|
| ≤5 | Alive | 88 (89.8) | 41 (87.2) | 0.65 |
| Dead | 10 (10.2) | 6 (12.8) | ||
| 5.1–10 | Alive | 112 (90.3) | 25 (86.2) | 0.51 |
| Dead | 12 (9.7) | 4 (13.8) | ||
| 10.1–15 | Alive | 78 (91.8) | 9 (81.8) | 0.29 |
| Dead | 7 (8.2) | 2 (18.2) | ||
| >15 | Alive | 31 (100) | 7 (100) | - |
| Dead | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matrone, A.; Gambale, C.; Prete, A.; Piaggi, P.; Cappagli, V.; Bottici, V.; Romei, C.; Ciampi, R.; Torregrossa, L.; De Napoli, L.; et al. Impact of Advanced Age on the Clinical Presentation and Outcome of Sporadic Medullary Thyroid Carcinoma. Cancers 2021, 13, 94. https://doi.org/10.3390/cancers13010094
Matrone A, Gambale C, Prete A, Piaggi P, Cappagli V, Bottici V, Romei C, Ciampi R, Torregrossa L, De Napoli L, et al. Impact of Advanced Age on the Clinical Presentation and Outcome of Sporadic Medullary Thyroid Carcinoma. Cancers. 2021; 13(1):94. https://doi.org/10.3390/cancers13010094
Chicago/Turabian StyleMatrone, Antonio, Carla Gambale, Alessandro Prete, Paolo Piaggi, Virginia Cappagli, Valeria Bottici, Cristina Romei, Raffaele Ciampi, Liborio Torregrossa, Luigi De Napoli, and et al. 2021. "Impact of Advanced Age on the Clinical Presentation and Outcome of Sporadic Medullary Thyroid Carcinoma" Cancers 13, no. 1: 94. https://doi.org/10.3390/cancers13010094
APA StyleMatrone, A., Gambale, C., Prete, A., Piaggi, P., Cappagli, V., Bottici, V., Romei, C., Ciampi, R., Torregrossa, L., De Napoli, L., Molinaro, E., Materazzi, G., Basolo, F., & Elisei, R. (2021). Impact of Advanced Age on the Clinical Presentation and Outcome of Sporadic Medullary Thyroid Carcinoma. Cancers, 13(1), 94. https://doi.org/10.3390/cancers13010094








