Connexins and cAMP Cross-Talk in Cancer Progression and Metastasis
Abstract
Simple Summary
Abstract
1. Introduction
2. Regulation of Cx Expression by cAMP/PKA Signaling in Cancer Cell Growth and Primary Cancer Progression
3. cAMP/PKA Signaling Mediates the Phosphorylation of Cxs to Promote Cancer Cell Migration and Malignant Transformation
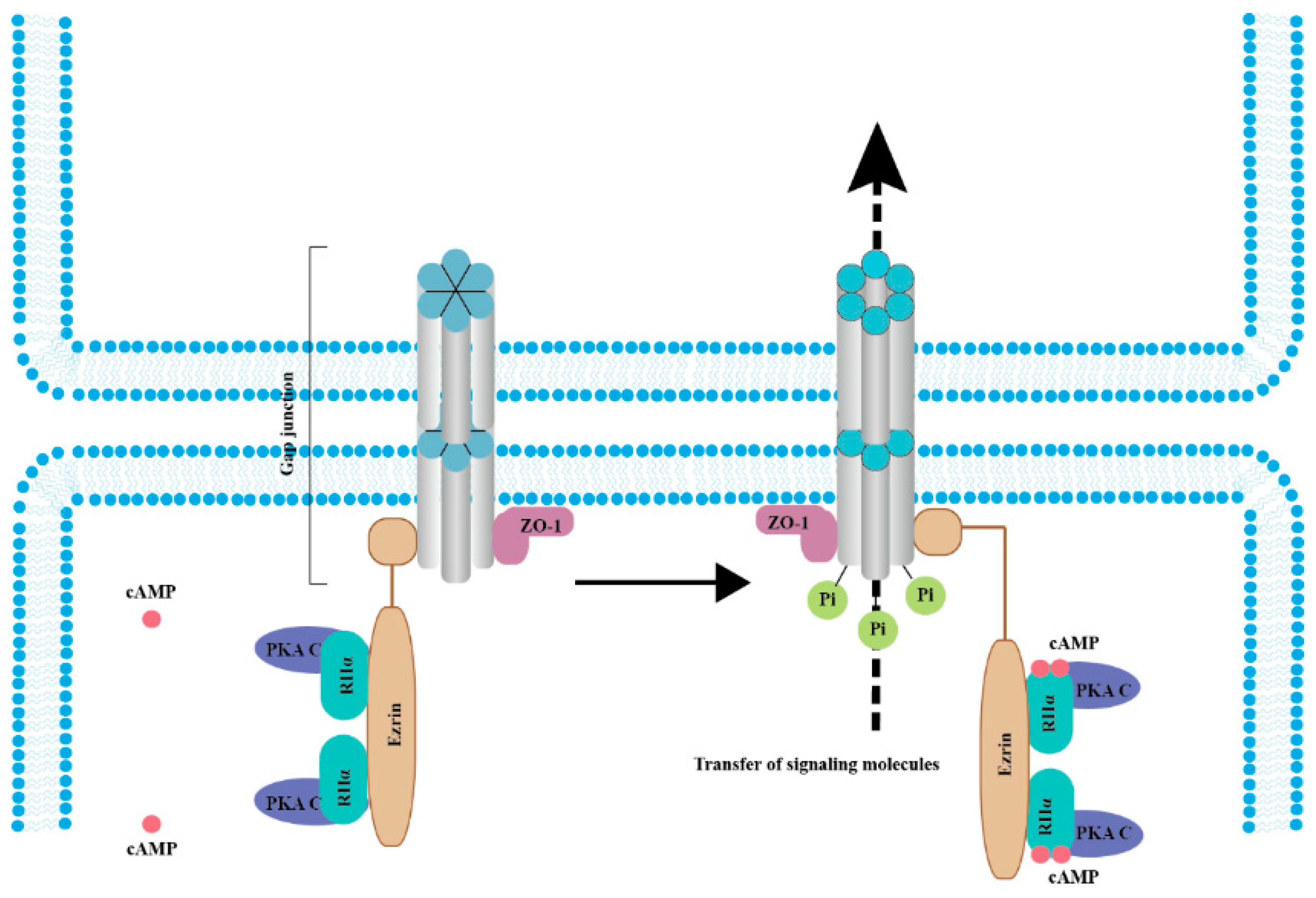
4. Gap Junction Channels Mediate Transfer of cAMP
5. Cxs and GJIC Regulate the Cell Cycle and Limit the Rate of Mitosis of the Tumor Cell Population
6. Role of Cx–cAMP in Cancer Metastasis
7. Potential Strategies Targeting cAMP and Cx for the Bystander Effect
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmidt, M.; Dekker, F.J.; Maarsingh, H. Exchange Protein Directly Activated by cAMP (epac): A Multidomain cAMP Mediator in the Regulation of Diverse Biological Functions. Pharmacol. Rev. 2013, 65, 670–709. [Google Scholar] [CrossRef]
- Sapio, L.; Gallo, M.; Illiano, M.; Chiosi, E.; Naviglio, D.; Spina, A.; Naviglio, S. The Natural cAMP Elevating Compound Forskolin in Cancer Therapy: Is It Time? J. Cell. Physiol. 2017, 232, 922–927. [Google Scholar] [CrossRef]
- Taylor, S.S.; Zhang, P.; Steichen, J.M.; Keshwani, M.M.; Kornev, A.P. PKA: Lessons learned after twenty years. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2013, 1834, 1271–1278. [Google Scholar] [CrossRef]
- Robichaux, W.G., 3rd; Cheng, X. Intracellular Camp Sensor Epac: Physiology, Pathophysiology, and Therapeutics Development. Physiol. Rev. 2018, 98, 919–1053. [Google Scholar] [CrossRef] [PubMed]
- Reggi, E.; Diviani, D. The role of A-kinase anchoring proteins in cancer development. Cell. Signal. 2017, 40, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinska, P.; Ptak, A.; Wrobel, A.; Gregoraszczuk, E.L. Hydroxylated Estrogens (2-Oh-E2 and 4-Oh-E2) Do Not Activate Camp/Pka and Erk1/2 Pathways Activation in a Breast Cancer Mcf-7 Cell Line. Endocr. Regul. 2012, 46, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Chen, Y.; Meng, F.; Zhang, Y.; Wu, H.; Wu, F. Roflumilast Restores Camp/Pka/Creb Signaling Axis for Ftmt-Mediated Tumor Inhibition of Ovarian Cancer. Oncotarget 2017, 8, 112341–112353. [Google Scholar] [CrossRef] [PubMed]
- Peverelli, E.; Giardino, E.; Mangili, F.; Treppiedi, D.; Catalano, R.; Ferrante, E.; Sala, E.; Locatelli, M.; Lania, A.G.; Arosio, M.; et al. Camp/Pka-Induced Filamin a (Flna) Phosphorylation Inhibits Sst2 Signal Transduction in Gh-Secreting Pituitary Tumor Cells. Cancer Lett. 2018, 435, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Z.; Qian, W.; Ji, D.; Wang, Q.; Ji, B.; Zhang, Y.; Zhang, C.; Sun, Y.; Zhu, C.; et al. Angiogenesis and Vasculogenic Mimicry Are Inhibited by 8-Br-Camp through Activation of the Camp/Pka Pathway in Colorectal Cancer. Onco. Targets Ther. 2018, 11, 3765–3774. [Google Scholar] [CrossRef]
