Secreting Germ Cell Tumors of the Central Nervous System: A Long-Term Follow-up Experience
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Diagnostic Workup and Staging
2.2. Treatment
2.2.1. Chemotherapy
2.2.2. Radiotherapy
2.3. High-Risk Patients
2.4. Follow-up
2.5. Neurocognitive Assessment
2.5.1. Intellectual Abilities
2.5.2. Attention
2.5.3. Executive Functioning
2.5.4. Memory
2.5.5. Visual-Motor Integration
2.6. Statistical Analysis
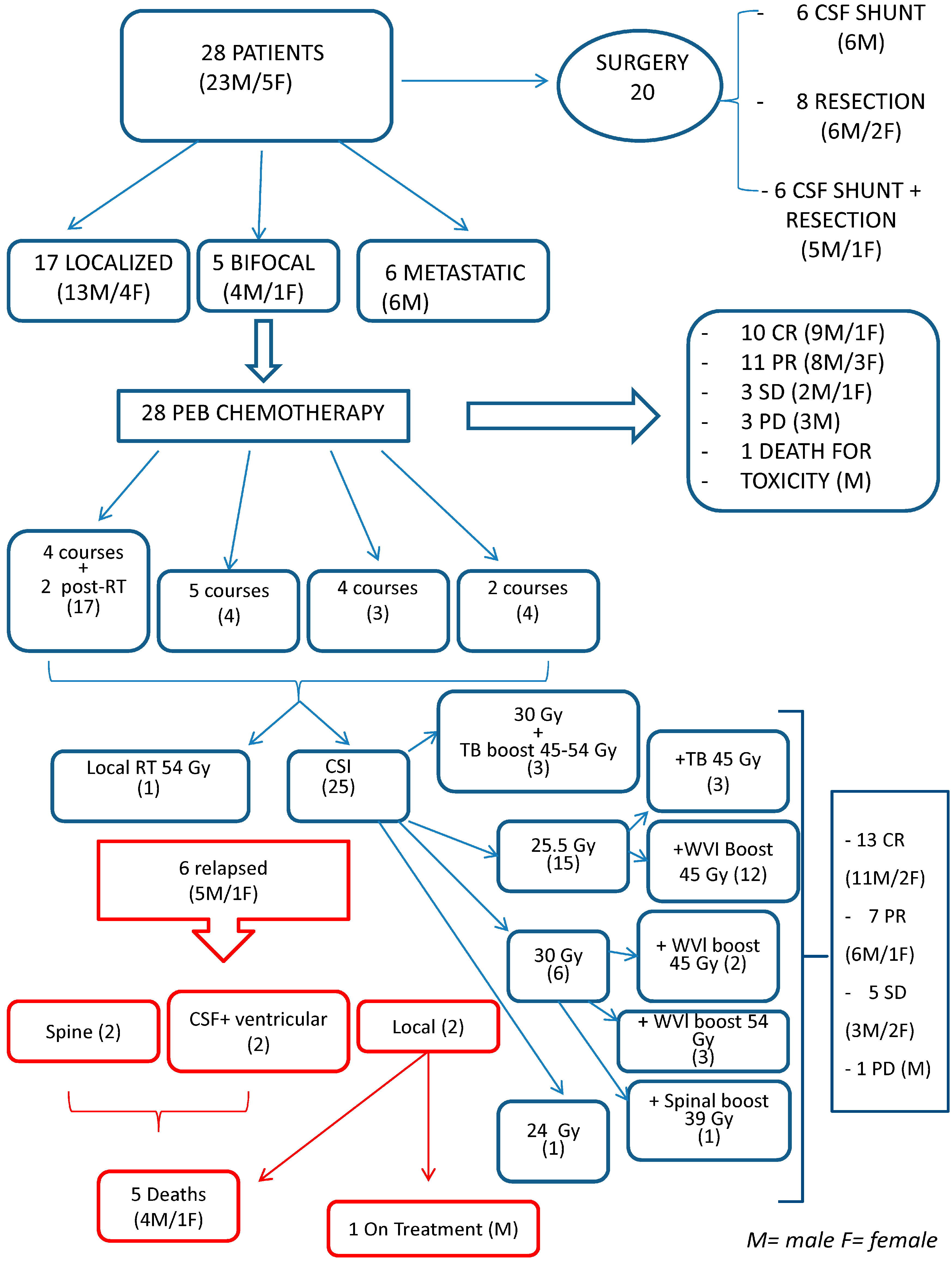
3. Results
3.1. Diagnosis
3.2. Treatment
3.3. Chemotherapy
3.4. Radiotherapy
3.5. Response
3.5.1. Results in High-Risk Patients
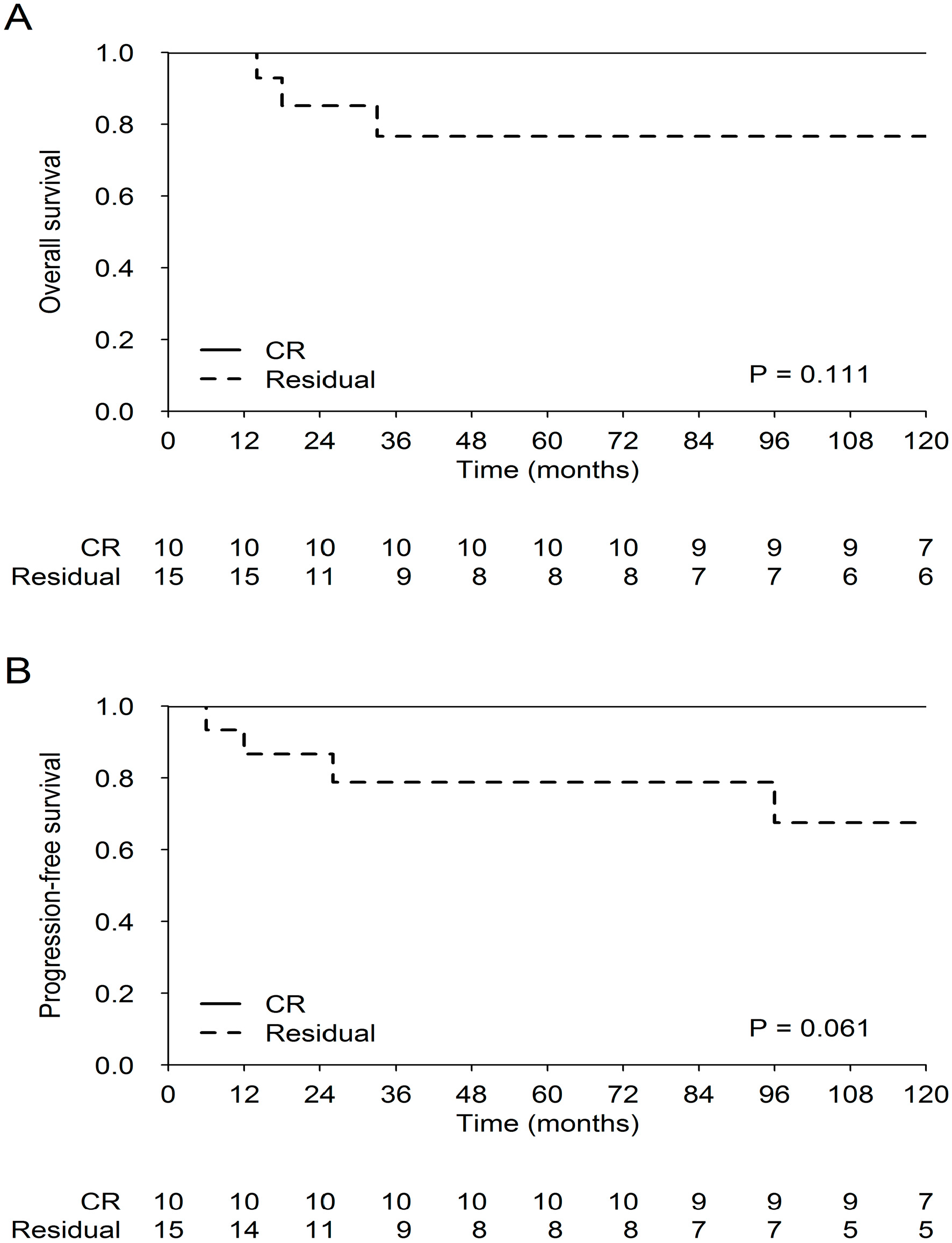
3.5.2. Survival End-Points
3.5.3. Patterns of Relapse/Progression
3.5.4. Surgery and Risk Dissemination
3.5.5. Long-Term Assessment
3.5.6. Neurocognitive Evaluation
4. Discussion
Of All GCTs, NGGCT Carry the Worst Prognosis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Informed Consent
References
- Jorsal, T.; Rørth, M. Intracranial germ cell tumours. A review with special reference to endocrine manifestations. Acta Oncol. 2012, 51, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Bendel, A.; Watterson, J.; Ries, L.; Bleyer, A. Epidemiology and outcome of CNS germ cell tumors in the United States, SEER (1975–2000), a disease of adolescents and young adults. Abstracts for the second international symposium on central nervous system germ cell tumors, November 18–21, 2005, Los Angeles, California. Neuro Oncol. 2005, 7, 513–533. [Google Scholar]
- Cheng, S.; Kilday, J.P.; Laperriere, N.; Janzen, L.; Drake, J.; Bouffet, E.; Bartels, U. Outcomes of children with central nervous system germinoma treated with multi-agent chemotherapy followed by reduced radiation. J. Neurooncol. 2016, 127, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Toita, T.; Nakamura, K.; Uno, T.; Onishi, H.; Itami, J.; Shikama, N.; Saeki, N.; Yoshii, Y.; Murayama, S. Treatment and prognosis of patients with intracranial nongerminomatous malignant germ cell tumors: A multiinstitutional retrospective analysis of 41 patients. Cancer 2003, 98, 369–376. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Prospective Trial for the Diagnosis and Treatment of Intracranial Germ Cell Tumors (SIOPCNSGCTII) ClinicalTrials.gov Identifier: NCT01424839. Available online: https://clinicaltrials.gov/ct2/show/NCT01424839 (accessed on 29 August 2011).
