Immunocyte Membrane-Coated Nanoparticles for Cancer Immunotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Immunocyte Membrane Molecules Contributing to Nanomaterials’ Anti-Tumor Immune Effects
3. Macrophage Membranes
3.1. Immune Evasion and Tumor Targeting
3.2. Penetrating the Blood–Brain Barrier (BBB) and Targeting Glioblastoma
3.3. Anti-Proliferation
3.4. Macrophage Hybrid Membrane
4. T-Cell Membranes
4.1. Targeting Tumors through TCRs
4.2. The Dual-Targeting Strategy
5. NK Cell Membranes
5.1. Targeting Tumors
5.2. M1 Polarization and Induction of Immunogenic Cell Death (ICD)
5.3. Penetrating the BBB
6. Dendritic Cell Membrane
6.1. Activation and Maintenance of Antigen-Specific T-cells
6.2. Intelligent Nano-DCs
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharma, P.; Wagner, K.; Wolchok, J.D.; Allison, J.P. Novel cancer immunotherapy agents with survival benefit: Recent successes and next steps. Nat. Rev. Cancer 2011, 11, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Finck, A.; Gill, S.I.; June, C.H. Cancer immunotherapy comes of age and looks for maturity. Nat. Commun. 2020, 11, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Policard, A. Cell Membranes and Their Role in the Cell Function. Pathol. Biol. 1968, 16, 973–977. [Google Scholar] [PubMed]
- Bentrup, F.W. Function of Cell Membranes in Cytomorphogenesis. Ber. Deut. Bot. Ges. 1968, 81, 311–314. [Google Scholar]
- Obrien, J.S. Cell Membranes—Composition—Structure—Function. J. Theor. Biol. 1967, 15, 307–324. [Google Scholar] [CrossRef]
- Murti, C.R.K. Biochemical Function of Cell Membranes. J. Sci. Ind. Res. India 1963, 22, 123–128. [Google Scholar]
- Zingaretti, G.; Nunez, C.; Rubiano, F.; Heymsfield, S.B. Cell membrane function modeled using bioimpedance analysis. Faseb. J. 2000, 14, A486. [Google Scholar]
- Whittaker, V.P. Structure and Function of Animal-Cell Membranes. Br. Med. Bull. 1968, 24, 101–106. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Sackmann, E. Thermo-elasticity and adhesion as regulators of cell membrane architecture and function. J. Phys. Condens. Matter 2006, 18, R785–R825. [Google Scholar] [CrossRef]
- Takakuwa, Y. Regulation of red cell membrane protein interactions: Implications for red cell function. Curr. Opin. Hematol. 2001, 8, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Brandley, B.K.; Schnaar, R.L. Cell-surface carbohydrates in cell recognition and response. J. Leukoc. Biol. 1986, 40, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Coers, J. Self and Non-self Discrimination of Intracellular Membranes by the Innate Immune System. PLoS Pathog. 2013, 9, e1003538. [Google Scholar] [CrossRef] [PubMed]
- Heyden, S.; Ortiz, M. Investigation of the influence of viscoelasticity on oncotripsy. Comput. Method Appl. Mech. Eng. 2017, 314, 314–322. [Google Scholar] [CrossRef]
- Biagiotti, S.; Paoletti, M.F.; Fraternale, A.; Rossi, L.; Magnani, M. Drug delivery by red blood cells. IUBMB Life 2011, 63, 621–631. [Google Scholar] [CrossRef]
- Kim, J.S.; Kang, M.; Bagyinszky, E.; Thanavel, R.; An, S.A. Mimicking red blood cells for drug delivery. Nanomedicine 2011, 6, 420. [Google Scholar]
- Piergiovanni, M.; Casagrande, G.; Taverna, F.; Corridori, I.; Frigerio, M.; Bianchi, E.; Arienti, F.; Mazzocchi, A.; Dubini, G.; Costantino, M.L. Shear-Induced Encapsulation into Red Blood Cells: A New Microfluidic Approach to Drug Delivery. Ann. Biomed. Eng. 2020, 48, 236–246. [Google Scholar] [CrossRef]
- Glassman, P.M.; Villa, C.H.; Ukidve, A.; Zhao, Z.; Smith, P.; Mitragotri, S.; Russell, A.J.; Brenner, J.S.; Muzykantov, V.R. Vascular Drug Delivery Using Carrier Red Blood Cells: Focus on RBC Surface Loading and Pharmacokinetics. Pharmaceutics 2020, 12, 440. [Google Scholar] [CrossRef]
- Kolesnikova, T.A.; Skirtach, A.G.; Mohwald, H. Red blood cells and polyelectrolyte multilayer capsules: Natural carriers versus polymer-based drug delivery vehicles. Expert Opin. Drug Deliv. 2013, 10, 47–58. [Google Scholar] [CrossRef]
