Immunocompetent Mouse Models in the Search for Effective Immunotherapy in Glioblastoma
Abstract
Simple Summary
Abstract
1. Introduction
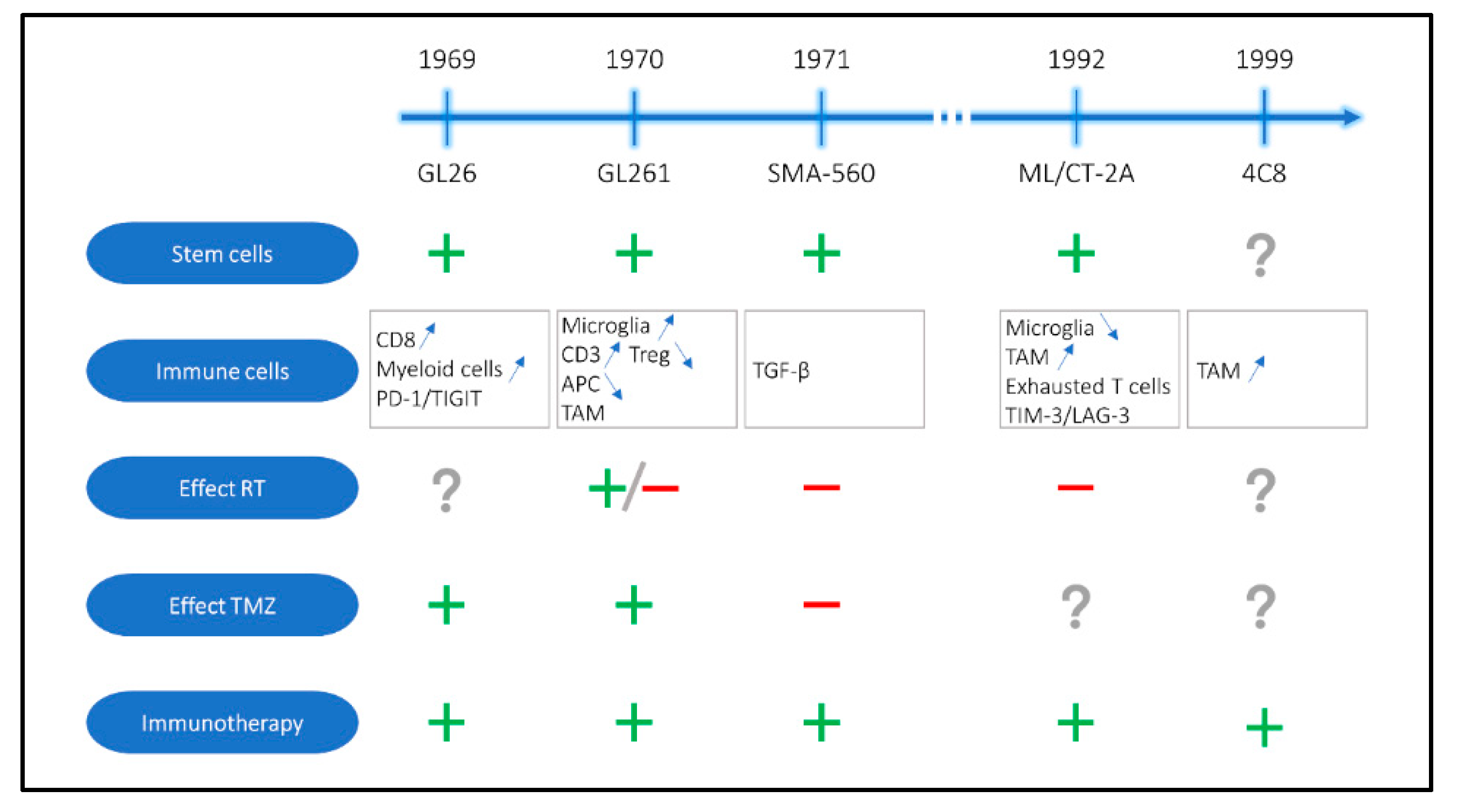
2. Oldest Available Immunocompetent Mouse Models for GBM
| Model | Host | Induction | Histology | Immune Composition | Stem Cells | Effect of Standard-of-Care Therapy | Response to Immunotherapy | Reference |
|---|---|---|---|---|---|---|---|---|
| GL261 | C57BL/6 | Chemical induction with methylcholanthrene | GBM, ependymoblastoma | Immunogenic profile with high of frequency activated microglia and CD3+ T cells, low frequency of Tregs, presence of TAMs, low frequency of APCs | Stem cell like phenotype with Nestin and CD133 expression | RT: +/− TMZ: + | Survival benefit with several immunotherapeutic strategies in single and combination treatment (ICB, vaccination, virotherapy, …) | Ausman 1970 [15,21,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115] |
| GL26 | C57BL/6 | Chemical induction with carcinogen implantation | GBM, ependymoblastoma | CD8+ T cell and myeloid cell infiltration with high expression of PD-1 and TIGIT immune checkpoints | Gene expression profile of glioma stem cells | TMZ: + | Generally positive | Sugiura 1969 [116,117,118,119,120,121,122,123,124,125,126,127,128,129,130] |
| ML/CT-2A | C57BL/6 | Chemical induction with methylcholanthrene | Anaplastic astrocytoma | Overall immune suppressive microenvironment with low numbers of microglia, high numbers of resident macrophages and exhausted CD8+ T cells with TIM-3 and LAG-3 expression | Positive for CD133, Nestin and Oct4 stem cell markers | RT: - | Generally positive | Seyfried 1992 [26,33,77,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147] |
| SMA-560 | VM/Dk | Spontaneous | Anaplastic astrocytoma | Upregulation immunoregulatory pathways, TGF-β signaling | CD44 and Nestin expression when cultured in spheres | RT: - TMZ: - | Generally positive | Fraser 1971 [134,148,149,150,151,152,153,154,155,156,157,158,159,160] |
| 4C8 | B6D2F1 | Clonal cell lines of a glial tumor from a transgenic mouse | Oligodendroglioma, astrocytoma | Large number of macrophages at the tumor periphery instead of in the tumor core | Not assessed | Not assessed | Generally positive (limited amount of data available) | Weiner 1999 [161,162,163,164,165] |
2.1. GL261
2.1.1. Origins and Tumor Characteristics
2.1.2. Effect of Standard-of-Care
2.1.3. Immunotherapeutic Approaches
2.2. GL26
2.2.1. Origins and Tumor Characteristics
2.2.2. Effect of Standard-of-Care
2.2.3. Immunotherapeutic Approaches
2.3. ML/CT-2A
2.3.1. Origins and Tumor Characteristics
2.3.2. Effect of Standard-of-Care
2.3.3. Immunotherapeutic Approaches
2.4. SMA-560
2.4.1. Origins and Tumor Characteristics
2.4.2. Effect of Standard-of-Care
2.4.3. Immunotherapeutic Approaches
2.5. C8
2.5.1. Origins and Tumor Characteristics
2.5.2. Immunotherapeutic Approaches
3. Recently Developed Immunocompetent Mouse Models for GBM
| Model | Host | Induction | Histology | Immune Composition | Stem Cells | Effect of Standard-of-Care Therapy | Response to Immunotherapy | Reference |
|---|---|---|---|---|---|---|---|---|
| KR158B | C57BL/6 | Spontaneous tumor development in Nf1 and p53 mutant mice | Secondary GBM | Not assessed | Not assessed | RT/TMZ: + | Resistance to ICB | Reilly 2000 [12,29,65,166,167,168] |
| Mut3 | C57BL/6 | Spontaneous tumor development in Nf1, p53 and Pten mutant mice | GBM, high-grade astrocytoma | High levels of classical and exhausted CD8+ T cells, CD4+ T cells, Tregs and resting microglia and low levels of DC infiltration | Increased GFAP and Nestin expression | Not assessed | Not assessed | Kwon 2008 [33,169,170] |
| 005 GSCs | C57BL/6 | Transduction in hippocampus of adult mice with vectors with activated HRas en AKT | GBM, heterogeneous | Relatively non-immunogenic, absence of MHC-I and down regulation of co-stimulatory molecules, limited T cell activation, strong correlation with human tumor immune microenvironment | Glioma stem cell tumor model | Not assessed | Resistance to ICB | Marumoto 2008 [24,33,168,171,172,173,174,175] |
| NSCL61 | BALB/c | HrasL61 overexpression in p53 deficient neural stem cells | GBM, heterogeneous | Not assessed | Tumor model is derived from neural stem cells | Not assessed | Generally positive (limited amount of data available) | Hide 2009 [27,68,72] |
| bRiTs-G3 | C57BL/6 | Overexpression of HRasV12 in neural stem cells from mice with homozygous deletion of the Ink4a/Arf locus | GBM, mesenchymal | Not assessed | Tumor model is derived from neural stem cells | RT: + RT resistance develops after repeated exposure | Generally positive (limited amount of data available) | Sampetrean 2011 [28,68,176] |
| NFpp10-GBM | C57BL/6 | Embryonic stem cells infected with shp53-shNf1 and shPten lentiviral vector | GBM | Lack of T cell infiltration | Tumor model is derived from neural stem cells | Not assessed | Resistance to ICB | Allen 2017 [13,24,25,177] |
| NS/CT-2A | C57BL/6 | Culturing of CT-2A cells in serum-free stem cell culture medium | Astrocytoma | Decrease in number of Tregs and increased CD8+ T cells compared to ML/CT-2A | Increased expression of Nestin and CD133 expression compared to ML/CT-2A | RT: + TMZ: + RT/TMZ: + | Resistance to ICB | Binello 2012 [21,26,30,133,178] |
| SB28 | C57BL/6 | Intraventricular transfection of Nras, PDGF and shp53 in neonates | GBM, proneural | Weakly immunogenic: few infiltrating T cells, abundant macrophage and microglial infiltration, absence of MHC-I and MHC-II expression | Not assessed | Not assessed | Resistance to ICB | Kosaka 2014 [9,12,23,58] |
| mGB2 | C57BL/6 | p53 and Pten deficient neural stem cells in adult mice | GBM, mesenchymal | Strong presence of myeloid cells and only few lymphocytes | Tumor model is derived from neural stem cells | Not assessed | Not assessed | Costa 2019 [22,179] |
3.1. KR158B
3.1.1. Origins and Tumor Characteristics
3.1.2. Effect of Standard-of-Care
3.1.3. Immunotherapeutic Approaches
3.2. Mut3
3.3. 005 GSCs
3.3.1. Origins and Tumor Characteristics
3.3.2. Effect of Standard-of-Care
3.3.3. Immunotherapeutic Approaches
3.4. NSCL61
3.4.1. Origins and Tumor Characteristics
3.4.2. Immunotherapeutic Approaches
3.5. bRiTs-G3
3.5.1. Origins and Tumor Characteristics
3.5.2. Effect of Standard-of-Care
3.5.3. Immunotherapeutic Approaches
3.6. NFpp10-GBM
3.6.1. Origins and Tumor Characteristics
3.6.2. Immunotherapeutic Approaches
3.7. NS/CT-2A
3.7.1. Origins and Tumor Characteristics
3.7.2. Effect of Standard-of-Care
3.7.3. Immunotherapeutic Approaches
3.8. SB28
3.8.1. Origins and Tumor Characteristics
3.8.2. Immunotherapeutic Approaches
3.9. mGB2
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cloughesy, T.F.; Mochizuki, A.Y.; Orpilla, J.R.; Hugo, W.; Lee, A.H.; Davidson, T.B.; Wang, A.C.; Ellingson, B.M.; Rytlewski, J.A.; Sanders, C.M.; et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat. Med. 2019, 25, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Bianco, J.; Bastiancich, C.; Jankovski, A.; Rieux, A.D.; Préat, V.; Danhier, F. On glioblastoma and the search for a cure: Where do we stand? Cell. Mol. Life Sci. 2017, 74, 2451–2466. [Google Scholar] [CrossRef] [PubMed]
