Pathophysiology and Imaging Findings of Bile Duct Necrosis: A Rare but Serious Complication of Transarterial Therapy for Liver Tumors
Simple Summary
Abstract
1. Introduction
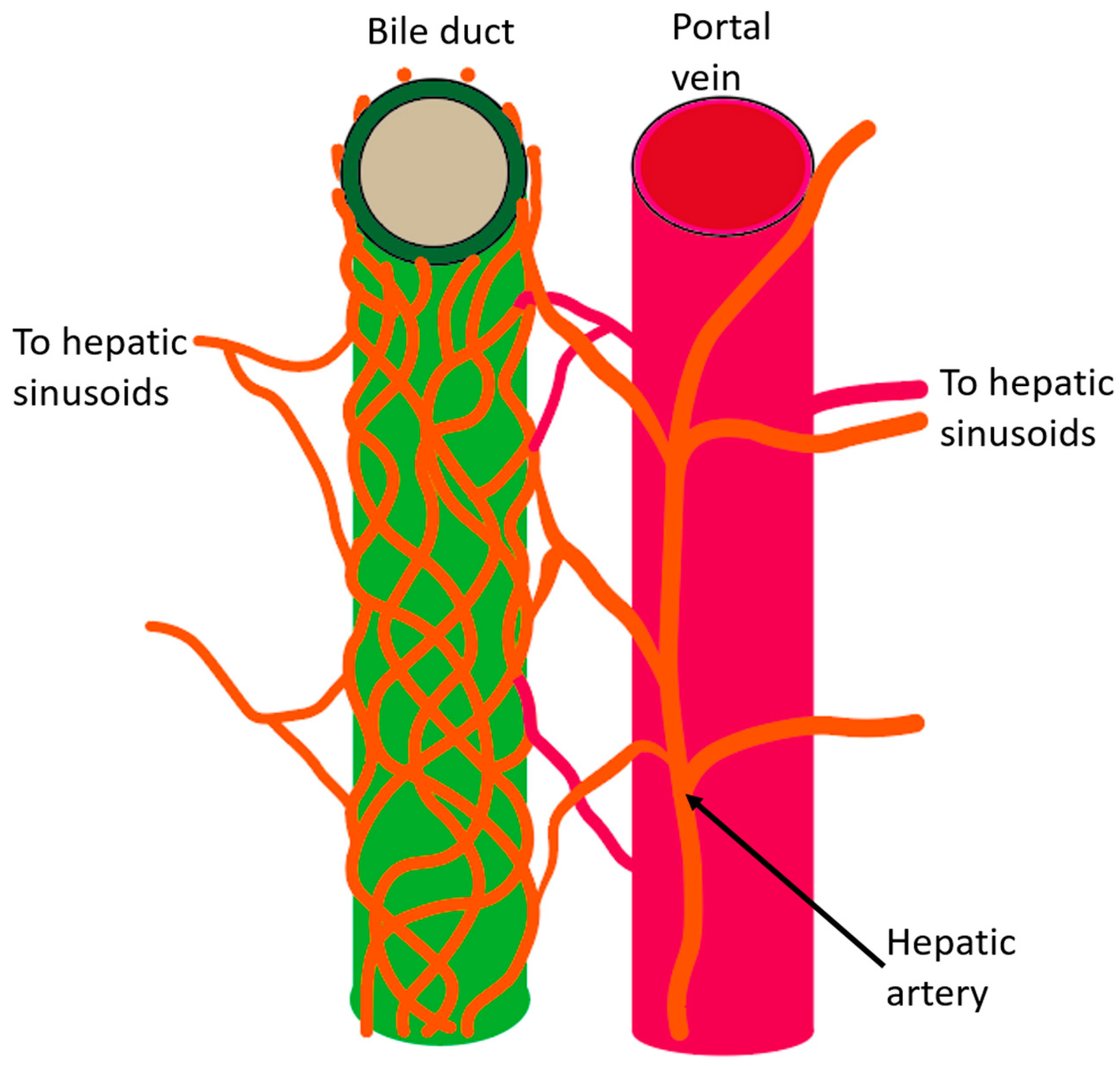
2. Anatomy of the Intrahepatic Bile Duct System
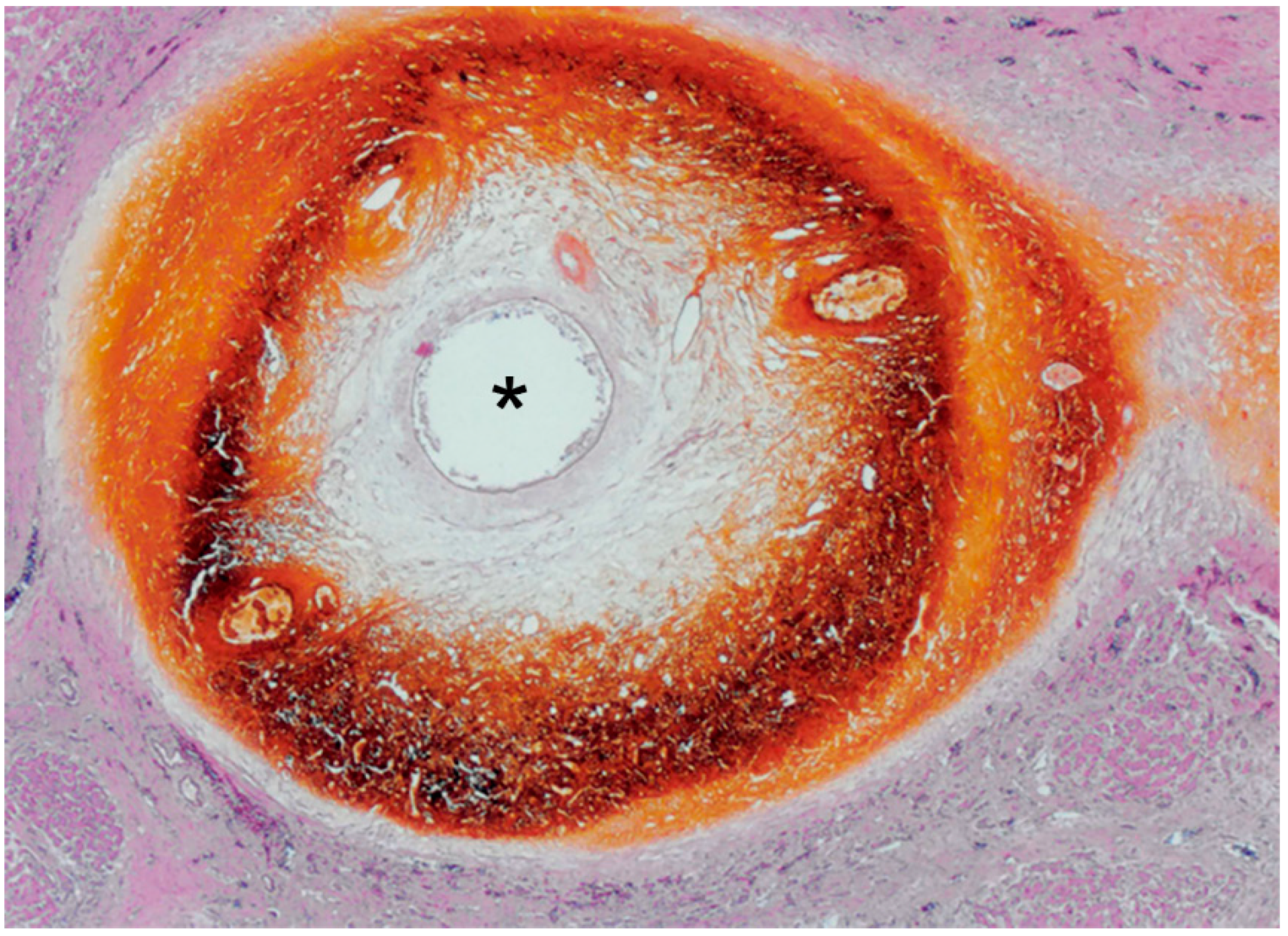
3. Function of the Bile Duct and the Significance of the PBP
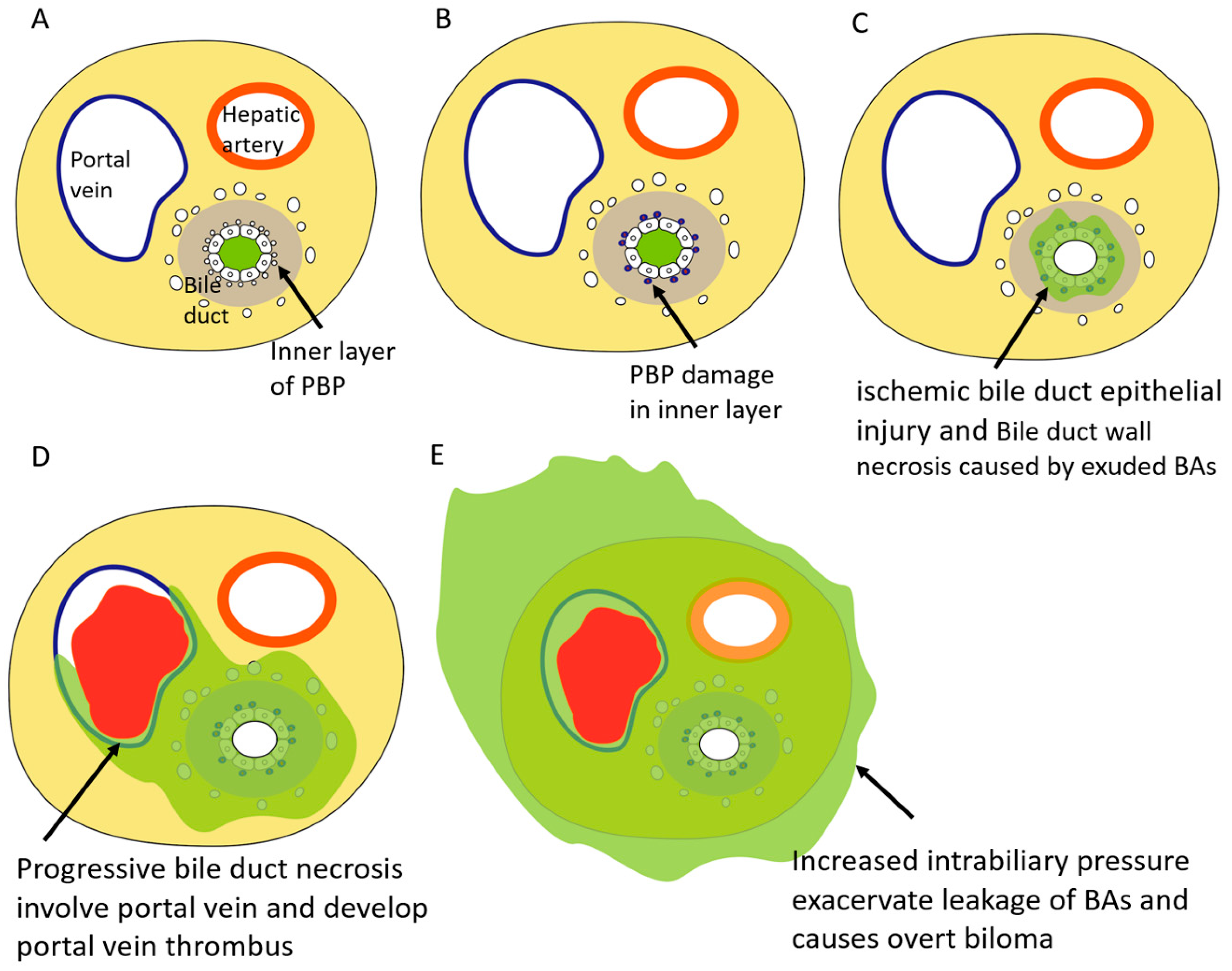
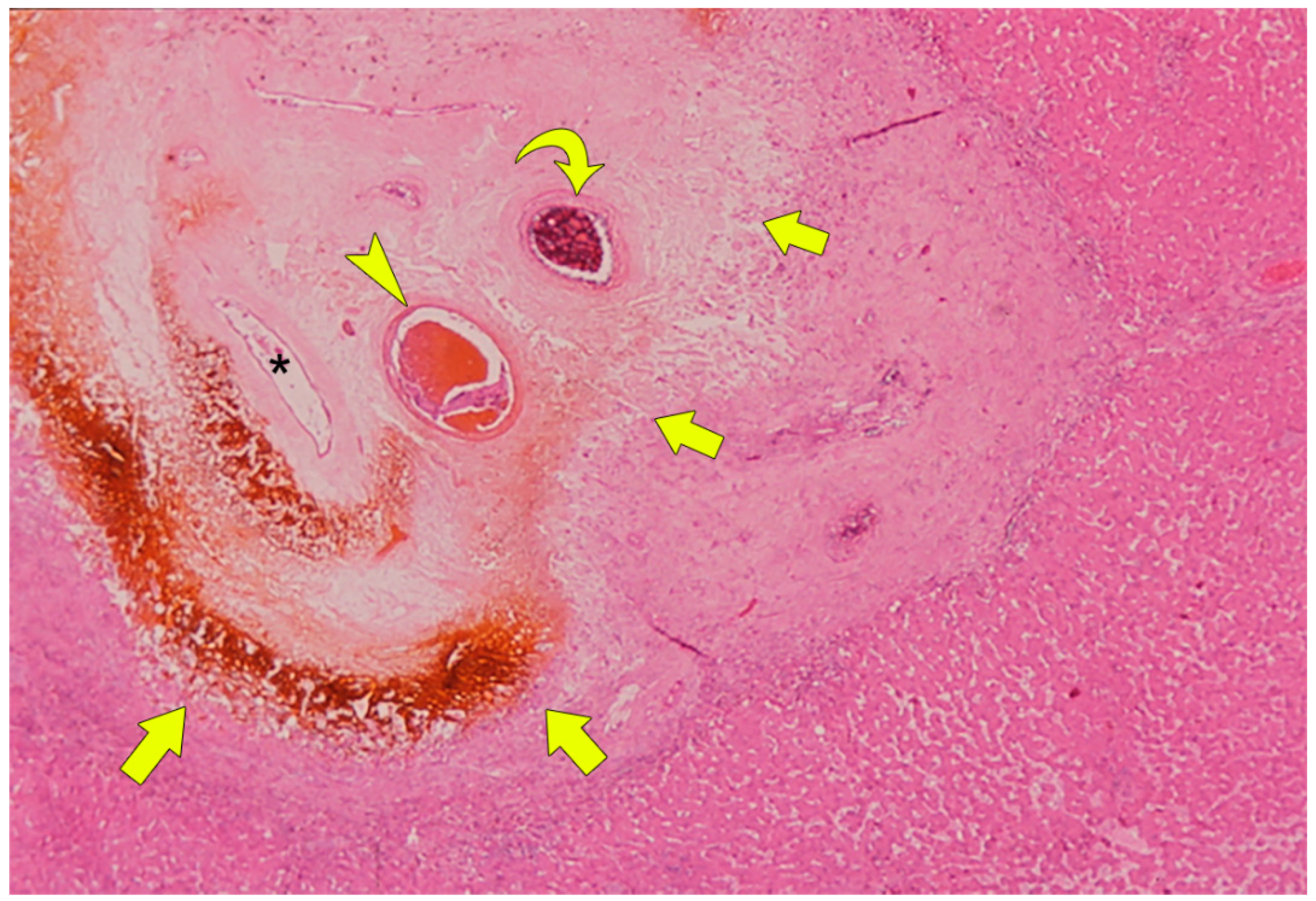
4. Pathophysiology of BDN after Transcatheter Therapy
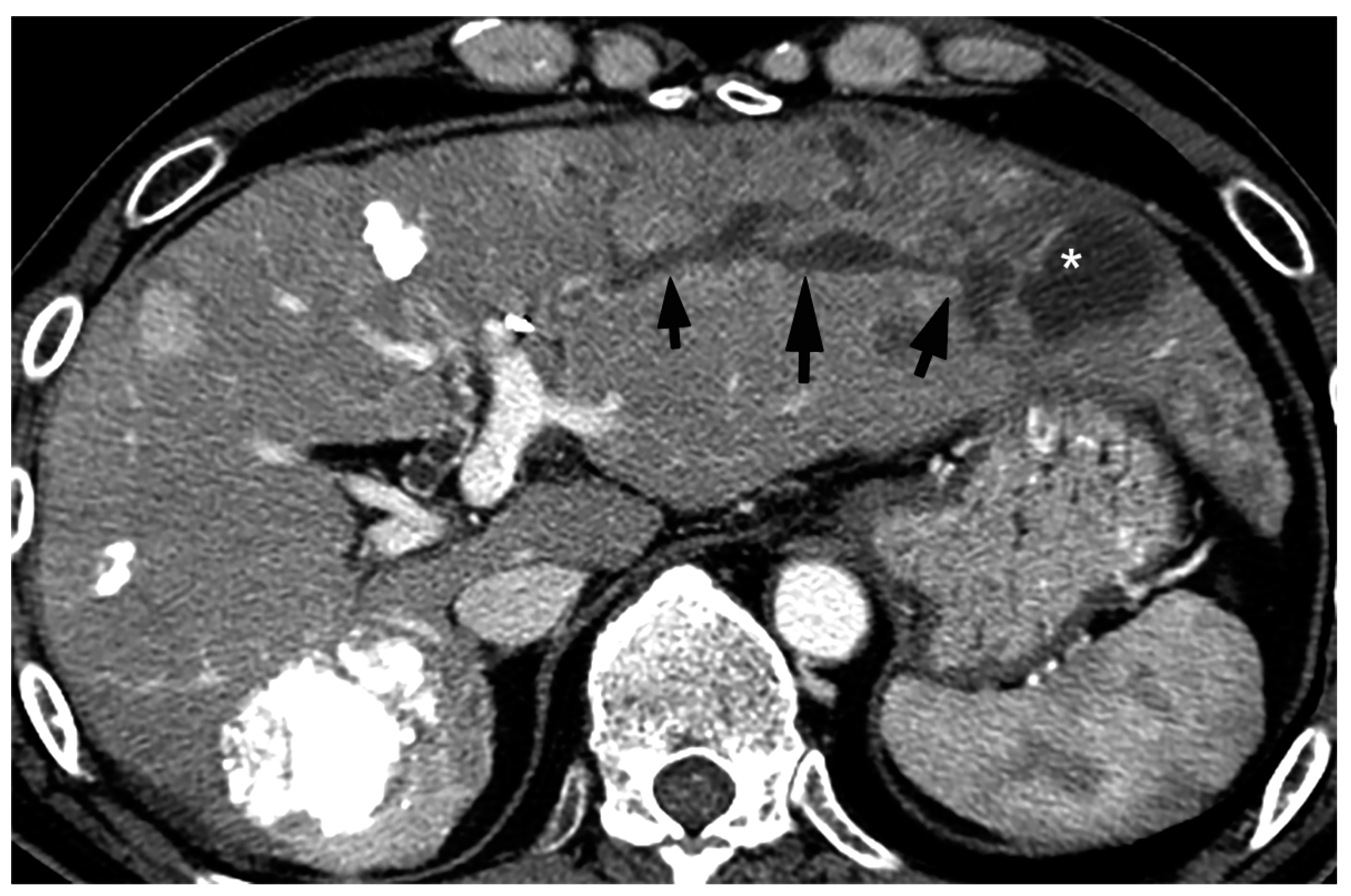
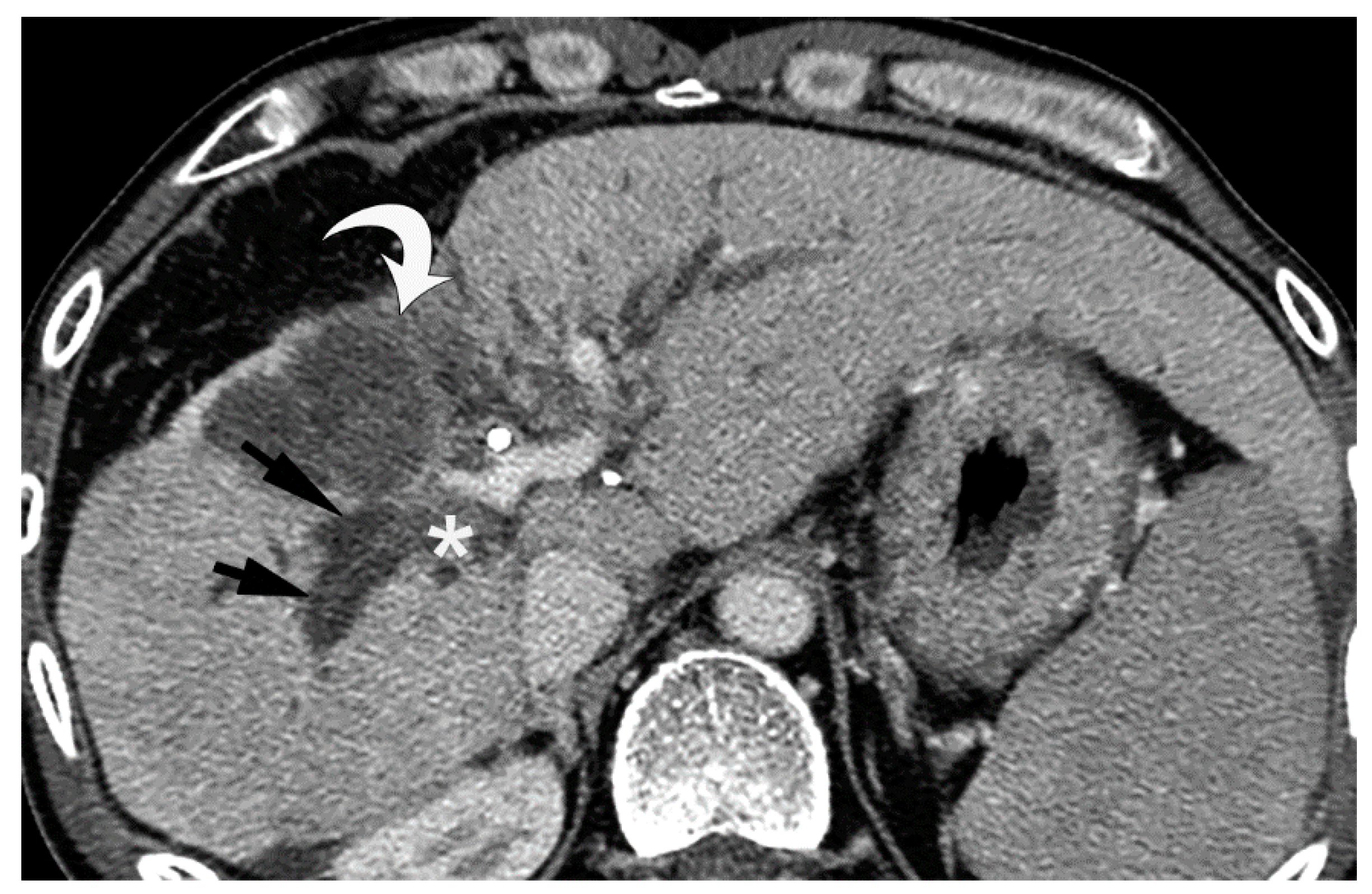
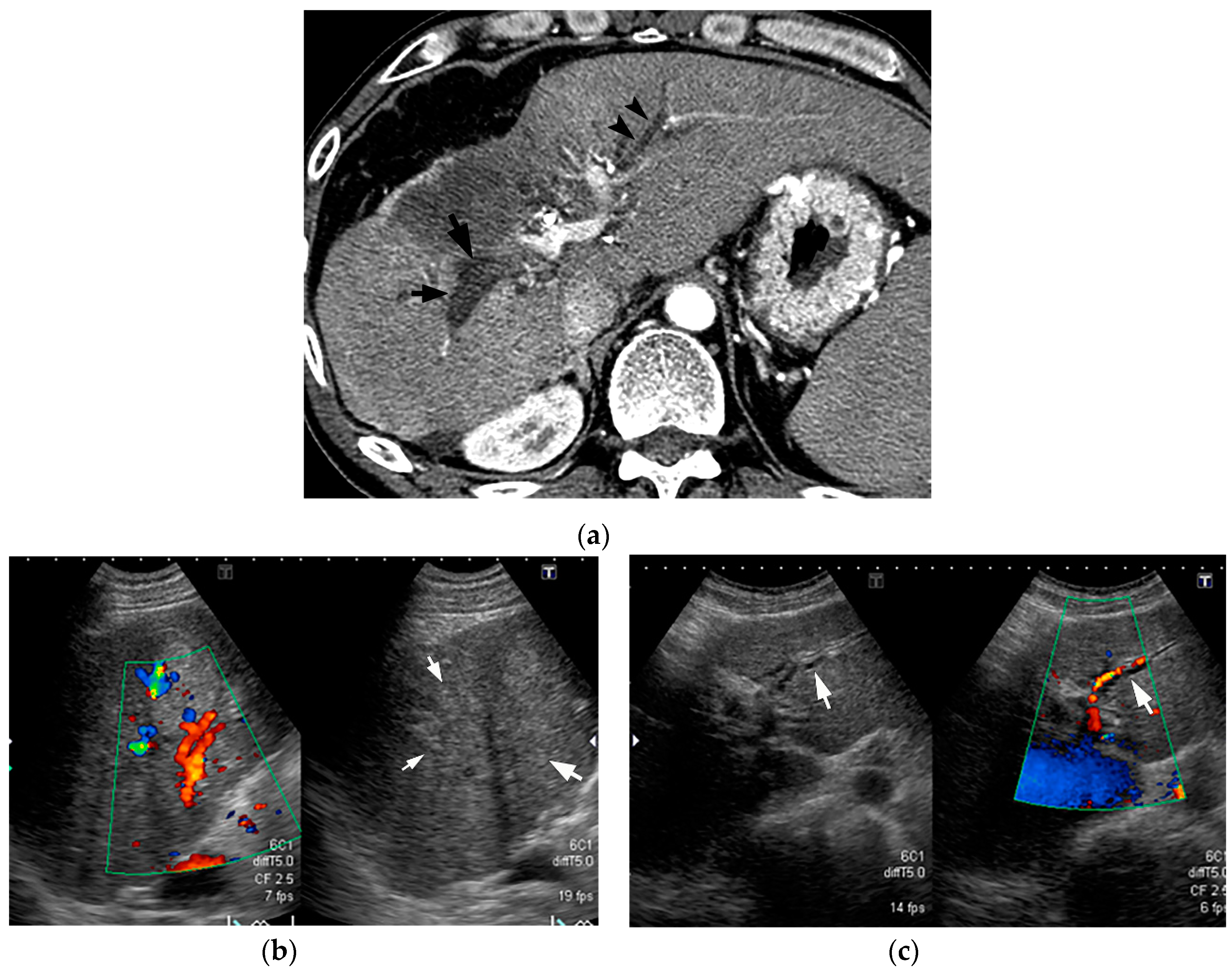
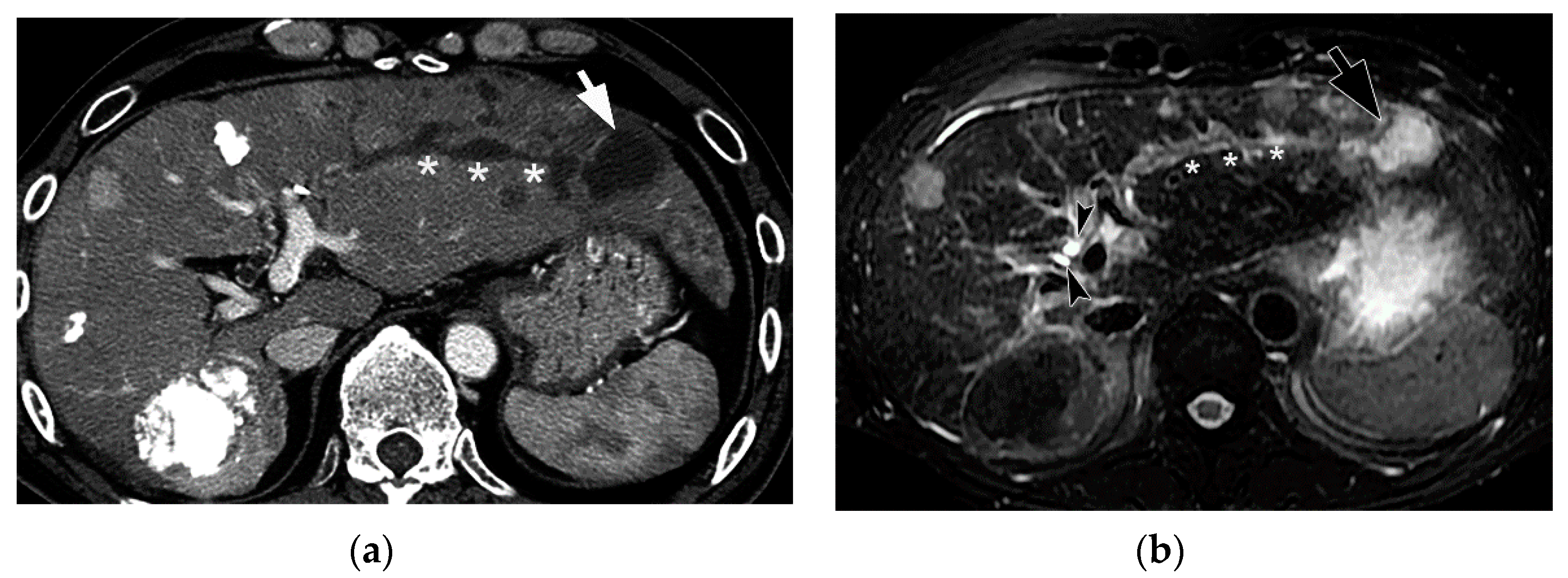
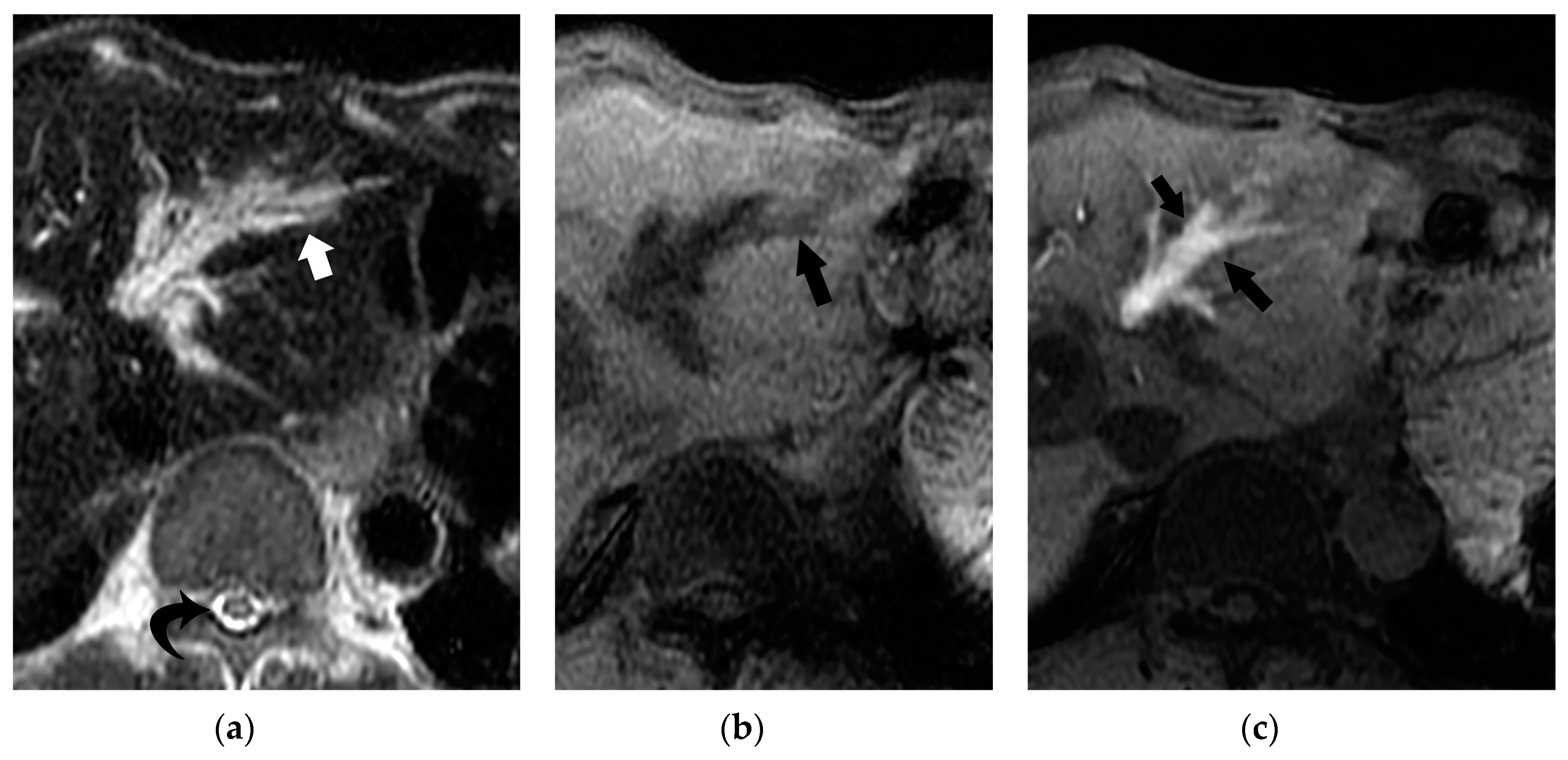
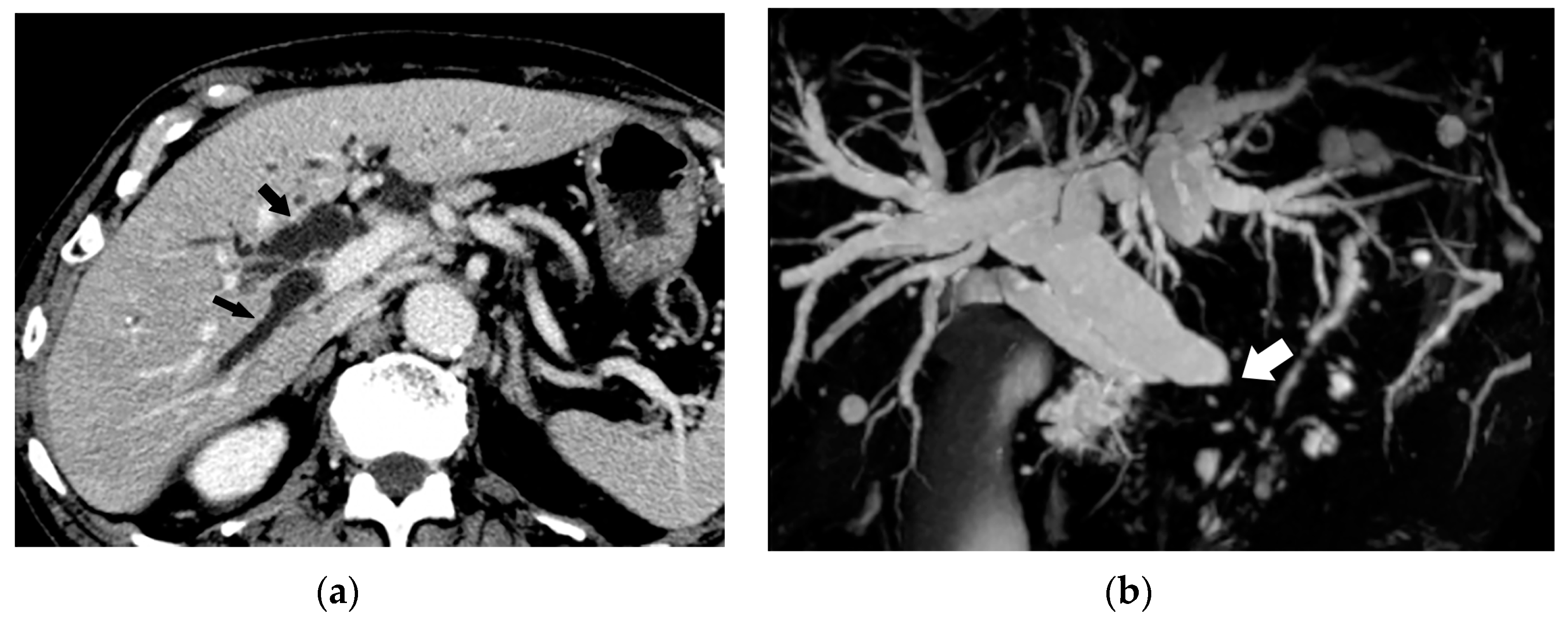
5. Imaging Findings of BDN
6. Progression of Bile Duct Necrosis on Sequential CT Images
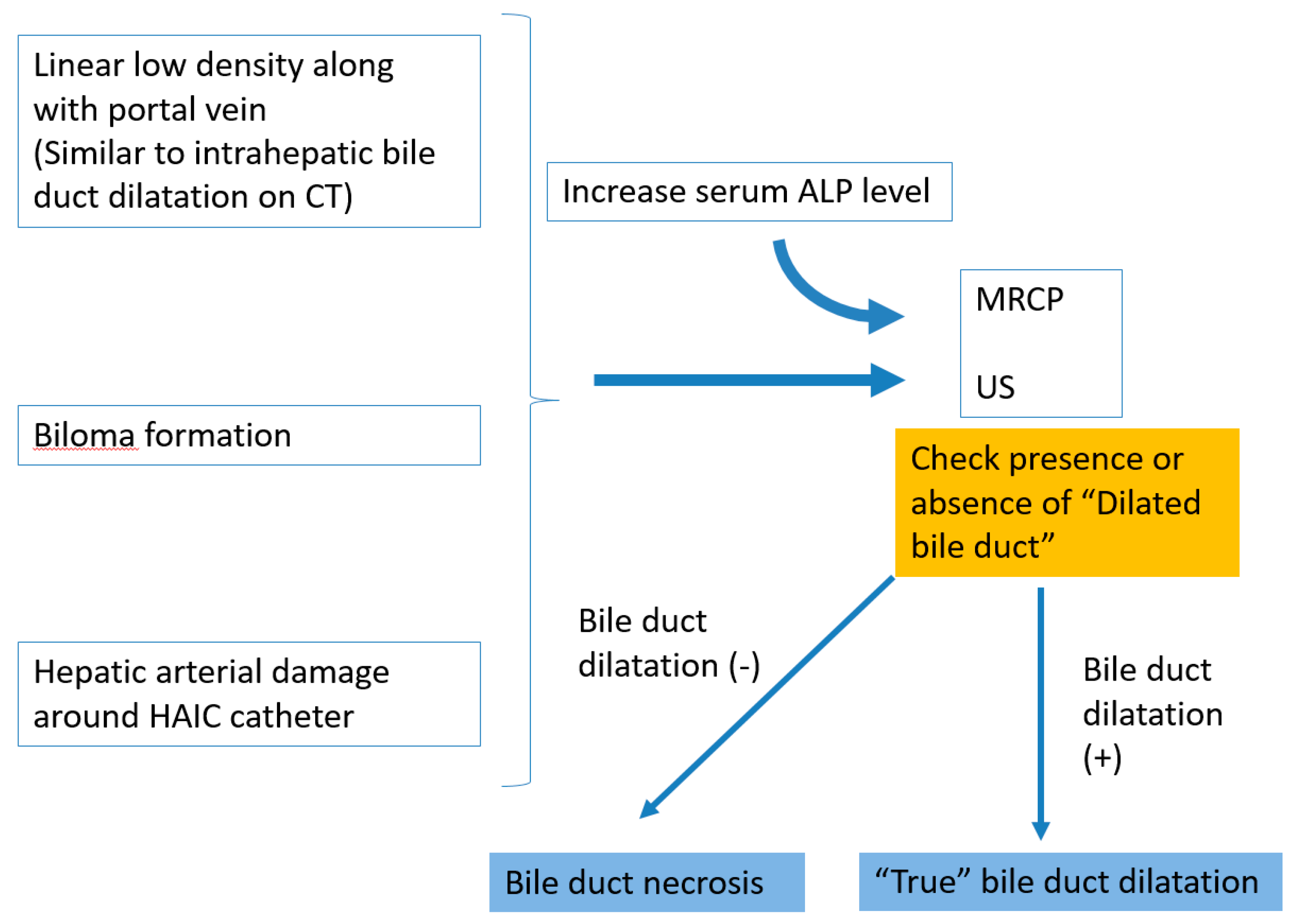
7. Mimickers of “Bile Duct Necrosis” on Imaging
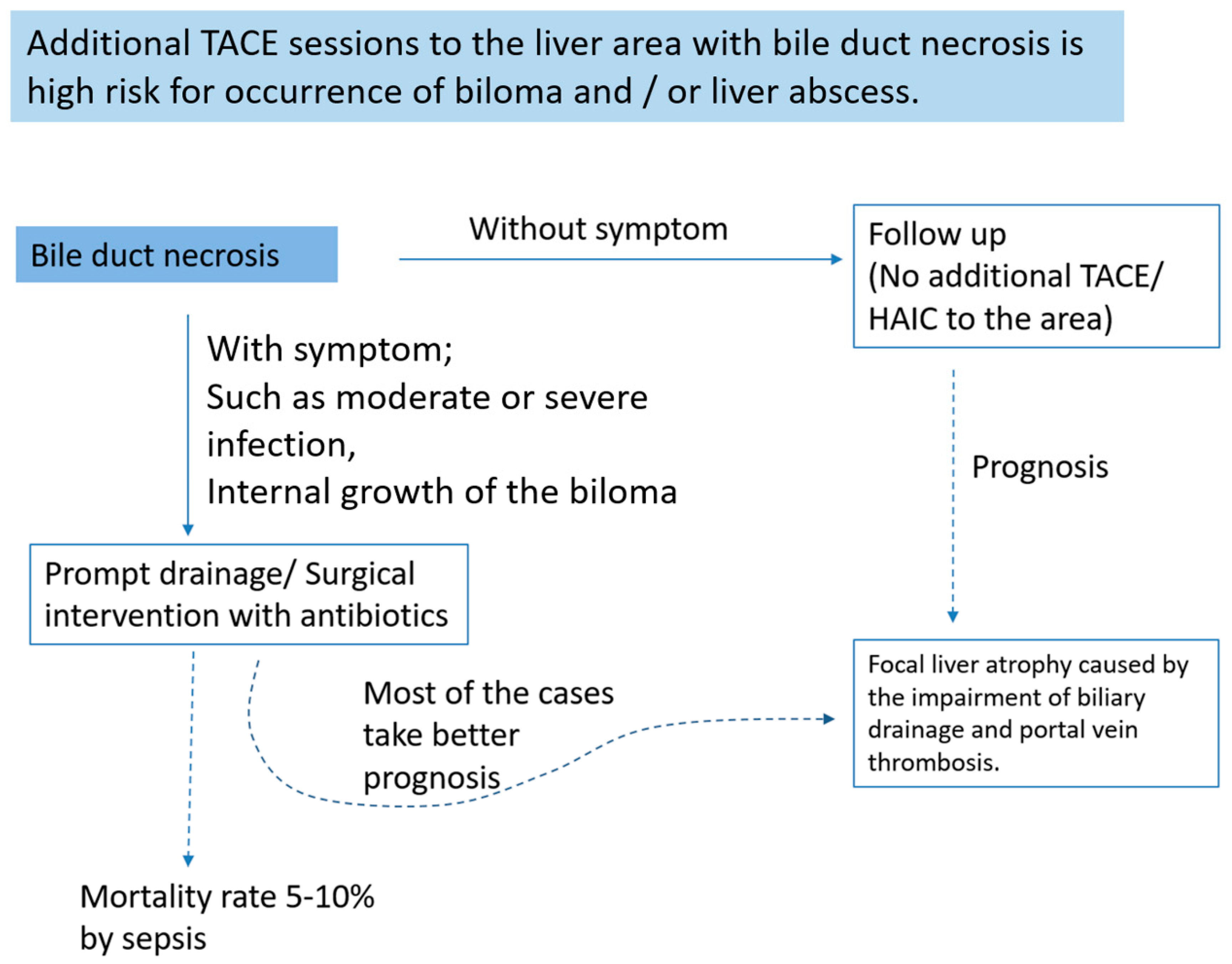
8. How to Treat Bile Duct Necrosis
9. Conclusions
Funding
Conflicts of Interest
References
- Deltenre, P.; Valla, D.C. Ischemic cholangiopathy. Semin. Liver Dis. 2008, 28, 235–246. [Google Scholar] [CrossRef]
- Makuuchi, M.; Sukigara, M.; Mori, T.; Kobayashi, J.; Yamazaki, S.; Hasegawa, H.; Moriyama, N.; Takayasu, K.; Hirohashi, S. Bile duct necrosis: Complication of transcatheter hepatic arterial embolization. Radiology 1985, 156, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Nakanuma, Y.; Terada, T.; Matsui, O. Postmortem survey of bile duct necrosis and biloma in hepatocellular carcinoma after transcatheter arterial chemoembolization therapy: Relevance to microvascular damages of peribiliary capillary plexus. Am. J. Gastroenterol. 1993, 88, 1410–1415. [Google Scholar] [PubMed]