- Hu, C.M.J.; Fang, R.H.; Wang, K.C.; Luk, B.T.; Thamphiwatana, S.; Dehaini, D.; Nguyen, P.; Angsantikul, P.; Wen, C.H.; Kroll, A.V.; et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 2015, 526, 118–121. [Google Scholar] [CrossRef]
- Zhen, X.; Cheng, P.; Pu, K. Recent Advances in Cell Membrane-Camouflaged Nanoparticles for Cancer Phototherapy. Small 2019, 15, e1804105. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.H.; Kroll, A.V.; Zhang, L. Nanoparticle-Based Manipulation of Antigen-Presenting Cells for Cancer Immunotherapy. Small 2015, 11, 5483–5496. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, Y.; Feng, N. Cell membrane-coated nanosized active targeted drug delivery systems homing to tumor cells: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110298. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Wang, B.; Wang, C.; Wang, Q.; Zhang, L. Cell membrane-coated nanoparticles: Research advances. Nanomedicine 2020, 15, 625–641. [Google Scholar] [CrossRef]
- Muzykantov, V.R. Drug delivery by red blood cells: Vascular carriers designed by mother nature. Expert Opin. Drug Deliv. 2010, 7, 403–427. [Google Scholar] [CrossRef]
- Magnani, M.; Rossi, L.; Casabianca, A.; Fraternale, A.; Schiavano, G.; Brandi, G.; Mannello, F.; Piedimonte, G. Red blood cells as advanced drug delivery systems for antiviral nucleoside analogues. Adv. Exp. Med. Biol. 1992, 326, 239–245. [Google Scholar] [CrossRef]
- Wang, S.; Gao, J.; Wang, Z. Outer membrane vesicles for vaccination and targeted drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1523. [Google Scholar] [CrossRef]
- Ai, X.; Wang, S.; Duan, Y.; Zhang, Q.; Chen, M.S.; Gao, W.; Zhang, L. Emerging Approaches to Functionalizing Cell Membrane-Coated Nanoparticles. Biochemistry 2020. [Google Scholar] [CrossRef]
- Xuan, M.J.; Shao, J.X.; Li, J.B. Cell membrane-covered nanoparticles as biomaterials. Natl. Sci. Rev. 2019, 6, 551–561. [Google Scholar] [CrossRef]
- Li, R.X.; He, Y.W.; Zhang, S.Y.; Qin, J.; Wang, J.X. Cell membrane-based nanoparticles: A new biomimetic platform for tumor diagnosis and treatment. Acta Pharm. Sin. B 2018, 8, 14–22. [Google Scholar] [CrossRef]
- Bose, R.J.C.; Paulmurugan, R.; Moon, J.; Lee, S.H.; Park, H. Cell membrane-coated nanocarriers: The emerging targeted delivery system for cancer theranostics. Drug Discov. Today 2018, 23, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Kim, H.S.; Palanikumar, L.; Go, E.M.; Jana, B.; Park, S.A.; Kim, H.Y.; Kim, K.; Seo, J.K.; Kwak, S.K.; et al. Cloaking nanoparticles with protein corona shield for targeted drug delivery. Nat. Commun. 2018, 9, 4548. [Google Scholar] [CrossRef] [PubMed]
- Tekie, F.S.M.; Hajiramezanali, M.; Geramifar, P.; Raoufi, M.; Dinarvand, R.; Soleimani, M.; Atyabi, F. Controlling evolution of protein corona: A prosperous approach to improve chitosan-based nanoparticle biodistribution and half-life. Sci. Rep. 2020, 10, 9664. [Google Scholar] [CrossRef] [PubMed]
- Ritz, S.; Schottler, S.; Kotman, N.; Baier, G.; Musyanovych, A.; Kuharev, J.; Landfester, K.; Schild, H.; Jahn, O.; Tenzer, S.; et al. Protein corona of nanoparticles: Distinct proteins regulate the cellular uptake. Biomacromolecules 2015, 16, 1311–1321. [Google Scholar] [CrossRef]
- Kroll, A.V.; Fang, R.H.; Zhang, L.F. Biointerfacing and Applications of Cell Membrane-Coated Nanoparticles. Bioconjugate Chem. 2017, 28, 23–32. [Google Scholar] [CrossRef]
- Chai, Z.L.; Hu, X.F.; Lu, W.Y. Cell membrane-coated nanoparticles for tumor-targeted drug delivery. Sci. China Mater. 2017, 60, 504–510. [Google Scholar] [CrossRef]
- Wang, H.J.; Liu, Y.; He, R.Q.; Xu, D.L.; Zang, J.; Weeranoppanant, N.; Dong, H.Q.; Li, Y.Y. Cell membrane biomimetic nanoparticles for inflammation and cancer targeting in drug delivery. Biomater. Sci. 2020, 8, 552–568. [Google Scholar] [CrossRef]
- Chow, A.; Brown, B.D.; Merad, M. Studying the mononuclear phagocyte system in the molecular age. Nat. Rev. Immunol. 2011, 11, 788–798. [Google Scholar] [CrossRef]