- Prados, M.D.; Byron, S.A.; Tran, N.L.; Phillips, J.J.; Molinaro, A.M.; Ligon, K.L.; Wen, P.Y.; Kuhn, J.G.; Mellinghoff, I.K.; de Groot, J.F.; et al. Toward precision medicine in glioblastoma: The promise and the challenges. Neuro. Oncol. 2015, 17, 1051–1063. [Google Scholar] [CrossRef]
- Jackson, C.M.; Choi, J.; Lim, M. Mechanisms of immunotherapy resistance: Lessons from glioblastoma. Nat. Immunol. 2019, 20, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fisher, J.L.; Langer, C.E.; Pekmezci, M.; Schwartzbaum, J.A.; Turner, M.C.; Walsh, K.M.; et al. The epidemiology of glioma in adults: A state of the science review. Neuro-Oncol. 2014, 16, 896–913. [Google Scholar] [CrossRef]
- Bagley, S.J.; Desai, A.S.; Linette, G.P.; June, C.H.; O’Rourke, D.M. CAR T-cell therapy for glioblastoma: Recent clinical advances and future challenges. Neuro. Oncol. 2018, 20, 1429–1438. [Google Scholar] [CrossRef]
- Wang, J.; Shen, F.; Yao, Y.; Wang, L.L.; Zhu, Y.; Hu, J. Adoptive Cell Therapy: A Novel and Potential Immunotherapy for Glioblastoma. Front. Oncol. 2020, 10, 59. [Google Scholar] [CrossRef]
- Lim, M.; Xia, Y.; Bettegowda, C.; Weller, M. Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol. 2018, 15, 422–442. [Google Scholar] [CrossRef]
- Liau, L.M.; Ashkan, K.; Tran, D.D.; Campian, J.L.; Trusheim, J.E.; Cobbs, C.S.; Heth, J.A.; Salacz, M.; Taylor, S.; D’Andre, S.D.; et al. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J. Transl. Med. 2018, 16, 142. [Google Scholar] [CrossRef]
- Wen, P.Y.; Reardon, D.A.; Armstrong, T.S.; Phuphanich, S.; Aiken, R.D.; Landolfi, J.C.; Curry, W.T.; Zhu, J.J.; Glantz, M.; Peereboom, D.M.; et al. A randomized double-blind placebo-controlled phase II trial of dendritic cell vaccine ICT-107 in newly diagnosed patients with glioblastoma. Clin. Cancer Res. 2019, 25, 5799–5807. [Google Scholar] [CrossRef]
- Sprooten, J.; Ceusters, J.; Coosemans, A.; Agostinis, P.; De Vleeschouwer, S.; Zitvogel, L.; Kroemer, G.; Galluzzi, L.; Garg, A.D. Trial watch: Dendritic cell vaccination for cancer immunotherapy. Oncoimmunology 2019, 8, 1638212. [Google Scholar] [CrossRef]
- Reardon, D.A.; Brandes, A.A.; Omuro, A.; Mulholland, P.; Lim, M.; Wick, A.; Baehring, J.; Ahluwalia, M.S.; Roth, P.; Bähr, O.; et al. Effect of Nivolumab vs Bevacizumab in Patients with Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.; Jabouille, A.; Rivera, L.B.; Lodewijckx, I.; Missiaen, R.; Steri, V.; Feyen, K.; Tawney, J.; Hanahan, D.; Michael, I.P.; et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci. Transl. Med. 2017, 9, eaak9679. [Google Scholar] [CrossRef]
- Verreault, M.; Schmitt, C.; Goldwirt, L.; Pelton, K.; Haidar, S.; Levasseur, C.; Guehennec, J.; Knoff, D.; Labussière, M.; Marie, Y.; et al. Preclinical efficacy of the MDM2 inhibitor RG7112 in MDM2-amplified and TP53 wild-type glioblastomas. Clin. Cancer Res. 2016, 22, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Maxwell, R.; Xia, Y.; Cardarelli, P.; Oyasu, M.; Belcaid, Z.; Kim, E.; Hung, A.; Luksik, A.S.; Garzon-Muvdi, T.; et al. Combination anti-CXCR4 and anti-PD-1 immunotherapy provides survival benefit in glioblastoma through immune cell modulation of tumor microenvironment. J. Neurooncol. 2019, 143, 241–249. [Google Scholar] [CrossRef]
- Gupta, S.K.; Kizilbash, S.H.; Carlson, B.L.; Mladek, A.C.; Boakye-Agyeman, F.; Bakken, K.K.; Pokorny, J.L.; Schroeder, M.A.; Decker, P.A.; Cen, L.; et al. Delineation of MGMT Hypermethylation as a Biomarker for Veliparib-Mediated Temozolomide-Sensitizing Therapy of Glioblastoma. J. Natl. Cancer Inst. 2016, 108, 1–10. [Google Scholar] [CrossRef]
- Zahonero, C.; Aguilera, P.; Ramírez-Castillejo, C.; Pajares, M.; Bolós, M.V.; Cantero, D.; Perez-Nuñez, A.; Hernández-Laín, A.; Sánchez-Gómez, P.; Sepúlveda, J.M. Preclinical test of dacomitinib, an irreversible EGFR inhibitor, confirms its effectiveness for glioblastoma. Mol. Cancer Ther. 2015, 14, 1548–1558. [Google Scholar] [CrossRef]
- Guishard, A.F.; Yakisich, J.S.; Azad, N.; Iyer, A.K.V. Translational gap in ongoing clinical trials for glioma. J. Clin. Neurosci. 2018, 47, 28–42. [Google Scholar] [CrossRef]
- Coosemans, A.; Vankerckhoven, A.; Baert, T.; Boon, L.; Ruts, H.; Riva, M.; Blagden, S.; Delforge, M.; Concin, N.; Mirza, M.R.; et al. Combining conventional therapy with immunotherapy: A risky business? Eur. J. Cancer 2019, 113, 41–44. [Google Scholar] [CrossRef]
- Kumar-Sinha, C.; Chinnaiyan, A.M. Precision oncology in the age of integrative genomics. Nat. Biotechnol. 2018, 36, 46–60. [Google Scholar] [CrossRef]
- Riva, M.; Wouters, R.; Sterpin, E.; Giovannoni, R.; Boon, L.; Himmelreich, U.; Gsell, W.; Van Ranst, M.; Coosemans, A. Radiotherapy, Temozolomide and anti-programmed cell death protein 1 treatments modulate the immune microenvironment in experimental high-grade glioma. Neurosurgery 2020. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Eisemann, T.; Strelau, J.; Spaan, I.; Korshunov, A.; Liu, H.K.; Bugert, P.; Angel, P.; Peterziel, H. Intratumoral platelet aggregate formation in a murine preclinical glioma model depends on podoplanin expression on tumor cells. Blood Adv. 2019, 3, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, A.; Ohkuri, T.; Okada, H. Combination of an agonistic anti-CD40 monoclonal antibody and the COX-2 inhibitor celecoxib induces anti-glioma effects by promotion of type-1 immunity in myeloid cells and T-cells. Cancer Immunol. Immunother. 2014, 63, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Marumoto, T.; Tashiro, A.; Friedmann-morvinski, D.; Soda, Y.; Gage, F.H.; Verma, I.M. Development of a novel mouse glioma model using lentiviral vectors. Nat. Med. 2009, 15, 110–116. [Google Scholar] [CrossRef]
- Friedmann-Morvinski, D.; Bushong, E.A.; Ke, E.; Soda, Y.; Marumoto, T.; Singer, O.; Ellisman, M.H.; Verma, I.M. Dedifferentiation of Neurons and Astrocytes by Oncogenes Can Induce Gliomas in Mice. Science 2012, 338, 1080–1084. [Google Scholar] [CrossRef]
- Binello, E.; Qadeer, Z.A.; Kothari, H.P.; Emdad, L.; Germano, I.M. Stemness of the CT-2A immunocompetent mouse brain tumor model: Characterization in vitro. J. Cancer 2012, 3, 166–174. [Google Scholar] [CrossRef]
- Hide, T.; Takezaki, T.; Nakatani, Y.; Nakamura, H.; Kuratsu, J.I.; Kondo, T. Sox11 prevents tumorigenesis of glioma-initiating cells by inducing neuronal differentiation. Cancer Res. 2009, 69, 7953–7959. [Google Scholar] [CrossRef]
- Sampetrean, O.; Saga, I.; Nakanishi, M.; Sugihara, E.; Fukaya, R.; Onishi, N.; Osuka, S.; Akahata, M.; Kai, K.; Sugimoto, H.; et al. Invasion precedes tumor mass formation in a malignant brain tumor model of genetically modified neural stem cells. Neoplasia 2011, 13, 784–791. [Google Scholar] [CrossRef]
- Reilly, K.M.; Loisel, D.A.; Bronson, R.T.; McLaughlin, M.E.; Jacks, T. Nf1;Trp53 mutant mice develop glioblastoma with evidence of strain-specific effects. Nat. Genet. 2000, 26, 109–113. [Google Scholar] [CrossRef]
- Oh, T.; Fakurnejad, S.; Sayegh, E.T.; Clark, A.J.; Ivan, M.E.; Sun, M.Z.; Safaee, M.; Bloch, O.; James, C.D.; Parsa, A.T. Immunocompetent murine models for the study of glioblastoma immunotherapy. J. Transl. Med. 2014, 12, 107. [Google Scholar] [CrossRef]
