1. Introduction
Ovarian cancer is characterized by a high fatality rate and is responsible for approximately 2–3% of all cancer deaths. The early-stage disease has a 5-year survival of 93%. Unfortunately, the majority of patients are diagnosed at the FIGO III or IV stage of the disease, for which the 5-year survival is much lower [
1]. Epithelial ovarian cancer (EOC) is the most common type of ovarian malignancy, as only 10% of tumors are of non- epithelial origin. Serous high-grade carcinomas (HGSOC) are the most prevalent type of EOC, which are characterized by TP53 mutations and relatively poor outcome [
2].
MicroRNAs (miRNAs) are small (approximately 18–25 nt), single-stranded non-coding RNAs that are evolutionarily conserved among species [
3]. MiRNA genes are transcribed by RNA polymerase II to pri-miRNAs, which are cleaved by the microprocessor complex (nuclease Drosha together with DGCR8 protein) to create hairpin-like pre-miRNAs. Pre-miRNAs bind to the Exportin- 5 (RanGTP-dependent transporter) and are exported to the cytoplasm. RNase III-type nuclease enzyme DICER splits pre-miRNAs into two single-stranded forms of miRNA (miRNA-3p and miRNA-5p). One strand creates the mature miRNA, and the second passenger strand is usually destroyed. However, it is possible that the passenger strand can also be selected as a mature form of miRNA. Mature forms of miRNAs are incorporated into the RNA-induced silencing complex (RISC) and bind to the 3′ untranslated regions (UTRs) of their mRNA targets, which causes posttranscriptional suppression/activation of translation or mRNA cleavage [
3,
4]. MiRNAs possess unique abilities to affect the expression of genes and take part in such cellular processes as proliferation, differentiation, invasion, migration, epithelial-to-mesenchymal transition (EMT), or apoptosis [
3,
4]. Numerous authors showed miRNAs to be dysregulated in multiple cancers. Aberrant expression of miRNAs has been reported in multiple neoplasms and related to the stage of the disease or clinical outcome [
5,
6]. A large body of evidence suggests that miRNAs play a crucial role in carcinogenesis, tumor progression, and metastasis. Several miRNAs may be up-regulated in specific neoplasms; however, a global miRNA reduction in human cancers seems to be a common phenomenon [
7]. A potential explanation for a global decrease in miRNA expression may be inhibition of DICER. Down-regulation of DICER has been detected in epithelial ovarian cancer (EOC). Meritt et al. reported that low DICER expression was associated with advanced-stage disease and reduced median survival [
8]. Martello et al. identified a miRNA family (miRNA-103/107) that inhibited miRNA biosynthesis by targeting DICER [
9]. They reported that high levels of miR-103/107 are associated with metastasis and poor outcome in breast cancer. Inhibition of miRNA-103/107 in malignant cells resulted in the attenuation of migratory and metastatic properties. Martello et al. concluded that the up-regulation of miRNA-103/107 is responsible for the induction of EMT, attained by down-regulating miRNA-200 levels. However, the role of miRNA-103/107 in EOC has not been elucidated yet, as the evidence is scarce. The Cancer Genome Atlas (TCGA) provided data on miRNA-103/107 expression levels in EOC [
10]. Yang et al. identified miRNA-103 as an oncogene in serous ovarian cancer, which promotes invasion and metastasis via the down-regulation of DICER1 [
11]. Apart from those two studies, there is no evidence on the clinical significance of both miRNAs. Several authors reported aberrant miRNA103/107 expression levels in other tumors. Yu et al. identified miRNA-103/107 up-regulation in bladder cancer specimens and revealed its oncogenic role in cell proliferation and PI3K/AKT signaling partially through PTEN dependent mechanism [
12]. However, some authors showed that miRNA-107 might also be considered as a tumor suppressor. Tang et al. described that ectopic expression of miRNA-107 suppressed cell proliferation and was associated with the down-regulation of cyclin E1 (CCNE1) expression [
13].
Although miRNA-103/107 may be one of the key-regulators of EOC carcinogenesis through the down-regulation of DICER, its prognostic and clinical significance has not been thoroughly evaluated. Thus, we decided to investigate miRNA-103/107 expression levels in primary HGSOC tissues and relate it to clinicopathological characteristics, with particular attention to overall survival (OS) and progression-free survival (PFS).
