The Role of CDK5 in Tumours and Tumour Microenvironments
Abstract
Simple Summary
Abstract
1. Introduction
2. Biology of CDK5
2.1. Basics of CDK5, Its Activators, and Inhibitors
2.1.1. p35
2.1.2. p25
2.1.3. p39 and p29
2.1.4. Other Activators and Inhibitors
2.2. CDK5 Regulation by Posttranslational Modification
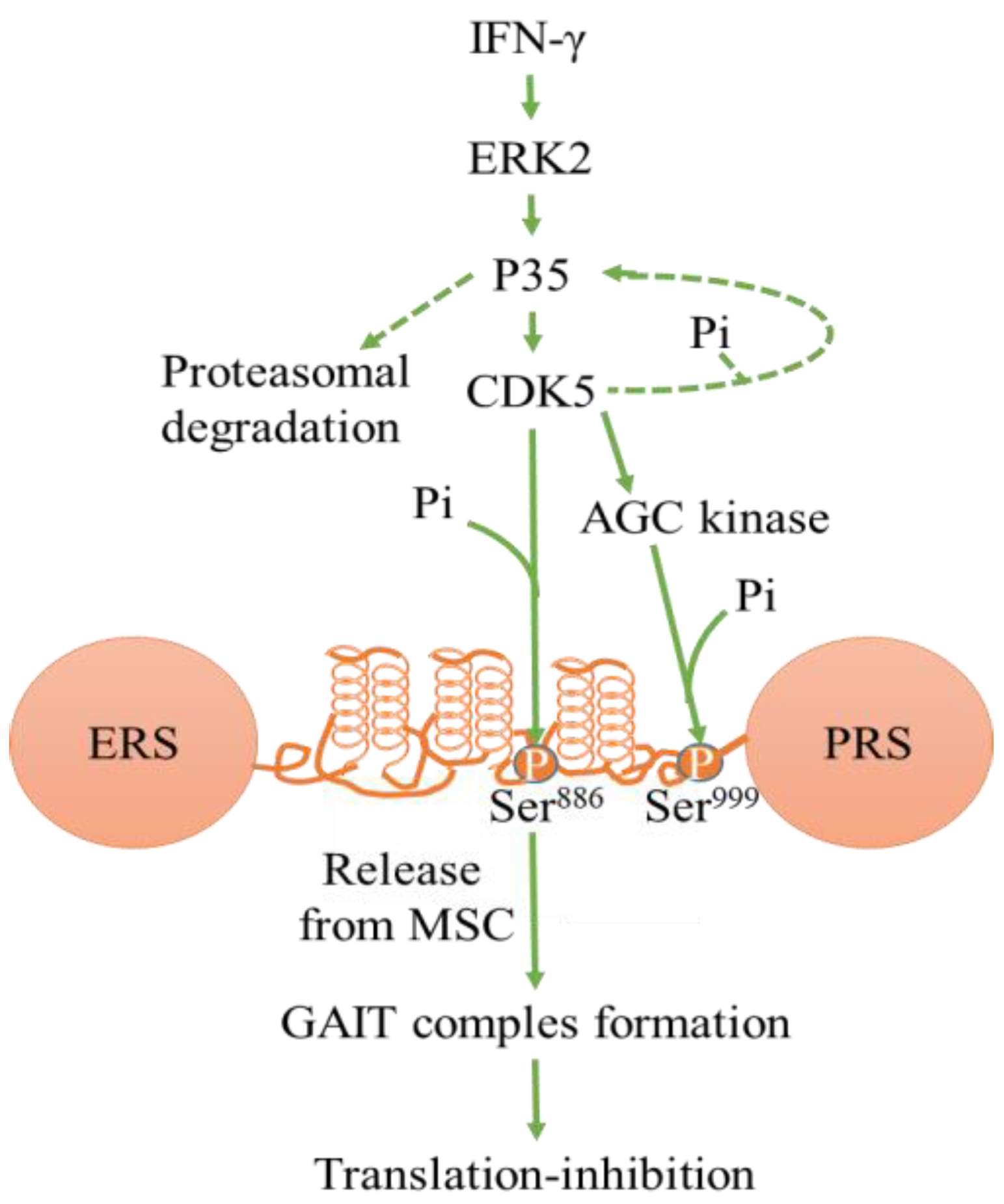
2.3. Regulation of Transcription and Translation by CDK5
2.3.1. Transcription Regulation
2.3.2. Translation Regulation
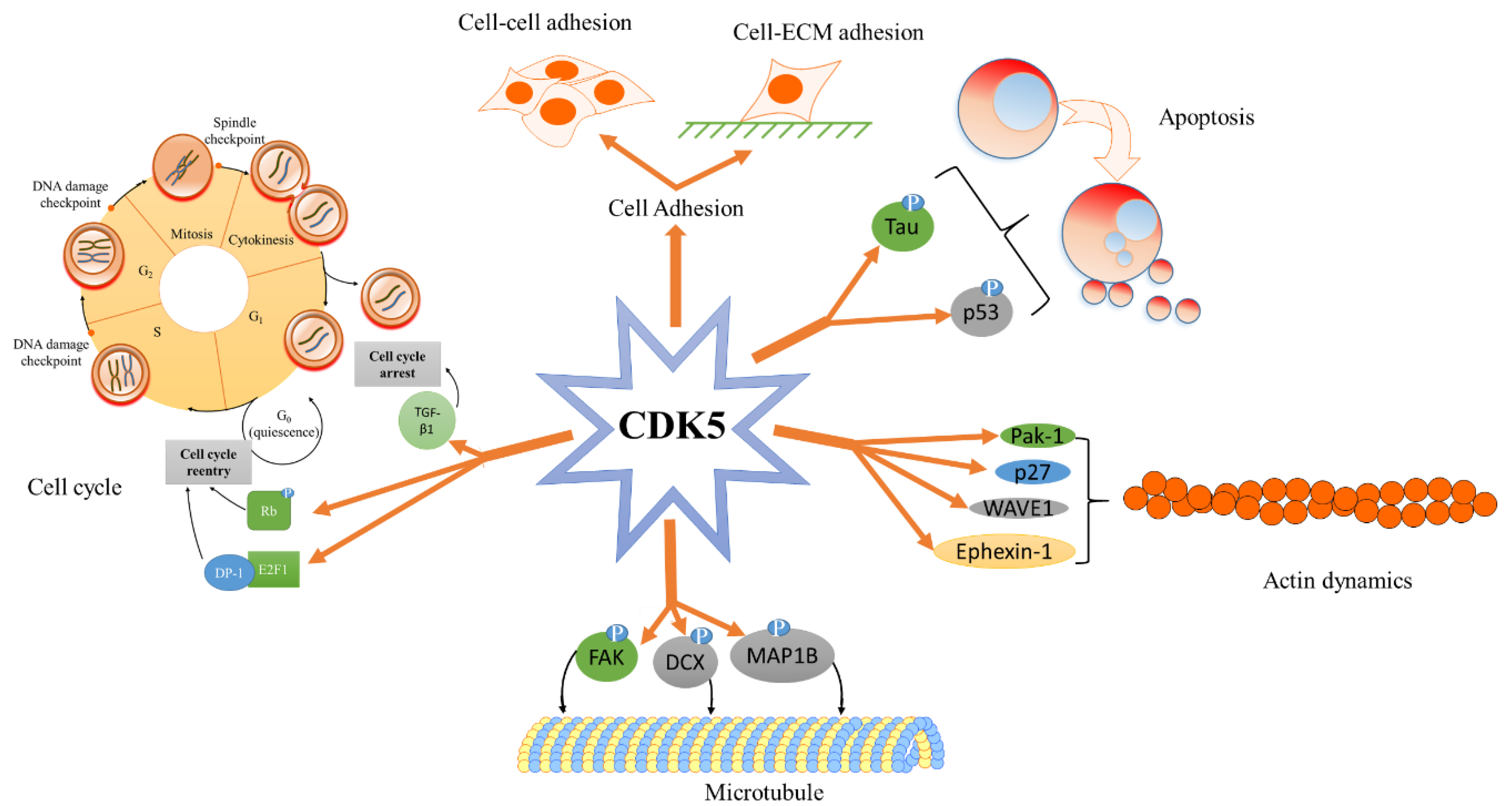
3. Role of CDK5 in Normal Cell Physiology
3.1. CDK5 and Cell Adhesion
3.2. CDK5 and Cytoskeleton
3.2.1. CDK5—Microtubule
3.2.2. CDK5—Intermediate Filaments
3.2.3. CDK5—Actin Cytoskeleton
3.3. CDK5 Cell Cycle and DNA Damage
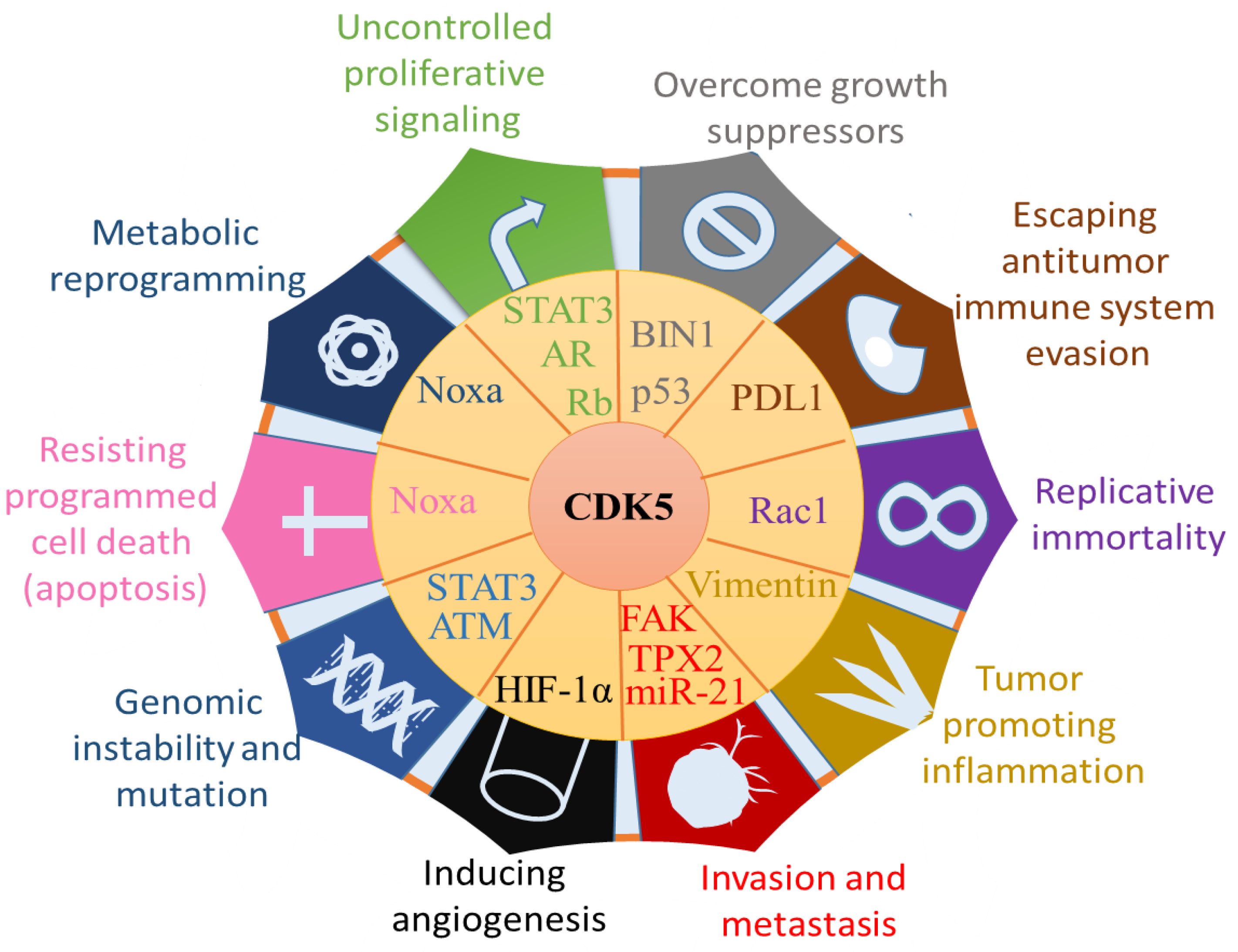
4. The Role of CDK5 in Cancer Cells
4.1. Effects of CDK5 on Cancer Hallmarks from Tumour Itself
4.1.1. Effect of CDK5 on the Proliferation and Growth of Cancer
4.1.2. Effect of CDK5 on the Migration and Invasion
4.1.3. Effects of CDK5 on the Genome Instability, Mutation, and Replicative Immortality
4.1.4. Effects of CDK5 on Cancer Cell Metabolism
4.2. Effects of CDK5 on Tumour Microenvironments
4.2.1. Effects of CDK5 on Angiogenesis
4.2.2. Effects of CDK5 on Inflammation and Immune Evasion
4.2.3. CDK5-Nerve and Cancer Connection
5. Potential Therapeutic Options
5.1. Early Pan CDK Inhibitors
5.2. Multitarget CDK5 Inhibitors
5.3. Selective CDK5 Inhibitors
6. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, W.Q.; Ip, J.P.; Li, R.; Ng, Y.P.; Lin, S.C.; Chen, Y.; Fu, A.K.; Ip, N.Y. Cdk5-mediated phosphorylation of Axin directs axon formation during cerebral cortex development. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 13613–13624. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Urushibara, T.; Yoshioka, N.; Saito, T.; Fukuda, M.; Tomomura, M.; Hisanaga, S. LMTK1 regulates dendritic formation by regulating movement of Rab11A-positive endosomes. Mol. Biol. Cell 2014, 25, 1755–1768. [Google Scholar] [CrossRef] [PubMed]
- Patzke, H.; Tsai, L.H. Calpain-mediated cleavage of the cyclin-dependent kinase-5 activator p39 to p29. J. Biol. Chem. 2002, 277, 8054–8060. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.U.; Kim, C.; Lee, J.; Kim, D.; Kim, Y.; Yun, N.; Oh, Y.J. Site-specific phosphorylation of Fbxw7 by Cdk5/p25 and its resulting decreased stability are linked to glutamate-induced excitotoxicity. Cell Death Dis. 2019, 10, 579. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Oba, T.; Shimizu, S.; Asada, A.; Iijima, K.M.; Ando, K. Cdk5 increases MARK4 activity and augments pathological tau accumulation and toxicity through tau phosphorylation at Ser262. Hum. Mol. Genet. 2019, 28, 3062–3071. [Google Scholar] [CrossRef]
- Shea, T.B.; Yabe, J.T.; Ortiz, D.; Pimenta, A.; Loomis, P.; Goldman, R.D.; Amin, N.; Pant, H.C. Cdk5 regulates axonal transport and phosphorylation of neurofilaments in cultured neurons. J. Cell Sci. 2004, 117, 933–941. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Jia, Y.; Liu, T.; Wang, M.; Lv, W.; Zhang, R.; Shi, J.; Liu, L. CDK5 neutralizes the tumor suppressing effect of BIN1 via mediating phosphorylation of c-MYC at Ser-62 site in NSCLC. Cancer Cell Int. 2019, 19, 226. [Google Scholar] [CrossRef]
- Ruiz de Porras, V.; Bystrup, S.; Cabrero-de Las Heras, S.; Musulén, E.; Palomero, L.; Alonso, M.H.; Nieto, R.; Arango, D.; Moreno, V.; Queralt, C.; et al. Tumor Expression of Cyclin-Dependent Kinase 5 (Cdk5) Is a Prognostic Biomarker and Predicts Outcome of Oxaliplatin-Treated Metastatic Colorectal Cancer Patients. Cancers 2019, 11, 1540. [Google Scholar] [CrossRef]
- Liang, Q.; Li, L.; Zhang, J.; Lei, Y.; Wang, L.; Liu, D.X.; Feng, J.; Hou, P.; Yao, R.; Zhang, Y.; et al. CDK5 is essential for TGF-β1-induced epithelial-mesenchymal transition and breast cancer progression. Sci. Rep. 2013, 3, 2932. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, T.; Dang, Y.; Li, Z.; Li, P.; Chen, G. Aberrant expression of CDK5 infers poor outcomes for nasopharyngeal carcinoma patients. Int. J. Clin. Exp. Pathol. 2015, 8, 8066–8074. [Google Scholar]
- Sharma, S.; Sicinski, P. A kinase of many talents: Non-neuronal functions of CDK5 in development and disease. Open Biol. 2020, 10, 190287. [Google Scholar] [CrossRef] [PubMed]
- Hellmich, M.R.; Pant, H.C.; Wada, E.; Battey, J.F. Neuronal cdc2-like kinase: A cdc2-related protein kinase with predominantly neuronal expression. Proc. Natl. Acad. Sci. USA 1992, 89, 10867–10871. [Google Scholar] [CrossRef] [PubMed]
- Paglini, G.; Cáceres, A. The role of the Cdk5--p35 kinase in neuronal development. Eur. J. Biochem. 2001, 268, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Humbert, S.; Bronson, R.T.; Takahashi, S.; Kulkarni, A.B.; Li, E.; Tsai, L.H. p35 and p39 are essential for cyclin-dependent kinase 5 function during neurodevelopment. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 6758–6771. [Google Scholar] [CrossRef]
- Tarricone, C.; Dhavan, R.; Peng, J.; Areces, L.B.; Tsai, L.H.; Musacchio, A. Structure and regulation of the CDK5-p25(nck5a) complex. Mol. Cell 2001, 8, 657–669. [Google Scholar] [CrossRef]
- Morgan, D.O. Principles of CDK regulation. Nature 1995, 374, 131–134. [Google Scholar] [CrossRef]
- Dhariwala, F.A.; Rajadhyaksha, M.S. An Unusual Member of the Cdk Family: Cdk5. Cell. Mol. Neurobiol. 2008, 28, 351–369. [Google Scholar] [CrossRef]
- Dhavan, R.; Tsai, L.H. A decade of CDK5. Nat. Rev. Mol. Cell Biol. 2001, 2, 749–759. [Google Scholar] [CrossRef]
- Shupp, A.; Casimiro, M.C.; Pestell, R.G. Biological functions of CDK5 and potential CDK5 targeted clinical treatments. Oncotarget 2017, 8, 17373–17382. [Google Scholar] [CrossRef]
- Hagmann, H.; Taniguchi, Y.; Pippin, J.W.; Kauerz, H.M.; Benzing, T.; Shankland, S.J.; Brinkkoetter, P.T. Cyclin I and p35 determine the subcellular distribution of Cdk5. Am. J. Physiol. Cell Physiol. 2015, 308, C339–C347. [Google Scholar] [CrossRef]
- Arif, A. Extraneuronal activities and regulatory mechanisms of the atypical cyclin-dependent kinase Cdk5. Biochem. Pharmacol. 2012, 84, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.H.; Chang, K.H.; Clawson, S.; Ghosh, S.; Mirzaei, H.; Regnier, F.; Shah, K. Glutathione-S-transferase P1 is a critical regulator of Cdk5 kinase activity. J. Neurochem. 2011, 118, 902–914. [Google Scholar] [CrossRef] [PubMed]
- Asada, A.; Yamamoto, N.; Gohda, M.; Saito, T.; Hayashi, N.; Hisanaga, S. Myristoylation of p39 and p35 is a determinant of cytoplasmic or nuclear localization of active cyclin-dependent kinase 5 complexes. J. Neurochem. 2008, 106, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Patrick, G.N.; Zukerberg, L.; Nikolic, M.; de la Monte, S.; Dikkes, P.; Tsai, L.H. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 1999, 402, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Humbert, S.; Dhavan, R.; Tsai, L. p39 activates cdk5 in neurons, and is associated with the actin cytoskeleton. J. Cell Sci. 2000, 113 Pt 6, 975–983. [Google Scholar]
- Brinkkoetter, P.T.; Olivier, P.; Wu, J.S.; Henderson, S.; Krofft, R.D.; Pippin, J.W.; Hockenbery, D.; Roberts, J.M.; Shankland, S.J. Cyclin I activates Cdk5 and regulates expression of Bcl-2 and Bcl-XL in postmitotic mouse cells. J. Clin. Investig. 2009, 119, 3089–3101. [Google Scholar] [CrossRef]
- Baldin, V.; Lukas, J.; Marcote, M.J.; Pagano, M.; Draetta, G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993, 7, 812–821. [Google Scholar] [CrossRef]
- Modi, P.K.; Komaravelli, N.; Singh, N.; Sharma, P. Interplay between MEK-ERK signaling, cyclin D1, and cyclin-dependent kinase 5 regulates cell cycle reentry and apoptosis of neurons. Mol. Biol. Cell 2012, 23, 3722–3730. [Google Scholar] [CrossRef]
- Ohtsubo, M.; Theodoras, A.M.; Schumacher, J.; Roberts, J.M.; Pagano, M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol. Cell. Biol. 1995, 15, 2612–2624. [Google Scholar] [CrossRef]