- Hume, D.A. The mononuclear phagocyte system. Curr. Opin. Immunol. 2006, 18, 49–53. [Google Scholar] [CrossRef]
- Nezelof, C. Cells of the Mononuclear Phagocyte System—Origin, Lifetime, Function. Arch. Pediatrie 1995, 2, S28–S31. [Google Scholar] [CrossRef]
- Nie, S.M. Understanding and overcoming major barriers in cancer nanomedicine. Nanomedicine-UK 2010, 5, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Sathyamoorthy, N.; Dhanaraju, M.D. Shielding Therapeutic Drug Carriers from the Mononuclear Phagocyte System: A Review. Crit. Rev. Ther. Drug 2016, 33, 489–567. [Google Scholar] [CrossRef] [PubMed]
- Rothlein, R.; Dustin, M.L.; Marlin, S.D.; Springer, T.A. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J. Immunol. 1986, 137, 1270–1274. [Google Scholar] [PubMed]
- Barreiro, O.; Yanez-Mo, M.; Serrador, J.M.; Montoya, M.C.; Vicente-Manzanares, M.; Tejedor, R.; Furthmayr, H.; Sanchez-Madrid, F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J. Cell. Biol. 2002, 157, 1233–1245. [Google Scholar] [CrossRef] [PubMed]
- Polin, R.S.; Bavbek, M.; Shaffrey, M.E.; Billups, K.; Bogaev, C.A.; Kassell, N.F.; Lee, K.S. Detection of soluble E-selectin, ICAM-1, VCAM-1, and L-selectin in the cerebrospinal fluid of patients after subarachnoid hemorrhage. J. Neurosurg. 1998, 89, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Nordal, R.A.; Wong, C.S. Intercellular adhesion molecule-1 and blood-spinal cord barrier disruption in central nervous system radiation injury. J. Neuropathol. Exp. Neurol. 2004, 63, 474–483. [Google Scholar] [CrossRef]
- Vivier, E.; Raulet, D.H.; Moretta, A.; Caligiuri, M.A.; Zitvogel, L.; Lanier, L.L.; Yokoyama, W.M.; Ugolini, S. Innate or adaptive immunity? The example of natural killer cells. Science 2011, 331, 44–49. [Google Scholar] [CrossRef]
- Pfefferle, A.; Jacobs, B.; Haroun-Izquierdo, A.; Kveberg, L.; Sohlberg, E.; Malmberg, K.J. Deciphering Natural Killer Cell Homeostasis. Front. Immunol. 2020, 11, 812. [Google Scholar] [CrossRef]
- Parodi, M.; Favoreel, H.; Candiano, G.; Gaggero, S.; Sivori, S.; Mingari, M.C.; Moretta, L.; Vitale, M.; Cantoni, C. NKp44-NKp44 Ligand Interactions in the Regulation of Natural Killer Cells and Other Innate Lymphoid Cells in Humans. Front. Immunol. 2019, 10, 719. [Google Scholar] [CrossRef]
- Smyth, M.J.; Hayakawa, Y.; Takeda, K.; Yagita, H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat. Rev. Cancer 2002, 2, 850–861. [Google Scholar] [CrossRef]
- Terunuma, H.; Deng, X.W.; Dewan, Z.; Fujimoto, S.; Yamamoto, N. Potential role of NK cells in the induction of immune responses: Implications for NK cell-based immunotherapy for cancers and viral infections. Int. Rev. Immunol. 2008, 27, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.G.; Rabinovich, P.M. T Cell Reprogramming Against Cancer. Methods Mol. Biol. 2020, 2097, 3–44. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gogenur, I. Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. Br. J. Cancer 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Shi, T.; Zhang, H.; Hu, J.; Song, Y.; Wei, J.; Ren, S.; Zhou, C. Tumor neoantigens: From basic research to clinical applications. J. Hematol. Oncol. 2019, 12, 93. [Google Scholar] [CrossRef]
- Brunet, J.F.; Denizot, F.; Luciani, M.F.; Roux-Dosseto, M.; Suzan, M.; Mattei, M.G.; Golstein, P. A new member of the immunoglobulin superfamily—CTLA-4. Nature 1987, 328, 267–270. [Google Scholar] [CrossRef]
- Lorusso, D.; Ceni, V.; Muratore, M.; Salutari, V.; Nero, C.; Pietragalla, A.; Ciccarone, F.; Carbone, V.; Daniele, G.; Scambia, G. Emerging role of immune checkpoint inhibitors in the treatment of ovarian cancer. Expert Opin. Emerg. Drugs 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Xiang, Y.; Xin, V.W.; Wang, X.W.; Peng, X.C.; Liu, X.Q.; Wang, D.; Li, N.; Cheng, J.T.; Lyv, Y.N.; et al. Dendritic cell biology and its role in tumor immunotherapy. J. Hematol. Oncol. 2020, 13, 107. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Thalhammer, T.; Tzakos, A.G.; Stojanovska, L. Targeting antigens to dendritic cell receptors for vaccine development. J. Drug. Deliv. 2013, 2013, 869718. [Google Scholar] [CrossRef]