- Jiang, K.; Yao, G.; Hu, L.; Yan, Y.; Liu, J.; Shi, J.; Chang, Y.; Zhang, Y.; Liang, D.; Shen, D.; et al. MOB2 suppresses GBM cell migration and invasion via regulation of FAK/Akt and cAMP/PKA signaling. Cell Death Dis. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Huang, F.; Ma, G.; Zhou, X.; Zhu, X.; Yu, X.; Ding, F.; Cao, X.; Liu, Z. Depletion of LAMP3 enhances PKA-mediated VASP phosphorylation to suppress invasion and metastasis in esophageal squamous cell carcinoma. Cancer Lett. 2020, 479, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Tonucci, F.M.; Almada, E.; Borini-Etichetti, C.; Pariani, A.; Hidalgo, F.; Rico, M.J.; Girardini, J.; Favre, C.; Goldenring, J.R.; Menacho-Márquez, M.; et al. Identification of a CIP4 PKA phosphorylation site involved in the regulation of cancer cell invasiveness and metastasis. Cancer Lett. 2019, 461, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Howell, G.M.; Rajput, A.; Teggart, C.A.; Brattain, L.E.; Weber, H.R.; Chowdhury, A.; Brattain, M.G. Identification of a Novel Tgfbeta/Pka Signaling Transduceome in Mediating Control of Cell Survival and Metastasis in Colon Cancer. PLoS ONE 2011, 6, e19335. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.F.; Faucz, F.R.; Bimpaki, E.; Horvath, A.; Levy, I.; De Alexandre, R.B.; Ahmad, F.; Manganiello, V.; Stratakis, C.A. Clinical and Molecular Genetics of the Phosphodiesterases (PDEs). Endocr. Rev. 2014, 35, 195–233. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.H.; Martin, P.E.M. Gap junctions: Structure and function (Review). Mol. Membr. Biol. 2002, 19, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Willecke, K.; Eiberger, J.; Degen, J.; Eckardt, D.; Romualdi, A.; Güldenagel, M.; Deutsch, U.; Söhl, G. Structural and Functional Diversity of Connexin Genes in the Mouse and Human Genome. Biol. Chem. 2002, 383, 725–737. [Google Scholar] [CrossRef]
- Söhl, G. Gap junctions and the connexin protein family. Cardiovasc. Res. 2004, 62, 228–232. [Google Scholar] [CrossRef]
- Rackauskas, M.; Neverauskas, V.; Skeberdis, V.A. Diversity and properties of connexin gap junction channels. Medicine 2010, 46, 1–12. [Google Scholar] [CrossRef]
- Aasen, T.; Mesnil, M.; Naus, C.C.; Lampe, P.D.; Laird, D. Gap junctions and cancer: Communicating for 50 years. Nat. Rev. Cancer 2016, 16, 775–788. [Google Scholar] [CrossRef]
- Laird, D.W.; Lampe, P.D. Therapeutic strategies targeting connexins. Nat. Rev. Drug Discov. 2018, 17, 905–921. [Google Scholar] [CrossRef]