3. Discussion
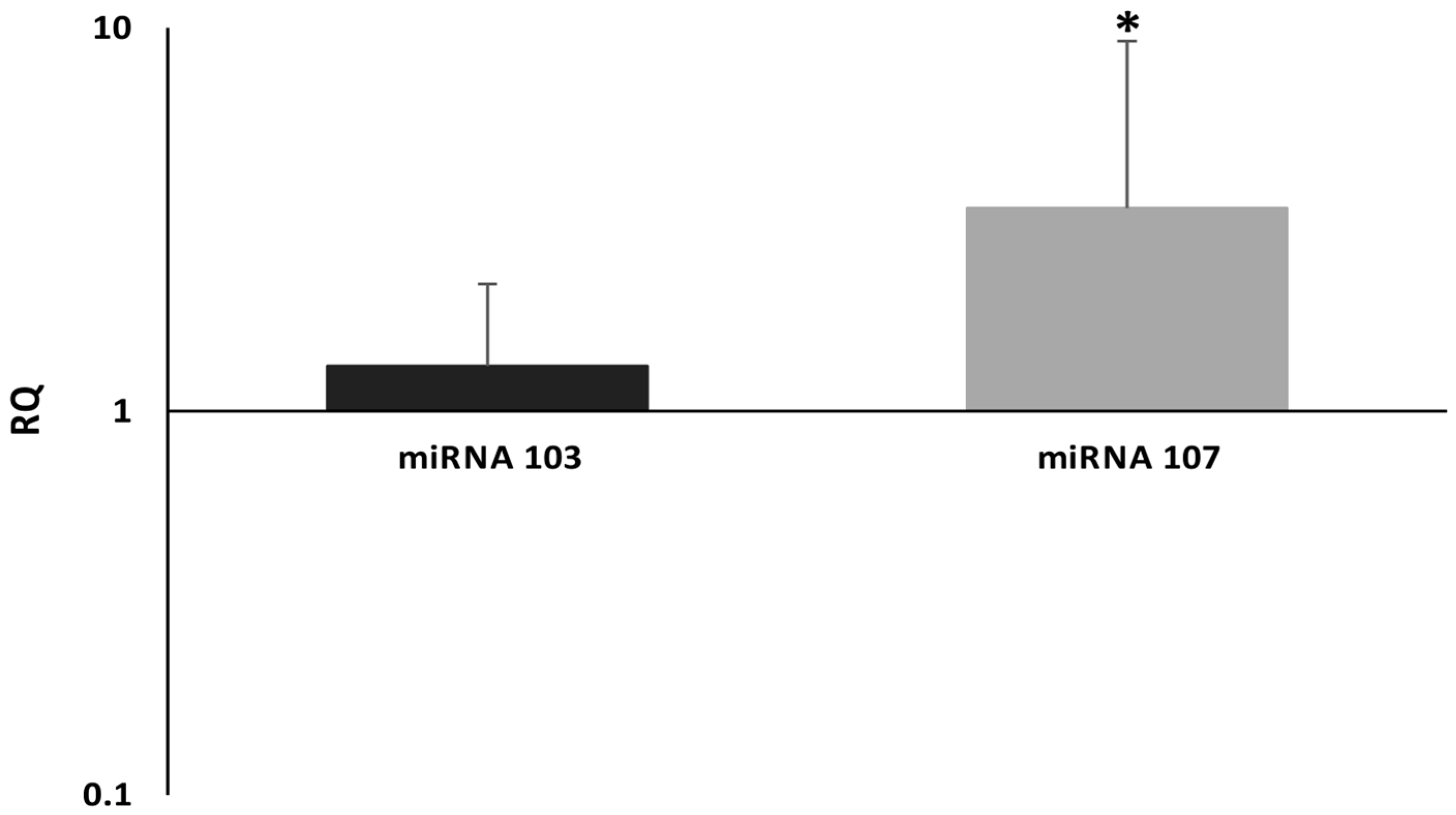
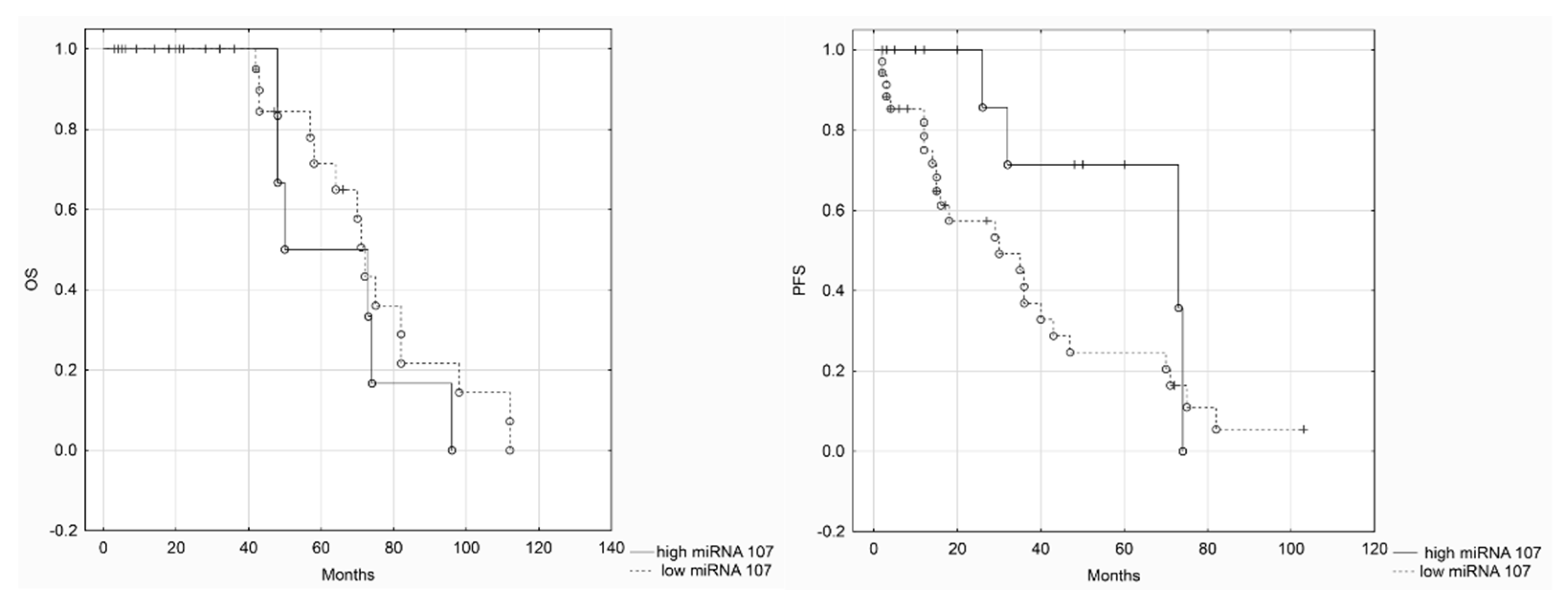
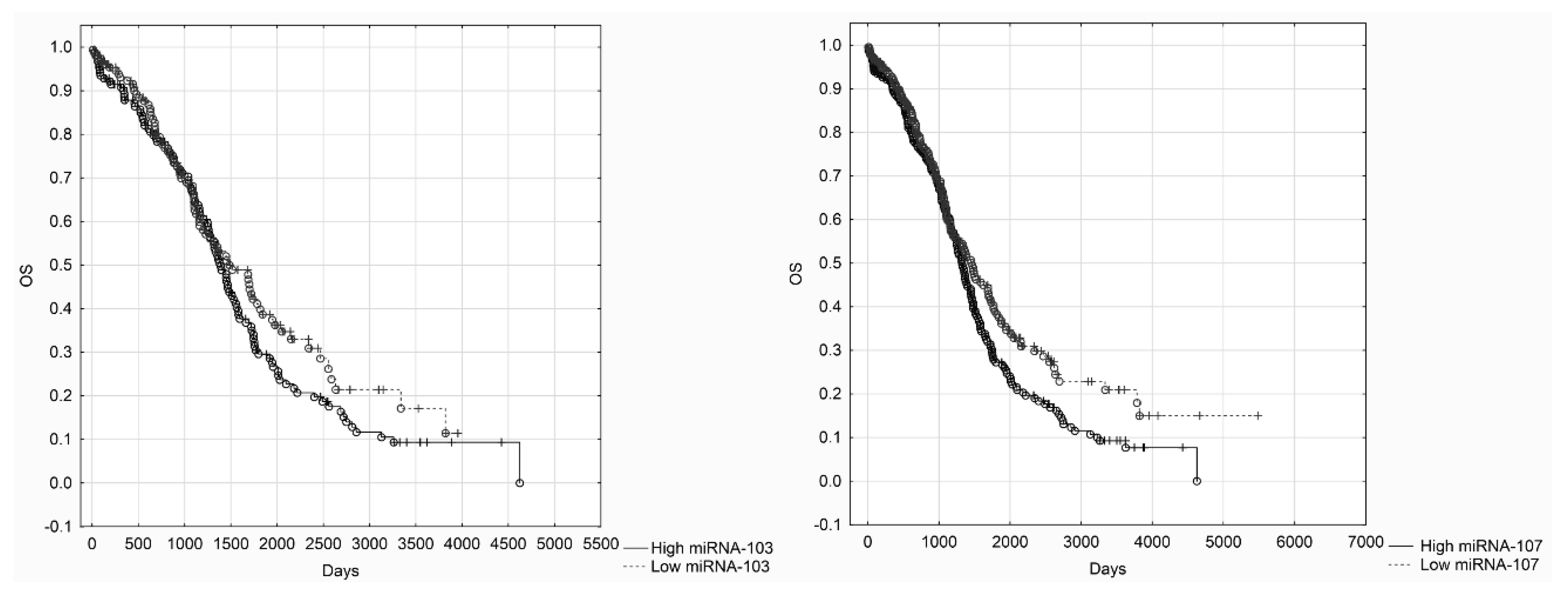
Our results showed that miRNA-107 is up-regulated in primary high-grade serous ovarian cancer tissues in comparison to normal epithelium derived from the Fallopian tube’s fimbriae. Nevertheless, we did not find any clinical correlations with miRNA-107 expression levels. We also did not confirm that miRNA-103 is up-regulated in cancerous tissues. Such results may be caused by a small number of samples (i.e., 50 patients). However, we believe that our investigation is not without merit. To the best of our knowledge, there have not been any studies on ovarian cancer that evaluated miRNA-103/107 expression levels in regard to clinicopathological data. The only data on miRNA-103/107 survival comes from TCGA research. We assessed the OS in TCGA cohort of patients. Kaplan–Meier analysis indicated that low miRNA-107 expression levels were associated with improved survival.
Expression levels of both miRNAs were also determined in other types of tumors, such as gastric or bladder cancers. MiRNA-103/107 expression was assessed by Yu et al. in bladder cancer samples. Both miRNAs were up-regulated in the bladder cancer specimens and positively correlated with the tumor stage [
12]. Li et al. demonstrated that overexpression of miRNA-107 in gastric cancer might be associated with gastric cancer metastasis. Their results suggested that miRNA-107 promotes cancer metastasis through the down-regulation of DICER1 [
19].