- Steinman, R.M. Dendritic cells and the control of immunity: Enhancing the efficiency of antigen presentation. Mt. Sinai. J. Med. 2001, 68, 160–166. [Google Scholar]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Rhee, I.; Zhong, M.C.; Reizis, B.; Cheong, C.; Veillette, A. Control of dendritic cell migration, T cell-dependent immunity, and autoimmunity by protein tyrosine phosphatase PTPN12 expressed in dendritic cells. Mol. Cell. Biol. 2014, 34, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Pattern recognition receptors: Doubling up for the innate immune response. Cell 2002, 111, 927–930. [Google Scholar] [CrossRef]
- Liu, H.; Mi, Z.; Wang, Z.; Zhang, F. Revealing of Pattern recognition receptors mediated macrophage immune response network induced by Mycobacterium leprae. J. Invest. Dermatol. 2020, 140, S42. [Google Scholar] [CrossRef]
- Taylor, P.R.; Martinez-Pomares, L.; Stacey, M.; Lin, H.H.; Brown, G.D.; Gordon, S. Macrophage receptors and immune recognition. Annu. Rev. Immunol. 2005, 23, 901–944. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.J.; Yu, W.J.; Ji, B.; Chen, C.B.; Yang, H.M.; Du, Y.Y.; Song, M.Y.; Cai, H.Q.; Yan, F.; Su, R. Saikosaponin D loaded macrophage membrane-biomimetic nanoparticles target angiogenic signaling for breast cancer therapy. Appl. Mater. Today 2020, 18, 100505. [Google Scholar] [CrossRef]
- Chen, L.J.; Zhao, X.; Liu, Y.Y.; Yan, X.P. Macrophage membrane coated persistent luminescence nanoparticle@MOF-derived mesoporous carbon core-shell nanocomposites for autofluorescence-free imaging-guided chemotherapy. J. Mater. Chem. B 2020, 8, 8071–8083. [Google Scholar] [CrossRef]
- Cao, H.; Dan, Z.; He, X.; Zhang, Z.; Yu, H.; Yin, Q.; Li, Y. Liposomes Coated with Isolated Macrophage Membrane Can Target Lung Metastasis of Breast Cancer. ACS Nano 2016, 10, 7738–7748. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, K.; Li, C.; Guo, Q.; Chen, Q.; He, X.; Liu, L.; Zhang, Y.; Lu, Y.; Chen, X.; et al. Macrophage-Membrane-Coated Nanoparticles for Tumor-Targeted Chemotherapy. Nano Lett. 2018, 18, 1908–1915. [Google Scholar] [CrossRef]
- Meng, Q.F.; Rao, L.; Zan, M.; Chen, M.; Yu, G.T.; Wei, X.; Wu, Z.; Sun, Y.; Guo, S.S.; Zhao, X.Z.; et al. Macrophage membrane-coated iron oxide nanoparticles for enhanced photothermal tumor therapy. Nanotechnology 2018, 29, 134004. [Google Scholar] [CrossRef]
- Zhao, H.; Li, L.; Zhang, J.; Zheng, C.; Ding, K.; Xiao, H.; Wang, L.; Zhang, Z. C-C Chemokine Ligand 2 (CCL2) Recruits Macrophage-Membrane-Camouflaged Hollow Bismuth Selenide Nanoparticles To Facilitate Photothermal Sensitivity and Inhibit Lung Metastasis of Breast Cancer. ACS Appl. Mater. Interfaces 2018, 10, 31124–31135. [Google Scholar] [CrossRef]
- Liang, B.; Deng, T.; Li, J.; Ouyang, X.; Na, W.; Deng, D. Biomimetic theranostic strategy for anti-metastasis therapy of breast cancer via the macrophage membrane camouflaged superparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 115, 111097. [Google Scholar] [CrossRef] [PubMed]
- Xuan, M.; Shao, J.; Dai, L.; He, Q.; Li, J. Macrophage Cell Membrane Camouflaged Mesoporous Silica Nanocapsules for In Vivo Cancer Therapy. Adv. Healthc. Mater. 2015, 4, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; He, Z.; Meng, Q.F.; Zhou, Z.; Bu, L.L.; Guo, S.S.; Liu, W.; Zhao, X.Z. Effective cancer targeting and imaging using macrophage membrane-camouflaged upconversion nanoparticles. J. Biomed. Mater. Res. A 2017, 105, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Tan, T.; Zhu, D.; Yu, H.; Liu, Y.; Zhou, H.; Jin, Y.; Xia, Q. Paclitaxel-Loaded Macrophage Membrane Camouflaged Albumin Nanoparticles for Targeted Cancer Therapy. Int. J. Nanomed. 2020, 15, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Deng, G.; Sun, Z.; Peng, X.; Li, J.; Gong, P.; Zhang, P.; Cai, L. Scaffolds biomimicking macrophages for a glioblastoma NIR-Ib imaging guided photothermal therapeutic strategy by crossing Blood-Brain Barrier. Biomaterials 2019, 211, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, T.; Mitoma, H.; Harashima, S.; Tsukamoto, H.; Shimoda, T. Transmembrane TNF-alpha: Structure, function and interaction with anti-TNF agents. Rheumatology 2010, 49, 1215–1228. [Google Scholar] [CrossRef]