- Calaminus, G.; Bamberg, M.; Baranzelli, M.C.; Benoit, Y.; di Montezemolo, L.C.; Fossati-Bellani, F.; Jürgens, H.; Kühl, H.J.; Lenard, H.G.; Lo Curto, M.; et al. Intracranial germ cell tumors: A comprehensive update of the European data. Neuropediatrics 1994, 25, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.; Bouffet, E.; Fisher, P.G.; Allen, J.C.; Robertson, P.L.; Chuba, P.J.; Donahue, B.; Kretschmar, C.S.; Zhou, T.; Buxton, A.B.; et al. Phase II trial assessing the ability of neoadjuvant chemotherapy with or without second-look surgery to eliminate measurable disease for nongerminomstous germ cell tumors: A Children’s Oncology Group study. J. Clin. Oncol. 2015, 33, 2464–2471. [Google Scholar] [CrossRef] [PubMed]
- Robertson, P.L.; DARosso, R.C.; Allen, J.C. Improved prognosis of intracranial non germinoma germ cell tumors with multimodality therapy. J. Neurooncol. 1997, 32, 71–80. [Google Scholar] [CrossRef]
- Takahashi, S.; Yoshida, K.; Kawase, T. Intracranial Germ Cell Tumors: Efficacy of Neoadjuvant Chemo-radiotherapy without Surgical Biopsy. Keio J. Med. 2011, 60, 56–64. [Google Scholar] [CrossRef]
- Sawamura, Y.; Shirato, H.; Ikeda, J.; Tada, M.; Ishii, N.; Kato, T.; Abe, H.; Fujieda, K. Induction chemotherapy followed by reduced-volume radiation therapy for newly diagnosed central nervous system germinoma. J. Neurosurg. 1998, 88, 66–72. [Google Scholar] [CrossRef]
- Kyritsis, A.P. Management of primary intracranial germ cell tumors. J. Neurooncol. 2010, 96, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.; Birch, R.; Einhorn, L.H.; Irwin, L.; Greco, F.A.; Loehrer, P.J. Treatment of disseminated germ cell tumors with cisplatin, bleomycin and either vinblastin or etoposide. N. Engl. J. Med. 1987, 316, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Göbel, U.; Bamberg, M.; Budach, V.; Haas, R.J.; Janka-Schaub, G.; Kühl, J.; Lenard, H.G.; Rister, M.; Spaar, H.J. Intracranial germ cell tumors: Analysis of the therapy study MAKEI 83/86 and changes in protocol for the follow-up study. Klin. Padiatr. 