- Chandrasekhar, A.; Kalmykov, E.A.; Polusani, S.R.; Mathis, S.A.; Zucker, S.N.; Nicholson, B.J. Intercellular Redistribution of cAMP Underlies Selective Suppression of Cancer Cell Growth by Connexin26. PLoS ONE 2013, 8, e82335. [Google Scholar] [CrossRef] [PubMed]
- Massimi, M.; Ragusa, F.; Cardarelli, S.; Giorgi, M. Targeting Cyclic AMP Signalling in Hepatocellular Carcinoma. Cells 2019, 8, 1511. [Google Scholar] [CrossRef] [PubMed]
- Ionta, M.; Rosa, M.C.; Almeida, R.B.; Freitas, V.M.; Rezende-Teixeira, P.; Machado-Santelli, G.M. Retinoic acid and cAMP inhibit rat hepatocellular carcinoma cell proliferation and enhance cell differentiation. Braz. J. Med Biol. Res. 2012, 45, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Faucheux, N.; Dufresne, M.; Nagel, M.-D. Organization of cyclic AMP-dependent connexin 43 in Swiss 3T3 cells attached to a cellulose substratum. Biomaterials 2002, 23, 413–421. [Google Scholar] [CrossRef]
- Haefliger, J.A.; Rohner-Jeanrenaud, F.; Caille, D.; Charollais, A.; Meda, P.; Allagnat, F. Hyperglycemia Downregulates Connexin36 in Pancreatic Islets Via the Upregulation of Icer-1/Icer-1gamma. J. Mol. Endocrinol. 2013, 51, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chuang, A.Z.; O’Brien, J. Regulation of photoreceptor gap junction phosphorylation by adenosine in zebrafish retina. Vis. Neurosci. 2014, 31, 237–243. [Google Scholar] [CrossRef]
- Yogo, K.; Ogawa, T.; Akiyama, M.; Ishida-Kitagawa, N.; Sasada, H.; Sato, E.; Takeya, T. PKA Implicated in the Phosphorylation of Cx43 Induced by Stimulation with FSH in Rat Granulosa Cells. J. Reprod. Dev. 2006, 52, 321–328. [Google Scholar] [CrossRef]
- Pidoux, G.; Gerbaud, P.; Dompierre, J.; Lygren, B.; Solstad, T.; Evain-Brion, D.; Taskén, K. A PKA-ezrin-Cx43 signaling complex controls gap junction communication and thereby trophoblast cell fusion. J. Cell Sci. 2014, 127, 4172–4185. [Google Scholar] [CrossRef]
- Loewenstein, W.R.; Kanno, Y. Intercellular Communication and the Control of Tissue Growth: Lack of Communication between Cancer Cells. Nature 1966, 209, 1248–1249. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Wu, X.; Li, C.; Huang, Y.; Zhou, H.; Cui, Y. Resveratrol Sensitizes Colorectal Cancer Cells to Cetuximab by Connexin 43 Upregulation-Induced Akt Inhibition. Front. Oncol. 2020, 10, 383. [Google Scholar] [CrossRef]
- Busby, M.; Hallett, M.T.; Plante, I. The Complex Subtype-Dependent Role of Connexin 43 (GJA1) in Breast Cancer. Int. J. Mol. Sci. 2018, 19, 693. [Google Scholar] [CrossRef] [PubMed]