- Decker, T.; Lohmannmatthes, M.L.; Gifford, G.E. Cell-Associated Tumor-Necrosis-Factor (Tnf) as a Killing Mechanism of Activated Cytotoxic Macrophages. J. Immunol. 1987, 138, 957–962. [Google Scholar]
- Peck, R.; Brockhaus, M.; Frey, J.R. Cell-Surface Tumor Necrosis Factor (Tnf) Accounts for Monocyte-Mediated and Lymphocyte-Mediated Killing of Tnf-Resistant Target-Cells. Cell. Immunol. 1989, 122, 1–10. [Google Scholar] [CrossRef]
- Fishman, M. Cytolytic Activities of Activated Macrophages Versus Paraformaldehyde-Fixed Macrophages—Soluble Versus Membrane-Associated Tnf. Cell. Immunol. 1991, 137, 164–174. [Google Scholar] [CrossRef]
- Caron, G.; Delneste, Y.; Aubry, J.P.; Magistrellli, G.; Herbault, N.; Blaecke, A.; Meager, A.; Bonnefoy, J.Y.; Jeannin, P. Human NK cells constitutively express membrane TNF-alpha (mTNF alpha) and present mTNF alpha-dependent cytotoxic activity. Eur. J. Immunol. 1999, 29, 3588–3595. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Ghosh, S.S. Transmembrane TNFalpha-Expressed Macrophage Membrane-Coated Chitosan Nanoparticles as Cancer Therapeutics. ACS Omega 2020, 5, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Wu, L.; Liu, Z.; Tian, R.; Yu, G.; Zhou, Z.; Yang, K.; Xiong, H.G.; Zhang, A.; Yu, G.T.; et al. Hybrid cellular membrane nanovesicles amplify macrophage immune responses against cancer recurrence and metastasis. Nat. Commun. 2020, 11, 4909. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Yu, X.; You, B.; Wu, Y.; Wang, R.; Han, L.; Wang, Y.; Gao, S.; Yuan, Y. Macrophage-cancer hybrid membrane-coated nanoparticles for targeting lung metastasis in breast cancer therapy. J. Nanobiotechnol. 2020, 18, 92. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.F.; Rosenberg, S.A. Human tumor antigens recognized by T lymphocytes: Implications for cancer therapy. J. Leukoc. Biol. 1996, 60, 296–309. [Google Scholar] [CrossRef]
- Boon, T.; van der Bruggen, P. Human tumor antigens recognized by T lymphocytes. J. Exp. Med. 1996, 183, 725–729. [Google Scholar] [CrossRef]
- Boon, T.; Cerottini, J.C.; Van den Eynde, B.; van der Bruggen, P.; Van Pel, A. Tumor antigens recognized by T lymphocytes. Annu. Rev. Immunol. 1994, 12, 337–365. [Google Scholar] [CrossRef]
- Van der Bruggen, P.; Van den Eynde, B. Molecular definition of tumor antigens recognized by T lymphocytes. Curr. Opin. Immunol. 1992, 4, 608–612. [Google Scholar] [CrossRef]
- Plata, F.; Langlade-Demoyen, P.; Abastado, J.P.; Berbar, T.; Kourilsky, P. Retrovirus antigens recognized by cytolytic T lymphocytes activate tumor rejection in vivo. Cell 1987, 48, 231–240. [Google Scholar] [CrossRef]
- Reinherz, E.L. alphabeta TCR-mediated recognition: Relevance to tumor-antigen discovery and cancer immunotherapy. Cancer Immunol. Res. 2015, 3, 305–312. [Google Scholar] [CrossRef]
- He, Q.H.; Jiang, X.H.; Zhou, X.K.; Weng, J.S. Targeting cancers through TCR-peptide/MHC interactions. J. Hematol. Oncol. 2019, 12, 1–17. [Google Scholar] [CrossRef]
- Yaman, S.; Ramachandramoorthy, H.; Oter, G.; Zhukova, D.; Nguyen, T.; Sabnani, M.K.; Weidanz, J.A.; Nguyen, K.T. Melanoma Peptide MHC Specific TCR Expressing T-Cell Membrane Camouflaged PLGA Nanoparticles for Treatment of Melanoma Skin Cancer. Front Bioeng. Biotech. 2020, 8, 943. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.J.; Zhu, D.M.; Li, J.H.; Chen, X.; Xie, W.; Jiang, X.; Wu, L.; Wang, G.G.; Xiao, Y.S.; Liu, Z.S.; et al. Coating biomimetic nanoparticles with chimeric antigen receptor T cell-membrane provides high specificity for hepatocellular carcinoma photothermal therapy treatment. Theranostics 2020, 10, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Van der Schans, J.J.; van de Donk, N.W.C.J.; Mutis, T. Dual Targeting to Overcome Current Challenges in Multiple Myeloma CAR T-Cell Treatment. Front. Oncol. 2020, 10, 1362. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.T.; Pan, H.; Li, W.J.; Chen, Z.; Ma, A.Q.; Yin, T.; Liang, R.J.; Chen, F.M.; Ma, N.; Jin, Y.; et al. T Cell Membrane Mimicking Nanoparticles with Bioorthogonal Targeting and Immune Recognition for Enhanced Photothermal Therapy. Adv. Sci. 2019, 6, 1362. [Google Scholar] [CrossRef] [PubMed]