MiRNA-103/107- DICER axis has been described in breast cancer cells by Martello et al. According to them, invasive and metastatic properties of cancer cells are empowered by the up-regulation of miRNA-103/107 [
9]. High levels of miRNA-103/107 affect DICER expression and cause its attenuation. Martello et al. associated the miRNA-103/107-DICER axis with epithelial to mesenchymal transition (EMT), identifying miRNA-200 family members as downstream mediators of the axis. Thus, high levels of both miRNAs may lead to more invasive and metastatic abilities of cancer cells. ZEB1 and ZEB2 are miRNA-200 targets and crucial genes for mesenchymality. According to Martello et al., both of them are down-regulated in antagomir-103/107-treated cells to about 50%. Additionally, the authors performed Kaplan–Meier survival analysis, which showed that high levels of miR-103/107 are associated with metastasis and poor outcome.
Identification of miRNAs that may control DICER expression levels is crucial in full understanding of miRNA-dependent carcinogenesis, tumor growth, and metastasis. Only a few authors have assessed MiRNA-103 expression levels in ovarian cancer. Wilczynski et al. assessed miRNA-103 by qRT-PCR in 48 samples derived from advanced serous ovarian cancer patients and found no differences between primary tumor and healthy ovarian tissue [
20]. Yang et al. performed qRT-PCR to compare miR-103 expression levels in ovarian cancer and healthy ovarian tissues, however, they used only five fresh tissue samples of serous ovarian cancer [
11]. Yang et al. showed that miRNA-103 was significantly up-regulated in ovarian cancer samples in comparison to healthy ovarian tissues. Furthermore, they reported that overexpression of miRNA-103 in cancer cell lines led to the enhancement of migration or invasion and a significant reduction of DICER1 levels.
DICER seems to be one of the key regulators of miRNA’s expression and action in cancerous cells. Several authors reported that DICER expression might be associated with patients’ prognosis. Meritt et al. performed qRT-PCR with validation by immunohistochemistry in 111 samples of EOC (2 endometrioid, 109 serous) and reported that low DICER expression was significantly associated with advanced tumor stage [