- Atassi, B.; Bangash, A.K.; Lewandowski, R.J.; Ibrahim, S.; Kulik, L.; Mulcahy, M.F.; Murthy, R.; Ryu, R.K.; Sato, K.T.; Miller, F.H. Biliary sequelae following radioembolization with Yttrium-90 microspheres. J. Vasc. Interv. Radiol. 2008, 19, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Adam, G.; Knuechel, R.; Vorwerk, D.; Held, C.; Guenther, R.W. Tissue response of the biliary and digestive system of rabbits after MTBE infusion into the gallbladder. Investig. Radiol. 1990, 25, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Hohn, D.C.; Rayner, A.A.; Economou, J.S.; Ignoffo, R.J.; Lewis, B.J.; Stagg, R.J. Toxicities and complications of implanted pump hepatic arterial and intravenous floxuridine infusion. Cancer 1986, 57, 465–470. [Google Scholar] [CrossRef]
- Shrikhande, S.; Friess, H.; Kleeff, J.; Tempia, A. Bile duct infarction following intraarterial hepatic chemotherapy mimicking multiple liver metastasis: Report of a case and review of the literature. Dig. Dis. Sci. 2002, 47, 338–344. [Google Scholar] [CrossRef]
- Chung, J.; Yu, J.S.; Chung, J.J.; Kim, J.H.; Kim, K.W. Haemodynamic events and localised parenchymal changes following transcatheter arterial chemoembolisation for hepatic malignancy: Interpretation of imaging findings. Br. J. Radiol. 2010, 83, 71–81. [Google Scholar] [CrossRef]
- Alabdulghani, F.; Healy, G.M.; Cantwell, C.P. Radiological findings in ischaemic cholangiopathy. Clin. Radiol. 2020, 75, 161–168. [Google Scholar] [CrossRef]
