Use of Palliative Chemotherapy and ICU Admissions in Gastric and Esophageal Cancer Patients in the Last Phase of Life: A Nationwide Observational Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population Selection
2.2. Outcomes and Characteristics
2.3. Statistical Analyses
3. Results
3.1. Population
3.2. ICU Admission and Chemotherapy
3.3. Outcomes in Relation to Age and Gender
3.4. Outcomes in Relation to Hospital Experience
4. Discussion
4.1. Main Findings
4.2. Clinical Relevance
4.3. Methodological Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer Today—Estimated Number of New Cases in 2018. Available online: https://gco.iarc.fr/today/online-analysis-table (accessed on 4 April 2020).
- Cancer Today—Stomach Cancer Fact Sheet. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/7-Stomach-fact-sheet.pdf (accessed on 4 April 2020).
- Cancer Today—Esophageal Cancer Fact Sheet. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/6-Oesophagus-fact-sheet.pdf (accessed on 4 April 2020).
- Van Putten, M.; De Vos-Geelen, J.; Nieuwenhuijzen, G.A.P.; Siersema, P.D.; Lemmens, V.E.P.P.; Rosman, C.; Van Der Sangen, M.J.C.; Verhoeven, R.H.A. Long-term survival improvement in oesophageal cancer in the Netherlands. Eur. J. Cancer 2018, 94, 138–147. [Google Scholar] [CrossRef]
- Nelen, S.D.; Verhoeven, R.H.A.; Lemmens, V.E.P.P.; De Wilt, J.H.W.; Bosscha, K. Increasing survival gap between young and elderly gastric cancer patients. Gastric Cancer 2017, 20, 919–928. [Google Scholar] [CrossRef]
- Van Kleef, J.J.; Ter Veer, E.; Boorn, H.G.V.D.; Schokker, S.; Ngai, L.L.; Prins, M.J.; Mohammad, N.H.; Van De Poll-Franse, L.V.; Zwinderman, A.H.; Van Oijen, M.G.H.; et al. Quality of Life During Palliative Systemic Therapy for Esophagogastric Cancer: Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2019, 112, 12–29. [Google Scholar] [CrossRef]
- Wright, A.A.; Zhang, B.; Ray, A.; Mack, J.W.; Trice, E.; Balboni, T.; Mitchell, S.L.; Jackson, V.A.; Block, S.D.; Maciejewski, P.K.; et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 2008, 300, 1665–1673. [Google Scholar] [CrossRef]
- Gade, G.; Venohr, I.; Conner, D.; McGrady, K.; Beane, J.; Richardson, R.H.; Williams, M.P.; Liberson, M.; Blum, M.; Della Penna, R. Impact of an Inpatient Palliative Care Team: A Randomized Controlled Trial. J. Palliat. Med. 2008, 11, 180–190. [Google Scholar] [CrossRef]
- Brumley, R.; Enguidanos, S.; Jamison, P.; Seitz, R.; Morgenstern, N.; Saito, S.; McIlwane, J.; Hillary, K.; Gonzalez, J. Increased Satisfaction with Care and Lower Costs: Results of a Randomized Trial of In-Home Palliative Care. J. Am. Geriatr. Soc. 2007, 55, 993–1000. [Google Scholar] [CrossRef]
- Maetens, A.; Beernaert, K.; De Schreye, R.; Faes, K.; Annemans, L.; Pardon, K.; Deliens, L.; Cohen, J. Impact of palliative home care support on the quality and costs of care at the end of life: A population-level matched cohort study. BMJ Open 2019, 9, e025180. [Google Scholar] [CrossRef]