- Choucair, K.; Duff, J.R.; Cassidy, C.S.; Albrethsen, M.T.; Kelso, J.D.; Lenhard, A.; Staats, H.; Patel, R.; Brunicardi, F.C.; Dworkin, L.; et al. Natural killer cells: A review of biology, therapeutic potential and challenges in treatment of solid tumors. Future Oncol. 2019, 15, 3053–3069. [Google Scholar] [CrossRef] [PubMed]
- Chiossone, L.; Dumas, P.Y.; Vienne, M.; Vivier, E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 2018, 18, 671–688. [Google Scholar] [CrossRef]
- Kiessling, R.; Klein, E.; Wigzell, H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur. J. Immunol. 1975, 5, 112–117. [Google Scholar] [CrossRef]
- Herberman, R.B.; Nunn, M.E.; Lavrin, D.H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int. J. Cancer 1975, 16, 216–229. [Google Scholar] [CrossRef]
- Koch, J.; Steinle, A.; Watzl, C.; Mandelboim, O. Activating natural cytotoxicity receptors of natural killer cells in cancer and infection. Trends Immunol. 2013, 34, 182–191. [Google Scholar] [CrossRef]
- Moretta, A.; Bottino, C.; Vitale, M.; Pende, D.; Cantoni, C.; Mingari, M.C.; Biassoni, R.; Moretta, L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 2001, 19, 197–223. [Google Scholar] [CrossRef]
- Schlecker, E.; Fiegler, N.; Arnold, A.; Altevogt, P.; Rose-John, S.; Moldenhauer, G.; Sucker, A.; Paschen, A.; von Strandmann, E.P.; Textor, S.; et al. Metalloprotease-mediated tumor cell shedding of B7-H6, the ligand of the natural killer cell-activating receptor NKp30. Cancer Res. 2014, 74, 3429–3440. [Google Scholar] [CrossRef] [PubMed]
- Pessino, A.; Sivori, S.; Bottino, C.; Malaspina, A.; Morelli, L.; Moretta, L.; Biassoni, R.; Moretta, A. Molecular cloning of NKp46: A novel member of the immunoglobulin superfamily involved in triggering of natural cytotoxicity. J. Exp. Med. 1998, 188, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Stannard, K.A.; Lemoine, S.; Waterhouse, N.J.; Vari, F.; Chatenoud, L.; Gandhi, M.K.; Martinet, L.; Smyth, M.J.; Guillerey, C. Human peripheral blood DNAM-1(neg) NK cells are a terminally differentiated subset with limited effector functions. Blood Adv. 2019, 3, 1681–1694. [Google Scholar] [CrossRef] [PubMed]
- Fionda, C.; Soriani, A.; Zingoni, A.; Santoni, A.; Cippitelli, M. NKG2D and DNAM-1 Ligands: Molecular Targets for NK Cell-Mediated Immunotherapeutic Intervention in Multiple Myeloma. BioMed Res. Int. 2015, 2015, 178698. [Google Scholar] [CrossRef] [PubMed]
- Soriani, A.; Fionda, C.; Ricci, B.; Iannitto, M.L.; Cippitelli, M.; Santoni, A. Chemotherapy-elicited upregulation of NKG2D and DNAM-1 ligands as a therapeutic target in multiple myeloma. Oncoimmunology 2013, 2, e26663. [Google Scholar] [CrossRef] [PubMed]
- Zingoni, A.; Ardolino, M.; Santoni, A.; Cerboni, C. NKG2D and DNAM-1 activating receptors and their ligands in NK-T cell interactions: Role in the NK cell-mediated negative regulation of T cell responses. Front. Immunol. 2012, 3, 408. [Google Scholar] [CrossRef] [PubMed]
- Pitchaimani, A.; Nguyen, T.D.T.; Aryal, S. Natural killer cell membrane infused biomimetic liposomes for targeted tumor therapy. Biomaterials 2018, 160, 124–137. [Google Scholar] [CrossRef]
- Deng, G.; Sun, Z.; Li, S.; Peng, X.; Li, W.; Zhou, L.; Ma, Y.; Gong, P.; Cai, L. Cell-Membrane Immunotherapy Based on Natural Killer Cell Membrane Coated Nanoparticles for the Effective Inhibition of Primary and Abscopal Tumor Growth. ACS Nano 2018, 12, 12096–12108. [Google Scholar] [CrossRef]