- Sakamoto, I.; Iwanaga, S.; Nagaoki, K.; Matsuoka, Y.; Ashizawa, K.; Uetani, M.; Fukuda, T.; Okimoto, T.; Okudaira, S.; Omagari, K. Intrahepatic biloma formation (bile duct necrosis) after transcatheter arterial chemoembolization. Am. J. Roentgenol. 2003, 181, 79–87. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, M.; Duan, F.; Song, P.; Liu, F. Bile Duct Injury after Transcatheter Arterial Chemoembolization: Risk Factors and Clinical Implications. Hepatogastroenterology 2014, 61, 947–953. [Google Scholar] [PubMed]
- Zhang, B.; Guo, Y.; Wu, K.; Shan, H. Intrahepatic biloma following transcatheter arterial chemoembolization for hepatocellular carcinoma: Incidence, imaging features and management. Mol. Clin. Oncol. 2017, 6, 937–943. [Google Scholar] [CrossRef]
- Yu, J.S.; Kim, K.W.; Jeong, M.G.; Lee, D.H.; Park, M.S.; Yoon, S.W. Predisposing factors of bile duct injury after transcatheter arterial chemoembolization (TACE) for hepatic malignancy. Cardiovasc. Intervent. Radiol. 2002, 25, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Vadvala, H.V.; Arellano, R.S. Imaging and Intervention of Biliary Leaks and Bilomas. Dig. Dis. Interv. 2017, 1, 14–21. [Google Scholar]
- Strazzabosco, M.; Fabris, L. Functional anatomy of normal bile ducts. Anat. Rec. 2008, 291, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Kozaka, K.; Matsui, O.; Nakanuma, Y.; Uesaka, K.; Inoue, D.; Yoneda, N.; Yoshida, K.; Kitao, A.; Yokka, A. Peribiliary glands: Development, dysfunction, related conditions and imaging findings. Abdom. Radiol. 2020, 45, 416–436. [Google Scholar] [CrossRef]
- Cho, K.J.; Lunderquist, A. The peribiliary vascular plexus: The microvascular architecture of the bile duct in the rabbit and in clinical cases. Radiology 1983, 147, 357–364. [Google Scholar] [CrossRef]
- Kobayashi, S.; Nakanuma, Y.; Matsui, O. Intrahepatic peribiliary vascular plexus in various hepatobiliary diseases: A histological survey. Hum. Pathol. 1994, 25, 940–946. [Google Scholar] [CrossRef]