- Merchant, S.J.; Lajkosz, K.; Brogly, S.B.; Booth, C.M.; Nanji, S.; Patel, S.V.; Baxter, N.N. The Final 30 Days of Life: A Study of Patients with Gastrointestinal Cancer in Ontario, Canada. J. Palliat. Care 2017, 32, 92–100. [Google Scholar] [CrossRef]
- Tramontano, A.C.; Nipp, R.; Kong, C.Y.; Yerramilli, D.; Gainor, J.F.; Hur, C. Hospice use and end-of-life care among older patients with esophageal cancer. Health Sci. Rep. 2018, 1, e76. [Google Scholar] [CrossRef]
- Mohammad, N.H.; Bernards, N.; Van Putten, M.; Lemmens, V.E.P.P.; Van Oijen, M.G.H.; Van Laarhoven, H.W.M. Volume-outcome relation in palliative systemic treatment of metastatic oesophagogastric cancer. Eur. J. Cancer 2017, 78, 28–36. [Google Scholar] [CrossRef]
- Kempf, E.; Tournigand, C.; Rochigneux, P.; Aubry, R.; Morin, L. Discrepancies in the use of chemotherapy and artificial nutrition near the end of life for hospitalised patients with metastatic gastric or oesophageal cancer. A countrywide, register-based study. Eur. J. Cancer 2017, 79, 31–40. [Google Scholar] [CrossRef]
- Hong, J.H.; Rho, S.-Y.; Hong, Y.S. Trends in the Aggressiveness of End-of-Life Care for Advanced Stomach Cancer Patients. Cancer Res. Treat. 2013, 45, 270–275. [Google Scholar] [CrossRef]
- Dijksterhuis, W.P.M.; Verhoeven, R.H.A.; Meijer, S.L.; Slingerland, M.; Mohammad, N.H.; De Vos-Geelen, J.; Beerepoot, L.V.; Van Voorthuizen, T.; Creemers, G.-J.; Van Oijen, M.G.H.; et al. Increased assessment of HER2 in metastatic gastroesophageal cancer patients: A nationwide population-based cohort study. Gastric Cancer 2020, 23, 579–590. [Google Scholar] [CrossRef]
- Evans, N.; Pasman, H.R.; Alonso, T.V.; Block, L.V.D.; Miccinesi, G.; Van Casteren, V.; Donker, G.; Bertolissi, S.; Zurriaga, O.; Deliens, L.; et al. End-of-Life Decisions: A Cross-National Study of Treatment Preference Discussions and Surrogate Decision-Maker Appointments. PLoS ONE 2013, 8, e57965. [Google Scholar] [CrossRef]
- Evans, N.; Costantini, M.; Pasman, H.R.; Block, L.V.D.; Donker, G.A.; Miccinesi, G.; Bertolissi, S.; Gil, M.; Boffin, N.; Zurriaga, O.; et al. End-of-Life Communication: A Retrospective Survey of Representative General Practitioner Networks in Four Countries. J. Pain Symptom Manag. 2014, 47, 604–619.e3. [Google Scholar] [CrossRef]
- Bestvina, C.M.; Wroblewski, K.E.; Daly, B.; Beach, B.; Chow, S.; Hantel, A.; Malec, M.; Huber, M.T.; Polite, B.N. A Rules-Based Algorithm to Prioritize Poor Prognosis Cancer Patients in Need of Advance Care Planning. J. Palliat. Med. 2018, 21, 846–849. [Google Scholar] [CrossRef]
- Turpin, M.H.; Meyers, E.A.; Fugelsang, J.A.; Friedman, O.; Białek, M. Sunk Cost Bias and Withdrawal Aversion. Am. J. Bioeth. 2019, 19, 57–59. [Google Scholar] [CrossRef]
- Meltzer, D.; Manning, W.G.; Morrison, J.; Shah, M.N.; Jin, L.; Guth, T.; Levinson, W. Effects of Physician Experience on Costs and Outcomes on an Academic General Medicine Service: Results of a Trial of Hospitalists. Ann. Intern. Med. 2002, 137, 866–874. [Google Scholar] [CrossRef]
