Comparative Efficacy and Safety of Anti-PD-1/PD-L1 Immune Checkpoint Inhibitors for Refractory or Relapsed Advanced Non-Small-Cell Lung Cancer—A Systematic Review and Network Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
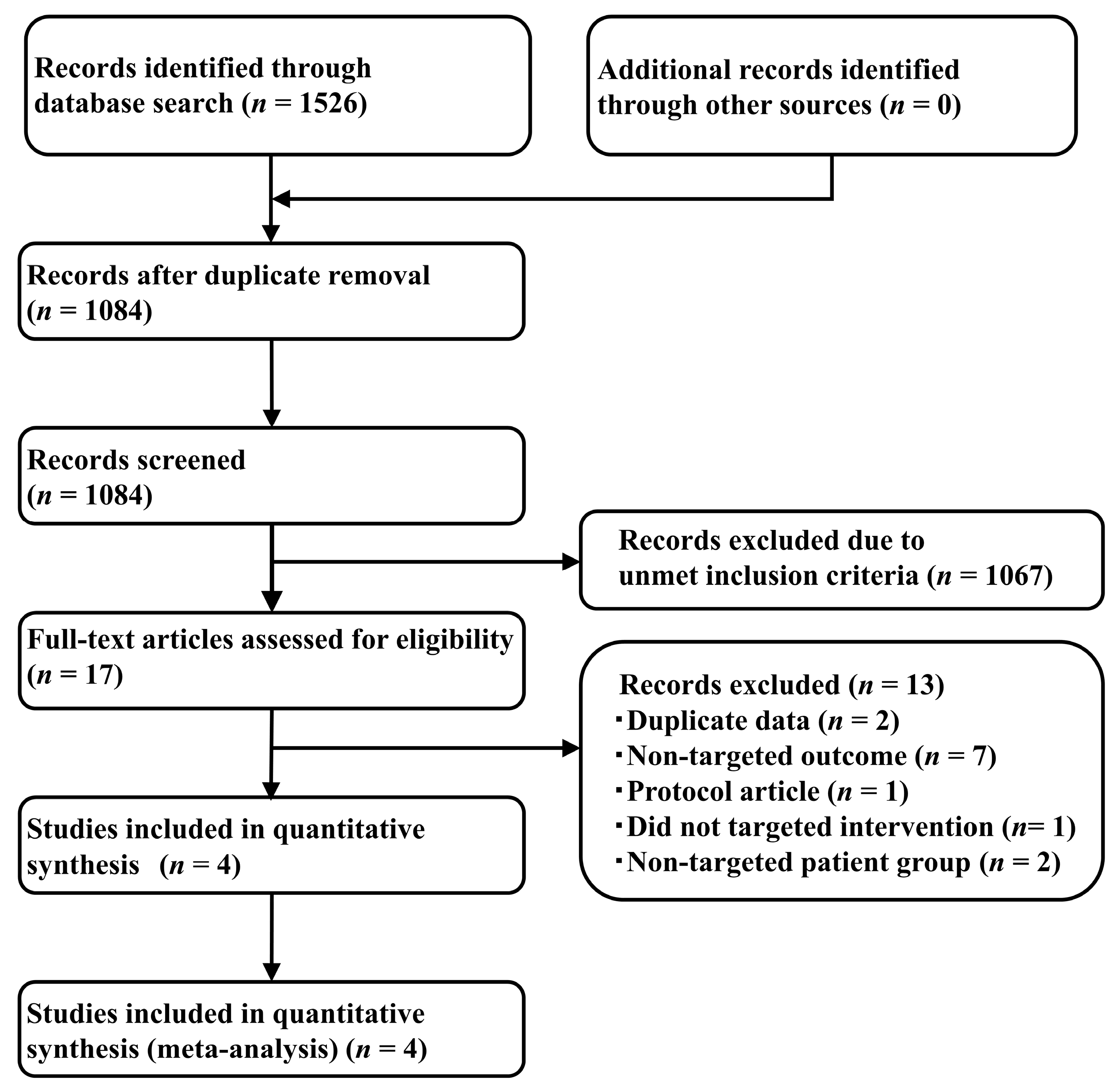
2.1. Systematic Review
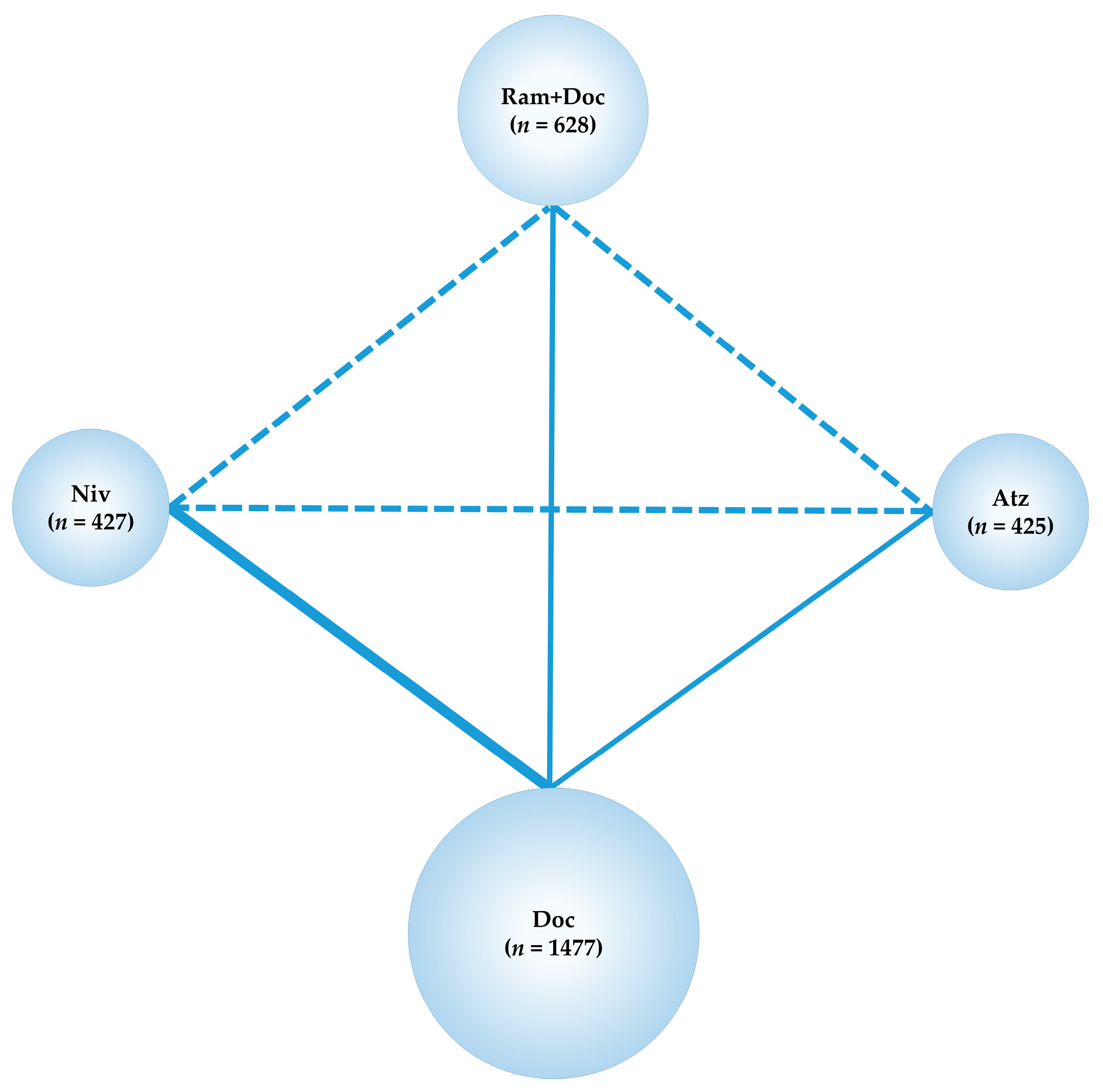
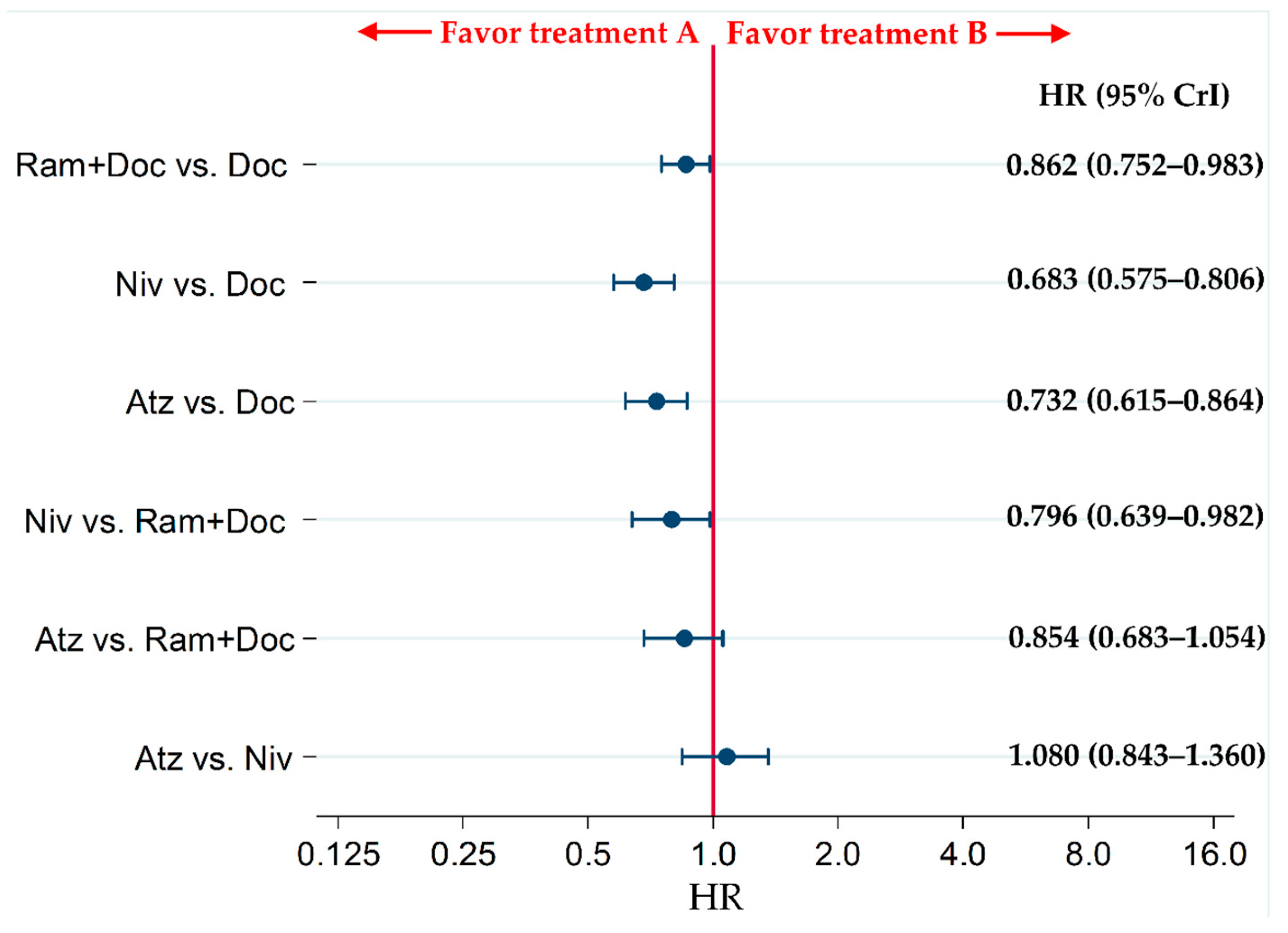
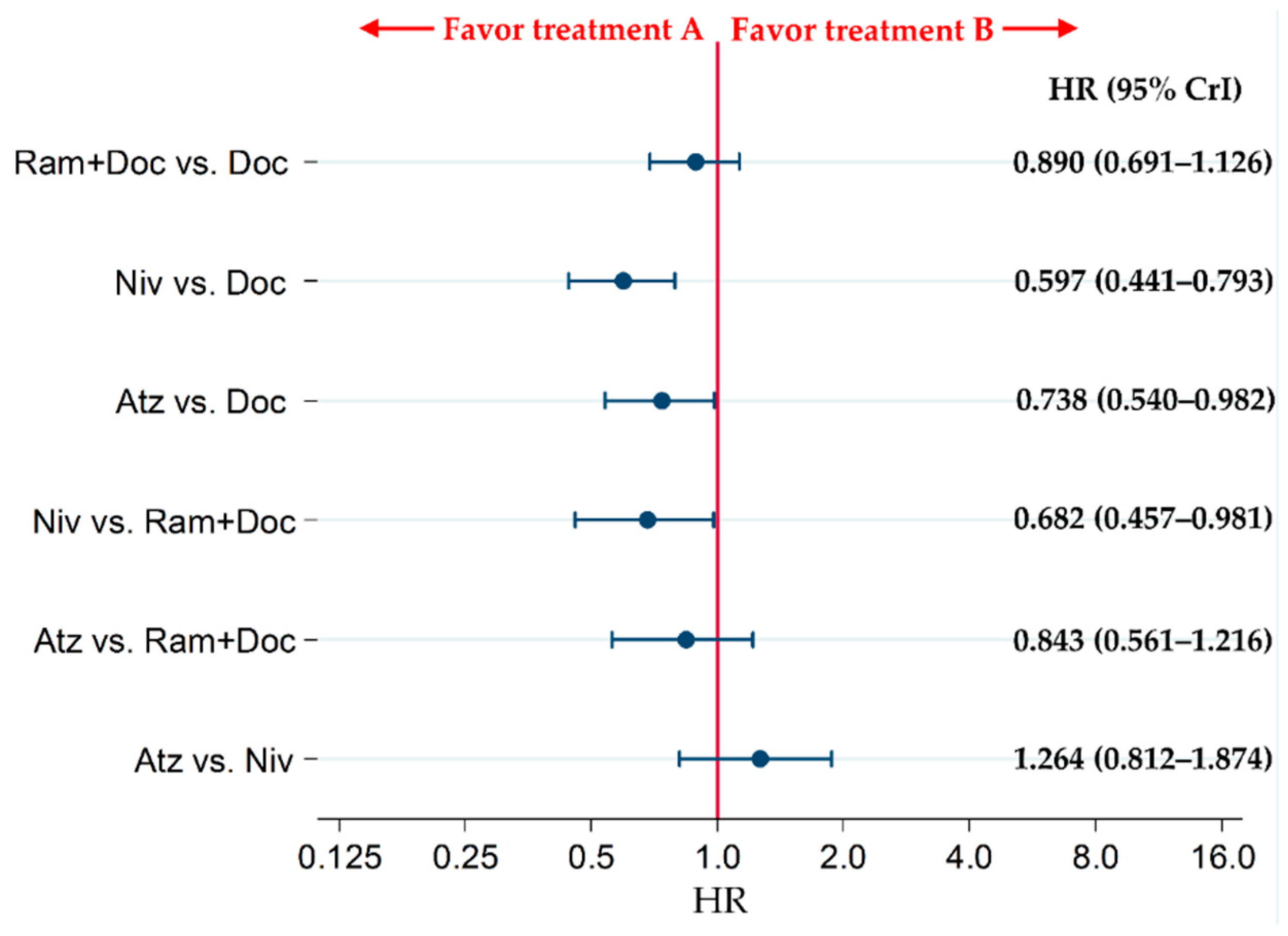
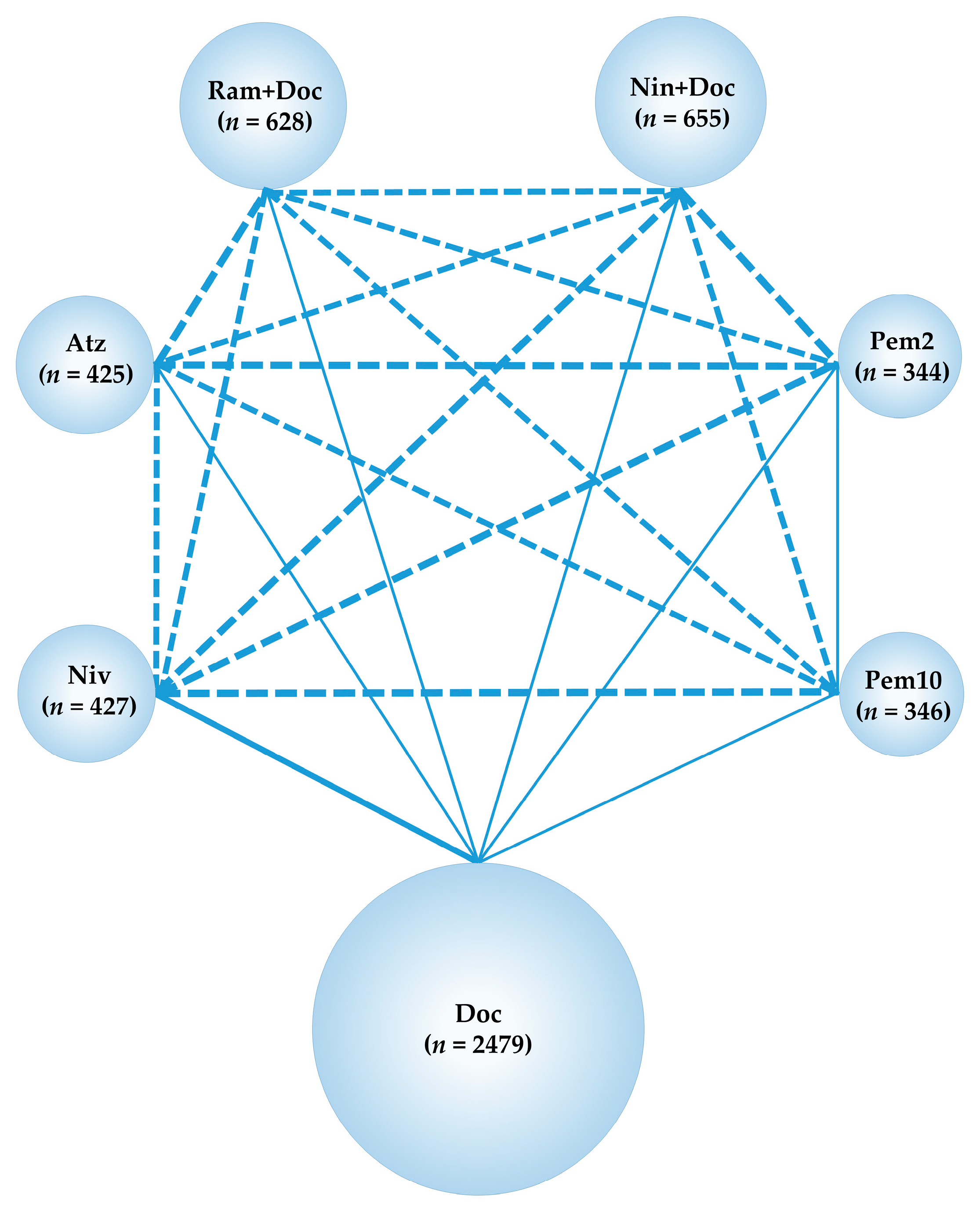
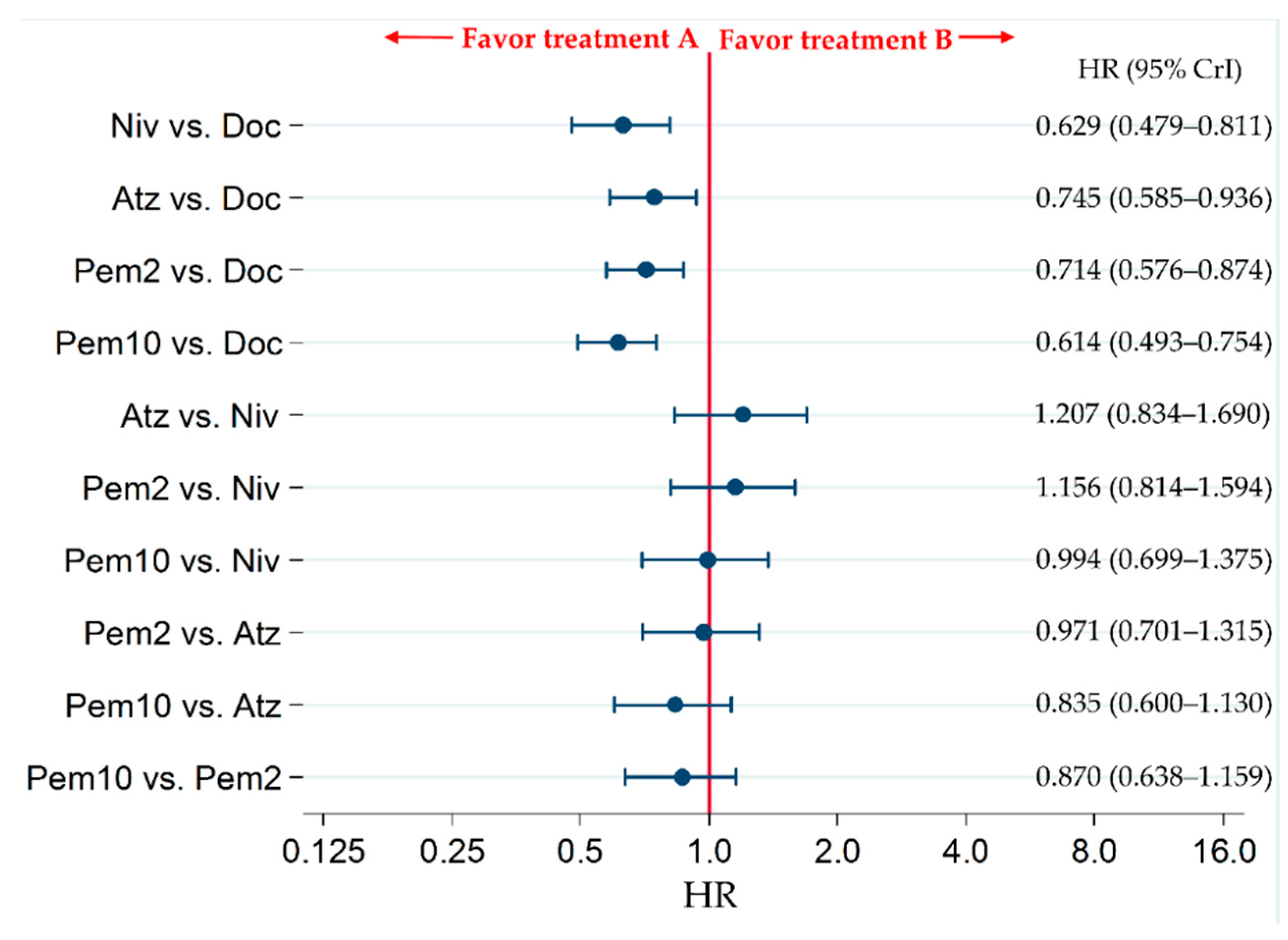
2.2. Primary Efficacy Endpoint: OS
2.3. Subgroup Analysis of the OS of Patients with Non-Squamous NSCLC
2.4. Subgroup Analysis of OS of Patients with Squamous NSCLC
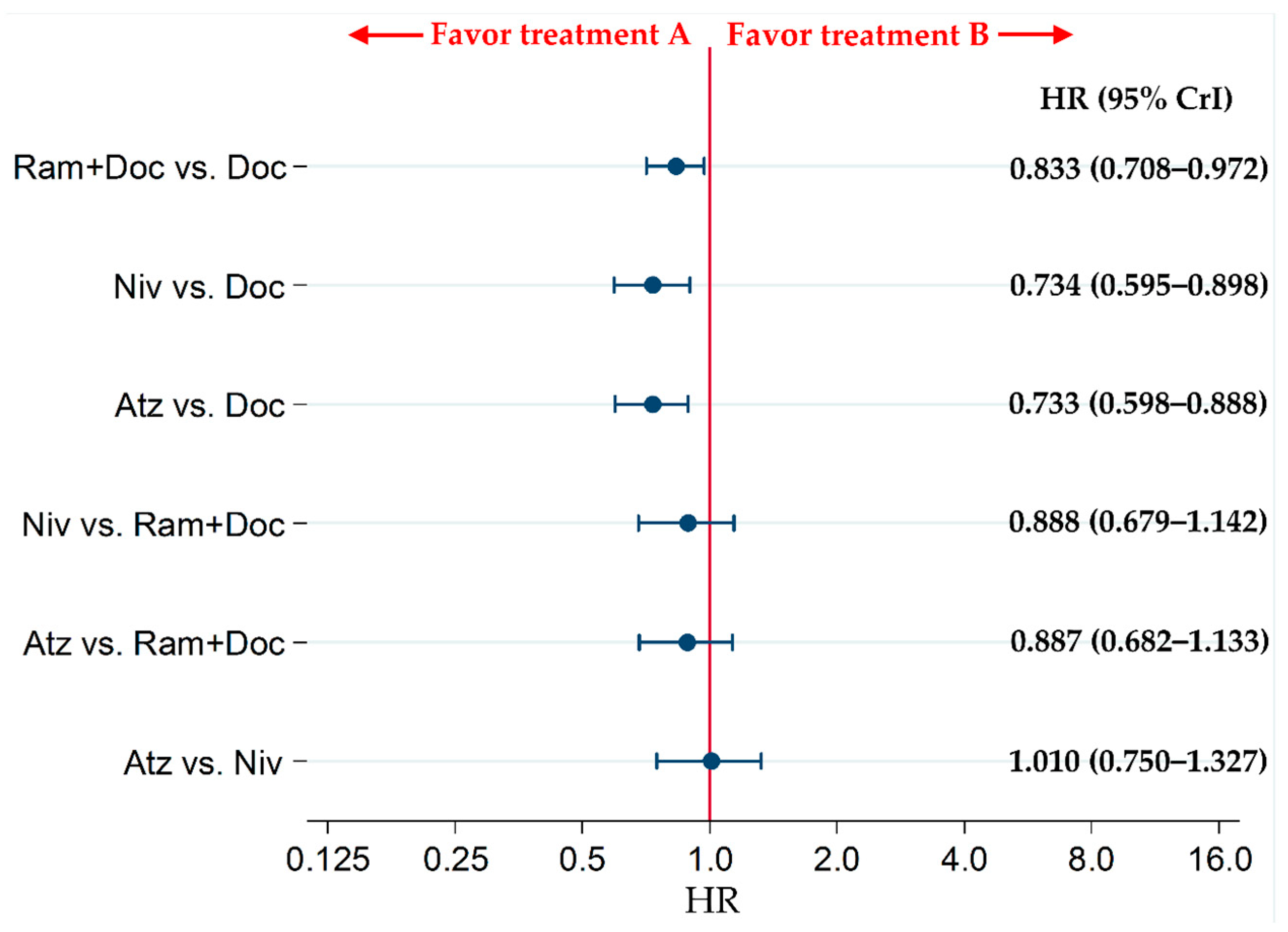
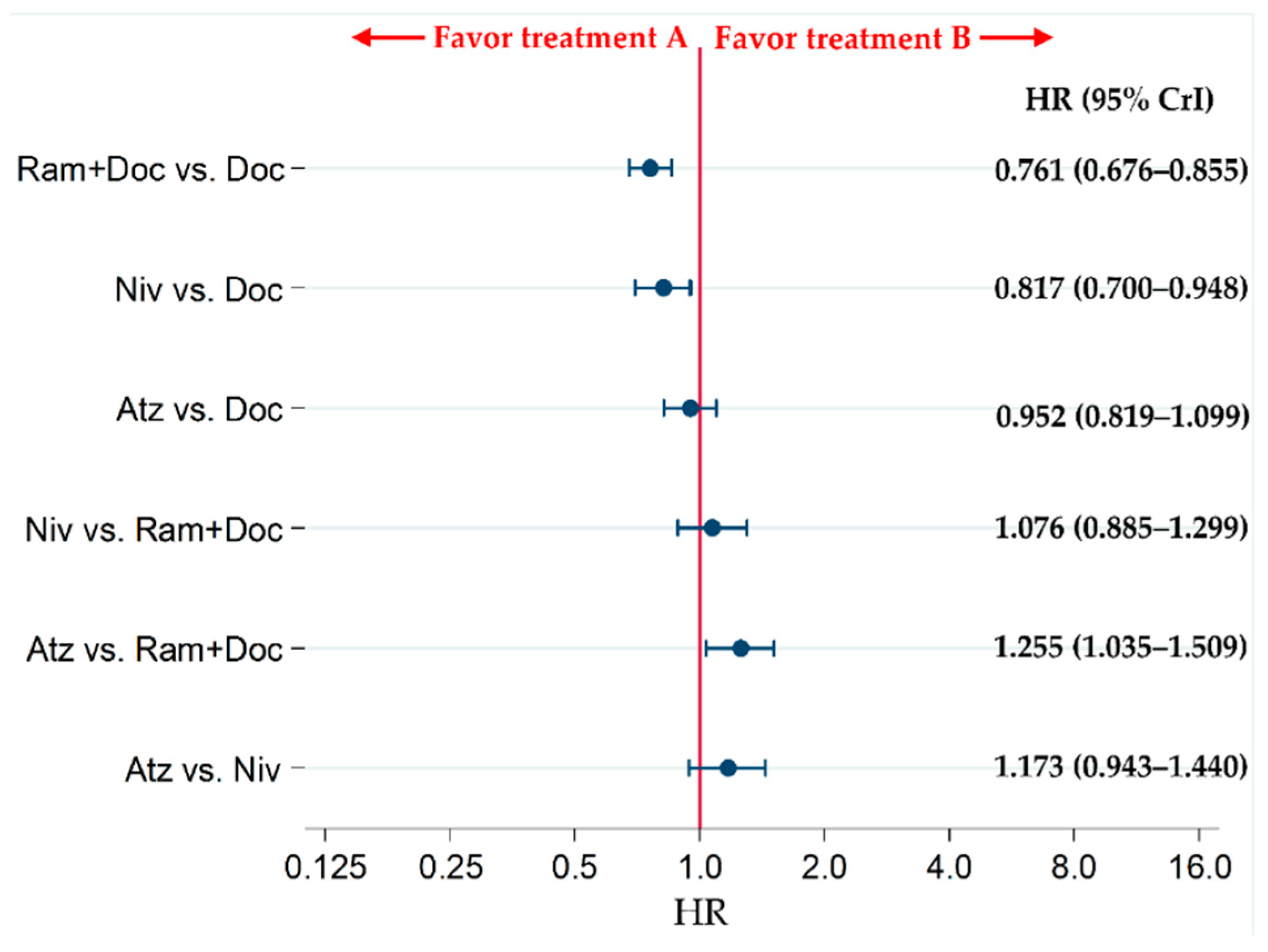
2.5. Secondary Efficacy Endpoint: PFS
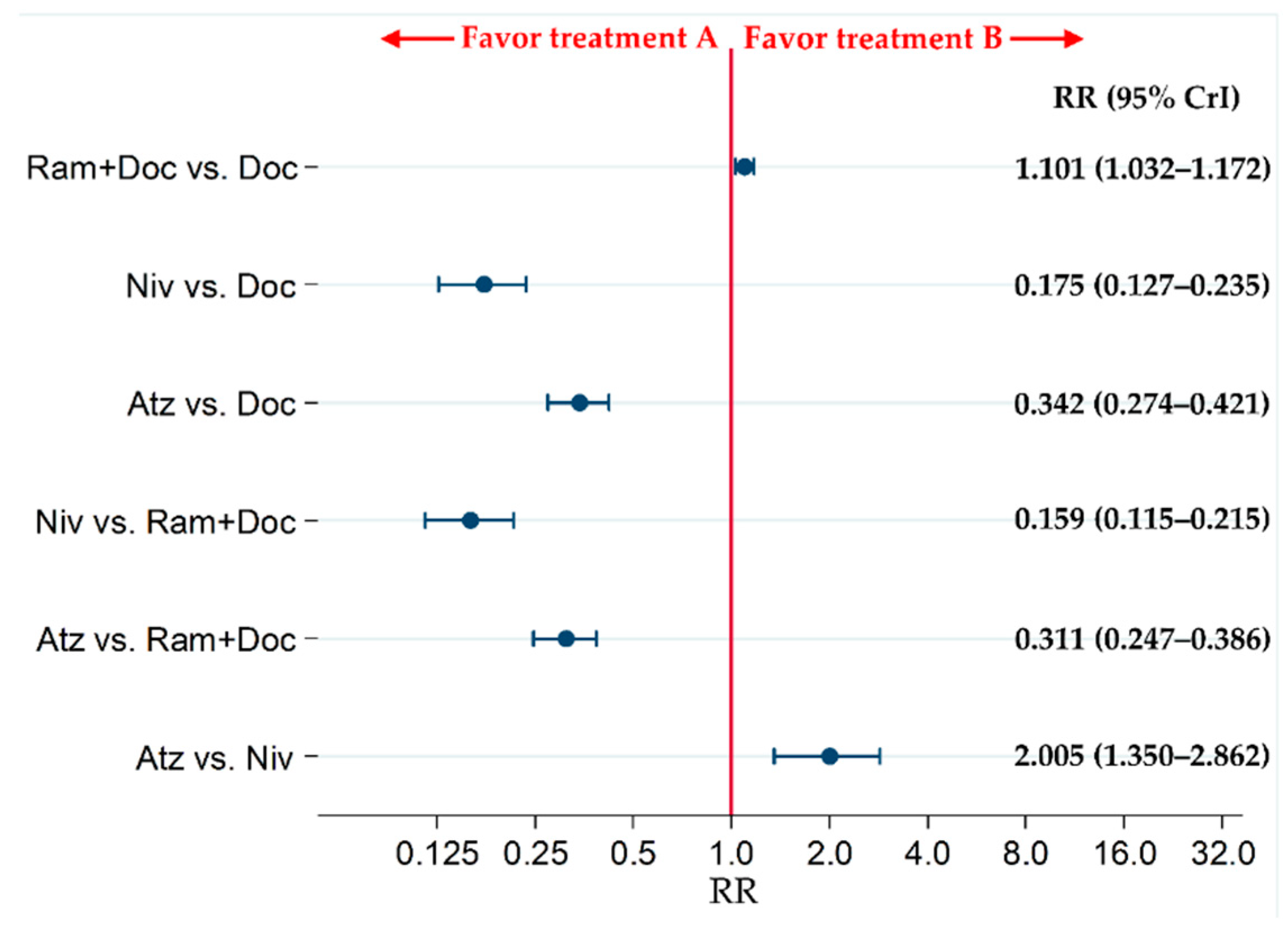
2.6. Primary Safety Endpoint: Grade 3–5 Treatment-Related Adverse Events (G3–5AEs)
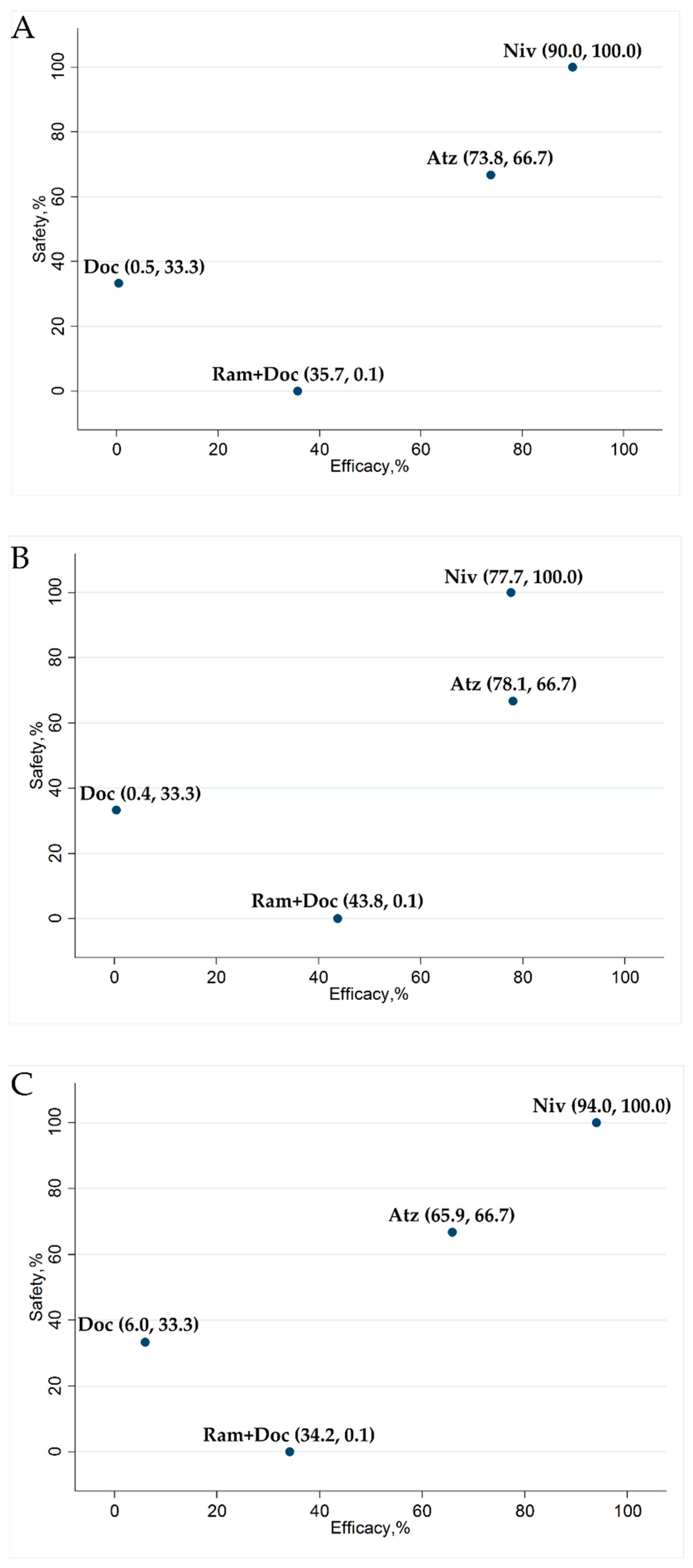