9]. Moreover, they found that high DICER expression was associated with increased survival among EOC patients. Flavin et al. reported similar results and showed that high DICER expression might be associated with low metastatic lesions. However, survival analysis revealed that DICER expression did not affect the survival rates [
21]. Such results stay in corcondance with our hypothesis. The results of survival analysis in TCGA cohort of patients showed that low miRNA-107 levels were associated with improved survival. The analysis of TCGA patients failed to show a possible impact of miRNA-103 levels of survival, but we believe it might have been changed if more patients had been included. Low miRNA-103/107 expression levels should be associated with high DICER levels. The up-regulation of DICER has been identified by Meritt et al. as a favorable factor in EOC patients. It seems that low levels of miRNA-107 and high levels of DICER should be both considered as a mark of improved prognosis among EOC patients.
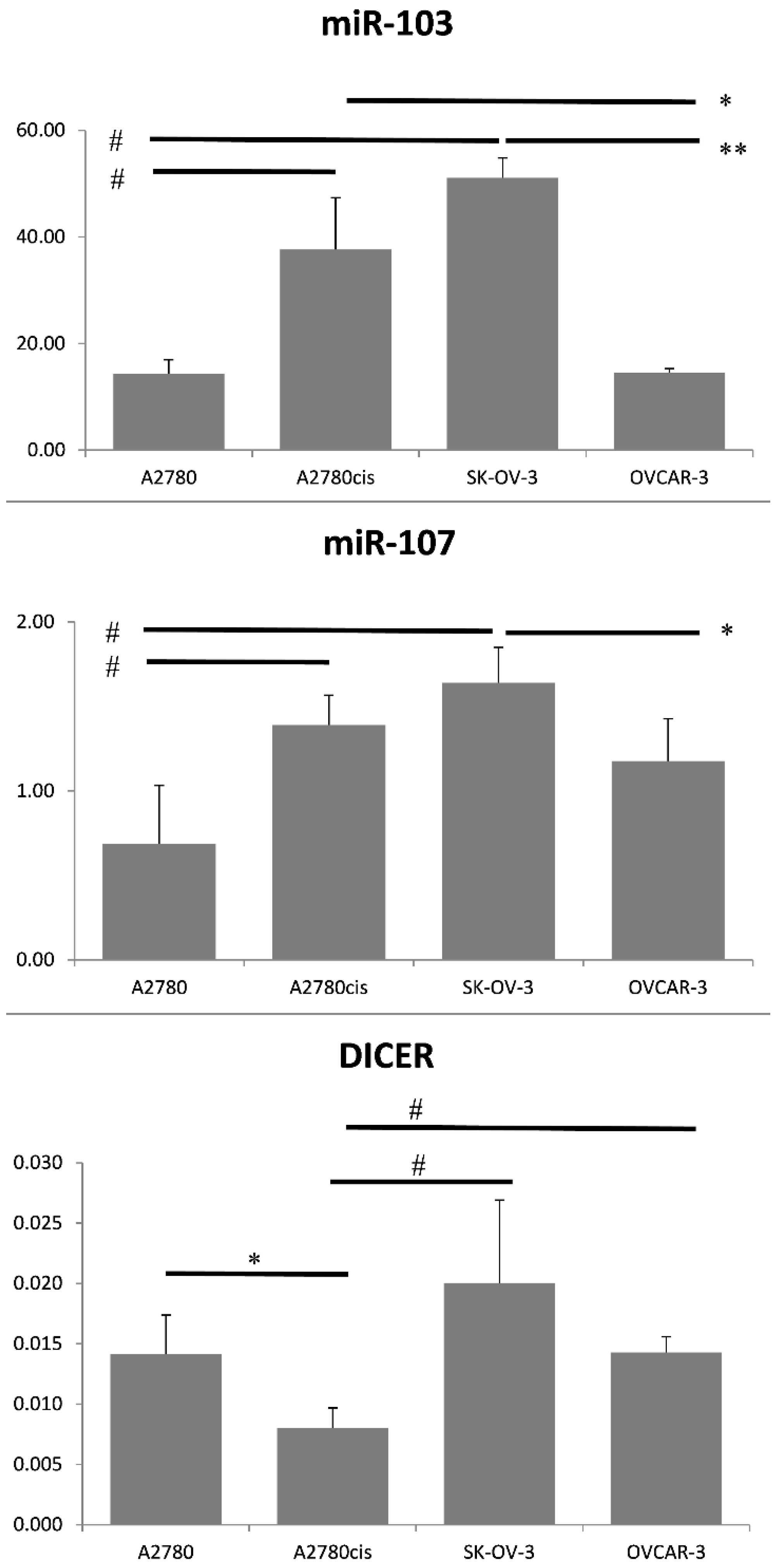
Different DICER expression levels characterize ovarian cancer cell lines. Wang et al. reported low DICER levels in cisplatin-resistant A2780 cells in comparison to A2780 cisplatin-sensitive line. Furthermore, the down-regulation of DICER decreased the sensitivity of A2780 cancer cells and inhibited cisplatin-induced apoptosis [
22]. In the study by Kuang et al., DICER down-regulation promoted cell proliferation and was significantly decreased in cisplatin-resistant A2780 cells compared with parental A2780 cells [
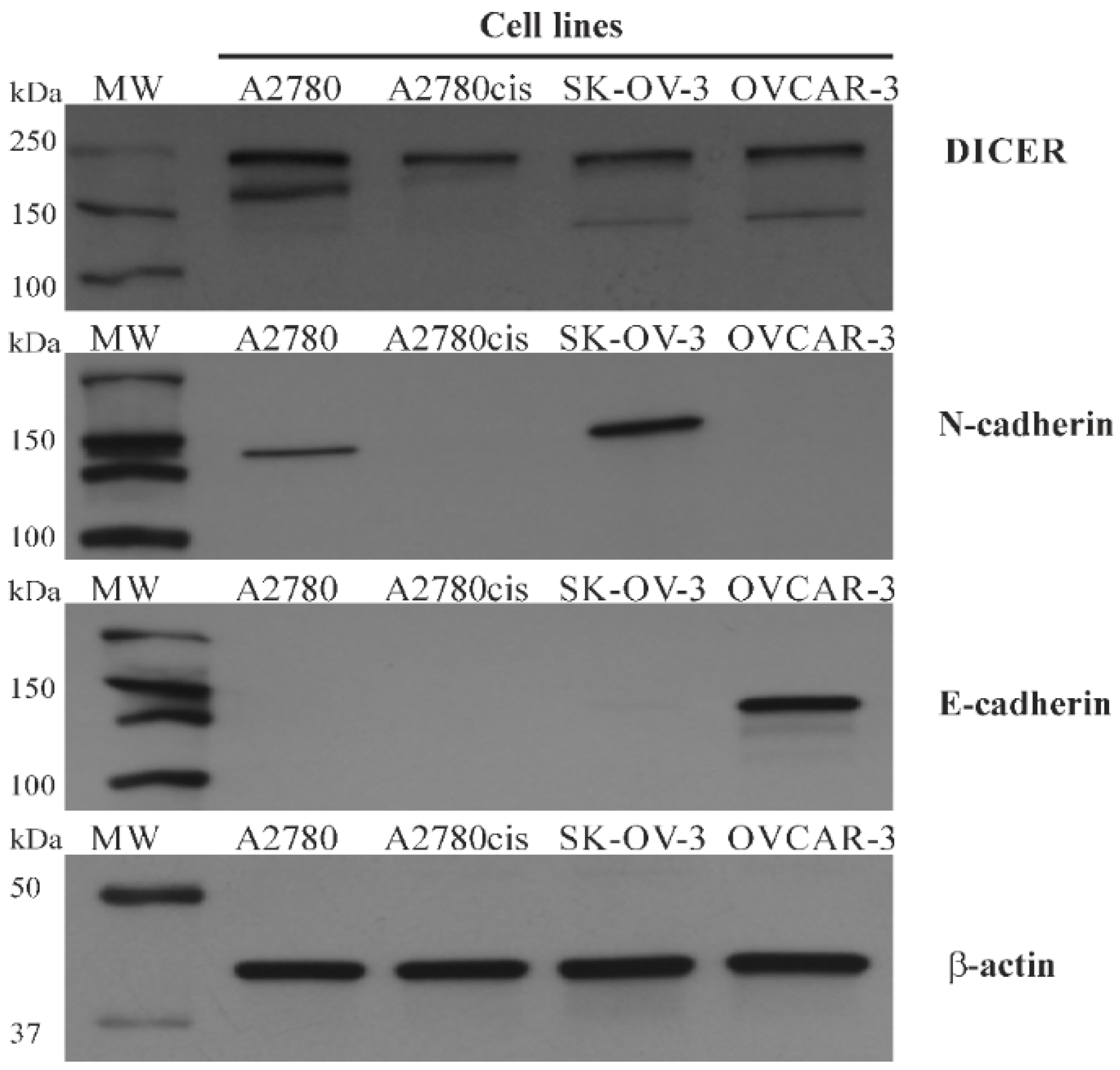
23]. Our results showed that A2780cis is characterized by lower DICER expression levels than parental A2780 ovarian cancer cells. Moreover, we demonstrated that A2780cis cells presented a significantly higher expression of miRNA-103 and miRNA-107 values in comparison to A2780 cells. Such results indicate the possible existence of the miRNA-103/107-DICER axis in the case of ovarian cancer. High levels of both miRNAs were coexistent with DICER down-regulation in A2780cis cells. Furthermore, we found that SK-OV-3 cancer cells were characterized by the highest miRNA-103/107 expression levels and relatively low level of DICER protein among four selected ovarian cancer cell lines. It is worth noticing that SK-OV-3 cells also presented high levels of N-cadherin, which may be evidence for the possible shift of these cells towards the mesenchymal phenotype.