- Takasaki, S.; Hano, H. Three-dimensional observations of the human hepatic artery (Arterial system in the liver). J. Hepatol. 2001, 34, 455–466. [Google Scholar] [CrossRef]
- Gaudio, E.; Franchitto, A.; Pannarale, L.; Carpino, G.; Alpini, G.; Francis, H.; Glaser, S.; Alvaro, D.; Onori, P. Cholangiocytes and blood supply. World J. Gastroenterol. 2006, 12, 3546–3552. [Google Scholar] [CrossRef]
- Kobayashi, S.; Nakanuma, Y.; Matsui, O. Histopathology of portal tracts in livers after transcatheter arterial chemo-embolization therapy for hepatocellular carcinoma. J. Gastroenterol. Hepatol. 1994, 9, 45–54. [Google Scholar] [CrossRef]
- Rao, R.K.; Samak, G. Bile duct epithelial tight junctions and barrier function. Tissue Barriers 2013, 1, e25718. [Google Scholar] [CrossRef]
- Perez, M.J.; Briz, O. Bile-acid-induced cell injury and protection. World J. Gastroenterol. 2009, 15, 1677–1689. [Google Scholar] [CrossRef]
- Xia, X.; Francis, H.; Glaser, S.; Alpini, G.; LeSage, G. Bile acid interactions with cholangiocytes. World J. Gastroenterol. 2006, 12, 3553–3563. [Google Scholar] [CrossRef] [PubMed]
- Beuers, U.; Hohenester, S.; de Buy Wenniger, L.J.; Kremer, A.E.; Jansen, P.L.; Elferink, R.P. The biliary HCO(3)(-) umbrella: A unifying hypothesis on pathogenetic and therapeutic aspects of fibrosing cholangiopathies. Hepatology 2010, 52, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Melero, S.; Spirlì, C.; Zsembery, A.; Medina, J.F.; Joplin, R.E.; Duner, E.; Zuin, M.; Neuberger, J.M.; Prieto, J.; Strazzabosco, M. Defective regulation of cholangiocyte Cl-/HCO3(-) and Na+/H+ exchanger activities in primary biliary cirrhosis. Hepatology 2002, 35, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, S.; Bie, P.; Li, X.; Dong, J. Endogenous bile salts are associated with bile duct injury in the rat liver transplantation model. Transplantation 2009, 87, 330–339. [Google Scholar] [CrossRef]