2.7. Ranking Assessment
2.8. Sensitivity Analysis
2.9. Bias Assessment
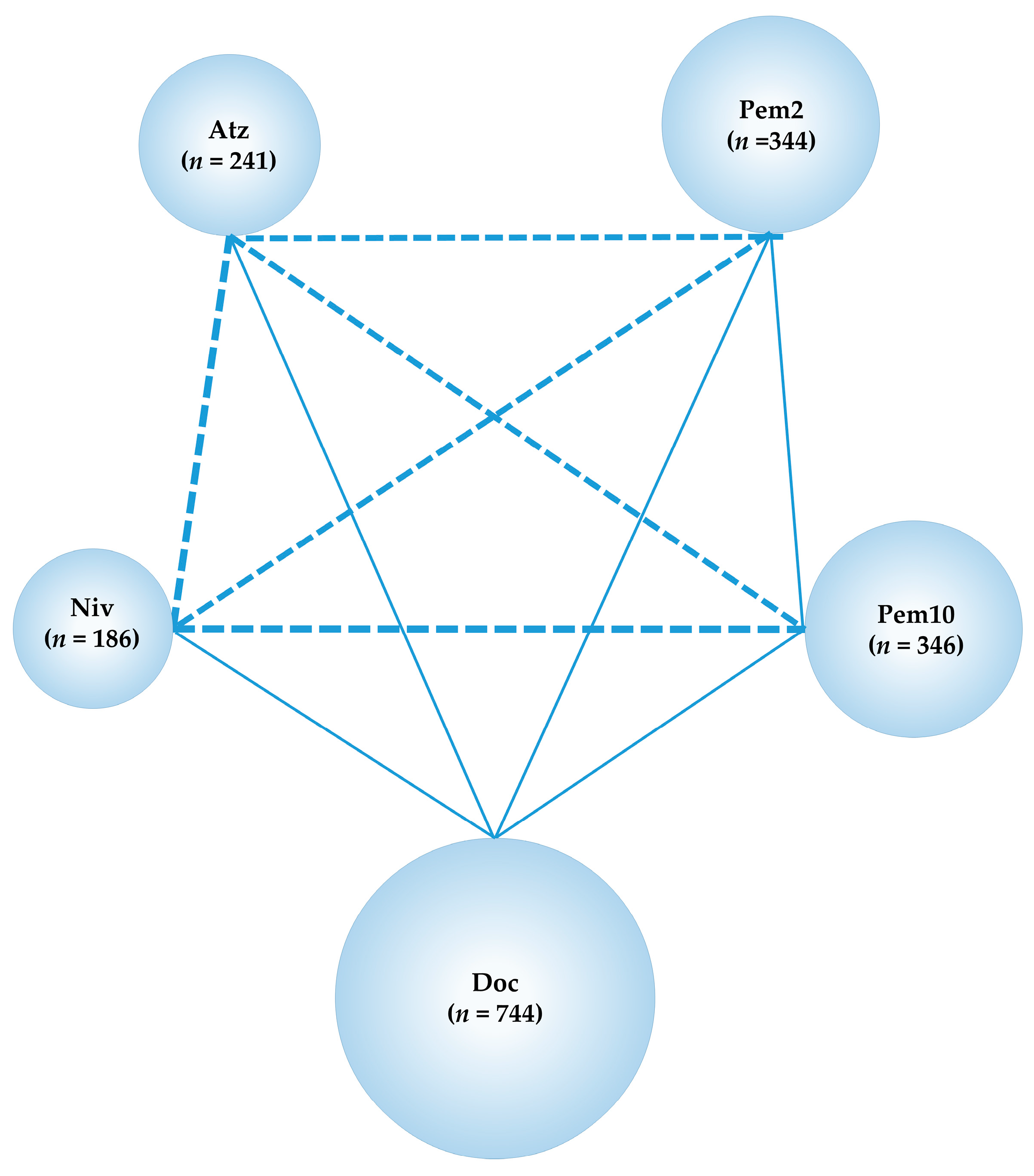
2.10. Comparison between the Effect of ICIs on Refractory or Relapse PD-L1-Positive (≥1%) Advanced NSCLC
2.10.1. Network Meta-Analysis of Six RCTs from REVEL, CheckMate057, CheckMate017, OAK, LUME-lung 1 and KEYNOTE-010, for Predefined Efficacy and Safety Outcomes (OS, PFS, and G3-5AEs)
2.10.2. Subgroup Analysis for Refractory or Relapse PD-L1-Positive (≥1%) Advanced NSCLC
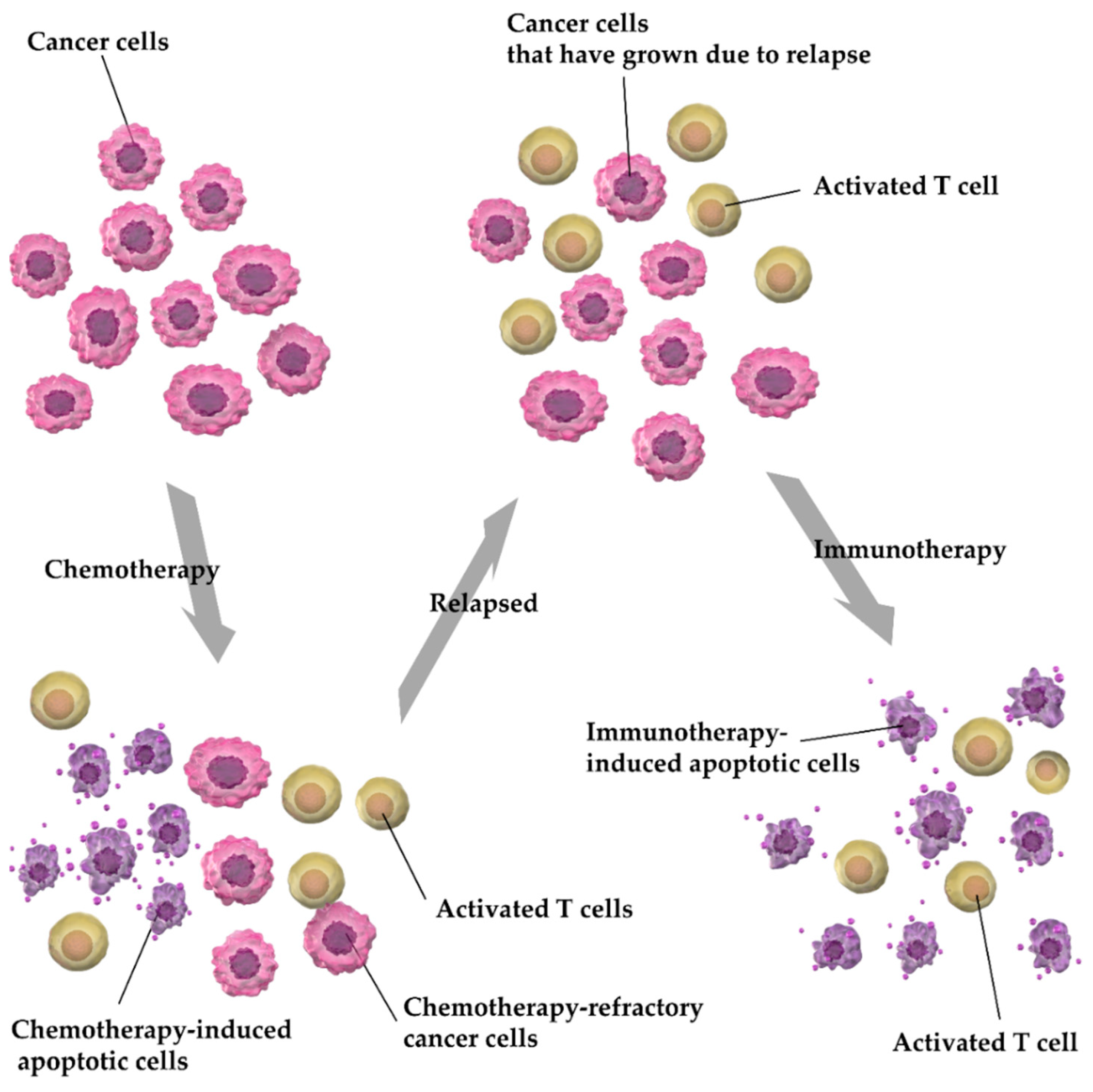
3. Discussion
4. Materials and Methods
4.1. Systematic Review
4.2. Quality Evaluation
4.3. Inclusion and Exclusion Criteria (Predefined PICOS)
4.3.1. Patients
4.3.2. Interventions/Comparisons
4.3.3. Outcomes
4.3.4. Study Design
4.4. Statistical Method of NMA
4.5. Ethical Aspects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Search Strategies in PubMed
References