- Odajima, J.; Wills, Z.P.; Ndassa, Y.M.; Terunuma, M.; Kretschmannova, K.; Deeb, T.Z.; Geng, Y.; Gawrzak, S.; Quadros, I.M.; Newman, J.; et al. Cyclin E constrains Cdk5 activity to regulate synaptic plasticity and memory formation. Dev. Cell 2011, 21, 655–668. [Google Scholar] [CrossRef]
- Amin, N.D.; Zheng, Y.; Bk, B.; Shukla, V.; Skuntz, S.; Grant, P.; Steiner, J.; Bhaskar, M.; Pant, H.C. The interaction of Munc 18 (p67) with the p10 domain of p35 protects in vivo Cdk5/p35 activity from inhibition by TFP5, a peptide derived from p35. Mol. Biol. Cell 2016, 27, 3221–3232. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, A.I.; Shuang, R.; Giovannucci, D.R.; Zhang, L.; Bittner, M.A.; Stuenkel, E.L. Regulation of exocytosis by cyclin-dependent kinase 5 via phosphorylation of Munc18. J. Biol. Chem. 1999, 274, 4027–4035. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Lahiri, D.K. Cdk5 activity in the brain—Multiple paths of regulation. J. Cell Sci. 2014, 127, 2391–2400. [Google Scholar] [CrossRef] [PubMed]
- Patrick, G.N.; Zhou, P.; Kwon, Y.T.; Howley, P.M.; Tsai, L.H. p35, the neuronal-specific activator of cyclin-dependent kinase 5 (Cdk5) is degraded by the ubiquitin-proteasome pathway. J. Biol. Chem. 1998, 273, 24057–24064. [Google Scholar] [CrossRef] [PubMed]
- Hisanaga, S.; Saito, T. The regulation of cyclin-dependent kinase 5 activity through the metabolism of p35 or p39 Cdk5 activator. Neuro-Signals 2003, 12, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, H.; Yabut, O.; Fitzpatrick, H.; D’Arcangelo, G.; Herrup, K. Cdk5 suppresses the neuronal cell cycle by disrupting the E2F1-DP1 complex. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 5219–5228. [Google Scholar] [CrossRef]
- Fu, X.; Choi, Y.K.; Qu, D.; Yu, Y.; Cheung, N.S.; Qi, R.Z. Identification of nuclear import mechanisms for the neuronal Cdk5 activator. J. Biol. Chem. 2006, 281, 39014–39021. [Google Scholar] [CrossRef]
- Harada, T.; Morooka, T.; Ogawa, S.; Nishida, E. ERK induces p35, a neuron-specific activator of Cdk5, through induction of Egr1. Nat. Cell Biol. 2001, 3, 453–459. [Google Scholar] [CrossRef]
- Bogush, A.; Pedrini, S.; Pelta-Heller, J.; Chan, T.; Yang, Q.; Mao, Z.; Sluzas, E.; Gieringer, T.; Ehrlich, M.E. AKT and CDK5/p35 mediate brain-derived neurotrophic factor induction of DARPP-32 in medium size spiny neurons in vitro. J. Biol. Chem. 2007, 282, 7352–7359. [Google Scholar] [CrossRef]
- Kamei, H.; Saito, T.; Ozawa, M.; Fujita, Y.; Asada, A.; Bibb, J.A.; Saido, T.C.; Sorimachi, H.; Hisanaga, S. Suppression of calpain-dependent cleavage of the CDK5 activator p35 to p25 by site-specific phosphorylation. J. Biol. Chem. 2007, 282, 1687–1694. [Google Scholar] [CrossRef]
- Asada, A.; Saito, T.; Hisanaga, S. Phosphorylation of p35 and p39 by Cdk5 determines the subcellular location of the holokinase in a phosphorylation-site-specific manner. J. Cell Sci. 2012, 125, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Vosler, P.S.; Brennan, C.S.; Chen, J. Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol. Neurobiol. 2008, 38, 78–100. [Google Scholar] [CrossRef] [PubMed]
- Pozo, K.; Bibb, J.A. The Emerging Role of Cdk5 in Cancer. Trends Cancer 2016, 2, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Z.H.; Ip, N.Y. Cyclin-Dependent Kinase 5—An Emerging Player in Parkinson’s Disease Pathophysiology. In Mechanisms in Parkinson’s Disease—Models and Treatments; Books on Demand: Norderstedt, Germany, 2012. [Google Scholar]
- Roufayel, R.; Murshid, N. CDK5: Key Regulator of Apoptosis and Cell Survival. Biomedicines 2019, 7, 88. [Google Scholar] [CrossRef]
- Kimura, T.; Ishiguro, K.; Hisanaga, S. Physiological and pathological phosphorylation of tau by Cdk5. Front. Mol. Neurosci. 2014, 7, 65. [Google Scholar] [CrossRef]
- Piedrahita, D.; Hernández, I.; López-Tobón, A.; Fedorov, D.; Obara, B.; Manjunath, B.S.; Boudreau, R.L.; Davidson, B.; Laferla, F.; Gallego-Gómez, J.C.; et al. Silencing of CDK5 reduces neurofibrillary tangles in transgenic alzheimer’s mice. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 13966–13976. [Google Scholar] [CrossRef]
- Tang, D.; Yeung, J.; Lee, K.Y.; Matsushita, M.; Matsui, H.; Tomizawa, K.; Hatase, O.; Wang, J.H. An isoform of the neuronal cyclin-dependent kinase 5 (Cdk5) activator. J. Biol. Chem. 1995, 270, 26897–26903. [Google Scholar] [CrossRef]
- Ledee, D.R.; Gao, C.Y.; Seth, R.; Fariss, R.N.; Tripathi, B.K.; Zelenka, P.S. A specific interaction between muskelin and the cyclin-dependent kinase 5 activator p39 promotes peripheral localization of muskelin. J. Biol. Chem. 2005, 280, 21376–21383. [Google Scholar] [CrossRef]
- Brinkkoetter, P.T.; Pippin, J.W.; Shankland, S.J. Cyclin I-Cdk5 governs survival in post-mitotic cells. Cell Cycle 2010, 9, 1729–1731. [Google Scholar] [CrossRef][Green Version]
- Shetty, K.T.; Kaech, S.; Link, W.T.; Jaffe, H.; Flores, C.M.; Wray, S.; Pant, H.C.; Beushausen, S. Molecular characterization of a neuronal-specific protein that stimulates the activity of Cdk5. J. Neurochem. 1995, 64, 1988–1995. [Google Scholar] [CrossRef]
- Bhaskar, K.; Shareef, M.M.; Sharma, V.M.; Shetty, A.P.; Ramamohan, Y.; Pant, H.C.; Raju, T.R.; Shetty, K.T. Co-purification and localization of Munc18-1 (p67) and Cdk5 with neuronal cytoskeletal proteins. Neurochem. Int. 2004, 44, 35–44. [Google Scholar] [CrossRef]
- Ji, Y.B.; Zhuang, P.P.; Ji, Z.; Wu, Y.M.; Gu, Y.; Gao, X.Y.; Pan, S.Y.; Hu, Y.F. TFP5 peptide, derived from CDK5-activating cofactor p35, provides neuroprotection in early-stage of adult ischemic stroke. Sci. Rep. 2017, 7, 40013. [Google Scholar] [CrossRef]
- Sasaki, Y.; Cheng, C.; Uchida, Y.; Nakajima, O.; Ohshima, T.; Yagi, T.; Taniguchi, M.; Nakayama, T.; Kishida, R.; Kudo, Y.; et al. Fyn and Cdk5 mediate semaphorin-3A signaling, which is involved in regulation of dendrite orientation in cerebral cortex. Neuron 2002, 35, 907–920. [Google Scholar] [CrossRef]
- Zukerberg, L.R.; Patrick, G.N.; Nikolic, M.; Humbert, S.; Wu, C.L.; Lanier, L.M.; Gertler, F.B.; Vidal, M.; Van Etten, R.A.; Tsai, L.H. Cables links Cdk5 and c-Abl and facilitates Cdk5 tyrosine phosphorylation, kinase upregulation, and neurite outgrowth. Neuron 2000, 26, 633–646. [Google Scholar] [CrossRef]
- Fu, W.Y.; Chen, Y.; Sahin, M.; Zhao, X.S.; Shi, L.; Bikoff, J.B.; Lai, K.O.; Yung, W.H.; Fu, A.K.; Greenberg, M.E.; et al. Cdk5 regulates EphA4-mediated dendritic spine retraction through an ephexin1-dependent mechanism. Nat. Neurosci. 2007, 10, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Z.H.; Chin, W.H.; Chen, Y.; Ng, Y.P.; Ip, N.Y. Cdk5 is involved in BDNF-stimulated dendritic growth in hippocampal neurons. PLoS Biol. 2007, 5, e63. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sharma, M.; Amin, N.D.; Albers, R.W.; Pant, H.C. Regulation of cyclin-dependent kinase 5 catalytic activity by phosphorylation. Proc. Natl. Acad. Sci. USA 1999, 96, 11156–11160. [Google Scholar] [CrossRef] [PubMed]
- Rosales, J.; Han, B.; Lee, K.Y. Cdk7 functions as a cdk5 activating kinase in brain. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2003, 13, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Roach, B.L.; Ngo, J.M.; Limso, C.; Oloja, K.B.; Bhandari, D. Identification and characterization of a novel phosphoregulatory site on cyclin-dependent kinase 5. Biochem. Biophys. Res. Commun. 2018, 504, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, I.; Wang, J.H. Demonstration of cyclin-dependent kinase inhibitory serine/threonine kinase in bovine thymus. J. Biol. Chem. 1996, 271, 5443–5450. [Google Scholar] [CrossRef]
- Pollan, S.G.; Huang, F.; Sperger, J.M.; Lang, J.M.; Morrissey, C.; Cress, A.E.; Chu, C.Y.; Bhowmick, N.A.; You, S.; Freeman, M.R.; et al. Regulation of inside-out β1-integrin activation by CDCP1. Oncogene 2018, 37, 2817–2836. [Google Scholar] [CrossRef]
- Qu, J.; Nakamura, T.; Cao, G.; Holland, E.A.; McKercher, S.R.; Lipton, S.A. S-Nitrosylation activates Cdk5 and contributes to synaptic spine loss induced by beta-amyloid peptide. Proc. Natl. Acad. Sci. USA 2011, 108, 14330–14335. [Google Scholar] [CrossRef]