1989, 201, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Calaminus, G.; Garré, M.L.; Kortmann, R.D.; Schober, R.; Göbel, U. Secreting germ cell tumors of the central nervous system (CNS). First results of the cooperative German/Italian pilot study (CNS sGCT). Klin. Padiatr. 1997, 209, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Seregni, E.; Massimino, M.; Nerini Molteni, S.; Pallotti, F.; van der Hiel, B.; Cefalo, G.; Spreafico, F.; Fossati Bellani, F.; Bombardieri, E. Serum and cerebrospinal fluid human chorionic gonadotropin (hCG) and alpha-fetoprotein (AFP) in intracranial germ cell tumors. Int. J. Biol. Markers 2002, 17, 112–118. [Google Scholar] [CrossRef]
- Denyer, S.; Bhimani, A.D.; Patil, S.N.; Mudreac, A.; Behbahani, M.; Metha, A.I. Treatment and serviva of primary intracranial germ cell tumors: A population-based study using SEER database. J. Cancer Res. Clin. Oncol. 2020, 146, 67–685. [Google Scholar] [CrossRef]
- Gittelman, H.; Cioffi, G.; Vecchione-Coval, T.; Ostrom, Q.T.; Kruchko, C.; Osorio, D.S.; Finlay, J.L.; Barnholtz-Sloan, J.S. Descriptive epidemiology of germ cell tumors of the central nervous system diagnosed in the United States from 2006 to 2015. J. Neuro-Oncol. 2019, 143, 251–260. [Google Scholar] [CrossRef]
- Phi, J.H.; Kim, S.K.; Lee, Y.A.; Shin, C.H.; Cheon, J.E.; Kim, I.O.; Yang, S.W.; Wang, K.C. Latency of intracranial germ cell tumors and diagnosis delay. Childs Nerv. Syst. 2013, 29, 1871–1881. [Google Scholar] [CrossRef]
- Sethi, R.V.; Marino, R.; Niemierko, A.; Tarbell, N.J.; Yock, T.I.; MacDonald, S.M. Delayed diagnosis in children with intracranial germ cell tumors. J. Pediatr. 2013, 163, 1448–1453. [Google Scholar] [CrossRef]
- Souweidane, M.M.; Krieger, M.D.; Weiner, H.L.; Finlay, J.L. Surgical management of primary central nervous system germ cell tumors: Proceedings from the Second International Symposium on Central Nervous System Germ Cell Tumors. J. Neurosurg. Pediatr. 2010, 6, 125–130. [Google Scholar] [CrossRef]