- Wu, A.; Oh, S.; Wiesner, S.M.; Ericson, K.; Chen, L.; Hall, W.A.; Champoux, P.E.; Low, W.C.; Ohlfest, J.R. Persistence of CD133+ cells in human and mouse glioma cell lines: Detailed characterization of GL261 glioma cells with cancer stem cell-like properties. Stem Cells Dev. 2008, 17, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Behnan, J.; Isakson, P.; Joel, M.; Cilio, C.; Langmoen, I.A.; Vik-Mo, E.O.; Badn, W. Recruited brain tumor-derived mesenchymal stem cells contribute to brain tumor progression. Stem Cells 2014, 32, 1110–1123. [Google Scholar] [CrossRef] [PubMed]
- Khalsa, J.K.; Cheng, N.; Keegan, J.; Chaudry, A.; Driver, J.; Bi, W.L.; Lederer, J.; Shah, K. Immune phenotyping of diverse syngeneic murine brain tumors identifies immunologically distinct types. Nat. Commun. 2020, 11, 3912. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.S.; Didier, S.; Murray, D.W.; Turner, T.H.; Issaivanan, M.; Ruggieri, R.; Al-Abed, Y.; Symons, M. Semapimod sensitizes glioblastoma tumors to ionizing radiation by targeting microglia. PLoS ONE 2014, 9, e95885. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.A.T.; Kim, Y.H.; Duong, T.H.; Jung, S.; Kim, I.Y.; Moon, K.S.; Jang, W.Y.; Lee, H.J.; Lee, J.J.; Jung, T.Y. Peptide Vaccine Combined Adjuvants Modulate Anti-tumor Effects of Radiation in Glioblastoma Mouse Model. Front. Immunol. 2020, 11, 1165. [Google Scholar] [CrossRef]
- Durant, S.T.; Zheng, L.; Wang, Y.; Chen, K.; Zhang, L.; Zhang, T.; Yang, Z.; Riches, L.; Trinidad, A.G.; Fok, J.H.L.; et al. The brain-penetrant clinical ATM inhibitor AZD1390 radiosensitizes and improves survival of preclinical brain tumor models. Sci. Adv. 2018, 4, eaat1719. [Google Scholar] [CrossRef]
- Zeng, J.; See, A.P.; Phallen, J.; Jackson, C.M.; Belcaid, Z.; Ruzevick, J.; Durham, N.; Meyer, C.; Harris, T.J.; Albesiano, E.; et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 343–349. [Google Scholar] [CrossRef]
- Dai, B.; Qi, N.; Li, J.; Zhang, G. Temozolomide combined with PD-1 Antibody therapy for mouse orthotopic glioma model. Biochem. Biophys. Res. Commun. 2018, 501, 871–876. [Google Scholar] [CrossRef]
- Ferrer-Font, L.; Arias-Ramos, N.; Lope-Piedrafita, S.; Julià-Sapé, M.; Pumarola, M.; Arús, C.; Candiota, A.P. Metronomic treatment in immunocompetent preclinical GL261 glioblastoma: Effects of cyclophosphamide and temozolomide. NMR Biomed. 2017, 30, e3748. [Google Scholar] [CrossRef]
- Hanihara, M.; Kawataki, T.; Oh-Oka, K.; Mitsuka, K.; Nakao, A.; Kinouchi, H. Synergistic antitumor effect with indoleamine 2,3-dioxygenase inhibition and temozolomide in a murine glioma model. J. Neurosurg. 2016, 124, 1594–1601. [Google Scholar] [CrossRef]
- Malo, C.S.; Renner, D.N.; Huseby Kelcher, A.M.; Jin, F.; Hansen, M.J.; Pavelko, K.D.; Johnson, A.J. The effect of vector silencing during picornavirus vaccination against experimental melanoma and glioma. PLoS ONE 2016, 11, e0162064. [Google Scholar] [CrossRef] [PubMed]
- Bu, N.; Wu, H.; Zhang, G.; Zhan, S.; Zhang, R.; Sun, H.; Du, Y.; Yao, L.; Wang, H. Exosomes from Dendritic Cells Loaded with Chaperone-Rich Cell Lysates Elicit a Potent T Cell Immune Response Against Intracranial Glioma in Mice. J. Mol. Neurosci. 2015, 56, 631–643. [Google Scholar] [CrossRef]
- Yan, Y.; Fang, M.; Xuan, W.; Wu, X.; Meng, X.; Wang, L.; Yu, Y. The therapeutic potency of HSP65-GTL in GL261 Glioma-bearing Mice. J. Immunother. 2015, 38, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Durant, S.T.; Zheng, L.; Wang, Y.; Chen, K.; Zhang, L.; Zhang, T.; Yang, Z.; Riches, L.; Trinidad, A.G.; Fok, J.H.; et al. Survivin Monoclonal Antibodies Detect Survivin Cell Surface Expression and Inhibit Tumor Growth in vivo. Clin. Cancer Res. 2018, 176, 139–148. [Google Scholar]
- Chen, M.; Sun, R.; Shi, B.; Wang, Y.; Di, S.; Luo, H.; Sun, Y.; Li, Z.; Zhou, M.; Jiang, H. Antitumor efficacy of chimeric antigen receptor T cells against EGFRvIII-expressing glioblastoma in C57BL/6 mice. Biomed. Pharmacother. 2019, 113, 108734. [Google Scholar] [CrossRef]
- Cockle, J.V.; Rajani, K.; Zaidi, S.; Kottke, T.; Thompson, J.; Diaz, R.M.; Shim, K.; Peterson, T.; Parney, I.F.; Short, S.; et al. Combination viroimmunotherapy with checkpoint inhibition to treat glioma, based on location-specific tumor profiling. Neuro. Oncol. 2016, 18, 518–527. [Google Scholar] [CrossRef]
- Jiang, H.; Clise-Dwyer, K.; Ruisaard, K.E.; Fan, X.; Tian, W.; Gumin, J.; Lamfers, M.L.; Kleijn, A.; Lang, F.F.; Yung, W.K.; et al. Delta-24-RGD oncolytic adenovirus elicits anti-glioma immunity in an immunocompetent mouse model. PLoS ONE 2014, 9, e97407. [Google Scholar] [CrossRef]
- Tang, B.; Guo, Z.S.; Bartlett, D.L.; Yan, D.Z.; Schane, C.P.; Thomas, D.L.; Liu, J.; McFadden, G.; Shisler, J.L.; Roy, E.J. Synergistic Combination of Oncolytic Virotherapy and Immunotherapy for Glioma. Clin. Cancer Res. 2020, 26, 2216–2230. [Google Scholar] [CrossRef]
- Koks, C.A.; Garg, A.D.; Ehrhardt, M.; Riva, M.; Vandenberk, L.; Boon, L.; De Vleeschouwer, S.; Agostinis, P.; Graf, N.; Van Gool, S.W. Newcastle disease virotherapy induces long-term survival and tumor-specific immune memory in orthotopic glioma through the induction of immunogenic cell death. Int. J. Cancer 2015, 136, E313–E325. [Google Scholar] [CrossRef]
- Kleijn, A.; van den Bossche, W.; Haefner, E.S.; Belcaid, Z.; Burghoorn-Maas, C.; Kloezeman, J.J.; Pas, S.D.; Leenstra, S.; Debets, R.; de Vrij, J.; et al. The Sequence of Delta24-RGD and TMZ Administration in Malignant Glioma Affects the Role of CD8+T Cell Anti-tumor Activity. Mol. Ther. Oncolytics 2017, 5, 11–19. [Google Scholar] [CrossRef]
- Zhu, S.; Lv, X.; Zhang, X.; Li, T.; Zang, G.; Yang, N.; Wang, X.; Wu, J.; Chen, W.; Liu, Y.J.; et al. An effective dendritic cell-based vaccine containing glioma stem-like cell lysate and CpG adjuvant for an orthotopic mouse model of glioma. Int. J. Cancer 2019, 144, 2867–2879. [Google Scholar] [CrossRef] [PubMed]
- Hardcastle, J.; Mills, L.; Malo, C.S.; Jin, F.; Kurokawa, C.; Geekiyanage, H.; Schroeder, M.; Sarkaria, J.; Johnson, A.J.; Galanis, E. Immunovirotherapy with measles virus strains in combination with anti-PD-1 antibody blockade enhances antitumor activity in glioblastoma treatment. Neuro. Oncol. 2017, 19, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Jahan, N.; Talat, H.; Alonso, A.; Saha, D.; Curry, W.T. Triple combination immunotherapy with GVAX, anti-PD-1 monoclonal antibody, and agonist anti-OX40 monoclonal antibody is highly effective against murine intracranial glioma. Oncoimmunology 2019, 8, e1577108. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.A.; Kim, J.E.; Theodros, D.; Tam, A.; Velarde, E.; Kochel, C.M.; Francica, B.; Nirschl, T.R.; Ghasemzadeh, A.; Mathios, D.; et al. Agonist anti-GITR monoclonal antibody and stereotactic radiation induce immune-mediated survival advantage in murine intracranial glioma. J. Immunother. Cancer 2016, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Belcaid, Z.; Phallen, J.A.; Zeng, J.; See, A.P.; Mathios, D.; Gottschalk, C.; Nicholas, S.; Kellett, M.; Ruzevick, J.; Jackson, C.; et al. Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS ONE 2014, 9, e101764. [Google Scholar] [CrossRef] [PubMed]
- Hung, A.L.; Maxwell, R.; Theodros, D.; Belcaid, Z.; Mathios, D.; Luksik, A.S.; Kim, E.; Wu, A.; Xia, Y.; Garzon-Muvdi, T.; et al. TIGIT and PD-1 dual checkpoint blockade enhances antitumor immunity and survival in GBM. Oncoimmunology 2018, 7, e1466769. [Google Scholar] [CrossRef]
- Kim, J.E.; Patel, M.A.; Mangraviti, A.; Kim, E.S.; Theodros, D.; Velarde, E.; Liu, A.; Sankey, E.W.; Tam, A.; Xu, H.; et al. Combination Therapy with Anti-PD-1, Anti-TIM-3, and Focal Radiation Results in Regression of Murine Gliomas. Clin. Cancer Res. 2017, 23, 124–136. [Google Scholar] [CrossRef]