- Huang, R.; Wang, X.; Zhou, Y.; Xiao, Y. RANKL-induced M1 macrophages are involved in bone formation. Bone Res. 2017, 5, 17019. [Google Scholar] [CrossRef]
- Wan, L.; Lin, H.J.; Huang, C.C.; Chen, Y.C.; Hsu, Y.A.; Lin, C.H.; Lin, H.C.; Chang, C.Y.; Huang, S.H.; Lin, J.M.; et al. Galectin-12 enhances inflammation by promoting M1 polarization of macrophages and reduces insulin sensitivity in adipocytes. Glycobiology 2016, 26, 732–744. [Google Scholar] [CrossRef]
- Wang, D.; Lou, J.; Ouyang, C.; Chen, W.; Liu, Y.; Liu, X.; Cao, X.; Wang, J.; Lu, L. Ras-related protein Rab10 facilitates TLR4 signaling by promoting replenishment of TLR4 onto the plasma membrane. Proc. Natl. Acad. Sci. USA 2010, 107, 13806–13811. [Google Scholar] [CrossRef] [PubMed]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, A.; Khan, R.; Ghosh, M.K. Blood brain barrier: A challenge for effectual therapy of brain tumors. Biomed Res. Int. 2015, 2015, 320941. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, Y.; Ransohoff, R.M. Inflammatory cell trafficking across the blood-brain barrier: Chemokine regulation and in vitro models. Immunol. Rev. 2012, 248, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Von Wedel-Parlow, M.; Schrot, S.; Lemmen, J.; Treeratanapiboon, L.; Wegener, J.; Galla, H.J. Neutrophils cross the BBB primarily on transcellular pathways: An in vitro study. Brain Res. 2011, 1367, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Peng, X.; Sun, Z.; Zheng, W.; Yu, J.; Du, L.; Chen, H.; Gong, P.; Zhang, P.; Cai, L.; et al. Natural-Killer-Cell-Inspired Nanorobots with Aggregation-Induced Emission Characteristics for Near-Infrared-II Fluorescence-Guided Glioma Theranostics. ACS Nano 2020, 14, 11452–11462. [Google Scholar] [CrossRef]
- Lee, H.K.; Iwasaki, A. Innate control of adaptive immunity: Dendritic cells and beyond. Semin. Immunol. 2007, 19, 48–55. [Google Scholar] [CrossRef]
- Schroder, J.M.; Reich, K.; Kabashima, K.; Liu, F.T.; Romani, N.; Metz, M.; Kerstan, A.; Lee, P.H.; Loser, K.; Schon, M.P.; et al. Who is really in control of skin immunity under physiological circumstances—Lymphocytes, dendritic cells or keratinocytes? Exp. Dermatol. 2006, 15, 913–929. [Google Scholar] [CrossRef]
- Zitvogel, L. Dendritic and natural killer cells cooperate in the control/switch of innate immunity. J. Exp. Med. 2002, 195, F9–F14. [Google Scholar] [CrossRef]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef]
- Fucikova, J.; Palova-Jelinkova, L.; Bartunkova, J.; Spisek, R. Induction of Tolerance and Immunity by Dendritic Cells: Mechanisms and Clinical Applications. Front. Immunol. 2019, 10, 2393. [Google Scholar] [CrossRef] [PubMed]
- Ochyl, L.J.; Moon, J.J. Dendritic Cell Membrane Vesicles for Activation and Maintenance of Antigen-Specific T Cells. Adv. Healthc Mater. 2019, 8, e1801091. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.L.; Zou, M.Z.; Liu, T.; Zeng, J.Y.; Li, X.; Yu, W.Y.; Li, C.X.; Ye, J.J.; Song, W.; Feng, J.; et al. Expandable Immunotherapeutic Nanoplatforms Engineered from Cytomembranes of Hybrid Cells Derived from Cancer and Dendritic Cells. Adv. Mater. 2019, 31, e1900499. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Deng, G.; Peng, X.; Xu, X.; Liu, L.; Xu, Z.; Peng, J.; Ma, Y.; Zhang, P.; Wang, Y.; et al. Intelligent Photothermal Dendritic Cells Restart the Cancer Immunity Cycle. Cell Rep. 2020. under review. [Google Scholar] [CrossRef]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.W.; Yuan, H.B.; Sun, D.X. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef]
- Shenoda, B.B.; Ajit, S.K. Modulation of Immune Responses by Exosomes Derived from Antigen-Presenting Cells. Clin. Med. Insights Pathol. 2016, 9, 1–8. [Google Scholar] [CrossRef]
- Escudier, B.; Dorval, T.; Chaput, N.; Andre, F.; Caby, M.P.; Novault, S.; Flament, C.; Leboulaire, C.; Borg, C.; Amigorena, S.; et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of thefirst phase I clinical trial. J. Transl. Med. 2005, 3, 10. [Google Scholar] [CrossRef]