- Birkmeyer, J.D.; Dimick, J.B. Understanding and Reducing Variation in Surgical Mortality. Annu. Rev. Med. 2009, 60, 405–415. [Google Scholar] [CrossRef]
- Chan, B.A.; Larkins, S.L.; Evans, R.; Watt, K.; Sabesan, S. Do teleoncology models of care enable safe delivery of chemotherapy in rural towns? Med. J. Aust. 2015, 203, 406. [Google Scholar] [CrossRef]
- Salami, A.C.; Barden, G.M.; Castillo, D.L.; Hanna, M.; Petersen, N.J.; Davila, J.A.; Naik, A.D.; Anaya, D.A. Establishment of a Regional Virtual Tumor Board Program to Improve the Process of Care for Patients with Hepatocellular Carcinoma. J. Oncol. Pr. 2015, 11, e66–e74. [Google Scholar] [CrossRef] [PubMed]
- Indini, A.; Aschele, C.; Cavanna, L.; Clerico, M.; Daniele, B.; Fiorentini, G.; Fioretto, L.; Giordano, M.; Montesarchio, V.; Ortega, C.; et al. Reorganisation of medical oncology departments during the novel coronavirus disease-19 pandemic: A nationwide Italian survey. Eur. J. Cancer 2020, 132, 17–23. [Google Scholar] [CrossRef]
- Van Erning, F.N.; Van Steenbergen, L.N.; Broek, W.V.D.; Rutten, H.J.T.; Lemmens, V.E.P.P. No difference between lowest and highest volume hospitals in outcome after colorectal cancer surgery in the southern Netherlands. Eur. J. Surg. Oncol. EJSO 2013, 39, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Jonker, F.H.W.; Hagemans, J.A.W.; Verhoef, C.; Burger, J.W.A. The impact of hospital volume on perioperative outcomes of rectal cancer. Eur. J. Surg. Oncol. EJSO 2017, 43, 1894–1900. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.; Blaauwgeers, H.G.T.; Ho, V.K.Y.; Van Houdt, W.J.; Van Der Hage, J.A.; Been, L.B.; Bonenkamp, J.J.; Bemelmans, M.H.A.; Van Dalen, T.; Haas, R.L.; et al. Increased survival of non low-grade and deep-seated soft tissue sarcoma after surgical management in high-volume hospitals: A nationwide study from the Netherlands. Eur. J. Cancer 2019, 110, 98–106. [Google Scholar] [CrossRef]
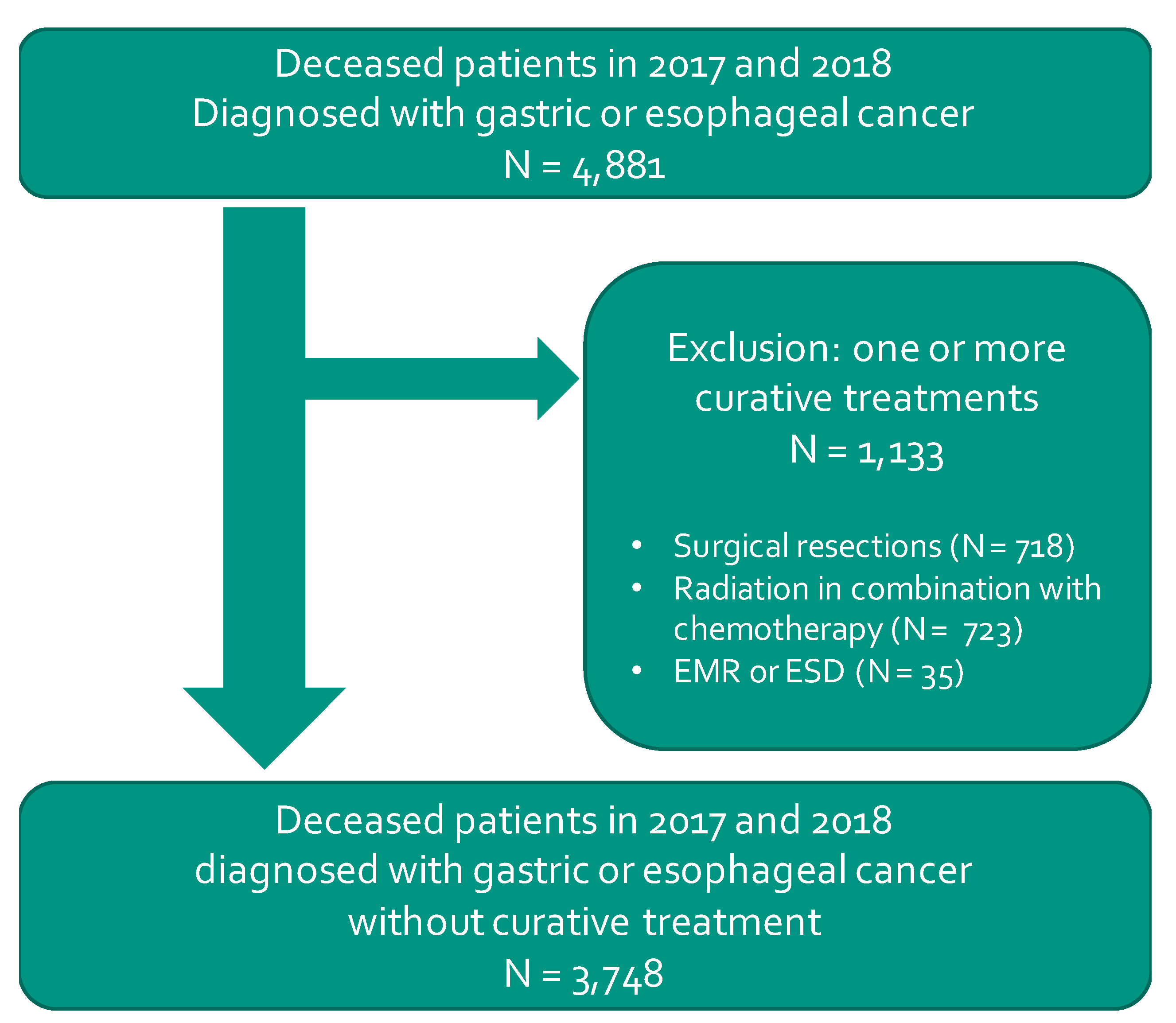
| Demography and Cancer Characteristics | Findings |
|---|---|
| Men—no. (%) | 2676 (71.4%) |
| Age at time of death—mean (SD) | 71.4 (11.1) |
| Esophageal cancer—no. (%) | 2448 (65.3%) |
| Gastric cancer—no. (%) | 1300 (34.7%) |
| Comorbidities | |
| Hypertension—no. (%) | 2413 (64.4%) |
| Diabetes—no. (%) | 766 (20.4%) |
| COPD—no. (%) | 515 (13.7%) |
| Clinical descriptives | |
| Radiation therapy in the final 3 months—no. (%) | 739 (19.7%) |
| Deceased in hospital—no. (%) | 865 (23.1%) |
| Outcome of Interest | 3 Months before Death | Final Month before Death | ||||
|---|---|---|---|---|---|---|
| Patients (no.) | Population (no.) | % | Patients (no.) | Population (no.) | % | |
| ICU admission | 209 | 3748 | 5.6% | 156 | 3748 | 4.2% |
| Chemotherapy | 795 | 3748 | 21.2% | 299 | 3748 | 8.0% |
| Chemotherapy within subpopulations | ||||||
| With previous chemotherapy | 488 | 1017 | 48.0% | 191 | 1216 | 15.7% |