- Genoud, V.; Marinari, E.; Nikolaev, S.I.; Castle, J.C.; Bukur, V.; Dietrich, P.Y.; Okada, H.; Walker, P.R. Responsiveness to anti-PD-1 and anti-CTLA-4 immune checkpoint blockade in SB28 and GL261 mouse glioma models. Oncoimmunology 2018, 7, e1501137. [Google Scholar] [CrossRef]
- Qian, J.; Wang, C.; Wang, B.; Yang, J.; Wang, Y.; Luo, F.; Xu, J.; Zhao, C.; Liu, R.; Chu, Y. The IFN-γ/PD-L1 axis between T cells and tumor microenvironment: Hints for glioma anti-PD-1/PD-L1 therapy. J. Neuroinflamm. 2018, 15, 290. [Google Scholar] [CrossRef]
- Garg, A.D.; Vandenberk, L.; Van Woensel, M.; Belmans, J.; Schaaf, M.; Boon, L.; De Vleeschouwer, S.; Agostinis, P. Preclinical efficacy of immune-checkpoint monotherapy does not recapitulate corresponding biomarkers-based clinical predictions in glioblastoma. Oncoimmunology 2017, 6, e1295903. [Google Scholar] [CrossRef]
- Shevtsov, M.; Pitkin, E.; Ischenko, A.; Stangl, S.; Khachatryan, W.; Galibin, O.; Edmond, S.; Lobinger, D.; Multhoff, G. Ex vivo Hsp70-activated NK cells in combination with PD-1 inhibition significantly increase overall survival in preclinical models of glioblastoma and lung cancer. Front. Immunol. 2019, 10, 454. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Kane, J.R.; Panek, W.K.; Young, J.S.; Rashidi, A.; Yu, D.; Kanojia, D.; Hasan, T.; Miska, J.; Gómez-Lim, M.A.; et al. A Dendritic Cell-Targeted Adenoviral Vector Facilitates Adaptive Immune Response Against Human Glioma Antigen (CMV-IE) and Prolongs Survival in a Human Glioma Tumor Model. Neurotherapeutics 2018, 15, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Maggio, D.; Ho, W.S.; Breese, R.; Walbridge, S.; Wang, H.; Cui, J.; Heiss, J.D.; Gilbert, M.R.; Kovach, J.S.; Lu, R.O.; et al. Inhibition of protein phosphatase-2A with LB-100 enhances antitumor immunity against glioblastoma. J. Neurooncol. 2020, 148, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Mathios, D.; Park, C.K.; Marcus, W.D.; Alter, S.; Rhode, P.R.; Jeng, E.K.; Wong, H.C.; Pardoll, D.M.; Lim, M. Therapeutic administration of IL-15 superagonist complex ALT-803 leads to long-term survival and durable antitumor immune response in a murine glioblastoma model. Int. J. Cancer 2016, 138, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Karachi, A.; Yang, C.; Dastmalchi, F.; Sayour, E.J.; Huang, J.; Azari, H.; Long, Y.; Flores, C.; Mitchell, D.A.; Rahman, M. Modulation of temozolomide dose differentially affects T-cell response to immune checkpoint inhibition. Neuro. Oncol. 2019, 21, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, X.; Duan, Y.; Liu, Y.; Wang, H.; Lian, S.; Zhuang, G.; Fan, Y. Combined blockade of Ts cell immunoglobulin and mucin domain 3 and carcinoembryonic antigen-related cell adhesion molecule 1 results in durable therapeutic efficacy in mice with intracranial gliomas. Med. Sci. Monit. 2017, 23, 3593–3602. [Google Scholar] [CrossRef]
- Jahan, N.; Talat, H.; Curry, W.T. Agonist OX40 immunotherapy improves survival in glioma-bearing mice and is complementary with vaccination with irradiated GM-CSF-expressing tumor cells. Neuro. Oncol. 2018, 20, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Saito, R.; Chonan, M.; Shibahara, I.; Sato, A.; Kanamori, M.; Sonoda, Y.; Kondo, T.; Ishii, N.; Tominaga, T. Local convection-enhanced delivery of an anti-CD40 agonistic monoclonal antibody induces antitumor effects in mouse glioma models. Neuro. Oncol. 2016, 18, 1120–1128. [Google Scholar] [CrossRef]
- Eberstå, S.; Sandén, E.; Fritzell, S.; Darabi, A.; Visse, E.; Siesjö, P. Intratumoral COX-2 inhibition enhances GM-CSF immunotherapy against established mouse GL261 brain tumors. Int. J. Cancer 2014, 134, 2748–2753. [Google Scholar] [CrossRef]
- Miska, J.; Rashidi, A.; Chang, A.L.; Muroski, M.E.; Han, Y.; Zhang, L.; Lesniak, M.S. Anti-GITR therapy promotes immunity against malignant glioma in a murine model. Cancer Immunol. Immunother. 2016, 65, 1555–1567. [Google Scholar] [CrossRef]
- Zheng, H.; Yang, B.; Xu, D.; Wang, W.; Tan, J.; Sun, L.; Li, Q.; Sun, L.; Xia, X. Induction of specific T helper-9 cells to inhibit glioma cell growth. Oncotarget 2017, 8, 4864–4874. [Google Scholar] [CrossRef] [PubMed]
- Chonan, M.; Saito, R.; Shoji, T.; Shibahara, I.; Kanamori, M.; Sonoda, Y.; Watanabe, M.; Kikuchi, T.; Ishii, N.; Tominaga, T. CD40/CD40L expression correlates with the survival of patients with glioblastomas and an augmentation in CD40 signaling enhances the efficacy of vaccinations against glioma models. Neuro. Oncol. 2015, 17, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Vandenberk, L.; Garg, A.D.; Verschuere, T.; Koks, C.; Belmans, J.; Beullens, M.; Agostinis, P.; De Vleeschouwer, S.; Van Gool, S.W. Irradiation of necrotic cancer cells, employed for pulsing dendritic cells (DCs), potentiates DC vaccine-induced antitumor immunity against high-grade glioma. Oncoimmunology 2016, 5, e1083669. [Google Scholar] [CrossRef] [PubMed]
- Moertel, C.L.; Xia, J.; LaRue, R.; Waldron, N.N.; Andersen, B.M.; Prins, R.M.; Okada, H.; Donson, A.M.; Foreman, N.K.; Hunt, M.A.; et al. CD200 in CNS tumor-induced immunosuppression: The role for CD200 pathway blockade in targeted immunotherapy. J. Immunother. Cancer 2014, 2, 46. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.; Waxman, D.J. CpG-1826 immunotherapy potentiates chemotherapeutic and anti-tumor immune responses to metronomic cyclophosphamide in a preclinical glioma model. Physiol. Behav. 2018, 176, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, F.; Li, A.; Qian, J.; Yao, Z.; Feng, X.; Chu, Y. Systemic injection of TLR1/2 agonist improves adoptive antigen-specific T cell therapy in glioma-bearing mice. Clin. Immunol. 2014, 154, 26–36. [Google Scholar] [CrossRef]
- Ladomersky, E.; Zhai, L.; Lauing, K.L.; Bell, A.; Xu, J.; Kocherginsky, M.; Zhang, B.; Wu, J.D.; Podojil, J.R.; Platanias, L.C.; et al. Advanced Age Increases Immunosuppression in the Brain and Decreases Immunotherapeutic Efficacy in Subjects with Glioblastoma. Clin. Cancer Res. 2020, 26, 5232–5245. [Google Scholar] [CrossRef]
- Azambuja, J.H.; da Silveira, E.F.; de Carvalho, T.R.; Oliveira, P.S.; Pacheco, S.; do Couto, C.T.; Beira, F.T.; Stefanello, F.M.; Spanevello, R.M.; Braganhol, E. Glioma sensitive or chemoresistant to temozolomide differentially modulate macrophage protumor activities. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 2652–2662. [Google Scholar] [CrossRef]
- Wu, S.; Calero-Pérez, P.; Villamañan, L.; Arias-Ramos, N.; Pumarola, M.; Ortega-Martorell, S.; Julià-Sapé, M.; Arús, C.; Candiota, A.P. Anti-tumour immune response in GL261 glioblastoma generated by Temozolomide Immune-Enhancing Metronomic Schedule monitored with MRSI-based nosological images. NMR Biomed. 2020, 33, e4229. [Google Scholar] [CrossRef]
- Dey, M.; Chang, A.L.; Miska, J.; Wainwright, D.A.; Ahmed, A.U.; Balyasnikova, I.V.; Pytel, P.; Han, Y.; Tobias, A.; Zhang, L.; et al. Dendritic Cell-Based Vaccines that Utilize Myeloid Rather than Plasmacytoid Cells Offer a Superior Survival Advantage in Malignant Glioma. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Kindy, M.S.; Yu, J.; Zhu, H.; Smith, M.T.; Gattoni-Celli, S. A therapeutic cancer vaccine against GL261 murine glioma. J. Transl. Med. 2016, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Wang, L.; Nie, S.; Li, X.; Xiao, Y.; Yang, L.; Meng, X.; Zhao, P.; Cui, C.; Tu, L.; et al. Anti-glioma effect of intracranial vaccination with tumor cell lysate plus flagellin in mice. Vaccine 2018, 36, 8148–8157. [Google Scholar] [CrossRef]