- Naoi, Y.; Miyoshi, Y.; Taguchi, T.; Kim, S.J.; Arai, T.; Tamaki, Y.; Noguchi, S. Connexin26 expression is associated with lymphatic vessel invasion and poor prognosis in human breast cancer. Breast Cancer Res. Treat. 2007, 106, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Lamiche, C.; Clarhaut, J.; Strale, P.-O.; Crespin, S.; Pedretti, N.; Bernard, F.-X.; Naus, C.C.; Chen, V.C.; Foster, L.J.; Defamie, N.; et al. The gap junction protein Cx43 is involved in the bone-targeted metastatic behaviour of human prostate cancer cells. Clin. Exp. Metastasis 2011, 29, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Stoletov, K.; Strnadel, J.; Zardouzian, E.; Momiyama, M.; Park, F.D.; Kelber, J.A.; Pizzo, D.P.; Hoffman, R.; Vandenberg, S.R.; Klemke, R.L. Role of connexins in metastatic breast cancer and melanoma brain colonization. J. Cell Sci. 2013, 126, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Teleki, I.; Szasz, A.M.; Maros, M.E.; Gyorffy, B.; Kulka, J.; Meggyeshazi, N.; Kiszner, G.; Balla, P.; Samu, A.; Krenács, T. Correlations of Differentially Expressed Gap Junction Connexins Cx26, Cx30, Cx32, Cx43 and Cx46 with Breast Cancer Progression and Prognosis. PLoS ONE 2014, 9, e112541. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ma, D.; Zhang, Y.; Li, Z.; Geng, L. Cervical squamous cancer mRNA profiles reveal the key genes of metastasis and invasion. Eur. J. Gynaecol. Oncol. 2015, 36, 309–317. [Google Scholar]
- El-Sabban, M.E.; Pauli, B.U. Cytoplasmic dye transfer between metastatic tumor cells and vascular endothelium. J. Cell Biol. 1991, 115, 1375–1382. [Google Scholar] [CrossRef]
- Elzarrad, M.K.; Haroon, A.; Willecke, K.; Dobrowolski, R.; Gillespie, M.N.; Al-Mehdi, A.-B. Connexin-43 upregulation in micrometastases and tumor vasculature and its role in tumor cell attachment to pulmonary endothelium. BMC Med. 2008, 6, 20. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Chen, C.-Z.; Jin, L.-P.; Ji, Q.-Q.; Chen, Y.-Z.; Li, Q.; Zhang, X.-H.; Qu, J.-M. Effect and mechanism of the metastasis suppressor gene BRMS1 on the migration of breast cancer cells. Int. J. Clin. Exp. Med. 2013, 6, 908–916. [Google Scholar]
- Friedl, P.; Mayor, R. Tuning Collective Cell Migration by Cell–Cell Junction Regulation. Cold Spring Harb. Perspect. Biol. 2017, 9, a029199. [Google Scholar] [CrossRef]