- Nicholson, J.C.; Punt, J.; Hale, J.; Saran, F.; Calaminus, G. Neurosurgical management of paediatric germ cell tumours of the central nervous system—A multi-disciplinary team approach for the new millennium; Germ Cell Tumour Working Groups of the United Kingdom Children’s Cancer Study Group (UKCCSG) and International Society of Paediatric Oncology (SIOP). Br. J. Neurosurg. 2002, 16, 93–95. [Google Scholar] [PubMed]
- Balmaceda, C.; Heller, G.; Diez, B.; Villablanca, J.G.; Kellie, S.; Maher, P.; Vlamis, V.; Walker, R.W.; Leibel, S.; Finlay, J.L. Chemotherapy without irradiation—A novel approach for newly diagnosed CNS germ cell tumors: Results of an international cooperative trial. The First International Central Nervous System Germ Cell Tumor Study. J. Clin. Oncol. 1996, 14, 2908–2915. [Google Scholar] [CrossRef]
- Baranzelli, M.C.; Patte, C.; Bouffet, E.; Portas, M.; Mechinaud-Lacroix, F.; Sariban, E.; Roche, H.; Kalifa, C. An attempt to treat pediatric intracranial alphaFP and betaHCG secreting germ cell tumors with chemotherapy alone. SFOP experience with 18 cases. Société Française d’Oncologie Pédiatrique. J. Neurooncol. 1998, 37, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.T.; Gelman, R.; Hochberg, F. Intracranial germ-cell tumors: Natural history and pathogenesis. J. Neurosurg. 1985, 63, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Calaminus, G.; Frappaz, D.; Kortmann, R.D.; Krefeld, B.; Saran, F.; Pietsch, T.; Vasiljevic, A.; Garré, M.L.; Ricardi, U.; Mann, J.R.; et al. Outcome of patients with intracranial non-germinomatous germ cell tumors—lessons from the SIOP-CNS-GCT-96 trial. Neuro-Oncology 2017, 19, 1661–1672. [Google Scholar] [CrossRef] [PubMed]
- Harnden, P.; Joffe, J.K.; Jones, W.G. Germ cell tumours V. In Proceedings of the Fifth Germ Cell Tumour Conference Devonshire Hall, University of Leeds, London, UK, 13–15 September 2001; Springer: London, UK, 2002. [Google Scholar]
- De B Cahlon, O.; Dunkel, I.J.; De Braganca, K.C.; Khakoo, Y.; Gilheeney, S.W.; Souweidane, M.M.; Wolden, S.L. Reduced-volume radiotherapy for patients with localized intracranial nongerminoma germ cell tumors. J. Neurooncol. 2017, 134, 349–356. [Google Scholar]