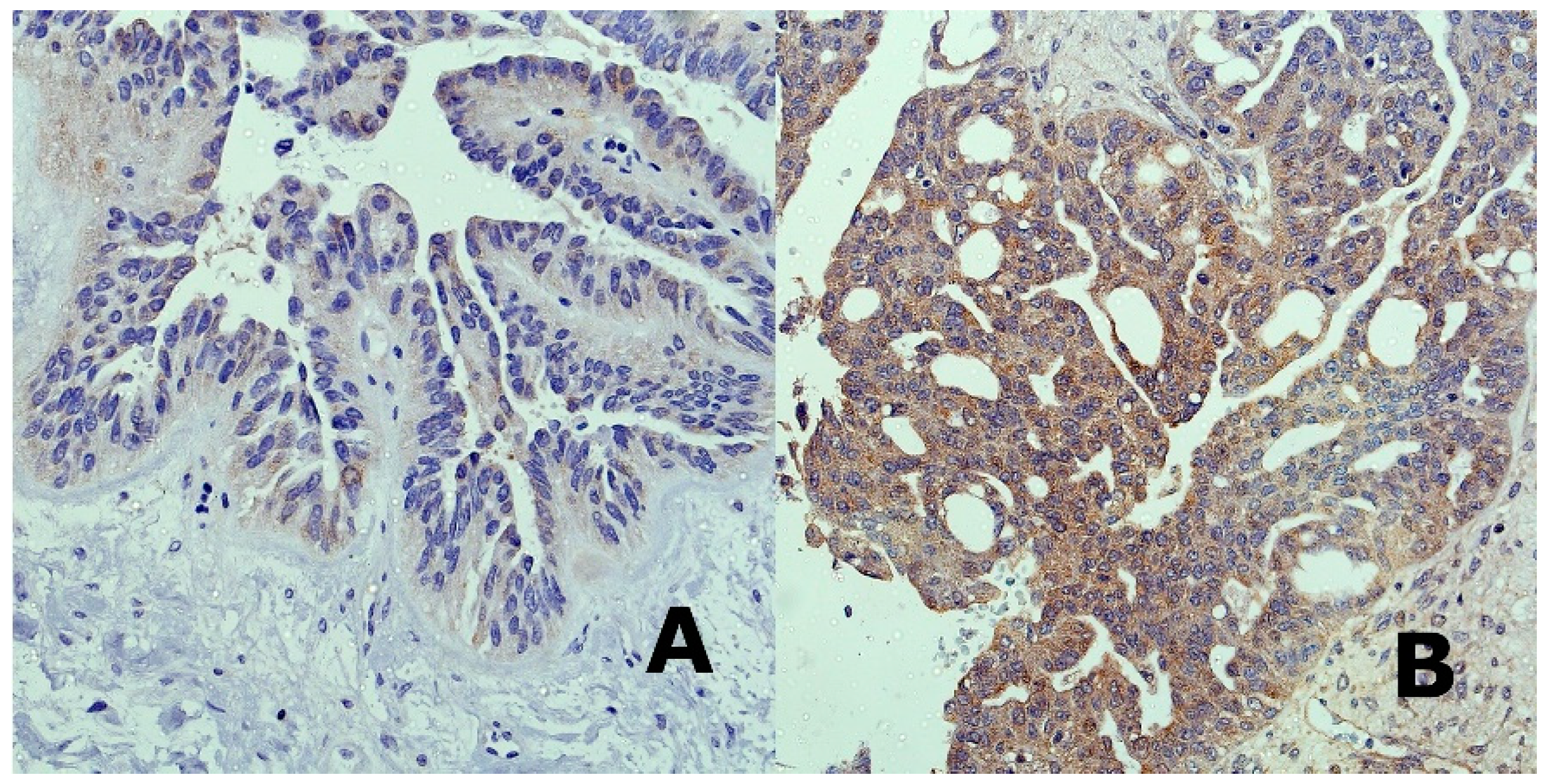
The possible existence of miRNA-103/107-DICER axis, that had previously been described in the case of breast cancer, convinced us to perform a study dedicated to the clinical usefulness of miRNA-103/107 expression levels in high-grade serous ovarian cancer patients. We did not aim to define the exact molecular ways of action of both miRNAs. MiRNA-103/107 expression levels have never been related to prognostic clinicopathological tumor characteristics. The results of the study indicated that miRNA-103/107 did not have any clinical or prognostic significance in our sample of patients. Furthermore, evaluation of DICER in FFPE tissues was not possible, due to the high amount of degraded mRNA. We found that majority of our serous high-grade ovarian cancer samples were characterized by reduced levels of DICER protein in immunohistochemistry, and it is what we expected in regard to the potential existence of the miRNA-103/107-DICER axis. Therefore, we aimed to perform a preliminary evaluation of SK-OV-3, OVCAR-3, A2780, and A2780cis cancer cell lines in order to verify a possible existence of the miRNA-103/107-DICER axis in the case of ovarian cancer. We selected various ovarian cancer cell lines that are derived from different histotypes of EOC. We expected that selected cells should vary between each other in regard to DICER and miRNA-103/103 expression levels. The cells with the highest miRNA-103/107 expression levels should be characterized by the lowest DICER expression at the same time. Our results confirmed that the miRNA-103/107-DICER axis might exist in the case of ovarian cancer, however, we are far from drawing any final conclusions.
Lack of clinical significance of both miRNAs might be related to the small set of patients or the fact that up-regulation of miRNA-103/107 may happen only in few ovarian cancer cells that gain migratory potential and form metastases.
4. Materials and Methods