- Polusani, S.R.; Kalmykov, E.A.; Chandrasekhar, A.; Zucker, S.N.; Nicholson, B.J. Cell coupling mediated by connexin 26 selectively contributes to reduced adhesivity and increased migration. J. Cell Sci. 2016, 129, 4399–4410. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yao, J.; Chi, Y.; Sawada, N.; Araki, I.; Kitamura, M.; Takeda, M. Eviprostat Activates cAMP Signaling Pathway and Suppresses Bladder Smooth Muscle Cell Proliferation. Int. J. Mol. Sci. 2013, 14, 12107–12122. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.L.; Lai, S.Y.; Lai, W.A.; Lee, M.T.; Liao, C.F.; Ke, F.C.; Hwang, J.J. Crtc2 and Nedd4 Ligase Involvement in Fsh and Tgfbeta1 Upregulation of Connexin43 Gap Junction. J. Mol. Endocrinol. 2015, 55, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.P.; Park, S.S.; Ryu, J.M.; Park, J.H.; Kim, M.O.; Lee, J.-H.; Han, H.J. Mechanism of PKA-Dependent and Lipid-Raft Independent Stimulation of Connexin43 Expression by Oxytoxin in Mouse Embryonic Stem Cells. Mol. Endocrinol. 2012, 26, 1144–1157. [Google Scholar] [CrossRef][Green Version]
- Wang, S.; Qiu, J.; Liu, L.; Su, C.; Qi, L.; Huang, C.; Chen, X.; Zhang, Y.; Ye, Y.; Ding, Y.; et al. CREB5 promotes invasiveness and metastasis in colorectal cancer by directly activating MET. J. Exp. Clin. Cancer Res. 2020, 39, 168. [Google Scholar] [CrossRef]
- van De Merbel, A.F.; van Der Horst, G.; Buijs, J.T.; van Der Pluijm, G. Protocols for Migration and Invasion Studies in Prostate Cancer. In Methods in Molecular Biology; Culig, Z., Ed.; Humana: New York, NY, USA, 2018; Volume 1786, pp. 67–79. [Google Scholar] [CrossRef]
- Warn-Cramer, B.J.; Lampe, P.D.; Kurata, W.E.; Kanemitsu, M.Y.; Loo, L.W.M.; Eckhart, W.; Lau, A.F. Characterization of the Mitogen-activated Protein Kinase Phosphorylation Sites on the Connexin-43 Gap Junction Protein. J. Biol. Chem. 1996, 271, 3779–3786. [Google Scholar] [CrossRef]
- Warn-Cramer, B.J.; Cottrell, G.T.; Burt, J.M.; Lau, A.F. Regulation of Connexin-43 Gap Junctional Intercellular Communication by Mitogen-activated Protein Kinase. J. Biol. Chem. 1998, 273, 9188–9196. [Google Scholar] [CrossRef]
- Chen, V.C.; Gouw, J.W.; Naus, C.C.; Foster, L.J. Connexin multi-site phosphorylation: Mass spectrometry-based proteomics fills the gap. Biochim. Biophys. Acta (BBA) Biomembr. 2013, 1828, 23–34. [Google Scholar] [CrossRef]
- Solan, J.L.; Lampe, P.D. Specific Cx43 phosphorylation events regulate gap junction turnover in vivo. FEBS Lett. 2014, 588, 1423–1429. [Google Scholar] [CrossRef]
- Friis, U.G.; Madsen, K.; Stubbe, J.; Hansen, P.B.; Svenningsen, P.; Bie, P.; Skøtt, O.; Jensen, B.L. Regulation of renin secretion by renal juxtaglomerular cells. Pflügers Arch. Eur. J. Physiol. 2012, 465, 25–37. [Google Scholar] [CrossRef]