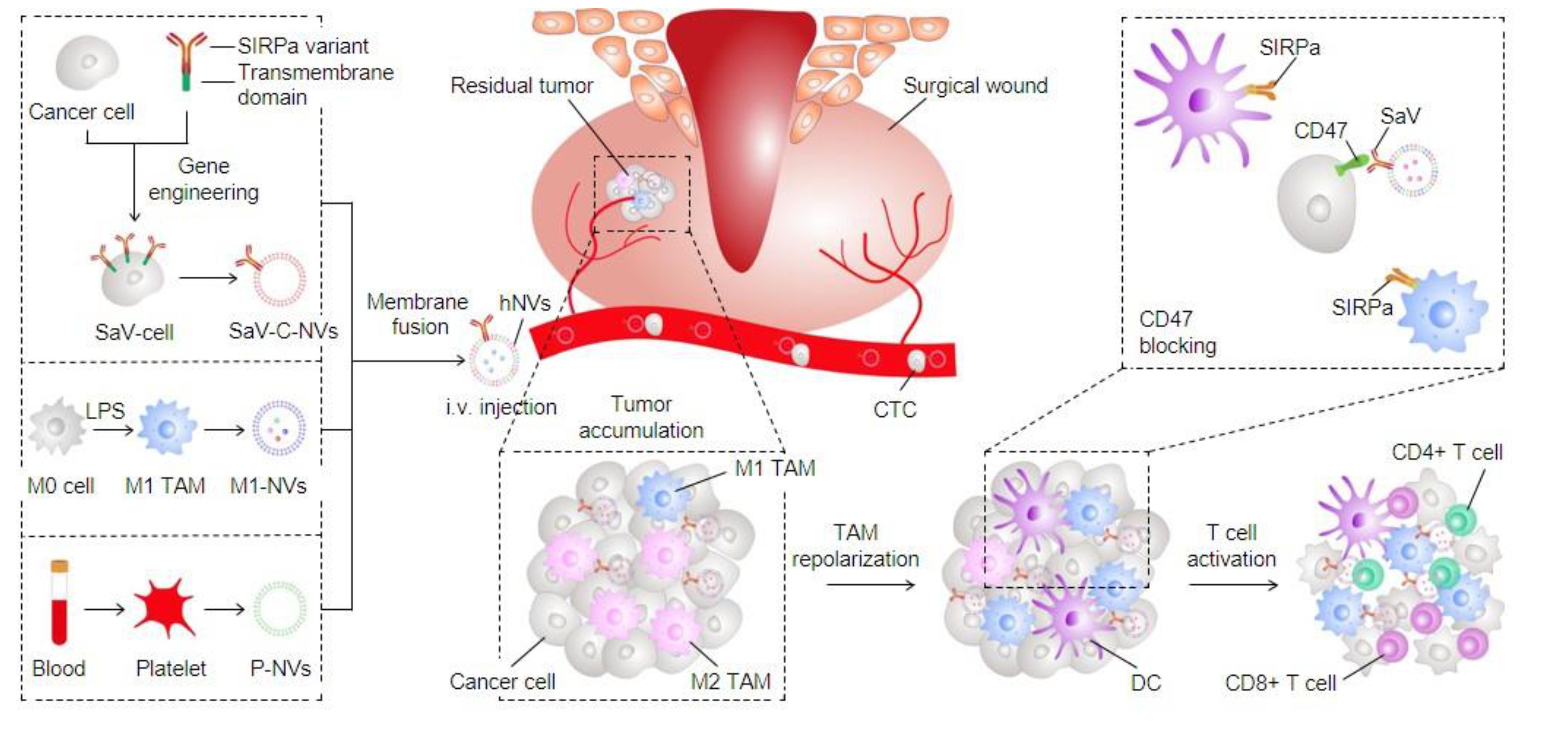
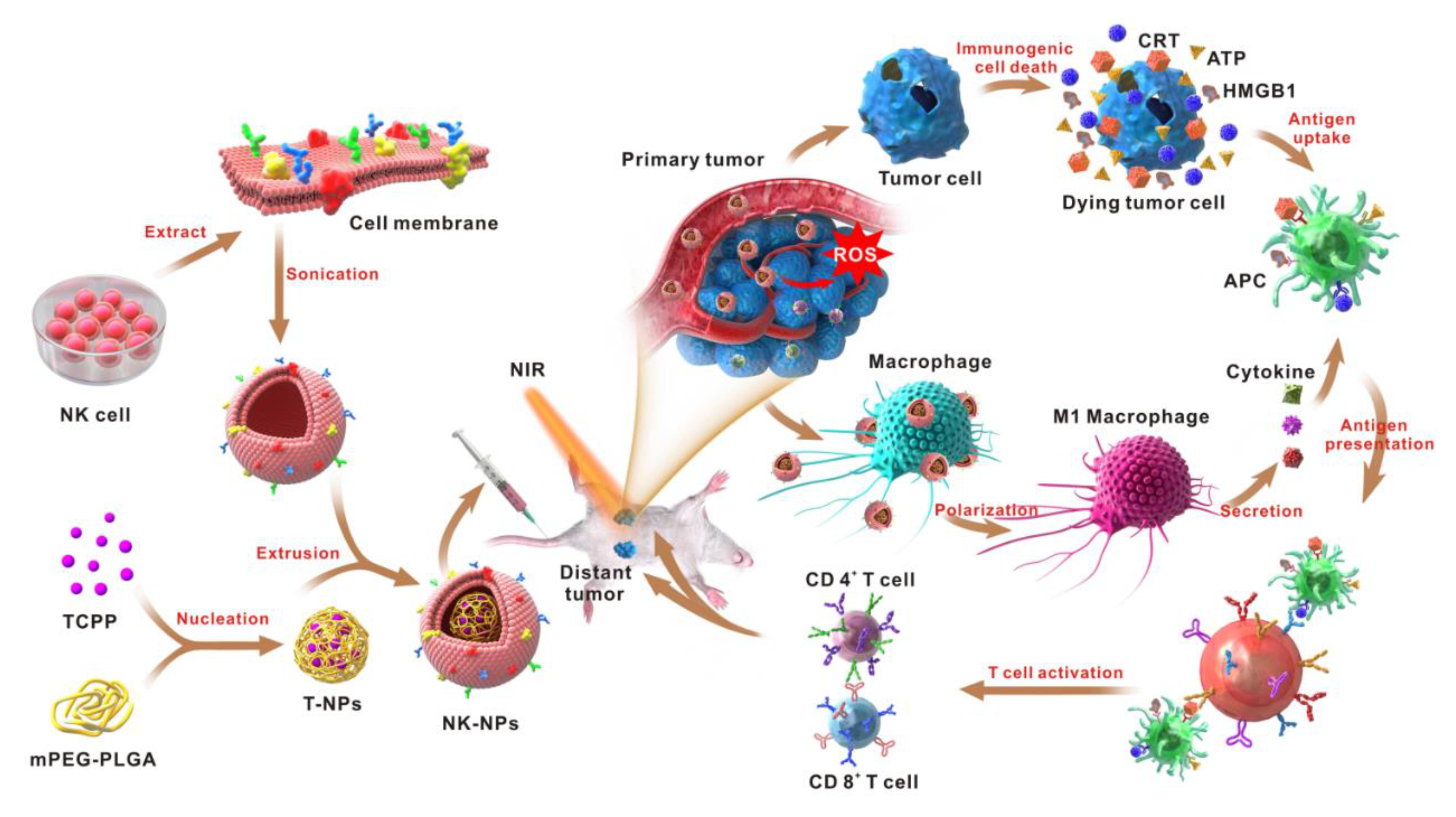
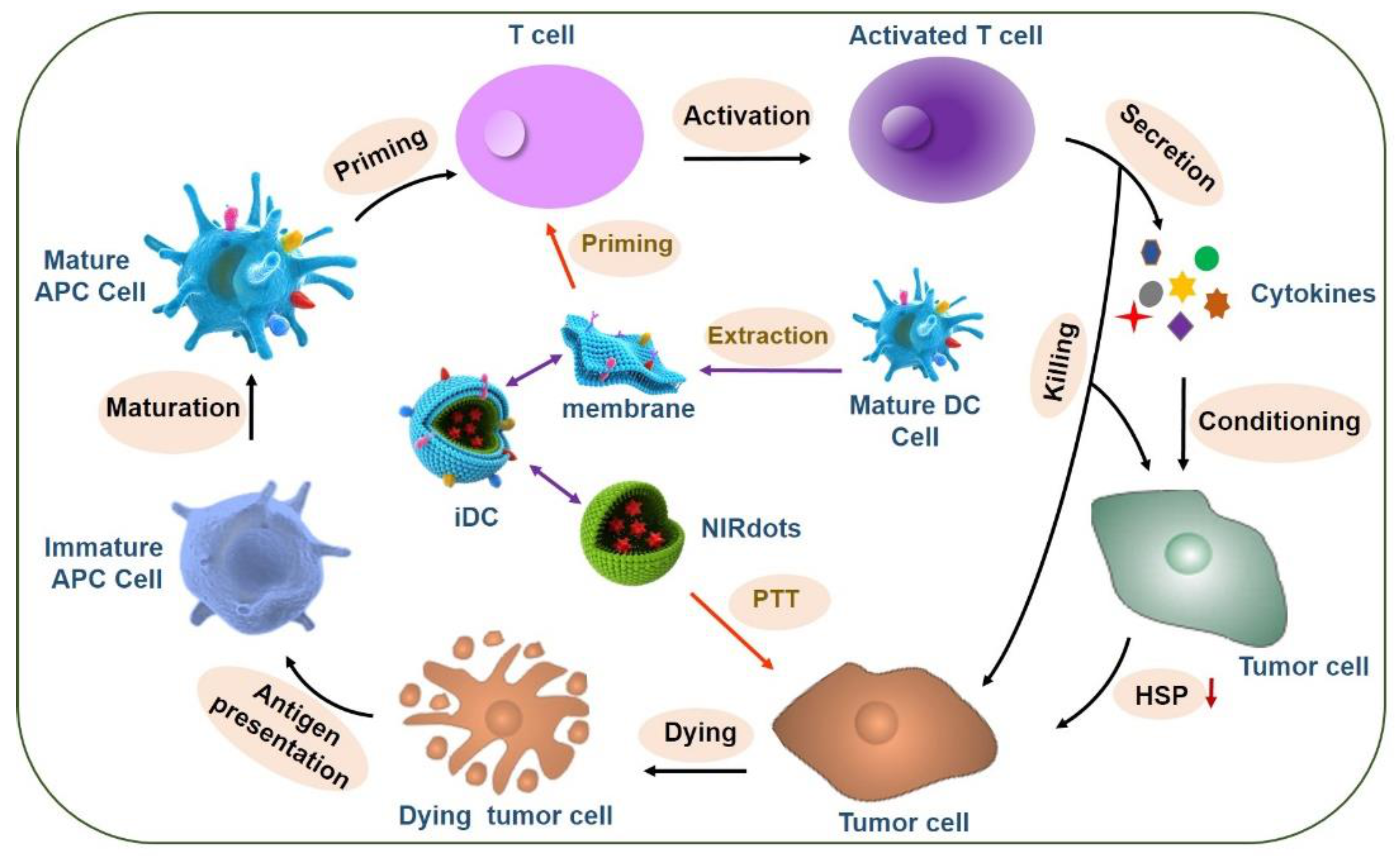
| Cell Type | Marker | Ligand | Function |
|---|---|---|---|
| Macrophage | CCR2 | CCL2 | Induces a strong chemotactic response, guides immune cells to inflammatory and tumor sites |
| VCAM-1 | 4VLA-4) or integrin α4β1 | Cell adhesion, cell signal transduction | |
| ICAM-1 | LFA-1, Mac-1 | Facilitates transmigration of leukocytes across vascular endothelia, intercellular adhesion | |
| T-cell | TCR | peptide/MHC complex | Antigen recognition and presentation |
| CD28 | CD80, CD86 | Brings T-cell and antigen-presenting cell membranes into close proximity | |
| CTLA-4 | CD80, CD86 | Immune checkpoint and down-regulates immune responses | |
| PD-1 | PD-l, B7 | Immune checkpoint and down-regulates immune responses | |
| LFA-1 | ICAM | Cell adhesion and co-stimulator | |
| LFA-2 | LFA-3, CD48 | Cell adhesion and co-stimulator | |
| NK cell | NK p46 | CD247, FCER1G. | Activates NK cells, mediates tumor cell lysis |
| NKp44 | NKp44L, 21spe-MLL5, PCNA, HSPGs | Activates NK cells, mediates tumor cell lysis. Transmembrane Signaling Receptor Activity | |
| NCAM1 | rabies virus glycoprotein | MAPK cascade, cell adhesion, host-virus interaction | |
| FCGR3 | immunoglobulin gamma Fc region | Binds to the Fc portion of igg antibodies and activates antibody-dependent cell mediated cytotoxicity (ADCC) | |
| DNAM-1 | PVR, NECTIN2 | Signal transducing adhesion involved in the adhesion of certain tumor cells to CTL and NK cells, mediates their cytotoxicity | |
| DC | peptide/MHC complex | TCR | Antigen recognition and presentation |
| INAM | IRF3 | Stimulates NK cell activation | |
| ICAM | LFA-1 | Cell adhesion and co-stimulator |
| Source of Cell Membranes | Functions | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Macrophage | Prolonged circulation time; penetrating the blood–brain barrier (BBB); anti-proliferation; tumor targeting; inflammation targeting | Immune evasion; targeting glioblastoma; enhanced intratumoral penetration | Only targeting to limited types of tumor | [66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82] |
| T-cell | Prolonged circulation time; targeting to specific tumors through TCRs; | Dual-targeting; improved tumoritropic accumulation of drug | MHC Restriction; only targeting to limited types of tumor | [92,95] |
| NK cell | Prolonged circulation time; tumor targeting; penetrating the blood–brain barrier (BBB); M1 polarization and induction of immunogenic cell death (ICD) | targeting glioblastoma; broad spectrum tumor targeting | Limited multiplication of primary NK cells | [108,109,117] |
| DC | Antigen-presenting; tumor vaccine; promote T-cells; lymph node targeting | Activation and maintenance of antigen-specific T-cells; providing immunological co-stimulatory molecules | MHC restriction; | [123,124,125] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, P.; Wang, Y.; Zhang, P.; Yang, Z.; Deng, W.; Sun, Z.; Yang, M.; Li, X.; Ma, G.; Deng, G.; et al. Immunocyte Membrane-Coated Nanoparticles for Cancer Immunotherapy. Cancers 2021, 13, 77. https://doi.org/10.3390/cancers13010077
Gong P, Wang Y, Zhang P, Yang Z, Deng W, Sun Z, Yang M, Li X, Ma G, Deng G, et al. Immunocyte Membrane-Coated Nanoparticles for Cancer Immunotherapy. Cancers. 2021; 13(1):77. https://doi.org/10.3390/cancers13010077
Chicago/Turabian StyleGong, Ping, Yifan Wang, Pengfei Zhang, Zhaogang Yang, Weiye Deng, Zhihong Sun, Mingming Yang, Xuefeng Li, Gongcheng Ma, Guanjun Deng, and et al. 2021. "Immunocyte Membrane-Coated Nanoparticles for Cancer Immunotherapy" Cancers 13, no. 1: 77. https://doi.org/10.3390/cancers13010077
APA StyleGong, P., Wang, Y., Zhang, P., Yang, Z., Deng, W., Sun, Z., Yang, M., Li, X., Ma, G., Deng, G., Dong, S., Cai, L., & Jiang, W. (2021). Immunocyte Membrane-Coated Nanoparticles for Cancer Immunotherapy. Cancers, 13(1), 77. https://doi.org/10.3390/cancers13010077