- Deltenre, P.; Valla, D.C. Ischemic cholangiopathy. J. Hepatol. 2006, 44, 806–817. [Google Scholar] [CrossRef] [PubMed]
- Phongkitkarun, S.; Kobayashi, S.; Varavithya, V.; Huang, X.; Curley, S.A.; Charnsangavej, C. Bile duct complications of hepatic arterial infusion chemotherapy evaluated by helical CT. Clin. Radiol. 2005, 60, 700–709. [Google Scholar] [CrossRef]
- Cameron, A.M.; Busuttil, R.W. Ischemic cholangiopathy after liver transplantation. Hepatobiliary Pancreat Dis. Int. 2005, 4, 495–501. [Google Scholar]
- Cheng, L.; Zhao, L.; Li, D.; Liu, Z.; Chen, G.; Tian, F.; Li, X.; Wang, S. Role of cholangiocyte bile Acid transporters in large bile duct injury after rat liver transplantation. Transplantation 2010, 90, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Boteon, A.P.C.d.; Boteon, Y.L.; Vinuela, E.F.; Derosas, C.; Mergental, H.; Isaac, J.R.; Muiesan, P.; Mehzard, H.; Ma, Y.T.; Shah, T. The impact of transarterial chemoembolization induced complications on outcomes after liver transplantation: A propensity-matched study. Clin. Transplant. 2018, 32, e13255. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.H.; Li, C.H.; Chen, Y.L.; Song, J.N.; Zhang, A.Q.; Zhou, C. Effects of partial portal vein arterialization on the hilar bile duct in a rat model. Hepatobiliary Pancreat Dis. Int. 2011, 10, 533–538. [Google Scholar] [CrossRef]