- Zhang, P.; Yu, P.C.; Tsang, A.H.; Chen, Y.; Fu, A.K.; Fu, W.Y.; Chung, K.K.; Ip, N.Y. S-nitrosylation of cyclin-dependent kinase 5 (cdk5) regulates its kinase activity and dendrite growth during neuronal development. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 14366–14370. [Google Scholar] [CrossRef]
- Lee, J.; Ko, Y.U.; Chung, Y.; Yun, N.; Kim, M.; Kim, K.; Oh, Y.J. The acetylation of cyclin-dependent kinase 5 at lysine 33 regulates kinase activity and neurite length in hippocampal neurons. Sci. Rep. 2018, 8, 13676. [Google Scholar] [CrossRef] [PubMed]
- Tuo, Q.Z.; Liuyang, Z.Y.; Lei, P.; Yan, X.; Shentu, Y.P.; Liang, J.W.; Zhou, H.; Pei, L.; Xiong, Y.; Hou, T.Y.; et al. Zinc induces CDK5 activation and neuronal death through CDK5-Tyr15 phosphorylation in ischemic stroke. Cell Death Dis. 2018, 9, 870. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Saito, T.; Sato, K.; Furusawa, K.; Hosokawa, T.; Tsutsumi, K.; Asada, A.; Kamada, S.; Ohshima, T.; Hisanaga, S. Phosphorylation of cyclin-dependent kinase 5 (Cdk5) at Tyr-15 is inhibited by Cdk5 activators and does not contribute to the activation of Cdk5. J. Biol. Chem. 2014, 289, 19627–19636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tan, V.B.; Lim, K.M.; Tay, T.E. The activation and inhibition of cyclin-dependent kinase-5 by phosphorylation. Biochemistry 2007, 46, 10841–10851. [Google Scholar] [CrossRef]
- Bórquez, D.A.; Olmos, C.; Álvarez, S.; Di Genova, A.; Maass, A.; González-Billault, C. Bioinformatic survey for new physiological substrates of Cyclin-dependent kinase 5. Genomics 2013, 101, 221–228. [Google Scholar] [CrossRef]
- Black, B.L.; Olson, E.N. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu. Rev. Cell Dev. Biol. 1998, 14, 167–196. [Google Scholar] [CrossRef]
- Camins, A.; Verdaguer, E.; Folch, J.; Canudas, A.M.; Pallàs, M. The role of CDK5/P25 formation/inhibition in neurodegeneration. Drug News Perspect. 2006, 19, 453–460. [Google Scholar] [CrossRef]
- Gong, X.; Tang, X.; Wiedmann, M.; Wang, X.; Peng, J.; Zheng, D.; Blair, L.A.; Marshall, J.; Mao, Z. Cdk5-mediated inhibition of the protective effects of transcription factor MEF2 in neurotoxicity-induced apoptosis. Neuron 2003, 38, 33–46. [Google Scholar] [CrossRef]
- Tang, X.; Wang, X.; Gong, X.; Tong, M.; Park, D.; Xia, Z.; Mao, Z. Cyclin-dependent kinase 5 mediates neurotoxin-induced degradation of the transcription factor myocyte enhancer factor 2. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 4823–4834. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.K.; Fu, W.Y.; Ng, A.K.; Chien, W.W.; Ng, Y.P.; Wang, J.H.; Ip, N.Y. Cyclin-dependent kinase 5 phosphorylates signal transducer and activator of transcription 3 and regulates its transcriptional activity. Proc. Natl. Acad. Sci. USA 2004, 101, 6728–6733. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.-N.; Chen, M.-C.; Lin, H.-Y.; Peng, Y.-T.; Li, P.-C.; Lin, E.; Chiang, M.-C.; Hsieh, J.-T.; Lin, H. Cyclin-dependent kinase 5 modulates STAT3 and androgen receptor activation through phosphorylation of Ser(727) on STAT3 in prostate cancer cells. Am. J. Physiol. Endocrinol. Metab. 2013, 305. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Chen, M.C.; Chiu, C.Y.; Song, Y.M.; Lin, S.Y. Cdk5 regulates STAT3 activation and cell proliferation in medullary thyroid carcinoma cells. J. Biol. Chem. 2007, 282, 2776–2784. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Zhong, Z.; Darnell, J.E., Jr. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell 1995, 82, 241–250. [Google Scholar] [CrossRef]
- Kino, T.; Jaffe, H.; Amin, N.D.; Chakrabarti, M.; Zheng, Y.L.; Chrousos, G.P.; Pant, H.C. Cyclin-dependent kinase 5 modulates the transcriptional activity of the mineralocorticoid receptor and regulates expression of brain-derived neurotrophic factor. Mol. Endocrinol. 2010, 24, 941–952. [Google Scholar] [CrossRef]
- Kino, T.; Ichijo, T.; Amin, N.D.; Kesavapany, S.; Wang, Y.; Kim, N.; Rao, S.; Player, A.; Zheng, Y.L.; Garabedian, M.J.; et al. Cyclin-dependent kinase 5 differentially regulates the transcriptional activity of the glucocorticoid receptor through phosphorylation: Clinical implications for the nervous system response to glucocorticoids and stress. Mol. Endocrinol. 2007, 21, 1552–1568. [Google Scholar] [CrossRef]
- Zhang, J.; Krishnamurthy, P.K.; Johnson, G.V. Cdk5 phosphorylates p53 and regulates its activity. J. Neurochem. 2002, 81, 307–313. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.S.; Lee, S.J.; Kim, K.T. Stabilization and activation of p53 induced by Cdk5 contributes to neuronal cell death. J. Cell Sci. 2007, 120, 2259–2271. [Google Scholar] [CrossRef]
- Gallazzini, M.; Heussler, G.E.; Kunin, M.; Izumi, Y.; Burg, M.B.; Ferraris, J.D. High NaCl-induced activation of CDK5 increases phosphorylation of the osmoprotective transcription factor TonEBP/OREBP at threonine 135, which contributes to its rapid nuclear localization. Mol. Biol. Cell 2011, 22, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Hanchate, N.K.; Tyagi, R.K.; Sharma, P. Cyclin dependent kinase 5 (Cdk5) mediated inhibition of the MAP kinase pathway results in CREB down regulation and apoptosis in PC12 cells. Biochem. Biophys. Res. Commun. 2007, 358, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; David, G.; Hung, K.W.; DePinho, R.A.; Fu, A.K.; Ip, N.Y. Cdk5/p35 phosphorylates mSds3 and regulates mSds3-mediated repression of transcription. J. Biol. Chem. 2004, 279, 54438–54444. [Google Scholar] [CrossRef] [PubMed]
- Arif, A.; Jia, J.; Moodt, R.A.; DiCorleto, P.E.; Fox, P.L. Phosphorylation of glutamyl-prolyl tRNA synthetase by cyclin-dependent kinase 5 dictates transcript-selective translational control. Proc. Natl. Acad. Sci. USA 2011, 108, 1415–1420. [Google Scholar] [CrossRef]
- Rosales, J.L.; Lee, K.Y. Extraneuronal roles of cyclin-dependent kinase 5. BioEssays News Rev. Mol. Cell. Dev. Biol. 2006, 28, 1023–1034. [Google Scholar] [CrossRef]
- Kwon, Y.T.; Gupta, A.; Zhou, Y.; Nikolic, M.; Tsai, L.H. Regulation of N-cadherin-mediated adhesion by the p35-Cdk5 kinase. Curr. Biol. CB 2000, 10, 363–372. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Song, Y.S.; Li, Y.; Jia, Y.H.; Zhao, H.D. Cdk5 links with DNA damage response and cancer. Mol. Cancer 2017, 16, 60. [Google Scholar] [CrossRef]
- Kawauchi, T. Cell adhesion and its endocytic regulation in cell migration during neural development and cancer metastasis. Int. J. Mol. Sci. 2012, 13, 4564–4590. [Google Scholar] [CrossRef]
- Kawauchi, T. Cdk5 regulates multiple cellular events in neural development, function and disease. Dev. Growth Differ. 2014, 56, 335–348. [Google Scholar] [CrossRef]
- Lin, S.; Wang, J.; Ye, Z.; Ip, N.Y.; Lin, S.C. CDK5 activator p35 downregulates E-cadherin precursor independently of CDK5. FEBS Lett. 2008, 582, 1197–1202. [Google Scholar] [CrossRef]
- Abdulghani, J.; Gu, L.; Dagvadorj, A.; Lutz, J.; Leiby, B.; Bonuccelli, G.; Lisanti, M.P.; Zellweger, T.; Alanen, K.; Mirtti, T.; et al. Stat3 promotes metastatic progression of prostate cancer. Am. J. Pathol. 2008, 172, 1717–1728. [Google Scholar] [CrossRef]
- Daval, M.; Gurlo, T.; Costes, S.; Huang, C.J.; Butler, P.C. Cyclin-dependent kinase 5 promotes pancreatic beta-cell survival via Fak-Akt signaling pathways. Diabetes 2011, 60, 1186–1197. [Google Scholar] [CrossRef]
- Ubeda, M.; Kemp, D.M.; Habener, J.F. Glucose-induced expression of the cyclin-dependent protein kinase 5 activator p35 involved in Alzheimer’s disease regulates insulin gene transcription in pancreatic beta-cells. Endocrinology 2004, 145, 3023–3031. [Google Scholar] [CrossRef]
- Negash, S.; Wang, H.S.; Gao, C.; Ledee, D.; Zelenka, P. Cdk5 regulates cell-matrix and cell-cell adhesion in lens epithelial cells. J. Cell Sci. 2002, 115, 2109–2117. [Google Scholar] [PubMed]