- Renner, D.N.; Jin, F.; Litterman, A.J.; Balgeman, A.J.; Hanson, L.M.; Gamez, J.D.; Chae, M.; Carlson, B.L.; Sarkaria, J.N.; Parney, I.F.; et al. Effective treatment of established GL261 murine gliomas through picornavirus vaccination-enhanced tumor antigen-specific CD8+ T cell responses. PLoS ONE 2015, 10, e0125565. [Google Scholar] [CrossRef] [PubMed]
- Gattoni-Celli, S.; Young, M.R.I. Restoration of immune responsiveness to glioma by vaccination of mice with established brain gliomas with a semi-allogeneic vaccine. Int. J. Mol. Sci. 2016, 17, 1465. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.A.; Cai, H.; Miao, J.; Khare, P.D.; Gonzalez, P.; Dalsing-Hernandez, J.; Sharma, G.; Chan, T.; Cooper, L.J.N.; Lebel, F. Regulated intratumoral expression of IL-12 using a RheoSwitch Therapeutic System® (RTS®) gene switch as gene therapy for the treatment of glioma. Cancer Gene Ther. 2018, 25, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Riccadonna, C.; Yacoub Maroun, C.; Vuillefroy de Silly, R.; Boehler, M.; Calvo Tardón, M.; Jueliger, S.; Taverna, P.; Barba, L.; Marinari, E.; Pellegatta, S.; et al. Decitabine treatment of glioma-initiating cells enhances immune recognition and killing. PLoS ONE 2016, 11, e0162105. [Google Scholar] [CrossRef]
- Hsu, S.P.C.; Chen, Y.C.; Chiang, H.C.; Huang, Y.C.; Huang, C.C.; Wang, H.E.; Wang, Y.S.; Chi, K.H. Rapamycin and hydroxychloroquine combination alters macrophage polarization and sensitizes glioblastoma to immune checkpoint inhibitors. J. Neurooncol. 2020, 146, 417–426. [Google Scholar] [CrossRef]
- Ciesielski, M.J.; Bu, Y.; Munich, S.A.; Teegarden, P.; Smolinski, M.P.; Clements, J.L.; Lau, J.Y.N.; Hangauer, D.G.; Fenstermaker, R.A. KX2-361: A novel orally bioavailable small molecule dual Src/tubulin inhibitor that provides long term survival in a murine model of glioblastoma. J. Neurooncol. 2018, 140, 519–527. [Google Scholar] [CrossRef]
- Su, Y.-T.; Butler, M.; Zhang, M.; Zhang, W.; Song, H.; Hwang, L.; Tran, A.D.; Bash, R.E.; Schorzman, A.N.; Pang, Y.; et al. MerTK inhibition decreases immune suppressive glioblastoma-associated macrophages and neoangiogenesis in glioblastoma microenvironment. Neuro-Oncol. Adv. 2020, 2, 1–13. [Google Scholar] [CrossRef]
- McFarland, B.C.; Marks, M.P.; Rowse, A.L.; Fehling, S.C.; Gerigk, M.; Qin, H.; Benveniste, E.N. Loss of SOCS3 in myeloid cells prolongs survival in a syngeneic model of glioma. Oncotarget 2016, 7, 20621–20635. [Google Scholar] [CrossRef]
- Leten, C.; Trekker, J.; Struys, T.; Dresselaers, T.; Gijsbers, R.; Vande Velde, G.; Lambrichts, I.; Van Der Linden, A.; Verfaillie, C.M.; Himmelreich, U. Assessment of bystander killing-mediated therapy of malignant brain tumors using a multimodal imaging approach. Stem Cell Res. Ther. 2015, 6, 163. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Li, J.; Li, Q.; Wang, X.; Medikonda, R.; Zhao, T.; Li, T.; Ma, H.; Yi, L.; Liu, P.; et al. ACT001 reduces the expression of PD-L1 by inhibiting the phosphorylation of STAT3 in glioblastoma. Theranostics 2020, 10, 5943–5956. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Waxman, D.J. Metronomic cyclophosphamide eradicates large implanted GL261 gliomas by activating antitumor Cd8+ T-cell responses and immune memory. Oncoimmunology 2015, 4, e1005521. [Google Scholar] [CrossRef]
- Wu, J.; Jordan, M.; Waxman, D.J. Metronomic cyclophosphamide activation of anti-tumor immunity: Tumor model, mouse host, and drug schedule dependence of gene responses and their upstream regulators. BMC Cancer 2016, 16, 623. [Google Scholar] [CrossRef]
- Wu, J.; Waxman, D.J. Metronomic cyclophosphamide schedule-dependence of innate immune cell recruitment and tumor regression in an implanted glioma model. Cancer Lett. 2014, 353, 272–280. [Google Scholar] [CrossRef]
- Pérez, J.E.; Fritzell, S.; Kopecky, J.; Visse, E.; Darabi, A.; Siesjö, P. The effect of locally delivered cisplatin is dependent on an intact immune function in an experimental glioma model. Sci. Rep. 2019, 9, 5632. [Google Scholar] [CrossRef]
- Wei, J.; Nduom, E.K.; Kong, L.Y.; Hashimoto, Y.; Xu, S.; Gabrusiewicz, K.; Ling, X.; Huang, N.; Qiao, W.; Zhou, S.; et al. MiR-138 exerts anti-glioma efficacy by targeting immune checkpoints. Neuro. Oncol. 2016, 18, 639–648. [Google Scholar] [CrossRef]
- Strong, A.D.; Indart, M.C.; Hill, N.R.; Daniels, R.L. GL261 glioma tumor cells respond to ATP with an intracellular calcium rise and glutamate release. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Han, C.J.; Zheng, J.Y.; Sun, L.; Yang, H.C.; Cao, Z.Q.; Zhang, X.H.; Zheng, L.T.; Zhen, X.C. The oncometabolite 2-hydroxyglutarate inhibits microglial activation via the AMPK/mTOR/NF-κB pathway. Acta Pharmacol. Sin. 2019, 40, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Panek, W.K.; Pituch, K.C.; Miska, J.; Kim, J.W.; Rashidi, A.; Kanojia, D.; Lopez-Rosas, A.; Han, Y.; Yu, D.; Chang, C.L.; et al. Local Application of Autologous Platelet Rich Fibrin Patch (PRF- P) Suppresses Regulatory T cell Recruitment in a Murine Glioma Model. Mol. Neurobiol. 2019, 176, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Bongiorno, E.K.; Garcia, S.A.; Sauma, S.; Hooper, D.C. Type 1 immune mechanisms driven by the response to infection with attenuated rabies virus result in changes in the immune bias of the tumor microenvironment and necrosis of mouse GL261 brain tumors. J. Immunol. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Roberts, N.B.; Alqazzaz, A.; Hwang, J.R.; Qi, X.; Keegan, A.D.; Kim, A.J.; Winkles, J.A.; Woodworth, G.F. Oxaliplatin disrupts pathological features of glioma cells and associated macrophages independent of apoptosis induction. J. Neurooncol. 2018, 140, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wei, J.; Wang, F.; Kong, L.Y.; Ling, X.Y.; Nduom, E.; Gabrusiewicz, K.; Doucette, T.; Yang, Y.; Yaghi, N.K.; et al. Effect of miR-142-3p on the M2 macrophage and therapeutic efficacy against murine glioblastoma. J. Natl. Cancer Inst. 2014, 106, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, R.; Deng, Y.; Qian, J.; Lu, Z.; Wang, Y.; Zhang, D.; Luo, F.; Chu, Y. MiR-15a/16 deficiency enhances anti-tumor immunity of glioma-infiltrating CD8+ T cells through targeting mTOR. Int. J. Cancer 2017, 141, 2082–2092. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Baidoo, J.N.E.; Sampat, S.; Mancuso, A.; David, L.; Cohen, L.S.; Zhou, S.; Banerjee, P. Liposomal tricurin, a synergistic combination of curcumin, epicatechin gallate and resveratrol, repolarizes tumor-associated microglia/macrophages, and eliminates glioblastoma (GBM) and GBM Stem Cells. Molecules 2018, 23, 201. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Fried, A.; Hussaini, R.; White, R.; Baidoo, J.; Yalamanchi, S.; Banerjee, P. Phytosomal curcumin causes natural killer cell-dependent repolarization of glioblastoma (GBM) tumor-associated microglia/macrophages and elimination of GBM and GBM stem cells. J. Exp. Clin. Cancer Res. 2018, 37, 168. [Google Scholar] [CrossRef] [PubMed]