Ethical approval was obtained from the Polish Mother’s Memorial Hospital Research Institute Ethics Committee (21 May 2019, approval number 71/2019). All individuals who participated in the study provided their consent. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All patients were operated in the Department of Operative Gynecology, Endoscopy and Gynecologic Oncology, Polish Mother’s Memorial Hospital Research Institute, Lodz, Poland. Total hysterectomy with bilateral salpingo-oophorectomy, omentectomy, appendectomy was performed in all cases. The extent of the surgery was individually modified in order to obtain optimal cytoreduction. Standard platinum-taxane chemotherapy was introduced in all cases as a first-line treatment. We defined platinum-resistant tumors when there was a relapse/progression within six months after completion of the chemotherapy. Serum levels of CA125 (cancer antigen 125), serum human epididymis antigen-4 (HE4), and ROMA index (Risk of Malignancy Algorithm) were obtained from patients.
4.1. Assessment of Formalin-Fixed, Paraffin-Embedded Tissues
RNA isolation was performed with the use of a miRNeasy FFPE Kit (Qiagen, Hilden, Germany), according to the manufacturer’s protocol. Spectrophotometry (PicoDrop, 260/280 nm) was used in order to assess the quality of the samples. Reverse transcription was performed with the use of the TaqMan® MicroRNA Reverse Transcription Kit (Applied Biosystems, Waltham, MA, USA) and miRNA-specific primers (RT primer), according to the manufacturer’s protocol.
Quantitative Real-time PCR was performed with the use of standard TaqMan® MicroRNA Assays (Applied Biosystems): hsa-miR-103 (Assay ID: 000439), hsa-miR-107 (Assay ID: 000443) and RNU6B (endogenous control, Assay ID: 001093). The 10 μL qPCR reaction mixture included 0.7 μL RT product, 5 μL TaqMan Fast PCR Master Mix (Applied Biosystems) and 1 μL TaqMan miRNA Assay (20×). The reactions were incubated in a 96-well plate at 95 °C for 10 min, followed by 40 cycles of 95 °C for 5 s and 60 °C for 20 s. All reactions were run in duplicate (Applied Biosystems 7900HT Fast Real-Time PCR System). We used Detection System 2.3 Software (Applied Biosystems, Waltham, MA, USA) for the quantification of miRNA. Relative expression was calculated according to the Ct method 2−ΔΔCt.