- Begandt, D.; Bader, A.; Gerhard, L.; Lindner, J.; Dreyer, L.; Schlingmann, B.; Ngezahayo, A. Dipyridamole-related enhancement of gap junction coupling in the GM-7373 aortic endothelial cells correlates with an increase in the amount of connexin 43 mRNA and protein as well as gap junction plaques. J. Bioenerg. Biomembr. 2013, 45, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Thévenin, A.F.; Margraf, R.A.; Fisher, C.G.; Kells-Andrews, R.M.; Falk, M.M. Phosphorylation regulates connexin43/ZO-1 binding and release, an important step in gap junction turnover. Mol. Biol. Cell 2017, 28, 3595–3608. [Google Scholar] [CrossRef] [PubMed]
- Dukic, A.R.; Haugen, L.H.; Pidoux, G.; Leithe, E.; Bakke, O.; Taskén, K. A protein kinase A-ezrin complex regulates connexin 43 gap junction communication in liver epithelial cells. Cell. Signal. 2017, 32, 1–11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dukic, A.R.; Gerbaud, P.; Guibourdenche, J.; Thiede, B.; Taskén, K.; Pidoux, G. Ezrin-anchored PKA phosphorylates serine 369 and 373 on connexin 43 to enhance gap junction assembly, communication, and cell fusion. Biochem. J. 2018, 475, 455–476. [Google Scholar] [CrossRef]
- Gould, V.E.; Mosquera, J.M.; Leykauf, K.; Gattuso, P.; Dürst, M.; Alonso, A. The phosphorylated form of connexin43 is up-regulated in breast hyperplasias and carcinomas and in their neoformed capillaries. Hum. Pathol. 2005, 36, 536–545. [Google Scholar] [CrossRef]
- Ye, X.-Y.; Jiang, Q.-H.; Hong, T.; Zhang, Z.-Y.; Yang, R.-J.; Huang, J.-Q.; Hu, K.; Peng, Y.-P. Altered expression of connexin43 and phosphorylation connexin43 in glioma tumors. Int. J. Clin. Exp. Pathol. 2015, 8, 4296–4306. [Google Scholar]
- Riquelme, M.A.; Burra, S.; Kar, R.; Lampe, P.D.; Jiang, J.X. Mitogen-activated Protein Kinase (MAPK) Activated by Prostaglandin E2Phosphorylates Connexin 43 and Closes Osteocytic Hemichannels in Response to Continuous Flow Shear Stress. J. Biol. Chem. 2015, 290, 28321–28328. [Google Scholar] [CrossRef]
- Flores, C.E.; Cachope, R.; Nannapaneni, S.; Ene, S.; Nairn, A.C.; Pereda, A.E. Variability of Distribution of Ca2+/Calmodulin-Dependent Kinase II at Mixed Synapses on the Mauthner Cell: Colocalization and Association with Connexin 35. J. Neurosci. 2010, 30, 9488–9499. [Google Scholar] [CrossRef]
- Valiunas, V.; Brink, P.R.; White, T.W. Lens Connexin Channels Have Differential Permeability to the Second Messenger cAMP. Investig. Opthalmology Vis. Sci. 2019, 60, 3821–3829. [Google Scholar] [CrossRef]
- Bedner, P.; Niessen, H.; Odermatt, B.; Kretz, M.; Willecke, K.; Harz, H. Selective Permeability of Different Connexin Channels to the Second Messenger Cyclic AMP. J. Biol. Chem. 2006, 281, 6673–6681. [Google Scholar] [CrossRef]