- Lepore, F.; D’Alessandro, G.; Antonangeli, F.; Santoro, A.; Esposito, V.; Limatola, C.; Trettel, F. CXCL16/CXCR6 axis drives microglia/macrophages phenotype in physiological conditions and plays a crucial role in glioma. Front. Immunol. 2018, 9, 2750. [Google Scholar] [CrossRef]
- Yan, J.; Kong, L.Y.; Hu, J.; Gabrusiewicz, K.; Dibra, D.; Xia, X.; Heimberger, A.B.; Li, S. FGL2 as a Multimodality Regulator of Tumor-Mediated Immune Suppression and Therapeutic Target in Gliomas. J. Natl. Cancer Inst. 2015, 107, djv137. [Google Scholar] [CrossRef]
- Turubanova, V.D.; Balalaeva, I.V.; Mishchenko, T.A.; Catanzaro, E.; Alzeibak, R.; Peskova, N.N.; Efimova, I.; Bachert, C.; Mitroshina, E.V.; Krysko, O.; et al. Immunogenic cell death induced by a new photodynamic therapy based on photosens and photodithazine. J. Immunother. Cancer 2019, 7, 350. [Google Scholar] [CrossRef]
- Sun, S.; Du, G.; Xue, J.; Ma, J.; Ge, M.; Wang, H.; Tian, J. PCC0208009 enhances the anti-tumor effects of temozolomide through direct inhibition and transcriptional regulation of indoleamine 2,3-dioxygenase in glioma models. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418787991. [Google Scholar] [CrossRef]
- Ferrer-Font, L.; Villamañan, L.; Arias-Ramos, N.; Vilardell, J.; Plana, M.; Ruzzene, M.; Pinna, L.A.; Itarte, E.; Arús, C.; Candiota, A.P. Targeting protein kinase CK2: Evaluating CX-4945 potential for GL261 glioblastoma therapy in immunocompetent mice. Pharmaceuticals 2017, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Proske, J.; Walter, L.; Bumes, E.; Hutterer, M.; Vollmann-Zwerenz, A.; Eyüpoglu, I.Y.; Savaskan, N.E.; Seliger, C.; Hau, P.; Uhl, M. Adaptive immune response to and survival effect of temozolomide-and valproic acid-induced autophagy in glioblastoma. Anticancer Res. 2016, 36, 899–906. [Google Scholar] [PubMed]
- Ott, M.; Kassab, C.; Marisetty, A.; Hashimoto, Y.; Wei, J.; Zamler, D.; Leu, J.S.; Tomaszowski, K.H.; Sabbagh, A.; Fang, D.; et al. Radiation with STAT3 blockade triggers dendritic cell-T cell interactions in the glioma microenvironment and therapeutic efficacy. Clin. Cancer Res. 2020, 26, 4983–4994. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Bolduc, A.R.; Hoda, M.N.; Gamble, D.N.; Dolisca, S.B.; Bolduc, A.K.; Hoang, K.; Ashley, C.; McCall, D.; Rojiani, A.M.; et al. The indoleamine 2,3-dioxygenase pathway controls complement-dependent enhancement of chemo-radiation therapy against murine glioblastoma. J. Immunother. Cancer 2014, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Giles, A.J.; Hutchinson, M.N.D.; Sonnemann, H.M.; Jung, J.; Fecci, P.E.; Ratnam, N.M.; Zhang, W.; Song, H.; Bailey, R.; Davis, D.; et al. Dexamethasone-induced immunosuppression: Mechanisms and implications for immunotherapy. J. Immunother. Cancer 2018, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Ausman, J.I.; Shapiro, W.R.; Rall, D.P. Studies on the Chemotherapy of Experimental Brain Tumors: Development of an Experimental Model. Cancer Res. 1970, 30, 2394–2400. [Google Scholar] [PubMed]
- Crommentuijn, M.H.W.; Schetters, S.T.T.; Dusoswa, S.A.; Kruijssen, L.J.W.; Garcia-Vallejo, J.J.; van Kooyk, Y. Immune involvement of the contralateral hemisphere in a glioblastoma mouse model. J. Immunother. Cancer 2020, 8, e000323. [Google Scholar] [CrossRef]
- Baker, G.J.; Castro, M.G.; Lowenstein, P.R. Isolation and flow cytometric analysis of glioma-infiltrating peripheral blood mononuclear cells. J. Vis. Exp. 2015, 2015, e53676. [Google Scholar] [CrossRef]
- Baker, G.J.; Chockley, P.; Zamler, D.; Castro, M.G.; Lowenstein, P.R. Natural killer cells require monocytic Gr-1+/CD11b+ myeloid cells to eradicate orthotopically engrafted glioma cells. Oncoimmunology 2016, 5, e1163461. [Google Scholar] [CrossRef]
- Chang, C.Y.; Jeon, S.B.; Yoon, H.J.; Choi, B.K.; Kim, S.S.; Oshima, M.; Park, E.J. Glial TLR2-driven innate immune responses and CD8 + T cell activation against brain tumor. Glia 2019, 67, 1179–1195. [Google Scholar] [CrossRef]
- Irvin, D.K.; Jouanneau, E.; Duvall, G.; Zhang, X.X.; Zhai, Y.; Sarayba, D.; Seksenyan, A.; Panwar, A.; Black, K.L.; Wheeler, C.J. T cells enhance stem-like properties and conditional malignancy in gliomas. PLoS ONE 2010, 5, e10974. [Google Scholar] [CrossRef] [PubMed]
- Candolfi, M.; Yagiz, K.; Wibowo, M.; Ahlzadeh, G.E.; Puntel, M.; Ghiasi, H.; Kamran, N.; Paran, C.; Lowenstein, P.R.; Castro, M.G. Temozolomide does not impair gene therapy-mediated antitumor immunity in syngeneic brain tumor models. Clin. Cancer Res. 2014, 20, 1555–1565. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Guan, X.; Begum, G.; Ding, D.; Gayden, J.; Hasan, M.N.; Fiesler, V.M.; Dodelson, J.; Kohanbash, G.; Hu, B.; et al. Blockade of Cell Volume Regulatory Protein NKCC1 Increases TMZ-Induced Glioma Apoptosis and Reduces Astrogliosis. Mol. Cancer Ther. 2020, 19, 1550–1561. [Google Scholar] [CrossRef] [PubMed]
- Safdie, F.; Brandhorst, S.; Wei, M.; Wang, W.; Lee, C.; Hwang, S.; Conti, P.S.; Chen, T.C.; Longo, V.D. Fasting Enhances the Response of Glioma to Chemo- and Radiotherapy. PLoS ONE 2012, 7, e44603. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.G.; Kim, C.H.; Park, J.S.; Park, S.D.; Kim, C.K.; Chung, D.S.; Hong, Y.K. Immunological factors relating to the antitumor effect of temozolomide chemoimmunotherapy in a murine glioma model. Clin. Vaccine Immunol. 2010, 17, 143–153. [Google Scholar] [CrossRef]
- Guan, X.; Hasan, M.N.; Begum, G.; Kohanbash, G.; Carney, K.E.; Pigott, V.M.; Persson, A.I.; Castro, M.G.; Jia, W.; Sun, D. Blockade of Na/H exchanger stimulates glioma tumor immunogenicity and enhances combinatorial TMZ and anti-PD-1 therapy. Cell Death Dis. 2018, 9, 1010. [Google Scholar] [CrossRef]
- Yadav, V.N.; Zamler, D.; Baker, G.J.; Kadiyala, P.; Erdreich-Epstein, A.; DeCarvalho, A.C.; Mikkelsen, T.; Castro, M.G.; Lowenstein, P.R. CXCR4 increases in-vivo glioma perivascular invasion, and reduces radiation induced apoptosis: A genetic knockdown study. Oncotarget 2016, 7, 83701–83719. [Google Scholar] [CrossRef]
- Guan, X.; Luo, L.; Begum, G.; Kohanbash, G.; Song, Q.; Rao, A.; Amankulor, N.; Sun, B.; Sun, D.; Jia, W. Elevated Na/H exchanger 1 (SLC9A1) emerges as a marker for tumorigenesis and prognosis in gliomas. J. Exp. Clin. Cancer Res. 2018, 37, 255. [Google Scholar] [CrossRef]
- Mineharu, Y.; Kamran, N.; Lowenstein, P.R.; Castro, M.G. Blockade of mTOR Signaling via Rapamycin Combined with Immunotherapy Augments Anti-glioma Cytotoxic and Memory T cells’ Functions. Mol. Cancer Ther. 2014, 13, 3024–3036. [Google Scholar] [CrossRef]
- Park, J.H.; Ryu, C.H.; Kim, M.J.; Jeun, S.S. Combination therapy for gliomas using temozolomide and interferon-beta secreting human bone marrow derived mesenchymal stem cells. J. Korean Neurosurg. Soc. 2015, 57, 323–328. [Google Scholar] [CrossRef]
- Seyfried, T.N.; El-Abbadi, M.; Roy, M.L. Ganglioside distribution in murine neural tumors. Mol. Chem. Neuropathol. 1992, 17, 147–167. [Google Scholar] [CrossRef]
- Martínez-Murillo, R.; Martínez, A. Standardization of an orthotopic mouse brain tumor model following transplantation of CT-2A astrocytoma cells. Histol. Histopathol. 2007, 22, 1309–1326. [Google Scholar] [PubMed]