- Nakano, N.; Nakao, A.; Ishidoh, K.; Tsuboi, R.; Kominami, E.; Okumura, K.; Ogawa, H. CDK5 regulates cell-cell and cell-matrix adhesion in human keratinocytes. Br. J. Dermatol. 2005, 153, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Negash, S.; Guo, H.T.; Ledee, D.; Wang, H.S.; Zelenka, P. CDK5 regulates cell adhesion and migration in corneal epithelial cells. Mol. Cancer Res. MCR 2002, 1, 12–24. [Google Scholar] [PubMed]
- Huang, C.; Rajfur, Z.; Yousefi, N.; Chen, Z.; Jacobson, K.; Ginsberg, M.H. Talin phosphorylation by Cdk5 regulates Smurf1-mediated talin head ubiquitylation and cell migration. Nat. Cell Biol. 2009, 11, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef]
- Xu, J.; Tsutsumi, K.; Tokuraku, K.; Estes, K.A.; Hisanaga, S.; Ikezu, T. Actin interaction and regulation of cyclin-dependent kinase 5/p35 complex activity. J. Neurochem. 2011, 116, 192–204. [Google Scholar] [CrossRef][Green Version]
- Xie, Z.; Samuels, B.A.; Tsai, L.H. Cyclin-dependent kinase 5 permits efficient cytoskeletal remodeling--a hypothesis on neuronal migration. Cereb. Cortex 2006, 16 (Suppl. 1), i64–i68. [Google Scholar] [CrossRef]
- Strock, C.J.; Park, J.I.; Nakakura, E.K.; Bova, G.S.; Isaacs, J.T.; Ball, D.W.; Nelkin, B.D. Cyclin-dependent kinase 5 activity controls cell motility and metastatic potential of prostate cancer cells. Cancer Res. 2006, 66, 7509–7515. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Gu, R.X.; Zhou, X.S.; Zhou, F.Z.; Wu, G. Cyclin-dependent kinase 5 regulates the proliferation, motility and invasiveness of lung cancer cells through its effects on cytoskeletal remodeling. Mol. Med. Rep. 2015, 12, 3979–3985. [Google Scholar] [CrossRef] [PubMed]
- Su, C.Y.; Yan, R.L.; Hsu, W.H.; Chu, C.T.; Chang, H.C.; Lai, C.C.; Hsu, H.P.; Chen, H.C. Phosphorylation of adducin-1 by cyclin-dependent kinase 5 is important for epidermal growth factor-induced cell migration. Sci. Rep. 2019, 9, 13703. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Li, Q.; He, L.; Lim, H.Y.; Fu, X.; Cheung, N.S.; Qi, D.X.; Qi, R.Z. Microtubule association of the neuronal p35 activator of Cdk5. J. Biol. Chem. 2007, 282, 18666–18670. [Google Scholar] [CrossRef]
- He, L.; Hou, Z.; Qi, R.Z. Calmodulin binding and Cdk5 phosphorylation of p35 regulate its effect on microtubules. J. Biol. Chem. 2008, 283, 13252–13260. [Google Scholar] [CrossRef]
- Kawauchi, T.; Chihama, K.; Nishimura, Y.V.; Nabeshima, Y.; Hoshino, M. MAP1B phosphorylation is differentially regulated by Cdk5/p35, Cdk5/p25, and JNK. Biochem. Biophys. Res. Commun. 2005, 331, 50–55. [Google Scholar] [CrossRef]
- Xie, Z.; Sanada, K.; Samuels, B.A.; Shih, H.; Tsai, L.H. Serine 732 phosphorylation of FAK by Cdk5 is important for microtubule organization, nuclear movement, and neuronal migration. Cell 2003, 114, 469–482. [Google Scholar] [CrossRef]
- Tanaka, T.; Serneo, F.F.; Tseng, H.C.; Kulkarni, A.B.; Tsai, L.H.; Gleeson, J.G. Cdk5 phosphorylation of doublecortin ser297 regulates its effect on neuronal migration. Neuron 2004, 41, 215–227. [Google Scholar] [CrossRef]
- Miller, C.C.; Ackerley, S.; Brownlees, J.; Grierson, A.J.; Jacobsen, N.J.; Thornhill, P. Axonal transport of neurofilaments in normal and disease states. Cell. Mol. Life Sci. CMLS 2002, 59, 323–330. [Google Scholar] [CrossRef]
- Lindqvist, J.; Wistbacka, N.; Eriksson, J.E. Studying Nestin and its Interrelationship with Cdk5. Methods Enzymol. 2016, 568, 509–535. [Google Scholar] [CrossRef]
- Mokrý, J.; Cízková, D.; Filip, S.; Ehrmann, J.; Osterreicher, J.; Kolár, Z.; English, D. Nestin expression by newly formed human blood vessels. Stem Cell. Dev. 2004, 13, 658–664. [Google Scholar] [CrossRef]
- Mokrý, J.; Ehrmann, J.; Karbanová, J.; Cízková, D.; Soukup, T.; Suchánek, J.; Filip, S.; Kolár, Z. Expression of intermediate filament nestin in blood vessels of neural and non-neural tissues. Acta Med. (Hradec Kralove) 2008, 51, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Sahlgren, C.M.; Mikhailov, A.; Vaittinen, S.; Pallari, H.M.; Kalimo, H.; Pant, H.C.; Eriksson, J.E. Cdk5 regulates the organization of Nestin and its association with p35. Mol. Cell. Biol. 2003, 23, 5090–5106. [Google Scholar] [CrossRef] [PubMed]
- Pallari, H.M.; Lindqvist, J.; Torvaldson, E.; Ferraris, S.E.; He, T.; Sahlgren, C.; Eriksson, J.E. Nestin as a regulator of Cdk5 in differentiating myoblasts. Mol. Biol. Cell 2011, 22, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, M.; Dudek, H.; Kwon, Y.T.; Ramos, Y.F.; Tsai, L.H. The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev. 1996, 10, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, M.; Chou, M.M.; Lu, W.; Mayer, B.J.; Tsai, L.H. The p35/Cdk5 kinase is a neuron-specific Rac effector that inhibits Pak1 activity. Nature 1998, 395, 194–198. [Google Scholar] [CrossRef]
- Kawauchi, T.; Chihama, K.; Nabeshima, Y.; Hoshino, M. Cdk5 phosphorylates and stabilizes p27kip1 contributing to actin organization and cortical neuronal migration. Nat. Cell Biol. 2006, 8, 17–26. [Google Scholar] [CrossRef]
- Fu, A.K.; Ip, N.Y. Cyclin-dependent kinase 5 links extracellular cues to actin cytoskeleton during dendritic spine development. Cell Adhes. Migr. 2007, 1, 110–112. [Google Scholar] [CrossRef][Green Version]
- Sedarous, M.; Keramaris, E.; O’Hare, M.; Melloni, E.; Slack, R.S.; Elce, J.S.; Greer, P.A.; Park, D.S. Calpains mediate p53 activation and neuronal death evoked by DNA damage. J. Biol. Chem. 2003, 278, 26031–26038. [Google Scholar] [CrossRef]
- Lu, T.; Pan, Y.; Kao, S.Y.; Li, C.; Kohane, I.; Chan, J.; Yankner, B.A. Gene regulation and DNA damage in the ageing human brain. Nature 2004, 429, 883–891. [Google Scholar] [CrossRef]
- Tian, B.; Yang, Q.; Mao, Z. Phosphorylation of ATM by Cdk5 mediates DNA damage signalling and regulates neuronal death. Nat. Cell Biol. 2009, 11, 211–218. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cicero, S.; Herrup, K. Cyclin-dependent kinase 5 is essential for neuronal cell cycle arrest and differentiation. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 9658–9668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cicero, S.A.; Wang, L.; Romito-Digiacomo, R.R.; Yang, Y.; Herrup, K. Nuclear localization of Cdk5 is a key determinant in the postmitotic state of neurons. Proc. Natl. Acad. Sci. USA 2008, 105, 8772–8777. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, S.; Harlow, E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science 1993, 262, 2050–2054. [Google Scholar] [CrossRef]
- Park, S.J.; Yang, S.W.; Kim, B.C. Transforming growth factor-β1 induces cell cycle arrest by activating atypical cyclin-dependent kinase 5 through up-regulation of Smad3-dependent p35 expression in human MCF10A mammary epithelial cells. Biochem. Biophys. Res. Commun. 2016, 472, 502–507. [Google Scholar] [CrossRef]
- Zhang, J.; Herrup, K. Nucleocytoplasmic Cdk5 is involved in neuronal cell cycle and death in post-mitotic neurons. Cell Cycle 2011, 10, 1208–1214. [Google Scholar] [CrossRef]
- Hamdane, M.; Bretteville, A.; Sambo, A.V.; Schindowski, K.; Bégard, S.; Delacourte, A.; Bertrand, P.; Buée, L. p25/Cdk5-mediated retinoblastoma phosphorylation is an early event in neuronal cell death. J. Cell Sci. 2005, 118, 1291–1298. [Google Scholar] [CrossRef]
- Futatsugi, A.; Utreras, E.; Rudrabhatla, P.; Jaffe, H.; Pant, H.C.; Kulkarni, A.B. Cyclin-dependent kinase 5 regulates E2F transcription factor through phosphorylation of Rb protein in neurons. Cell Cycle 2012, 11, 1603–1610. [Google Scholar] [CrossRef][Green Version]
- Ianes, C.; Xu, P.; Werz, N.; Meng, Z.; Henne-Bruns, D.; Bischof, J.; Knippschild, U. CK1δ activity is modulated by CDK2/E- and CDK5/p35-mediated phosphorylation. Amino Acids 2016, 48, 579–592. [Google Scholar] [CrossRef]
- Knippschild, U.; Krüger, M.; Richter, J.; Xu, P.; García-Reyes, B.; Peifer, C.; Halekotte, J.; Bakulev, V.; Bischof, J. The CK1 Family: Contribution to Cellular Stress Response and Its Role in Carcinogenesis. Front. Oncol. 2014, 4, 96. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A.J.C. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Broertjes, J. The Ten Hallmarks of Cancer in Cutaneous Malignant Melanoma. UNAV J. Med. Stud. 2015, 1, 6–12. [Google Scholar]