- Keppler, U.; Moussavian, M.R.; Jeanmonod, P.; Strowitzki, M.J.; Wagner, M.; Scheuer, C.; Menger, M.D.; von Heesen, M. Neither Isolated Hepatic Arterial Clamping nor Hepatic Arterial Ligation Induce Ischemic Type Biliary Lesions in Rats. Ann. Transplant 2016, 21, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Miyayama, S.; Yamashiro, M.; Hashimoto, M.; Hashimoto, N.; Ikuno, M.; Okumura, K.; Yoshida, M.; Matsui, O. Blood supply of the main bile duct from the caudate artery and medial subsegmental artery of the hepatic artery: Evaluation using images obtained during transcatheter arterial chemoembolization for hepatocellular carcinoma. Hepatol. Res. 2013, 43, 1175–1181. [Google Scholar] [CrossRef]
- Yu, J.S.; Kim, K.W.; Park, M.S.; Yoon, S.W. Bile duct injuries leading to portal vein obliteration after transcatheter arterial chemoembolization in the liver: CT findings and initial observations. Radiology 2001, 221, 429–436. [Google Scholar] [CrossRef]
- Spina, J.C.; Ulla, M.; Yeyati, E.L.; Kucharczyk, M.C.; Irusta, H.; Savluk, J.L.; García-Mónaco, R. MDCT findings after hepatic chemoembolization with DC-beads: What the radiologist needs to know. Abdom. Imaging 2013, 38, 778–784. [Google Scholar] [CrossRef]
- Kitao, A.; Zen, Y.; Matsui, O.; Gabata, T.; Kobayashi, S.; Koda, W.; Kozaka, K.; Yoneda, N.; Yamashita, T.; Kaneko, S.; et al. Hepatocellular carcinoma: Signal intensity at gadoxetic acid-enhanced MR Imaging—correlation with molecular transporters and histopathologic features. Radiology 2010, 256, 817–826. [Google Scholar] [CrossRef]
- Geisel, D.; Lüdemann, L.; Hamm, B.; Denecke, T. Imaging-Based Liver Function Tests--Past, Present and Future. Rofo 2015, 187, 863–871. [Google Scholar] [CrossRef]