- Riva, M.; Wouters, R.; Weerasekera, A.; Belderbos, S.; Nittner, D.; Thal, D.R.; Baert, T.; Giovannoni, R.; Gsell, W.; Himmelreich, U.; et al. CT-2A neurospheres-derived high-grade glioma in mice: A new model to address tumor stem cells and immunosuppression. Biol. Open 2019, 8, bio044552. [Google Scholar] [CrossRef] [PubMed]
- Woroniecka, K.; Chongsathidkiet, P.; Rhodin, K.; Kemeny, H.; Dechant, C.; Farber, S.H.; Elsamadicy, A.A.; Cui, X.; Koyama, S.; Jackson, C.; et al. T-cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin. Cancer Res. 2018, 24, 4175–4186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Miska, J.; Lee-Chang, C.; Rashidi, A.; Panek, W.K.; An, S.; Zannikou, M.; Lopez-Rosas, A.; Han, Y.; Xiao, T.; et al. Therapeutic targeting of tumor-associated myeloid cells synergizes with radiation therapy for glioblastoma. Proc. Natl. Acad. Sci. USA 2019, 116, 23714–23723. [Google Scholar] [CrossRef] [PubMed]
- Mohme, M.; Maire, C.L.; Geumann, U.; Schliffke, S.; Dührsen, L.; Fita, K.; Akyüz, N.; Binder, M.; Westphal, M.; Guenther, C.; et al. Local Intracerebral Immunomodulation Using Interleukin-Expressing Mesenchymal Stem Cells in Glioblastoma. Clin. Cancer Res. 2020, 26, 2626–2639. [Google Scholar] [CrossRef]
- von Roemeling, C.A.; Wang, Y.; Qie, Y.; Yuan, H.; Zhao, H.; Liu, X.; Yang, Z.; Yang, M.; Deng, W.; Bruno, K.A.; et al. Therapeutic modulation of phagocytosis in glioblastoma can activate both innate and adaptive antitumour immunity. Nat. Commun. 2020, 11, 1508. [Google Scholar] [CrossRef]
- Lamano, J.B.; Lamano, J.B.; Li, Y.D.; DiDomenico, J.D.; Choy, W.; Veliceasa, D.; Oyon, D.E.; Fakurnejad, S.; Ampie, L.; Kesavabhotla, K.; et al. Glioblastoma-derived IL6 induces immunosuppressive peripheral myeloid cell PD-L1 and promotes tumor growth. Clin. Cancer Res. 2019, 25, 3643–3657. [Google Scholar] [CrossRef]
- Woroniecka, K.I.; Rhodin, K.E.; Dechant, C.; Cui, X.; Chongsathidkiet, P.; Wilkinson, D.; Waibl-Polania, J.; Sanchez-Perez, L.; Fecci, P.E. 4-1BB agonism averts Til exhaustion and licenses PD-1 blockade in glioblastoma and other intracranial cancers. Clin. Cancer Res. 2020, 26, 1349–1358. [Google Scholar] [CrossRef]
- Speranza, M.C.; Passaro, C.; Ricklefs, F.; Kasai, K.; Klein, S.R.; Nakashima, H.; Kaufmann, J.K.; Ahmed, A.K.; Nowicki, M.O.; Obi, P.; et al. Preclinical investigation of combined gene-mediated cytotoxic immunotherapy and immune checkpoint blockade in glioblastoma. Neuro. Oncol. 2018, 20, 225–235. [Google Scholar] [CrossRef]
- Passaro, C.; Alayo, Q.; DeLaura, I.; McNulty, J.; Grauwet, K.; Ito, H.; Bhaskaran, V.; Mineo, M.; Lawler, S.E.; Shah, K.; et al. Erratum: Arming an oncolytic herpes simplex virus type 1 with a single-chain fragment variable antibody against PD-1 for experimental glioblastoma therapy. Clin. Cancer Res. 2020, 26, 758. [Google Scholar] [CrossRef] [PubMed]
- Belcaid, Z.; Berrevoets, C.; Choi, J.; van Beelen, E.; Stavrakaki, E.; Pierson, T.; Kloezeman, J.; Routkevitch, D.; van der Kaaij, M.; van der Ploeg, A.; et al. Low-dose oncolytic adenovirus therapy overcomes tumor-induced immune suppression and sensitizes intracranial gliomas to anti-PD-1 therapy. Neuro-Oncology Adv. 2020, 2, vdaa011. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Schaettler, M.; Blaha, D.T.; Bowman-Kirigin, J.A.; Kobayashi, D.K.; Livingstone, A.J.; Bender, D.; Miller, C.A.; Kranz, D.M.; Johanns, T.M.; et al. Treatment of an aggressive orthotopic murine glioblastoma model with combination checkpoint blockade and a multivalent neoantigen vaccine. Neuro. Oncol. 2020, 22, 1276–1288. [Google Scholar] [CrossRef] [PubMed]
- Balathasan, L.; Tang, V.A.; Yadollahi, B.; Brun, J.; Labelle, M.; Lefebvre, C.; Swift, S.L.; Stojdl, D.F. Activating Peripheral Innate Immunity Enables Safe and Effective Oncolytic Virotherapy in the Brain. Mol. Ther. Oncolytics 2017, 7, 45–56. [Google Scholar] [CrossRef]
- Sarén, T.; Ramachandran, M.; Martikainen, M.; Yu, D. Insertion of the Type-I IFN Decoy Receptor B18R in a miRNA-Tagged Semliki Forest Virus Improves Oncolytic Capacity but Results in Neurotoxicity. Mol. Ther. Oncolytics 2017, 7, 67–75. [Google Scholar] [CrossRef]
- Ramachandran, M.; Yu, D.; Dyczynski, M.; Baskaran, S.; Zhang, L.; Lulla, A.; Lulla, V.; Saul, S.; Nelander, S.; Dimberg, A.; et al. Safe and effective treatment of experimental neuroblastoma and glioblastoma using systemically delivered triple microrna-detargeted oncolytic semliki forest virus. Clin. Cancer Res. 2017, 23, 1519–1530. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef]
- Fraser, H. Astrocytomas in an inbred mouse strain. J. Pathol. 1971, 103, 266–270. [Google Scholar] [CrossRef]
- Serano, R.D.; Pegram, C.N.; Bigner, D.D. Tumorigenic cell culture lines from a spontaneous VM/Dk murine astrocytoma (SMA). Acta Neuropathol. 1980, 51, 53–64. [Google Scholar] [CrossRef]
- Learn, C.A.; Grossi, P.M.; Schmittling, R.J.; Xie, W.; Mitchell, D.A.; Karikari, I.; Wei, Z.; Dressman, H.; Sampson, J.H. Genetic analysis of intracranial tumors in a murine model of glioma demonstrate a shift in gene expression in response to host immunity. J. Neuroimmunol. 2007, 182, 63–72. [Google Scholar] [CrossRef]
- Tran, T.T.; Uhl, M.; Ma, J.Y.; Janssen, L.; Sriram, V.; Aulwurm, S.; Kerr, I.; Lam, A.; Webb, H.K.; Kapoun, A.M.; et al. Inhibiting TGF-β signaling restores immune surveillance in the SMA-560 glioma model. Neuro. Oncol. 2007, 9, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Frei, K.; Willscher, E.; Stefanski, A.; Kaulich, K.; Roth, P.; Stühler, K.; Reifenberger, G.; Binder, H.; Weller, M. How stemlike are sphere cultures from long-term cancer cell lines? Lessons from mouse glioma models. J. Neuropathol. Exp. Neurol. 2014, 73, 1062–1077. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.; Lohmann, B.; Wirsching, H.G.; Hasenbach, K.; Rushing, E.J.; Frei, K.; Pruschy, M.; Tabatabai, G.; Weller, M. Age-associated and therapy-induced alterations in the cellular microenvironment of experimental gliomas. Oncotarget 2017, 8, 87124–87135. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schötterl, S.; Huber, S.M.; Lentzen, H.; Mittelbronn, M.; Naumann, U. Adjuvant Therapy Using Mistletoe Containing Drugs Boosts the T-Cell-Mediated Killing of Glioma Cells and Prolongs the Survival of Glioma Bearing Mice. Evidence-Based Complement. Altern. Med. 2018, 2018, 3928572. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.; Szabo, E.; Machado, R.A.; Broggini-Tenzer, A.; Walter, A.; Lobell, M.; Heldmann, D.; Süssmeier, F.; Grünewald, S.; Weller, M. Novel TIE-2 inhibitor BAY-826 displays in vivo efficacy in experimental syngeneic murine glioma models. J. Neurochem. 2017, 140, 170–182. [Google Scholar] [CrossRef]
- Seystahl, K.; Papachristodoulou, A.; Burghardt, I.; Schneider, H.; Hasenbach, K.; Janicot, M.; Roth, P.; Weller, M. Biological role and therapeutic targeting of TGF-β3 in glioblastoma. Mol. Cancer Ther. 2017, 16, 1177–1186. [Google Scholar] [CrossRef]
- Silginer, M.; Weller, M.; Ziegler, U.; Roth, P. Integrin inhibition promotes atypical anoikis in glioma cells. Cell Death Dis. 2014, 5, e1012–e1013. [Google Scholar] [CrossRef]
- Papachristodoulou, A.; Silginer, M.; Weller, M.; Schneider, H.; Hasenbach, K.; Janicot, M.; Roth, P. Therapeutic targeting of TGFb ligands in glioblastoma using novel antisense oligonucleotides reduces the growth of experimental gliomas. Clin. Cancer Res. 2019, 25, 7189–7201. [Google Scholar] [CrossRef]