- Wu, Z.; Man, S.; Sun, R.; Li, Z.; Wu, Y.; Zuo, D. Recent advances and challenges of immune checkpoint inhibitors in immunotherapy of non-small cell lung cancer. Int. Immunopharmacol. 2020, 85, 106613. [Google Scholar] [CrossRef]
- Nasim, F.; Sabath, B.F.; Eapen, G.A. Lung cancer. Med. Clin. N. Am. 2019, 103, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Wood, D.E.; Aggarwal, C.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; et al. NCCN guidelines insights: Non-Small Cell Lung Cancer, Version 1.2020. J. Natl. Compr. Cancer Netw. 2019, 17, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Onoi, K.; Chihara, Y.; Uchino, J.; Shimamoto, T.; Morimoto, Y.; Iwasaku, M.; Kaneko, Y.; Yamada, T.; Takayama, K. Immune checkpoint inhibitors for lung cancer treatment: A review. J. Clin. Med. 2020, 9, 1362. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Pasello, G.; Pavan, A.; Attili, I.; Bortolami, A.; Bonanno, L.; Menis, J.; Conte, P.; Guarneri, V. Real world data in the era of immune checkpoint inhibitors (ICIs): Increasing evidence and future applications in lung cancer. Cancer Treat. Rev. 2020, 87, 102031. [Google Scholar] [CrossRef] [PubMed]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-small cell lung cancer: Epidemiology, screening, diagnosis, and treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Arbour, K.C.; Riely, G.J. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: A review. JAMA 2019, 322, 764–774. [Google Scholar] [CrossRef]
- Garon, E.B.; Ciuleanu, T.E.; Arrieta, O.; Prabhash, K.; Syrigos, K.N.; Goksel, T.; Park, K.; Gorbunova, V.; Kowalyszyn, R.D.; Pikiel, J.; et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): A multicentre, double-blind, randomised phase 3 trial. Lancet 2014, 384, 665–773. [Google Scholar] [CrossRef]