- Stecca, B.; Rovida, E. Impact of ERK5 on the Hallmarks of Cancer. Int. J. Mol. Sci. 2019, 20, 1426. [Google Scholar] [CrossRef]
- Lenjisa, J.L.; Tadesse, S.; Khair, N.Z.; Kumarasiri, M.; Yu, M.; Albrecht, H.; Milne, R.; Wang, S. CDK5 in oncology: Recent advances and future prospects. Future Med. Chem. 2017, 9, 1939–1962. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, K.; Zhang, J.; Xiong, M.; Wang, X.; Luo, X.; Han, L.; Meng, Y.; Zhang, Y.; Liao, W.; Liu, S. CDK5 functions as a tumor promoter in human colorectal cancer via modulating the ERK5-AP-1 axis. Cell Death Dis. 2016, 7, e2415. [Google Scholar] [CrossRef]
- Zeng, J.; Xie, S.; Liu, Y.; Shen, C.; Song, X.; Zhou, G.L.; Wang, C. CDK5 Functions as a Tumor Promoter in Human Lung Cancer. J. Cancer 2018, 9, 3950–3961. [Google Scholar] [CrossRef]
- Wei, K.; Ye, Z.; Li, Z.; Dang, Y.; Chen, X.; Huang, N.; Bao, C.; Gan, T.; Yang, L.; Chen, G. An immunohistochemical study of cyclin-dependent kinase 5 (CDK5) expression in non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC): A possible prognostic biomarker. World J. Surg. Oncol. 2016, 14, 34. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, W.; Gao, Y.; Zhou, J.; Li, H.; Zhang, G.; Guo, D.; Xie, C.; Li, J.; Yin, Z.; et al. CDK5-mediated phosphorylation and stabilization of TPX2 promotes hepatocellular tumorigenesis. J. Exp. Clin. Cancer Res. CR 2019, 38, 286. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, Z.; Mao, W.; Ahmed, A.A.; Yang, H.; Zhou, J.; Jennings, N.; Rodriguez-Aguayo, C.; Lopez-Berestein, G.; Miranda, R.; et al. CDK5 Regulates Paclitaxel Sensitivity in Ovarian Cancer Cells by Modulating AKT Activation, p21Cip1- and p27Kip1-Mediated G1 Cell Cycle Arrest and Apoptosis. PLoS ONE 2015, 10, e0131833. [Google Scholar] [CrossRef]
- Ehrlich, S.M.; Liebl, J.; Ardelt, M.A.; Lehr, T.; De Toni, E.N.; Mayr, D.; Brandl, L.; Kirchner, T.; Zahler, S.; Gerbes, A.L.; et al. Targeting cyclin dependent kinase 5 in hepatocellular carcinoma--A novel therapeutic approach. J. Hepatol. 2015, 63, 102–113. [Google Scholar] [CrossRef]
- Lu, J.; Lin, J.X.; Zhang, P.Y.; Sun, Y.Q.; Li, P.; Xie, J.W.; Wang, J.B.; Chen, Q.Y.; Cao, L.L.; Lin, Y.; et al. CDK5 suppresses the metastasis of gastric cancer cells by interacting with and regulating PP2A. Oncol. Rep. 2019, 41, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhou, J.; Zhang, J.; Wu, S.; Yang, X.; Zhao, X.; Li, H.; Luo, M.; Yu, Q.; Lin, G.; et al. Cyclin-dependent kinase 5 decreases in gastric cancer and its nuclear accumulation suppresses gastric tumorigenesis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.Q.; Xie, J.W.; Chen, P.C.; Zheng, C.H.; Li, P.; Wang, J.B.; Lin, J.X.; Lu, J.; Chen, Q.Y.; Cao, L.L.; et al. Low Expression of CDK5 and p27 Are Associated with Poor Prognosis in Patients with Gastric Cancer. J. Cancer 2016, 7, 1049–1056. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Elliott, K.; Sakamuro, D.; Basu, A.; Du, W.; Wunner, W.; Staller, P.; Gaubatz, S.; Zhang, H.; Prochownik, E.; Eilers, M.; et al. Bin1 functionally interacts with Myc and inhibits cell proliferation via multiple mechanisms. Oncogene 1999, 18, 3564–3573. [Google Scholar] [CrossRef]
- Elliott, K.; Ge, K.; Du, W.; Prendergast, G.C. The c-Myc-interacting adaptor protein Bin1 activates a caspase-independent cell death program. Oncogene 2000, 19, 4669–4684. [Google Scholar] [CrossRef]
- Pineda-Lucena, A.; Ho, C.S.; Mao, D.Y.; Sheng, Y.; Laister, R.C.; Muhandiram, R.; Lu, Y.; Seet, B.T.; Katz, S.; Szyperski, T.; et al. A structure-based model of the c-Myc/Bin1 protein interaction shows alternative splicing of Bin1 and c-Myc phosphorylation are key binding determinants. J. Mol. Biol. 2005, 351, 182–194. [Google Scholar] [CrossRef]
- Sang, Y.; Li, Y.; Zhang, Y.; Alvarez, A.A.; Yu, B.; Zhang, W.; Hu, B.; Cheng, S.Y.; Feng, H. CDK5-dependent phosphorylation and nuclear translocation of TRIM59 promotes macroH2A1 ubiquitination and tumorigenicity. Nat. Commun. 2019, 10, 4013. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Wyckoff, J.; Condeelis, J. Cell migration in tumors. Curr. Opin. Cell Biol. 2005, 17, 559–564. [Google Scholar] [CrossRef]
- Jin, X.; Yang, C.; Fan, P.; Xiao, J.; Zhang, W.; Zhan, S.; Liu, T.; Wang, D.; Wu, H. CDK5/FBW7-dependent ubiquitination and degradation of EZH2 inhibits pancreatic cancer cell migration and invasion. J. Biol. Chem. 2017, 292, 6269–6280. [Google Scholar] [CrossRef]
- Demelash, A.; Rudrabhatla, P.; Pant, H.C.; Wang, X.; Amin, N.D.; McWhite, C.D.; Naizhen, X.; Linnoila, R.I. Achaete-scute homologue-1 (ASH1) stimulates migration of lung cancer cells through Cdk5/p35 pathway. Mol. Biol. Cell 2012, 23, 2856–2866. [Google Scholar] [CrossRef]
- Liu, R.; Tian, B.; Gearing, M.; Hunter, S.; Ye, K.; Mao, Z. Cdk5-mediated regulation of the PIKE-A-Akt pathway and glioblastoma cell invasion. Proc. Natl. Acad. Sci. USA 2008, 105, 7570–7575. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Liu, C.; Wu, D.; Li, Z.; Li, C.; Zhang, Y. Phosphorylation of kinase insert domain receptor by cyclin-dependent kinase 5 at serine 229 is associated with invasive behavior and poor prognosis in prolactin pituitary adenomas. Oncotarget 2016, 7, 50883–50894. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhandari, D.; Lopez-Sanchez, I.; To, A.; Lo, I.C.; Aznar, N.; Leyme, A.; Gupta, V.; Niesman, I.; Maddox, A.L.; Garcia-Marcos, M.; et al. Cyclin-dependent kinase 5 activates guanine nucleotide exchange factor GIV/Girdin to orchestrate migration-proliferation dichotomy. Proc. Natl. Acad. Sci. USA 2015, 112, E4874–E4883. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, W.; Kan, H. TPX2 is a prognostic marker and contributes to growth and metastasis of human hepatocellular carcinoma. Int. J. Mol. Sci. 2014, 15, 18148–18161. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Jia, C.; Huang, Y.; He, H.; Li, J.; Liao, H.; Liu, X.; Liu, X.; Bai, X.; Yang, D. TPX2 Level Correlates with Hepatocellular Carcinoma Cell Proliferation, Apoptosis, and EMT. Dig. Dis. Sci. 2015, 60, 2360–2372. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.S.; Zhou, X.; Huang, Y.Y.; Kong, L.P.; Mei, M.; Guo, W.Y.; Zhao, M.H.; Ren, Y.; Shen, Q.; Zhang, L. Targeting STAT3/miR-21 axis inhibits epithelial-mesenchymal transition via regulating CDK5 in head and neck squamous cell carcinoma. Mol. Cancer 2015, 14, 213. [Google Scholar] [CrossRef]
- Negrini, S.; Gorgoulis, V.G.; Halazonetis, T.D. Genomic instability--an evolving hallmark of cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 220–228. [Google Scholar] [CrossRef]
- Chiker, S.; Pennaneach, V.; Loew, D.; Dingli, F.; Biard, D.; Cordelières, F.P.; Gemble, S.; Vacher, S.; Bieche, I.; Hall, J.; et al. Cdk5 promotes DNA replication stress checkpoint activation through RPA-32 phosphorylation, and impacts on metastasis free survival in breast cancer patients. Cell Cycle 2015, 14, 3066–3078. [Google Scholar] [CrossRef]
- Turner, N.C.; Lord, C.J.; Iorns, E.; Brough, R.; Swift, S.; Elliott, R.; Rayter, S.; Tutt, A.N.; Ashworth, A. A synthetic lethal siRNA screen identifying genes mediating sensitivity to a PARP inhibitor. EMBO J. 2008, 27, 1368–1377. [Google Scholar] [CrossRef]
- Courapied, S.; Sellier, H.; de Carné Trécesson, S.; Vigneron, A.; Bernard, A.C.; Gamelin, E.; Barré, B.; Coqueret, O. The cdk5 kinase regulates the STAT3 transcription factor to prevent DNA damage upon topoisomerase I inhibition. J. Biol. Chem. 2010, 285, 26765–26778. [Google Scholar] [CrossRef]
- Bartkova, J.; Rezaei, N.; Liontos, M.; Karakaidos, P.; Kletsas, D.; Issaeva, N.; Vassiliou, L.V.; Kolettas, E.; Niforou, K.; Zoumpourlis, V.C.; et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 2006, 444, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Shen, W.H.; Liu, L. Senescence and Cancer. Cancer Transl. Med. 2018, 4, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Hinds, P.W. p35 is required for CDK5 activation in cellular senescence. J. Biol. Chem. 2010, 285, 14671–14680. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.; Yang, H.S.; Hinds, P.W. Cellular senescence requires CDK5 repression of Rac1 activity. Mol. Cell. Biol. 2004, 24, 2808–2819. [Google Scholar] [CrossRef]