- Pituch, K.C.; Miska, J.; Krenciute, G.; Panek, W.K.; Li, G.; Rodriguez-Cruz, T.; Wu, M.; Han, Y.; Lesniak, M.S.; Gottschalk, S.; et al. Adoptive Transfer of IL13Rα2-Specific Chimeric Antigen Receptor T Cells Creates a Pro-inflammatory Environment in Glioblastoma. Mol. Ther. 2018, 26, 986–995. [Google Scholar] [CrossRef]
- Kovacs, Z.; Werner, B.; Rassi, A.; Sass, J.O.; Martin-Fiori, E.; Bernasconi, M. Prolonged survival upon ultrasound-enhanced doxorubicin delivery in two syngenic glioblastoma mouse models. J. Control. Release 2014, 187, 74–82. [Google Scholar] [CrossRef]
- Weiner, N.; Pyles, R.B.; Chalk, C.L.; Balko, M.G.; Miller, M.A.; Dyer, C.A.; Warnick, R.E.; Parysek, L.M. A syngeneic mouse glioma model for study of glioblastoma therapy. J. Neuropathol. Exp. Neurol. 1999, 58, 54–60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gazdzinski, L.M.; Nieman, B.J. Cellularimaging and texture analysis distinguish differences in cellular dynamics in mouse brain tumors. Magn. Reson. Med. 2014, 71, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Lobo, M.R.; Wang, X.; Gillespie, G.Y.; Woltjer, R.L.; Pike, M.M. Combined efficacy of cediranib and quinacrine in glioma is enhanced by hypoxia and causally linked to autophagic vacuole accumulation. PLoS ONE 2014, 9, e114110. [Google Scholar] [CrossRef] [PubMed]
- Lobo, M.R.; Kukino, A.; Tran, H.; Schabel, M.C.; Springer, C.S., Jr.; Gillespie, G.Y.; Grafe, M.R.; Woltjer, R.L.; Pike, M.M. Synergistic antivascular and antitumor efficacy with combined cediranib and SC6889 in intracranial mouse glioma. PLoS ONE 2015, 10, e0144488. [Google Scholar] [CrossRef]
- Hellums, E.K.; Markert, J.M.; Parker, J.N.; He, B.; Perbal, B.; Roizman, B.; Whitley, R.J.; Langford, C.P.; Bharara, S.; Gillespie, G.Y. Increased efficacy of an interleukin-12-secreting herpes simplex virus in a syngeneic intracranial murine glioma model. Neuro. Oncol. 2005, 7, 213–224. [Google Scholar] [CrossRef]
- Flores, C.; Pham, C.; Snyder, D.; Yang, S.; Sanchez-Perez, L.; Sayour, E.; Cui, X.; Kemeny, H.; Friedman, H.; Bigner, D.D.; et al. Novel role of hematopoietic stem cells in immunologic rejection of malignant gliomas. Oncoimmunology 2015, 4, e994374. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.E.; Kopecky, J.; Visse, E.; Darabi, A.; Siesjö, P. Convection-enhanced delivery of temozolomide and whole cell tumor immunizations in GL261 and KR158 experimental mouse gliomas. BMC Cancer 2020, 20, 7. [Google Scholar]
- Flores-Toro, J.A.; Luo, D.; Gopinath, A.; Sarkisian, M.R.; Campbell, J.J.; Charo, I.F.; Singh, R.; Schall, T.J.; Datta, M.; Jain, R.K.; et al. CCR2 inhibition reduces tumor myeloid cells and unmasks a checkpoint inhibitor effect to slow progression of resistant murine gliomas. Proc. Natl. Acad. Sci. USA 2020, 117, 1129–1138. [Google Scholar] [CrossRef]
- Kwon, C.H.; Zhao, D.; Chen, J.; Alcantara, S.; Li, Y.; Burns, D.K.; Mason, R.P.; Lee, E.Y.; Wu, H.; Parada, L.F. Pten haploinsufficiency accelerates formation of high-grade astrocytomas. Cancer Res. 2008, 68, 3286–3294. [Google Scholar] [CrossRef]
- Llaguno, S.A.; Chen, J.; Kwon, C.H.; Jackson, E.L.; Li, Y.; Burns, D.K.; Alvarez-Buylla, A.; Parada, L.F. Malignant Astrocytomas Originate from Neural Stem/Progenitor Cells in a Somatic Tumor Suppressor Mouse Model. Cancer Cell 2009, 15, 45–56. [Google Scholar] [CrossRef]
- Soda, Y.; Marumoto, T.; Friedmann-Morvinski, D.; Soda, M.; Liu, F.; Michiue, H.; Pastorino, S.; Yang, M.; Hoffman, R.M.; Kesari, S.; et al. Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc. Natl. Acad. Sci. USA 2011, 108, 4274–4280. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Martuza, R.L.; Rabkin, S.D. Macrophage Polarization Contributes to Glioblastoma Eradication by Combination Immunovirotherapy and Immune Checkpoint Blockade. Cancer Cell 2017, 32, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Cheema, T.A.; Wakimoto, H.; Fecci, P.E.; Ning, J.; Kuroda, T.; Jeyaretna, D.S.; Martuza, R.L.; Rabkin, S.D. Multifaceted oncolytic virus therapy for glioblastoma in an immunocompetent cancer stem cell model. Proc. Natl. Acad. Sci. USA 2013, 110, 12006–12011. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Rabkin, S.D.; Martuza, R.L. Temozolomide antagonizes oncolytic immunovirotherapy in glioblastoma. J. Immunother. Cancer 2020, 8, 345. [Google Scholar] [CrossRef]
- Saha, D.; Wakimoto, H.; Peters, C.W.; Antoszczyk, S.J.; Rabkin, S.D.; Martuza, R.L. Combinatorial effects of vegfr kinase inhibitor axitinib and oncolytic virotherapy in mouse and human glioblastoma stem-like cell models. Clin. Cancer Res. 2018, 24, 3409–3422. [Google Scholar] [CrossRef]
- Osuka, S.; Sampetrean, O.; Shimizu, T.; Saga, I.; Onishi, N.; Sugihara, E.; Okubo, J.; Fujita, S.; Takano, S.; Matsumura, A.; et al. IGF1 receptor signaling regulates adaptive radioprotection in glioma stem cells. Stem Cells 2013, 31, 627–640. [Google Scholar] [CrossRef]
- He, B.; Jabouille, A.; Steri, V.; Johansson-Percival, A.; Michael, I.P.; Kotamraju, V.R.; Junckerstorff, R.; Nowak, A.K.; Hamzah, J.; Lee, G.; et al. Vascular targeting of LIGHT normalizes blood vessels in primary brain cancer and induces intratumoural high endothelial venules. J. Pathol. 2018, 245, 209–221. [Google Scholar] [CrossRef]
- Riva, M.; Wouters, R.; Nittner, D.; Ceuster, J.; Sterpin, E.; Giovannoni, R.; Himmelreich, U.; Gsell, W.; Van Ranst, M.; Coosemans, A. Radiation dose-escalation and dose-fractionation modulate the immune microenvironment, cancer stem cells and vasculature in experimental high-grade gliomas. J. Neurosurg. Sci. 2020. [Google Scholar] [CrossRef]
- Costa, B.; Fletcher, M.; Boskovic, P.; Ivanova, E.L.; Eisemann, T.; Lohr, S.; Bunse, L.; Löwer, M.; Burchard, S.; Korshunov, A.; et al. A novel neural stem cell-derived immunocompetent mouse model of glioblastoma for preclinical studies. bioRxiv 2020. [Google Scholar] [CrossRef]
- Aslan, K.; Turco, V.; Blobner, J.; Sonner, J.K.; Liuzzi, A.R.; Núñez, N.G.; De Feo, D.; Kickingereder, P.; Fischer, M.; Green, E.; et al. Heterogeneity of response to immune checkpoint blockade in hypermutated experimental gliomas. Nature Comm 2020, 11, 931. [Google Scholar] [CrossRef]
- Schafer, N.; Gielen, G.H.; Rauschenbach, L.; Kebir, S.; Till, A.; Reinartz, R.; Simon, M.; Niehusmann, P.; Kleinschnitz, C.; Herrlinger, U.; et al. Longitudinal heterogeneity in glioblastoma: Moving targets in recurrent versus primary tumors. J. Transl. Med. 2019, 17, 96. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wouters, R.; Bevers, S.; Riva, M.; De Smet, F.; Coosemans, A. Immunocompetent Mouse Models in the Search for Effective Immunotherapy in Glioblastoma. Cancers 2021, 13, 19. https://doi.org/10.3390/cancers13010019
Wouters R, Bevers S, Riva M, De Smet F, Coosemans A. Immunocompetent Mouse Models in the Search for Effective Immunotherapy in Glioblastoma. Cancers. 2021; 13(1):19. https://doi.org/10.3390/cancers13010019
Chicago/Turabian StyleWouters, Roxanne, Sien Bevers, Matteo Riva, Frederik De Smet, and An Coosemans. 2021. "Immunocompetent Mouse Models in the Search for Effective Immunotherapy in Glioblastoma" Cancers 13, no. 1: 19. https://doi.org/10.3390/cancers13010019
APA StyleWouters, R., Bevers, S., Riva, M., De Smet, F., & Coosemans, A. (2021). Immunocompetent Mouse Models in the Search for Effective Immunotherapy in Glioblastoma. Cancers, 13(1), 19. https://doi.org/10.3390/cancers13010019