- Cieszanowski, A.; Stadnik, A.; Lezak, A.; Maj, E.; Zieniewicz, K.; Rowinska-Berman, K.; Grudzinski, I.P.; Krawczyk, M.; Rowiński, O. Detection of active bile leak with Gd-EOB-DTPA enhanced MR cholangiography: Comparison of 20–25 min delayed and 60–180 min delayed images. Eur. J. Radiol. 2013, 82, 2176–2182. [Google Scholar] [CrossRef]
- Margini, C.; Berzigotti, A. Portal vein thrombosis: The role of imaging in the clinical setting. Dig. Liver Dis. 2017, 49, 113–120. [Google Scholar] [CrossRef]
- Elsayes, K.M.; Shaaban, A.M.; Rothan, S.M.; Javadi, S.; Madrazo, B.L.; Castillo, R.P.; Casillas, V.J.; Menias, C.O. A Comprehensive Approach to Hepatic Vascular Disease. Radiographics 2017, 37, 813–836. [Google Scholar] [CrossRef] [PubMed]
- Ide, T.; Ohno, Y.; Nakanuma, Y.; Kanematsu, T. Early development of cavernomatous vasculatures in portal venous thrombosis: Morphometric kinetics in rabbit model. Hepatol. Res. 2003, 27, 136–142. [Google Scholar] [CrossRef]
- Modha, K. Clinical Approach to Patients with Obstructive Jaundice. Tech. Vasc. Interv. Radiol. 2015, 18, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Hoshiba, K.; Matsui, O.; Kadoya, M.; Yoshikawa, J.; Gabata, T.; Terayama, N.; Takashima, T. Peribiliary cysts in cirrhotic liver: Observation on computed tomography. Abdom. Imaging 1996, 21, 228–232. [Google Scholar] [CrossRef]
- Brancatelli, G.; Federle, M.P.; Vilgrain, V.; Vullierme, M.P.; Marin, D.; Lagalla, R. Fibropolycystic liver disease: CT and MR imaging findings. Radiographics 2005, 25, 659–670. [Google Scholar] [CrossRef]
- Choi, B.I.; Yeon, K.M.; Kim, S.H.; Han, M.C. Caroli disease: Central dot sign in CT. Radiology 1990, 174, 161–163. [Google Scholar] [CrossRef]
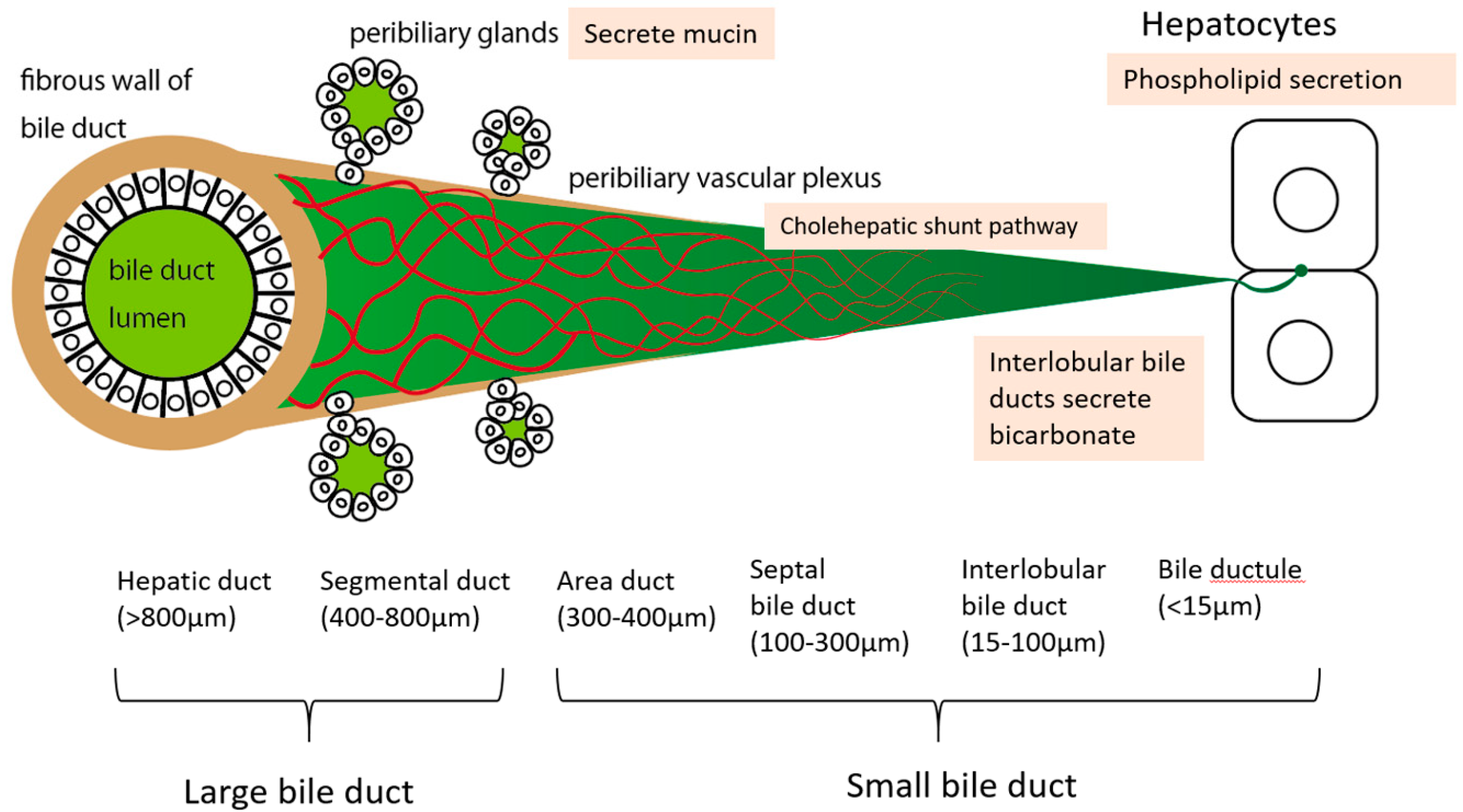









| Mechanism | Notes |
|---|---|
| Cholehepatic shunt pathway | Bile duct epithelium absorbs BAs through the BA transporter and excretes them into the peribiliary vascular plexus. They are then transported to hepatocytes |