- Lalioti, V.; Muruais, G.; Dinarina, A.; van Damme, J.; Vandekerckhove, J.; Sandoval, I.V. The atypical kinase Cdk5 is activated by insulin, regulates the association between GLUT4 and E-Syt1, and modulates glucose transport in 3T3-L1 adipocytes. Proc. Natl. Acad. Sci. USA 2009, 106, 4249–4253. [Google Scholar] [CrossRef]
- Wei, F.Y.; Nagashima, K.; Ohshima, T.; Saheki, Y.; Lu, Y.F.; Matsushita, M.; Yamada, Y.; Mikoshiba, K.; Seino, Y.; Matsui, H.; et al. Cdk5-dependent regulation of glucose-stimulated insulin secretion. Nat. Med. 2005, 11, 1104–1108. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Hao, J.; Zhou, Y.; Liu, W. High glucose increases Cdk5 activity in podocytes via transforming growth factor-β1 signaling pathway. Exp. Cell Res. 2014, 326, 219–229. [Google Scholar] [CrossRef]
- Griffin, S.V.; Hiromura, K.; Pippin, J.; Petermann, A.T.; Blonski, M.J.; Krofft, R.; Takahashi, S.; Kulkarni, A.B.; Shankland, S.J. Cyclin-dependent kinase 5 is a regulator of podocyte differentiation, proliferation, and morphology. Am. J. Pathol. 2004, 165, 1175–1185. [Google Scholar] [CrossRef]
- Lowman, X.H.; McDonnell, M.A.; Kosloske, A.; Odumade, O.A.; Jenness, C.; Karim, C.B.; Jemmerson, R.; Kelekar, A. The proapoptotic function of Noxa in human leukemia cells is regulated by the kinase Cdk5 and by glucose. Mol. Cell 2010, 40, 823–833. [Google Scholar] [CrossRef]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
- Thakkar, S.; Sharma, D.; Kalia, K.; Tekade, R.K. Tumor microenvironment targeted nanotherapeutics for cancer therapy and diagnosis: A review. Acta Biomater. 2020, 101, 43–68. [Google Scholar] [CrossRef] [PubMed]
- Servais, C.; Erez, N. From sentinel cells to inflammatory culprits: Cancer-associated fibroblasts in tumour-related inflammation. J. Pathol. 2013, 229, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Allinen, M.; Beroukhim, R.; Cai, L.; Brennan, C.; Lahti-Domenici, J.; Huang, H.; Porter, D.; Hu, M.; Chin, L.; Richardson, A.; et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell 2004, 6, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Erez, N.; Truitt, M.; Olson, P.; Arron, S.T.; Hanahan, D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell 2010, 17, 135–147. [Google Scholar] [CrossRef]
- Ren, Y.; Jia, H.H.; Xu, Y.Q.; Zhou, X.; Zhao, X.H.; Wang, Y.F.; Song, X.; Zhu, Z.Y.; Sun, T.; Dou, Y.; et al. Paracrine and epigenetic control of CAF-induced metastasis: The role of HOTAIR stimulated by TGF-ß1 secretion. Mol. Cancer 2018, 17, 5. [Google Scholar] [CrossRef]
- Reiter, V.; Matschkal, D.M.; Wagner, M.; Globisch, D.; Kneuttinger, A.C.; Müller, M.; Carell, T. The CDK5 repressor CDK5RAP1 is a methylthiotransferase acting on nuclear and mitochondrial RNA. Nucleic Acids Res. 2012, 40, 6235–6240. [Google Scholar] [CrossRef]
- Gupta, M.K.; Qin, R.Y. Mechanism and its regulation of tumor-induced angiogenesis. World J. Gastroenterol. 2003, 9, 1144–1155. [Google Scholar] [CrossRef]
- Liebl, J.; Weitensteiner, S.B.; Vereb, G.; Takács, L.; Fürst, R.; Vollmar, A.M.; Zahler, S. Cyclin-dependent kinase 5 regulates endothelial cell migration and angiogenesis. J. Biol. Chem. 2010, 285, 35932–35943. [Google Scholar] [CrossRef]
- Lampropoulou, E.; Logoviti, I.; Koutsioumpa, M.; Hatziapostolou, M.; Polytarchou, C.; Skandalis, S.S.; Hellman, U.; Fousteris, M.; Nikolaropoulos, S.; Choleva, E.; et al. Cyclin-dependent kinase 5 mediates pleiotrophin-induced endothelial cell migration. Sci. Rep. 2018, 8, 5893. [Google Scholar] [CrossRef]
- Papadimitriou, E.; Pantazaka, E.; Castana, P.; Tsalios, T.; Polyzos, A.; Beis, D. Pleiotrophin and its receptor protein tyrosine phosphatase beta/zeta as regulators of angiogenesis and cancer. Biochim. Biophys. Acta 2016, 1866, 252–265. [Google Scholar] [CrossRef]
- Herzog, J.; Ehrlich, S.M.; Pfitzer, L.; Liebl, J.; Fröhlich, T.; Arnold, G.J.; Mikulits, W.; Haider, C.; Vollmar, A.M.; Zahler, S. Cyclin-dependent kinase 5 stabilizes hypoxia-inducible factor-1α: A novel approach for inhibiting angiogenesis in hepatocellular carcinoma. Oncotarget 2016, 7, 27108–27121. [Google Scholar] [CrossRef] [PubMed]
- Liebl, J.; Zhang, S.; Moser, M.; Agalarov, Y.; Demir, C.S.; Hager, B.; Bibb, J.A.; Adams, R.H.; Kiefer, F.; Miura, N.; et al. Cdk5 controls lymphatic vessel development and function by phosphorylation of Foxc2. Nat. Commun. 2015, 6, 7274. [Google Scholar] [CrossRef]
- Sharma, M.R.; Tuszynski, G.P.; Sharma, M.C. Angiostatin-induced inhibition of endothelial cell proliferation/apoptosis is associated with the down-regulation of cell cycle regulatory protein cdk5. J. Cell. Biochem. 2004, 91, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Merk, H.; Zhang, S.; Lehr, T.; Müller, C.; Ulrich, M.; Bibb, J.A.; Adams, R.H.; Bracher, F.; Zahler, S.; Vollmar, A.M.; et al. Inhibition of endothelial Cdk5 reduces tumor growth by promoting non-productive angiogenesis. Oncotarget 2016, 7, 6088–6104. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Wang, H.; He, Y.; Li, D.; Gong, L.; Zhang, Y. CDK5 and its activator P35 in normal pituitary and in pituitary adenomas: Relationship to VEGF expression. Int. J. Biol. Sci. 2014, 10, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Candido, J.; Hagemann, T. Cancer-related inflammation. J. Clin. Immunol. 2013, 33 (Suppl. 1), S79–S84. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Lu, H.; Ouyang, W.; Huang, C. Inflammation, a key event in cancer development. Mol. Cancer Res. MCR 2006, 4, 221–233. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Lee, K.Y.; Liu, L.; Jin, Y.; Fu, S.B.; Rosales, J.L. Cdk5 mediates vimentin Ser56 phosphorylation during GTP-induced secretion by neutrophils. J. Cell. Physiol. 2012, 227, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Zhang, T.; Michowski, W.; Rebecca, V.W.; Xiao, M.; Ferretti, R.; Suski, J.M.; Bronson, R.T.; Paulo, J.A.; Frederick, D.; et al. Targeting the cyclin-dependent kinase 5 in metastatic melanoma. Proc. Natl. Acad. Sci. USA 2020, 117, 8001–8012. [Google Scholar] [CrossRef] [PubMed]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.; et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015, 35, S185–S198. [Google Scholar] [CrossRef] [PubMed]
- Dorand, R.D.; Nthale, J.; Myers, J.T.; Barkauskas, D.S.; Avril, S.; Chirieleison, S.M.; Pareek, T.K.; Abbott, D.W.; Stearns, D.S.; Letterio, J.J.; et al. Cdk5 disruption attenuates tumor PD-L1 expression and promotes antitumor immunity. Science 2016, 353, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.; Choi, S.H.; Pareek, T.K.; Kim, B.G.; Letterio, J.J. Cyclin-dependent kinase 5 represses Foxp3 gene expression and Treg development through specific phosphorylation of Stat3 at Serine 727. Mol. Immunol. 2015, 67, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.; Pareek, T.K.; Letterio, J.J. Cdk5 controls IL-2 gene expression via repression of the mSin3a-HDAC complex. Cell Cycle 2015, 14, 1327–1336. [Google Scholar] [CrossRef][Green Version]
- Lee, C.H.; Cho, J.; Lee, K. Tumour Regression via Integrative Regulation of Neurological, Inflammatory, and Hypoxic Tumour Microenvironment. Biomol. Ther. (Seoul) 2020, 28, 119–130. [Google Scholar] [CrossRef]
- Venkataramani, V.; Tanev, D.I.; Strahle, C.; Studier-Fischer, A.; Fankhauser, L.; Kessler, T.; Korber, C.; Kardorff, M.; Ratliff, M.; Xie, R.; et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 2019, 573, 532–538. [Google Scholar] [CrossRef]
- Arese, M.; Bussolino, F.; Pergolizzi, M.; Bizzozero, L.; Pascal, D. Tumor progression: The neuronal input. Ann. Transl. Med. 2018, 6, 89. [Google Scholar] [CrossRef]
- Cole, S.W.; Nagaraja, A.S.; Lutgendorf, S.K.; Green, P.A.; Sood, A.K. Sympathetic nervous system regulation of the tumour microenvironment. Nat. Rev. Cancer 2015, 15, 563–572. [Google Scholar] [CrossRef]