- Fangusaro, J.; Wu, S.; MacDonald, S.; Murphy, E.; Shaw, D.; Bartels, U.; Khatua, S.; Souweidane, M.; Lu, H.M.; Morris, D.; et al. Phase II trial of response-based radiation therapy for patients with localized CNS nongerminomatous germ cell tumors: A children’s oncology group study. J. Clin. Oncol. 2019, 37, 3283–3290. [Google Scholar] [CrossRef]
- Fetcko, K.; Dey, M. Primary Central Nervous System Germ Cell Tumors: A Review and Update. Med. Res. Arch. 2018, 6, 1719. [Google Scholar] [CrossRef]

| Patient | Male/Female | Age at Diagnosis (yrs, Days) | L = Localized; M = Metastatic; B = Bifocal | Shunt Placement | Diagnostic Surgery |
|---|---|---|---|---|---|
| 1 | M | 12.25 | B | Y | N |
| 2 | M | 13 | M | Y | Y |
| 3 | M | 12.17 | M | Y | Y |
| 4 | M | 14.5 | L | N | N |
| 5 | M | 13.92 | L | N | Y |
| 6 | M | 11.75 | B | Y | N |
| 7 | M | 10.33 | B | N | N |
| 8 | M | 12.17 | L | N | N |
| 9 | M | 18.42 | L | N | Y |
| 10 | M | 34 | L | N | Y |
| 11 | M | 9.75 | L | Y | N |
| 12 | M | 23.67 | L | Y | N |
| 13 | M | 23 | L | N | Y |
| 14 | F | 6 | L | N | Y |
| 15 | M | 8.75 | L | N | N |
| 16 | F | 10.67 | B | N | N |
| 17 | M | 35.67 | L | N | N |
| 18 | M | 8.33 | M | Y | Y |
| 19 | M | 15.67 | B | N | Y |
| 20 | M | 9.67 | M | Y | Y |
| 21 | M | 15.08 | L | N | N |
| 22 | M | 11.58 | M | N | Y |
| 23 | M | 15 | L | Y | Y |
| 24 | M | 17.33 | M | Y | N |
| 25 | F | 8.5 | L | N | N |
| 26 | F | 20.67 | L | N | Y |
| 27 | M | 15.75 | L | Y | N |
| 28 | F | 9.92 | L | Y | Y |
| Patient | Sieric AFP Absolute Values ng/mL | Sieric AFP | CSF AFP Absolute Values ng/mL | CSF AFP | Sieric βhcg Absolute Values IU/L | Sieric βhcg | CSF βhcg Absolute Values IU/L | CSF βhcg |
|---|---|---|---|---|---|---|---|---|
| 1 | 40 | 1 | 35 | 1 | 21,600 | 4 | >10,000 | 4 |
| 2 | 0 | 0 | 0 | 0 | 14,850 | 4 | 303 | 2 |
| 3 | 11 | 1 | 119 | 2 | 0 | 0 | 0 | 0 |
| 4 | 41 | 1 | 4.6 | 0 | 680 | 2 | 171 | 2 |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 432 | 2 | 207 | 2 | 0 | 0 | 8 | 1 |
| 7 | 62 | 1 | 22 | 1 | 0 | 0 | 77 | 1 |
| 8 | 0 | 0 | 0 | 0 | 8489 | 4 | 3010 | 4 |
| 9 | 394 | 2 | 145 | 2 | 450 | 2 | 372 | 2 |
| 10 | 0 | 0 | 0 | 0 | 90.98 | 1 | 147.56 | 2 |
| 11 | 0 | 0 | 0 | 0 | 148 | 2 | 280.9 | 2 |
| 12 | 11,069 | 4 | 5862 | 4 | 54 | 1 | 165 | 2 |
| 13 | 0 | 0 | 13 | 1 | 16 | 1 | 223 | 2 |