- Popat, S.; Grohé, C.; Corral, J.; Reck, M.; Novello, S.; Gottfried, M.; Radonjic, D.; Kaiser, R. Anti-angiogenic agents in the age of resistance to immune checkpoint inhibitors: Do they have a role in non-oncogene-addicted non-small cell lung cancer? Lung Cancer 2020, 144, 76–84. [Google Scholar] [CrossRef]
- Reck, M.; Kaiser, R.; Mellemgaard, A.; Douillard, J.Y.; Orlov, S.; Krzakowski, M.; Von Pawel, J.; Gottfried, M.; Bondarenko, I.; Liao, M.; et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): A phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014, 15, 143–155. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar] [PubMed]
- Chen, Y.; Zhou, Y.; Tang, L.; Peng, X.; Jiang, H.; Wang, G.; Zhuang, W. Immune-checkpoint inhibitors as the first line treatment of advanced non-small cell lung cancer: A meta-analysis of randomized controlled trials. J. Cancer 2019, 10, 6261–6268. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Ades, A.E. Combination of direct and indirect evidence in mixed treatment comparisons. Stat. Med. 2004, 23, 3105–3124. [Google Scholar] [CrossRef] [PubMed]
- Lumley, T. Network meta-analysis for indirect treatment comparisons. Stat. Med. 2002, 21, 2313–2324. [Google Scholar] [CrossRef] [PubMed]
- Bucher, H.C.; Guyatt, G.H.; Griffith, L.E.; Walter, S.D. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J. Clin. Epidemiol. 1997, 50, 683–691. [Google Scholar] [CrossRef]
- White, I.R. Network meta-analysis. Stata J. 2015, 15, 951–985. [Google Scholar] [CrossRef]
- Hoaglin, D.C.; Hawkins, N.; Jansen, J.P.; Scott, D.A.; Itzler, R.; Cappelleri, J.C.; Boersma, C.; Thompson, D.; Larholt, K.M.; Diaz, M.; et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: Report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: Part 2. Value Health 2011, 14, 429–437. [Google Scholar] [CrossRef]
- Jansen, J.P.; Crawford, B.; Bergman, G.; Stam, W. Bayesian meta-analysis of multiple treatment comparisons: An introduction to mixed treatment comparisons. Value Health 2008, 11, 956–964. [Google Scholar] [CrossRef]
- Tonin, F.S.; Rotta, I.; Mendes, A.M.; Pontarolo, R. Network meta-analysis: A technique to gather evidence from direct and indirect comparisons. Pharm. Pract. (Granada) 2017, 15, 943. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Salanti, G.; Ades, A.E.; Ioannidis, J.P. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 2011, 64, 163–171. [Google Scholar] [CrossRef] [PubMed]
- PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/ (accessed on 1 July 2020).
- Embase. Available online: https://www.embase.com/login (accessed on 1 July 2020).
- Cochrane Central Register of Controlled Trials [CENTRAL]. Available online: https://www.cochranelibrary.com/ (accessed on 1 July 2020).
- SCOPUS. Available online: https://www.scopus.com/home.uri (accessed on 1 July 2020).