- Gupta, A.; Anderson, H.; Buo, A.M.; Moorer, M.C.; Ren, M.; Stains, J.P. Communication of cAMP by connexin43 gap junctions regulates osteoblast signaling and gene expression. Cell. Signal. 2016, 28, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Wynn, J.; Shah, U.; Murray, S.A. Redistribution of connexin 43 by cAMP: A mechanism for growth control in adrenal cells. Endocr. Res. 2002, 28, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Meroni, S.B.; Galardo, M.N.; Rindone, G.; Gorga, A.; Riera, M.F.; Cigorraga, S.B. Molecular Mechanisms and Signaling Pathways Involved in Sertoli Cell Proliferation. Front. Endocrinol. 2019, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Ai, G.; Wang, D.; Chen, R.; Guo, D.; Yao, Y.; Wang, K.; Liang, G.; Qi, F.; Liu, W.; et al. PDE4 and Epac1 Synergistically Promote Rectal Carcinoma via the cAMP Pathway. Anal. Cell. Pathol. 2019, 2019, 1–5. [Google Scholar] [CrossRef]
- Seino, S.; Shibasaki, T. Pka-Dependent and Pka-Independent Pathways for Camp-Regulated Exocytosis. Physiol. Rev. 2005, 85, 1303–1342. [Google Scholar] [CrossRef]
- Gancedo, J.M. Biological roles of cAMP: Variations on a theme in the different kingdoms of life. Biol. Rev. 2013, 88, 645–668. [Google Scholar] [CrossRef]
- Ji, H.; Qiu, R.; Gao, X.; Zhang, R.; Li, X.; Hei, Z.; Yuan, D. Propofol attenuates monocyte-endothelial adhesion via modulating connexin43 expression in monocytes. Life Sci. 2019, 232, 116624. [Google Scholar] [CrossRef]
- Mehta, P.; Piao, X. Adhesion G-protein coupled receptors and extracellular matrix proteins: Roles in myelination and glial cell development. Dev. Dyn. 2017, 246, 275–284. [Google Scholar] [CrossRef]
- Scholz, N. Cancer Cell Mechanics: Adhesion G Protein-coupled Receptors in Action? Front. Oncol. 2018, 8, 59. [Google Scholar] [CrossRef]
- Xie, T.; Tang, Y.; Luo, R.; Zhang, X.; Wu, S.; Gu, Y.; Liu, T.; Hu, F. GPR64 promotes cAMP pathway in tumor aggressiveness in sparsely granulated growth hormone cell adenomas. Endocrine 2020, 68, 629–639. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, M.-F.; Yang, M.-L.; Wu, T.-Y.; Xu, C.-J.; Wang, J.-M.; Li, C.-J.; Li, X. G Protein–Coupled Receptor 30 Mediates the Anticancer Effects Induced by Eicosapentaenoic Acid in Ovarian Cancer Cells. Cancer Res. Treat. 2020, 52, 815. [Google Scholar] [CrossRef] [PubMed]
- Delaunay, M.; Osman, H.; Kaiser, S.; Diviani, D. The Role of Cyclic AMP Signaling in Cardiac Fibrosis. Cells 2019, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Kurrikoff, K.; Aphkhazava, D.; Langel, Ü. The future of peptides in cancer treatment. Curr. Opin. Pharmacol. 