| Mucin secretion from peribiliary glands | Limited to large bile ducts which have peribiliary glands around the duct wall |
| Bicarbonate secretion by bile duct epithelium (HCO3− umbrella) | Limited to small bile ducts |
| Phospholipid secretion from hepatocytes | May depend on hepatocyte functional state |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, S.; Kozaka, K.; Gabata, T.; Matsui, O.; Koda, W.; Okuda, M.; Okumura, K.; Sugiura, T.; Ogi, T. Pathophysiology and Imaging Findings of Bile Duct Necrosis: A Rare but Serious Complication of Transarterial Therapy for Liver Tumors. Cancers 2020, 12, 2596. https://doi.org/10.3390/cancers12092596
Kobayashi S, Kozaka K, Gabata T, Matsui O, Koda W, Okuda M, Okumura K, Sugiura T, Ogi T. Pathophysiology and Imaging Findings of Bile Duct Necrosis: A Rare but Serious Complication of Transarterial Therapy for Liver Tumors. Cancers. 2020; 12(9):2596. https://doi.org/10.3390/cancers12092596
Chicago/Turabian StyleKobayashi, Satoshi, Kazuto Kozaka, Toshifumi Gabata, Osamu Matsui, Wataru Koda, Miho Okuda, Kenichiro Okumura, Takumi Sugiura, and Takahiro Ogi. 2020. "Pathophysiology and Imaging Findings of Bile Duct Necrosis: A Rare but Serious Complication of Transarterial Therapy for Liver Tumors" Cancers 12, no. 9: 2596. https://doi.org/10.3390/cancers12092596
APA StyleKobayashi, S., Kozaka, K., Gabata, T., Matsui, O., Koda, W., Okuda, M., Okumura, K., Sugiura, T., & Ogi, T. (2020). Pathophysiology and Imaging Findings of Bile Duct Necrosis: A Rare but Serious Complication of Transarterial Therapy for Liver Tumors. Cancers, 12(9), 2596. https://doi.org/10.3390/cancers12092596