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; Von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Brooks, S.P.; Roberts, G.O. Convergence assessment techniques for Markov chain Monte Carlo. Stat. Comput. 1998, 8, 319–335. [Google Scholar] [CrossRef]
- Brooks, S.P.; Gelman, A. General methods for monitoring convergence of iterative simulations. J. Comput. Graph. Stat. 1996, 7, 434–455. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Dubos Arvis, C.; Ahn, M.J. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019. [Google Scholar]
- Chamoto, K.; Al-Habsi, M.; Honjo, T. Role of PD-1 in immunity and diseases. Curr. Top. Microbiol. Immunol. 2017, 410, 75–97. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Jansen, J.P.; Fleurence, R.; Devine, B.; Itzler, R.; Barrett, A.; Hawkins, N.; Lee, K.; Boersma, C.; Annemans, L.; Cappelleri, J.C. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: Report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: Part 1. Value Health 2011, 14, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Siemieniuk, R.A.; Bartoszko, J.J.; Ge, L.; Zeraatkar, D.; Izcovich, A.; Pardo-Hernandez, H.; Rochwerg, B.; Lamontagne, F.; Han, M.A.; Kum, E.; et al. Drug treatments for covid-19: Living systematic review and network meta-analysis. BMJ 2020, 370, m2980. [Google Scholar] [CrossRef] [PubMed]
- Hodkinson, A.; Bower, P.; Grigoroglou, C.; Zghebi, S.S.; Pinnock, H.; Kontopantelis, E.; Panagioti, M. Self-management interventions to reduce healthcare use and improve quality of life among patients with asthma: Systematic review and network meta-analysis. BMJ 2020, 370, m2521. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Sadeghirad, B.; Ball, G.D.C.; Da Costa, B.R.; Hitchcock, C.L.; Svendrovski, A.; Kiflen, R.; Quadri, K.; Kwon, H.Y.; Karamouzian, M.; et al. Comparison of dietary macronutrient patterns of 14 popular named dietary programmes for weight and cardiovascular risk factor reduction in adults: Systematic review and network meta-analysis of randomised trials. BMJ 2020, 369, m696. [Google Scholar] [CrossRef] [PubMed]
- Elaidi, R.; Phan, L.; Borchiellini, D.; Barthelemy, P.; Ravaud, A.; Oudard, S.; Vano, Y. Comparative efficacy of first-line immune-based combination therapies in metastatic renal cell carcinoma: A systematic review and network meta-analysis. Cancers 2020, 12, 1673. [Google Scholar] [CrossRef]
- Ando, K.; Kishino, Y.; Homma, T.; Kusumoto, S.; Yamaoka, T.; Tanaka, A.; Ohmori, T.; Ohnishi, T.; Sagara, H. Nivolumab plus ipilimumab versus existing immunotherapies in patients with PD-L1-positive advanced non-small cell lung cancer: A systematic review and network meta-analysis. Cancers 2020, 12, 1905. [Google Scholar] [CrossRef]
- Ando, K.; Akimoto, K.; Sato, H.; Manabe, R.; Kishino, Y.; Homma, T.; Kusumoto, S.; Yamaoka, T.; Tanaka, A.; Ohmori, T.; et al. Brigatinib and alectinib for ALK rearrangement-positive advanced non-small cell lung cancer with or without central nervous system metastasis: A systematic review and network meta-analysis. Cancers 2020, 12, 942. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Cai, X.; Pan, Z.; Liu, J.; Yin, W.; Chen, H.; Xie, Z.; Liang, H.; Wang, W.; et al. Efficacy and safety of first line treatments for patients with advanced epidermal growth factor receptor mutated, non-small cell lung cancer: Systematic review and network meta-analysis. BMJ 2019, 367, l5460. [Google Scholar] [CrossRef]
- Dias, S.; Welton, N.J.; Sutton, A.J.; Caldwell, D.M.; Lu, G.; Ades, A.E. Evidence synthesis for decision making 4: Inconsistency in networks of evidence based on randomized controlled trials. Med. Decis. Mak. 2013, 33, 641–656. [Google Scholar] [CrossRef]
- Dias, S.; Sutton, A.J.; Welton, N.J.; Ades, A.E. Evidence synthesis for decision making 3: Heterogeneity—subgroups, meta-regression, bias, and bias-adjustment. Med. Decis. Mak. 2013, 33, 618–640. [Google Scholar] [CrossRef]
- Dias, S.; Sutton, A.J.; Ades, A.E.; Welton, N.J. Evidence synthesis for decision making 2: A generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med. Decis. Mak. 2013, 33, 607–617. [Google Scholar] [CrossRef] [PubMed]
| Study Names | Key Inclusion Criteria |
|---|---|
| REVEL [9] |
|
| |
| |
| |
| CheckMate057 [28] |
|
| |
| |
| |
| CheckMate017 [27] |
|
| |
| |
| |
| OAK [29] |
|
| |
| |
|
| Study Names | Treatment Arms | N | Age, Year Median (Range) | Females No. (%) | ECOG PS No. (%) | Histological Type No. (%) |
|---|---|---|---|---|---|---|
| REVEL [9] | Ramucirumab (10 mg/kg) plus docetaxel (75 mg/m2) on day 1 of 21-day cycle | 628 | 62 (21–85) | 209 (33) | PS0: 207 (33) | Non-squamous: 465 (74) |
| PS1: 420 (67) | Squamous: 157 (25) | |||||
| Unknown: 6 (1) | ||||||
| Placebo plus docetaxel (75 mg/m2) | 625 | 61 (25–86) | 210 (34) | PS0: 199 (32) | Non-squamous: 447 (72) | |
| on day 1 of 21-day cycle | PS1: 425 (68) | Squamous: 171 (27) | ||||
| Unknown: 7 (1) | ||||||
| Total: 1253 | ||||||
| CheckMate057 [28] | Nivolumab (3 mg/kg e2w) | 292 | 61 (37–84) | 141 (48) | PS0: 84 (29) | Non-squamous: 292 (100) |
| PS1: 208 (71) | Squamous: 0 (0) | |||||
| NR: 0 | ||||||
| Docetaxel (75 mg/m2 e3w) | 290 | 64 (21–85) | 122 (42) | PS0: 95 (33) | Non-squamous: 290 (100) | |
| PS1: 194 (67) | Squamous: 0 (0) | |||||
| NR: 1 (<1) | ||||||
| Total: 582 | ||||||
| CheckMate017 [27] | Nivolumab (3 mg/kg e2w) | 135 | 62 (39–85) | 24 (18) | PS0: 27 (20) | Non-squamous: 0 (0) |
| PS1: 106 (79) | Squamous: 135 (100) | |||||
| NR: 2 (1) | ||||||
| Docetaxel (75 mg/m2 e3w) | 137 | 64 (42–84) | 40 (29) | PS0: 37 (27) | Non-squamous: 0 (0) | |
| PS1: 100 (73) | Squamous: 137 (100) | |||||
| NR: 0 (0) | ||||||
| Total: 272 | ||||||
| OAK [29] | Atezolizumab (1200 mg e3w) | 425 | 63.0 (33.0–82.0) | 164 (39) | PS0: 155 (36) | Non-squamous: 313 (74) |
| PS1: 270 (64) | Squamous: 112 (26) | |||||
| Docetaxel (75 mg/m2 e3w) | 425 | 64.0 (34.0–85.0) | 166 (39) | PS0: 160 (38) | Non-squamous: 315 (74) | |
| PS1: 265 (62) | Squamous: 110 (26) | |||||
| Total: 850 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ando, K.; Manabe, R.; Kishino, Y.; Kusumoto, S.; Yamaoka, T.; Tanaka, A.; Ohmori, T.; Ohnishi, T.; Sagara, H. Comparative Efficacy and Safety of Anti-PD-1/PD-L1 Immune Checkpoint Inhibitors for Refractory or Relapsed Advanced Non-Small-Cell Lung Cancer—A Systematic Review and Network Meta-Analysis. Cancers 2021, 13, 52. https://doi.org/10.3390/cancers13010052
Ando K, Manabe R, Kishino Y, Kusumoto S, Yamaoka T, Tanaka A, Ohmori T, Ohnishi T, Sagara H. Comparative Efficacy and Safety of Anti-PD-1/PD-L1 Immune Checkpoint Inhibitors for Refractory or Relapsed Advanced Non-Small-Cell Lung Cancer—A Systematic Review and Network Meta-Analysis. Cancers. 2021; 13(1):52. https://doi.org/10.3390/cancers13010052
Chicago/Turabian StyleAndo, Koichi, Ryo Manabe, Yasunari Kishino, Sojiro Kusumoto, Toshimitsu Yamaoka, Akihiko Tanaka, Tohru Ohmori, Tsukasa Ohnishi, and Hironori Sagara. 2021. "Comparative Efficacy and Safety of Anti-PD-1/PD-L1 Immune Checkpoint Inhibitors for Refractory or Relapsed Advanced Non-Small-Cell Lung Cancer—A Systematic Review and Network Meta-Analysis" Cancers 13, no. 1: 52. https://doi.org/10.3390/cancers13010052
APA StyleAndo, K., Manabe, R., Kishino, Y., Kusumoto, S., Yamaoka, T., Tanaka, A., Ohmori, T., Ohnishi, T., & Sagara, H. (2021). Comparative Efficacy and Safety of Anti-PD-1/PD-L1 Immune Checkpoint Inhibitors for Refractory or Relapsed Advanced Non-Small-Cell Lung Cancer—A Systematic Review and Network Meta-Analysis. Cancers, 13(1), 52. https://doi.org/10.3390/cancers13010052