| 14 | 52 | 1 | <1 | 0 | 1.1 | 0 | 0 | 0 |
| 15 | 0.27 | 0 | 0.44 | 0 | 10.28 | 1 | 53 | 1 |
| 16 | 1790 | 4 | 13.9 | 1 | 23 | 1 | 16 | 1 |
| 17 | 1.71 | 0 | 0.09 | 0 | 77.86 | 1 | 604 | 3 |
| 18 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 |
| 19 | 246 | 2 | 91 | 1 | 643 | 3 | 543 | 3 |
| 20 | 123 | 2 | 298 | 2 | 438 | 2 | 1395 | 4 |
| 21 | 0 | 0 | 0 | 0 | 46 | 1 | 257 | 2 |
| 22 | 0 | 0 | 0 | 0 | 34 | 1 | 60 | 1 |
| 23 | 221 | 2 | not done | 9 | 36 | 1 | not done | 9 |
| 24 | 1 | 0 | 46 | 1 | 49 | 1 | 500 | 2 |
| 25 | 138 | 2 | 66.5 | 1 | 360 | 2 | 414 | 2 |
| 26 | 1155 | 4 | 67.21 | 1 | 7.1 | 1 | 8.1 | 1 |
| 27 | 2614 | 4 | 1996 | 4 | 0.1 | 0 | 1.3 | 0 |
| 28 | 312.5 | 2 | 205.7 | 2 | 0.1 | 0 | 1.9 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biassoni, V.; Schiavello, E.; Gandola, L.; Pecori, E.; Poggi, G.; Spreafico, F.; Terenziani, M.; Meazza, C.; Podda, M.; Ferrari, A.; et al. Secreting Germ Cell Tumors of the Central Nervous System: A Long-Term Follow-up Experience. Cancers 2020, 12, 2688. https://doi.org/10.3390/cancers12092688
Biassoni V, Schiavello E, Gandola L, Pecori E, Poggi G, Spreafico F, Terenziani M, Meazza C, Podda M, Ferrari A, et al. Secreting Germ Cell Tumors of the Central Nervous System: A Long-Term Follow-up Experience. Cancers. 2020; 12(9):2688. https://doi.org/10.3390/cancers12092688
Chicago/Turabian StyleBiassoni, Veronica, Elisabetta Schiavello, Lorenza Gandola, Emilia Pecori, Geraldina Poggi, Filippo Spreafico, Monica Terenziani, Cristina Meazza, Marta Podda, Andrea Ferrari, and et al. 2020. "Secreting Germ Cell Tumors of the Central Nervous System: A Long-Term Follow-up Experience" Cancers 12, no. 9: 2688. https://doi.org/10.3390/cancers12092688
APA StyleBiassoni, V., Schiavello, E., Gandola, L., Pecori, E., Poggi, G., Spreafico, F., Terenziani, M., Meazza, C., Podda, M., Ferrari, A., Luksch, R., Casanova, M., Puma, N., Chiaravalli, S., Bergamaschi, L., Cefalo, G., Simonetti, F., Gattuso, G., Seregni, E. C., ... Massimino, M. (2020). Secreting Germ Cell Tumors of the Central Nervous System: A Long-Term Follow-up Experience. Cancers, 12(9), 2688. https://doi.org/10.3390/cancers12092688