- Makale, M.T.; McDonald, C.R.; Hattangadi-Gluth, J.A.; Kesari, S. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat. Rev. Neurol. 2017, 13, 52–64. [Google Scholar] [CrossRef]
- Pasquier, E.; Street, J.; Pouchy, C.; Carre, M.; Gifford, A.J.; Murray, J.; Norris, M.D.; Trahair, T.; Andre, N.; Kavallaris, M. beta-blockers increase response to chemotherapy via direct antitumour and anti-angiogenic mechanisms in neuroblastoma. Br. J. Cancer 2013, 108, 2485–2494. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sun, Y.; Gao, D. Role of the nervous system in cancer metastasis. Oncol. Lett. 2013, 5, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Demers, M.; Couillard, J.; Bélanger, S.; St-Pierre, Y. New roles for matrix metalloproteinases in metastasis. Crit. Rev. Immunol. 2005, 25, 493–523. [Google Scholar] [CrossRef] [PubMed]
- Deryugina, E.I.; Quigley, J.P. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006, 25, 9–34. [Google Scholar] [CrossRef]
- Shapiro, D.M.; Warren, S. Cancer innervation. Cancer Res. 1949, 9, 707–711, illust. [Google Scholar]
- Namgung, U.; Choi, B.H.; Park, S.; Lee, J.U.; Seo, H.S.; Suh, B.C.; Kim, K.T. Activation of cyclin-dependent kinase 5 is involved in axonal regeneration. Mol. Cell. Neurosci. 2004, 25, 422–432. [Google Scholar] [CrossRef]
- Grondin, R.; Gash, D.M. Glial cell line-derived neurotrophic factor (GDNF): A drug candidate for the treatment of Parkinson’s disease. J. Neurol. 1998, 245, P35–P42. [Google Scholar] [CrossRef]
- Okada, Y.; Eibl, G.; Duffy, J.P.; Reber, H.A.; Hines, O.J. Glial cell-derived neurotrophic factor upregulates the expression and activation of matrix metalloproteinase-9 in human pancreatic cancer. Surgery 2003, 134, 293–299. [Google Scholar] [CrossRef]
- Okada, Y.; Eibl, G.; Guha, S.; Duffy, J.P.; Reber, H.A.; Hines, O.J. Nerve growth factor stimulates MMP-2 expression and activity and increases invasion by human pancreatic cancer cells. Clin. Exp. Metastasis 2004, 21, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Paratcha, G.; Ibáñez, C.F.; Ledda, F. GDNF is a chemoattractant factor for neuronal precursor cells in the rostral migratory stream. Mol. Cell. Neurosci. 2006, 31, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.V.; Sood, A.K.; Chen, M.; Li, Y.; Eubank, T.D.; Marsh, C.B.; Jewell, S.; Flavahan, N.A.; Morrison, C.; Yeh, P.E.; et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 2006, 66, 10357–10364. [Google Scholar] [CrossRef] [PubMed]
- Lutgendorf, S.K.; Cole, S.; Costanzo, E.; Bradley, S.; Coffin, J.; Jabbari, S.; Rainwater, K.; Ritchie, J.M.; Yang, M.; Sood, A.K. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003, 9, 4514–4521. [Google Scholar]
- Kim, S.H.; Ryan, T.A. CDK5 serves as a major control point in neurotransmitter release. Neuron 2010, 67, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, S.R.; Mallinger, A.; Workman, P.; Clarke, P.A. Inhibitors of cyclin-dependent kinases as cancer therapeutics. Pharmacol. Ther. 2017, 173, 83–105. [Google Scholar] [CrossRef]
- Cicenas, J.; Valius, M. The CDK inhibitors in Cancer Res. and therapy. J. Cancer Res. Clin. Oncol. 2011, 137, 1409–1418. [Google Scholar] [CrossRef]
- Ivanchuk, S.M.; Rutka, J.T. Regulation of the cell cycle and interventional developmental therapeutics. In Handbook of Brain Tumor Chemotherapy; Elsevier: Amsterdam, The Netherlands, 2006; pp. 123–140. [Google Scholar]
- Edamatsu, H.; Gau, C.L.; Nemoto, T.; Guo, L.; Tamanoi, F. Cdk inhibitors, roscovitine and olomoucine, synergize with farnesyltransferase inhibitor (FTI) to induce efficient apoptosis of human cancer cell lines. Oncogene 2000, 19, 3059–3068. [Google Scholar] [CrossRef]
- Knillová, J.; Bouchal, J.; Hlobilková, A.; Strnad, M.; Kolár, Z. Synergic effects of the cyclin-dependent kinase (CDK) inhibitor olomoucine and androgen-antagonist bicalutamide on prostatic cancer cell lines. Neoplasma 2004, 51, 358–367. [Google Scholar]
- Meijer, L.; Borgne, A.; Mulner, O.; Chong, J.P.; Blow, J.J.; Inagaki, N.; Inagaki, M.; Delcros, J.G.; Moulinoux, J.P. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 1997, 243, 527–536. [Google Scholar] [CrossRef]
- Parry, D.; Guzi, T.; Shanahan, F.; Davis, N.; Prabhavalkar, D.; Wiswell, D.; Seghezzi, W.; Paruch, K.; Dwyer, M.P.; Doll, R.; et al. Dinaciclib (SCH 727965), a novel and potent cyclin-dependent kinase inhibitor. Mol. Cancer Ther. 2010, 9, 2344–2353. [Google Scholar] [CrossRef] [PubMed]
- Hoessel, R.; Leclerc, S.; Endicott, J.A.; Nobel, M.E.; Lawrie, A.; Tunnah, P.; Leost, M.; Damiens, E.; Marie, D.; Marko, D.; et al. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat. Cell Biol. 1999, 1, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Robb, C.M.; Kour, S.; Contreras, J.I.; Agarwal, E.; Barger, C.J.; Rana, S.; Sonawane, Y.; Neilsen, B.K.; Taylor, M.; Kizhake, S.; et al. Characterization of CDK(5) inhibitor, 20-223 (aka CP668863) for colorectal cancer therapy. Oncotarget 2018, 9, 5216–5232. [Google Scholar] [CrossRef] [PubMed]
- Dolman, M.E.; Poon, E.; Ebus, M.E.; den Hartog, I.J.; van Noesel, C.J.; Jamin, Y.; Hallsworth, A.; Robinson, S.P.; Petrie, K.; Sparidans, R.W.; et al. Cyclin-Dependent Kinase Inhibitor AT7519 as a Potential Drug for MYCN-Dependent Neuroblastoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 5100–5109. [Google Scholar] [CrossRef] [PubMed]
- Cicenas, J.; Kalyan, K.; Sorokinas, A.; Stankunas, E.; Levy, J.; Meskinyte, I.; Stankevicius, V.; Kaupinis, A.; Valius, M. Roscovitine in cancer and other diseases. Ann. Transl. Med. 2015, 3, 135. [Google Scholar] [CrossRef]
- Mapelli, M.; Massimiliano, L.; Crovace, C.; Seeliger, M.A.; Tsai, L.H.; Meijer, L.; Musacchio, A. Mechanism of CDK5/p25 binding by CDK inhibitors. J. Med. Chem. 2005, 48, 671–679. [Google Scholar] [CrossRef]
- De Azevedo, W.F.; Leclerc, S.; Meijer, L.; Havlicek, L.; Strnad, M.; Kim, S.H. Inhibition of cyclin-dependent kinases by purine analogues: Crystal structure of human cdk2 complexed with roscovitine. Eur. J. Biochem. 1997, 243, 518–526. [Google Scholar] [CrossRef]
- Nair, B.C.; Vallabhaneni, S.; Tekmal, R.R.; Vadlamudi, R.K. Roscovitine confers tumor suppressive effect on therapy-resistant breast tumor cells. Breast Cancer Res. BCR 2011, 13, R80. [Google Scholar] [CrossRef]
- Wiernik, P.H. Alvocidib (flavopiridol) for the treatment of chronic lymphocytic leukemia. Expert Opin. Investig. Drugs 2016, 25, 729–734. [Google Scholar] [CrossRef]
- Gojo, I.; Zhang, B.; Fenton, R.G. The cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in multiple myeloma cells through transcriptional repression and down-regulation of Mcl-1. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2002, 8, 3527–3538. [Google Scholar]
- Sedlacek, H.H. Mechanisms of action of flavopiridol. Crit. Rev. Oncol. Hematol. 2001, 38, 139–170. [Google Scholar] [CrossRef]
- Juric, V.; Murphy, B. Cyclin-dependent kinase inhibitors in brain cancer: Current state and future directions. Cancer Drug Resist. 2020, 3, 48–62. [Google Scholar] [CrossRef]
- Shapiro, G.I.; Koestner, D.A.; Matranga, C.B.; Rollins, B.J. Flavopiridol induces cell cycle arrest and p53-independent apoptosis in non-small cell lung cancer cell lines. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1999, 5, 2925–2938. [Google Scholar]
- Mihara, M.; Shintani, S.; Nakashiro, K.; Hamakawa, H. Flavopiridol, a cyclin dependent kinase (CDK) inhibitor, induces apoptosis by regulating Bcl-x in oral cancer cells. Oral Oncol. 2003, 39, 49–55. [Google Scholar] [CrossRef]
- Leclerc, S.; Garnier, M.; Hoessel, R.; Marko, D.; Bibb, J.A.; Snyder, G.L.; Greengard, P.; Biernat, J.; Wu, Y.Z.; Mandelkow, E.M.; et al. Indirubins inhibit glycogen synthase kinase-3 beta and CDK5/p25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer’s disease. A property common to most cyclin-dependent kinase inhibitors? J. Biol. Chem. 2001, 276, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Ichimaru, Y.; Sano, M.; Kajiwara, I.; Tobe, T.; Yoshioka, H.; Hayashi, K.; Ijichi, H.; Miyairi, S. Indirubin 3′-Oxime Inhibits Migration, Invasion, and Metastasis InVivo in Mice Bearing Spontaneously Occurring Pancreatic Cancer via Blocking the RAF/ERK, AKT, and SAPK/JNK Pathways. Transl. Oncol. 2019, 12, 1574–1582. [Google Scholar] [CrossRef]