4.2. Quantitative Real-Time PCR Method for Selected Ovarian Cancer Cell Lines
A2780 and A2780cis were purchased from ECACC General Cell Collection (UK), while SK-OV-3, and OVCAR-3 were purchased from ATCC (USA). All cell lines are of epithelial origin, have adherent growth as a monolayer, and defined resistance or sensitivity to cisplatin. The growth medium consists of RPMI 1640 with 10% FBS and the addition of penicillin and streptomycin (100 U/mL/100 μg/mL). Moreover, cisplatin in the concentration of 1µM was added to the A2780cis cell line every 2–3 passages in order to maintain its resistance to cisplatin. A2780, A2780cis, SK-OV-3, OVCAR3 ovarian cancer cell lines were cultured on culture flasks until they reached 90% of confluence. All cell lines were harvested, centrifuged, and total RNA or miRNA was isolated from cells using TRIzol® Reagent (Life Technologies, Waltham, MA, USA) or GeneMatrix Universal RNA/miRNA Purification Kit (EURX, Gdansk, Poland), respectively, according to the manufacturer procedure. Next, the quality control of isolated RNA and miRNA with the use of Nanodrop with ND 1000 Software (ThermoFisher Scientific, Waltham, MA, USA) was performed. RNA (5 μg) was processed directly to cDNA synthesis using Maxima First Strand cDNA Synthesis Kits for RT-qPCR (Life Technologies), according to the manufacturers manual. Similarly, the mature RNU6B, miR-103, and miR-107 were processed directly to cDNA with the use of the Taqman MicroRNA Reverse transcription kit. The human β-actin, DICER, RNU6B, miR-103, miR-107 expressions were quantified using TaqMan Gene Expression Assays (Applied Biosystems, CA, USA) by real-time PCR using ABI 7900-HT detection system (Applied Biosystems, Life Technologies), according to the manufacturer’s protocol. Controls with no template cDNA were performed with each assay. Relative quantitation of gene expression was calculated using the comparative CT (∆∆CT) method. The obtained data were analyzed with ABI 7900-HT (RQ manager software v1.2) and DataAssist software v3.01 and are presented as the RQ value- representing fold change in gene expression normalized to the reference genes (β-actin or RNU6B) and relative to the control. Additionally, data are presented as 2−ΔCT, which represents the absolute value of the mRNA level of each evaluated gene in a particular cell line.
4.3. Immunoblotting-ECL (Western Blotting Method)
A2780, A2780cis, SK-OV-3, OVCAR3 ovarian cancer cell lines were cultured on culture flasks until they reached 90% of confluence. All cell lines were harvested, centrifuged, and lysed with RIPA lysing buffer (ThermoFisher Scientific) with the addition of 1 mM PMSF and 1% of halt protease and phosphatase inhibition cocktail for 30 min on ice. The lysates were stored at −70°C until further analysis. The amount of protein in each sample was measured using a DC Protein Assay kit. The cell lysates containing equal levels of proteins were run on a 10% SDS-PAGE mini-protean precast TGX gel, and proteins were transferred to PVDF membranes using a Trans-Blot Turbo Transfer System (Bio-Rad, Hercules, CA, USA) at 2.5A for 10 min. Afterward, the membranes were blocked with SuperBlock Blocking Buffer for 30 min and blotted with mouse monoclonal anti-DICER (1:500, Thermo Fisher Scientific, catalog # MA5-31353,Waltham, MA, USA), rabbit monoclonal anti-N-cadherin (1:1000, Cell Signaling Technology, N-Cadherin (D4R1H) XP® Rabbit mAb #13116), rabbit monoclonal anti-E-cadherin (1:1000, Cell Signaling Technology, E-Cadherin (24E10) Rabbit mAb #3195, Danvers, MA, USA) or mouse IgG anti-βactin antibody (1:4000, Thermo Fisher Scientific, catalog # MA1-744) (1 h, room temperature). After washing the membranes (5 times in 2× TBS-Tween 20), they were incubated with secondary antibodies, HRP-conjugated goat anti-rabbit IgG (1:4000) or HRP-conjugated goat anti-mouse IgG (1:4000) (1 h, room temperature), and again washed five times. Proteins were detected by the incubation of membranes with ECL Western Blotting Substrate. Proteins in blots underwent densitometric analysis using a FluoroChem MultiImage FC Cabinet (Alpha Innotech Corporation, San Leandro, CA, USA) and Alpha Ease FC software 3.1.2 (Alpha Innotech Corporation, San Leandro, CA, USA). The results are presented as the optical density intensity (ODI) of the area under each band’s peak.
4.4. Immunohistochemistry
Immunohistochemical staining was performed in formalin-fixed, paraffin-embedded tissues, which were cut into 4 μm slices on a microtome. Deparaffinization and rehydration were performed in xylene and ethanol (standard protocol). Slices were boiled in 0.01 M citrate buffer (pH 6.0) for 20 min, and then incubated with 3% hydrogen peroxide for 5 min. After washings with PBS, sections were incubated for 0.5 h with mouse DICER monoclonal antibody (1:400, Thermo Fisher Scientific, CL0378, Catalog # MA5-31353). After washes, a horseradish peroxidase kit was used for antibody detection and diaminobenzidine for chromagen visualization (Dako EnVision Detection Systems).
4.5. Statistical Analysis
Statistical analysis was performed with Excel 2013 (Microsoft, Redmond, Washington) and STATISTICA 13.1 software (Statsoft, Tulsa, OK, USA). Statistical significance was defined by p value lower than 0.05. Kruskal–Wallis test, median test (Pearson’s chi-squared test), unpaired t-test, and Mann–Whitney U-test were used. Relative expression levels (RQ values) were determined with the use of the delta-delta CT method, adjusted to the expression of selected endogenous control.