| Without previous chemotherapy | 307 | 2731 | 11.2% | 108 | 2532 | 4.3% |
| Outcome of Interest | 3 Months before Death | Final Month before Death | |||
|---|---|---|---|---|---|
| Correlation r (Weighted) | Significance p (Two-Tailed) | Correlation r (Weighted) | Significance p (Two-Tailed) | Institutions (no.) | |
| ICU admission | 0.18 | 0.11 | 0.20 | 0.07 * | 81 |
| Chemotherapy | −0.12 | 0.29 | −0.23 | 0.04 ** | 81 |
| Chemotherapy within subpopulations | |||||
| With previous chemotherapy | −0.12 | 0.30 | −0.18 | 0.12 | 75 |
| Without previous chemotherapy | −0.21 | 0.06 * | −0.23 | 0.03 ** | 81 |
| Outcome of Interest | 3 Months before Death | Final Month before Death | |||
|---|---|---|---|---|---|
| Correlation r (Weighted) | Significance p (Two-Tailed) | Correlation r (Weighted) | Significance p (Two-Tailed) | Institutions (no.) | |
| ICU admission | 0.02 | 0.84 | 0.07 | 0.55 | 81 |
| Chemotherapy | −0.01 | 0.92 | −0.07 | 0.55 | 81 |
| Chemotherapy within subpopulations | |||||
| With previous chemotherapy | −0.23 | 0.04 ** | −0.11 | 0.33 | 75 |
| Without previous chemotherapy | −0.05 | 0.67 | −0.08 | 0.46 | 81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Besseling, J.; Reitsma, J.; Van Erkelens, J.A.; Schepens, M.H.J.; Siroen, M.P.C.; Ziedses des Plantes, C.M.P.; van Berge Henegouwen, M.I.; Beerepoot, L.V.; Van Voorthuizen, T.; Van Zuylen, L.; et al. Use of Palliative Chemotherapy and ICU Admissions in Gastric and Esophageal Cancer Patients in the Last Phase of Life: A Nationwide Observational Study. Cancers 2021, 13, 145. https://doi.org/10.3390/cancers13010145
Besseling J, Reitsma J, Van Erkelens JA, Schepens MHJ, Siroen MPC, Ziedses des Plantes CMP, van Berge Henegouwen MI, Beerepoot LV, Van Voorthuizen T, Van Zuylen L, et al. Use of Palliative Chemotherapy and ICU Admissions in Gastric and Esophageal Cancer Patients in the Last Phase of Life: A Nationwide Observational Study. Cancers. 2021; 13(1):145. https://doi.org/10.3390/cancers13010145
Chicago/Turabian StyleBesseling, Joost, Jan Reitsma, Judith A. Van Erkelens, Maike H. J. Schepens, Michiel P. C. Siroen, Cathelijne M. P. Ziedses des Plantes, Mark I. van Berge Henegouwen, Laurens V. Beerepoot, Theo Van Voorthuizen, Lia Van Zuylen, and et al. 2021. "Use of Palliative Chemotherapy and ICU Admissions in Gastric and Esophageal Cancer Patients in the Last Phase of Life: A Nationwide Observational Study" Cancers 13, no. 1: 145. https://doi.org/10.3390/cancers13010145
APA StyleBesseling, J., Reitsma, J., Van Erkelens, J. A., Schepens, M. H. J., Siroen, M. P. C., Ziedses des Plantes, C. M. P., van Berge Henegouwen, M. I., Beerepoot, L. V., Van Voorthuizen, T., Van Zuylen, L., Verhoeven, R. H. A., & van Laarhoven, H. (2021). Use of Palliative Chemotherapy and ICU Admissions in Gastric and Esophageal Cancer Patients in the Last Phase of Life: A Nationwide Observational Study. Cancers, 13(1), 145. https://doi.org/10.3390/cancers13010145