- Lo, W.Y.; Chang, N.W. An indirubin derivative, indirubin-3′-monoxime suppresses oral cancer tumorigenesis through the downregulation of survivin. PLoS ONE 2013, 8, e70198. [Google Scholar] [CrossRef]
- Fabre, C.; Gobbi, M.; Ezzili, C.; Zoubir, M.; Sablin, M.P.; Small, K.; Im, E.; Shinwari, N.; Zhang, D.; Zhou, H.; et al. Clinical study of the novel cyclin-dependent kinase inhibitor dinaciclib in combination with rituximab in relapsed/refractory chronic lymphocytic leukemia patients. Cancer Chemother. Pharmacol. 2014, 74, 1057–1064. [Google Scholar] [CrossRef]
- Kumar, S.K.; LaPlant, B.; Chng, W.J.; Zonder, J.; Callander, N.; Fonseca, R.; Fruth, B.; Roy, V.; Erlichman, C.; Stewart, A.K.; et al. Dinaciclib, a novel CDK inhibitor, demonstrates encouraging single-agent activity in patients with relapsed multiple myeloma. Blood 2015, 125, 443–448. [Google Scholar] [CrossRef]
- Wyatt, P.G.; Woodhead, A.J.; Berdini, V.; Boulstridge, J.A.; Carr, M.G.; Cross, D.M.; Davis, D.J.; Devine, L.A.; Early, T.R.; Feltell, R.E.; et al. Identification of N-(4-piperidinyl)-4-(2,6-dichlorobenzoylamino)-1H-pyrazole-3-carboxamide (AT7519), a novel cyclin dependent kinase inhibitor using fragment-based X-ray crystallography and structure based drug design. J. Med. Chem. 2008, 51, 4986–4999. [Google Scholar] [CrossRef]
- Xi, C.; Wang, L.; Yu, J.; Ye, H.; Cao, L.; Gong, Z. Inhibition of cyclin-dependent kinases by AT7519 is effective to overcome chemoresistance in colon and cervical cancer. Biochem. Biophys. Res. Commun. 2019, 513, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Arisan, E.D.; Obakan, P.; Coker-Gurkan, A.; Calcabrini, A.; Agostinelli, E.; Unsal, N.P. CDK inhibitors induce mitochondria-mediated apoptosis through the activation of polyamine catabolic pathway in LNCaP, DU145 and PC3 prostate cancer cells. Curr. Pharm. Des. 2014, 20, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Obakan, P.; Arısan, E.D.; Özfiliz, P.; Çoker-Gürkan, A.; Palavan-Ünsal, N. Purvalanol A is a strong apoptotic inducer via activating polyamine catabolic pathway in MCF-7 estrogen receptor positive breast cancer cells. Mol. Biol. Rep. 2014, 41, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Gürkan, A.C.; Arisan, E.D.; Obakan, P.; Palavan-Ünsal, N. Inhibition of polyamine oxidase prevented cyclin-dependent kinase inhibitor-induced apoptosis in HCT 116 colon carcinoma cells. Apoptosis Int. J. Program. Cell Death 2013, 18, 1536–1547. [Google Scholar] [CrossRef]
- Tabouret, E.; Wang, H.; Amin, N.; Jung, J.; Appay, R.; Cui, J.; Song, Q.; Cardone, A.; Park, D.M.; Gilbert, M.R.; et al. TP5, a Peptide Inhibitor of Aberrant and Hyperactive CDK5/p25: A Novel Therapeutic Approach against Glioblastoma. Cancers 2020, 12, 935. [Google Scholar] [CrossRef]
- Binukumar, B.K.; Zheng, Y.L.; Shukla, V.; Amin, N.D.; Grant, P.; Pant, H.C. TFP5, a peptide derived from p35, a Cdk5 neuronal activator, rescues cortical neurons from glucose toxicity. J. Alzheimer’s Dis. JAD 2014, 39, 899–909. [Google Scholar] [CrossRef]
- Binukumar, B.K.; Shukla, V.; Amin, N.D.; Grant, P.; Bhaskar, M.; Skuntz, S.; Steiner, J.; Pant, H.C. Peptide TFP5/TP5 derived from Cdk5 activator P35 provides neuroprotection in the MPTP model of Parkinson’s disease. Mol. Biol. Cell 2015, 26, 4478–4491. [Google Scholar] [CrossRef]
- Lopez, M.; Di Lauro, L.; Paoletti, G.; Santini, S.; Gandolfo, G.M.; Vitelli, G.; Frasca, A.M.; Ameglio, F.; Rasi, G.; Garaci, E. Sequential biochemotherapy for metastatic colorectal cancer using fluorouracil, folinic acid, thymopentin and interleukin-2: Clinical and immunological effects. Ann. Oncol. Off. J. Eur. Soc. Med Oncol. 1995, 6, 1011–1017. [Google Scholar] [CrossRef]
- Lopez, M.; Di Lauro, L.; Gionfra, T.; Gandolfo, G.; Ameglio, F.; Paoletti, G. Thymopentin and interleukin-2 in combination with 5-fluorouracil and leucovorin in metastatic colorectal adenocarcinoma: Preliminary results. J. Surg. Oncol. Suppl. 1991, 2, 108–111. [Google Scholar] [CrossRef]
- Khair, N.Z.; Lenjisa, J.L.; Tadesse, S.; Kumarasiri, M.; Basnet, S.K.C.; Mekonnen, L.B.; Li, M.; Diab, S.; Sykes, M.J.; Albrecht, H.; et al. Discovery of CDK5 Inhibitors through Structure-Guided Approach. ACS Med. Chem. Lett. 2019, 10, 786–791. [Google Scholar] [CrossRef]


| Protein | Position | Interaction with CDK5 |
|---|---|---|
| p35/p25 | p35 mainly localises in the plasma membrane, perinuclear region, and less in the nucleus [23], whereas p25 mostly exists in the cytosolic region and nucleus [24]. | p35 can activate CDK5 through binding to CDK5 [19]. However, the binding with p25 leads to the hyperactivation of CDK5 [24]. |
| p39/p29 | p39 localises in the plasma membrane and nucleus [25]. | p39/p29 can bind to CDK5 and then activate CDK5 [25]. |
| Cyclin I | Cyclin I could activate CDK5 by forming a complex with CDK5, and this complex acts as an anti-apoptotic factor [26]. | |
| Cyclin D1 | During G1 phase, cyclin D1 is synthesised and localises in the nucleus before entering S phase [27]. | Cyclin D1 competes with p35 to inhibit CDK5, contributing to neuronal apoptosis through the MEK-ERK pathway [28]. |
| Cyclin E | All cell cycle phase, cyclin E is synthesised and accumulated in the nucleus [29]. | Cyclin E binds to CDK5 to prevent the interaction between CDK5 and its activators, leading to effects on synapse function and memory [30]. |
| GSTP1 | GSTP1 inhibits the activity of CDK5 through two mechanisms: competing with p35 or p39 to bind to CDK5; depleting oxidative stress [22]. | |
| Munc18 | Munc18 binds to and protects the CDK5/p35 complex from the inhibitory effect of TFP5 [31,32]. |
| Site | Effect | Ref. |
|---|---|---|
| Phosphorylation | ||
| Tyr15 | Facilitates the activity of CDK5. | [54,55,56,57] |
| Ser159 | Is required for maximal activation of CDK5/p35 complex. Facilitates the activity of CDK5/p25 complex. | [58,59] |
| Ser47 | Suppresses the interaction between CDK5 and p35, leading to decreasing kinase activity of CDK5 and interfering with cell migration. | [60] |
| Thr14 | Inhibits the activity of CDK5. | [61] |
| Thr77 | Disrupts the interaction between CDK5 and p35, resulting in the inactivation of CDK5. | [62] |
| S-nitrosylation | ||
| Cys83 | Inhibits the activity of CDK5. | [63,64] |
| Cys157 | Inhibits the activity of CDK5. | [63,64] |
| Acetylation | ||
| Lys33 | Inhibits the activity of CDK5. | [65] |
| Transcription Factor | CDK5 Complex | Phosphorylation Site | Physiological Significance | Ref. |
|---|---|---|---|---|
| MEF2 | CDK5/p25 | Ser444 | Neuronal cell death | [70,71,72,73] |
| STAT3 | Ser727 | Cancer | [74,75,76,77] | |
| MR | CDK5/p35 CDK5/p25 | Ser128 Ser250 Thr159 | Neuron function | [78] |
| GR | CDK5/p35 | Ser203 Ser211 | Neuron function | [79] |
| CDK5/p25 | ||||
| p53 | CDK5/p35 | Ser15 Ser33 Ser46 | Neuronal cell death | [80,81] |
| TonEBP/OREBP | Thr135 | Osmotic stress. | [82] | |
| MEK1 | CDK5/p35 | Thr286 | Cell death | [83] |
| mSds3 | CDK5/p35 | Ser228 | Neuron and muscle development. | [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do, P.A.; Lee, C.H. The Role of CDK5 in Tumours and Tumour Microenvironments. Cancers 2021, 13, 101. https://doi.org/10.3390/cancers13010101
Do PA, Lee CH. The Role of CDK5 in Tumours and Tumour Microenvironments. Cancers. 2021; 13(1):101. https://doi.org/10.3390/cancers13010101
Chicago/Turabian StyleDo, Phuong Anh, and Chang Hoon Lee. 2021. "The Role of CDK5 in Tumours and Tumour Microenvironments" Cancers 13, no. 1: 101. https://doi.org/10.3390/cancers13010101
APA StyleDo, P. A., & Lee, C. H. (2021). The Role of CDK5 in Tumours and Tumour Microenvironments. Cancers, 13(1), 101. https://doi.org/10.3390/cancers13010101