2019, 47, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Michalides, R.; Griekspoor, A.; Balkenende, A.; Verwoerd, D.; Janssen, L.; Jalink, K.; Floore, A.; Velds, A.; van’t Veer, L.; Neefjes, J. Tamoxifen Resistance by a Conformational Arrest of the Estrogen Receptor Alpha after Pka Activation in Breast Cancer. Cancer Cell 2004, 5, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Liu, J.; She, L.; Chen, J.; Zhu, T.; Yin, J.; Li, X.; Li, X.; Zhou, H.; Liu, Z. A perspective profile of ADCY1 in cAMP signaling with drug-resistance in lung cancer. J. Cancer 2019, 10, 6848–6857. [Google Scholar] [CrossRef]
- Sun, P.; Liu, Y.; Ying, H.; Li, S. Action of Db-Camp on the Bystander Effect and Chemosensitivity through Connexin 43 and Bcl-2-Mediated Pathways in Medulloblastoma Cells. Oncol. Rep. 2012, 28, 969–976. [Google Scholar] [CrossRef]
- Wu, D.P.; Ding, C.H.; Bai, L.R.; Zhou, Y.; Yang, S.M.; Zhang, F.; Huang, J.L. Decreased Phototoxicity of Photodynamic Therapy by Cx32/Cx26-Composed Gjic: A “Good Samaritan” Effect. Lasers Surg. Med. 2019, 51, 301–308. [Google Scholar] [CrossRef]
- Ponsioen, B.; Van Zeijl, L.; Moolenaar, W.H.; Jalink, K. Direct measurement of cyclic AMP diffusion and signaling through connexin43 gap junctional channels. Exp. Cell Res. 2007, 313, 415–423. [Google Scholar] [CrossRef]
- Van Der Heyden, M.A.; Rook, M.B.; Hermans, M.M.; Rijksen, G.; Boonstra, J.; Defize, L.H.; Destrée, O.H. Identification of connexin43 as a functional target for Wnt signalling. J. Cell Sci. 1998, 111, 1741–1749. [Google Scholar]
- Carystinos, G.D.; Kandouz, M.; Alaoui-Jamali, M.A.; Batist, G. Unexpected induction of the human connexin 43 promoter by the ras signaling pathway is mediated by a novel putative promoter sequence. Mol. Pharmacol. 2003, 63, 821–831. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-X.; Luo, K.-J.; Yang, J.-P.; Huang, Y.-C.; Cardenas, E.R.; Nicholson, B.J.; Jiang, J.X. Connexins and cAMP Cross-Talk in Cancer Progression and Metastasis. Cancers 2021, 13, 58. https://doi.org/10.3390/cancers13010058
Chen C-X, Luo K-J, Yang J-P, Huang Y-C, Cardenas ER, Nicholson BJ, Jiang JX. Connexins and cAMP Cross-Talk in Cancer Progression and Metastasis. Cancers. 2021; 13(1):58. https://doi.org/10.3390/cancers13010058
Chicago/Turabian StyleChen, Chang-Xu, Kai-Jun Luo, Jia-Peng Yang, Yun-Chao Huang, Eduardo R. Cardenas, Bruce J. Nicholson, and Jean X. Jiang. 2021. "Connexins and cAMP Cross-Talk in Cancer Progression and Metastasis" Cancers 13, no. 1: 58. https://doi.org/10.3390/cancers13010058
APA StyleChen, C.-X., Luo, K.-J., Yang, J.-P., Huang, Y.-C., Cardenas, E. R., Nicholson, B. J., & Jiang, J. X. (2021). Connexins and cAMP Cross-Talk in Cancer Progression and Metastasis. Cancers, 13(1), 58. https://doi.org/10.3390/cancers13010058






