N-MYC Downstream Regulated Gene 4 (NDRG4), a Frequent Downregulated Gene through DNA Hypermethylation, plays a Tumor Suppressive Role in Esophageal Adenocarcinoma
Simple Summary
Abstract
1. Introduction
2. Results
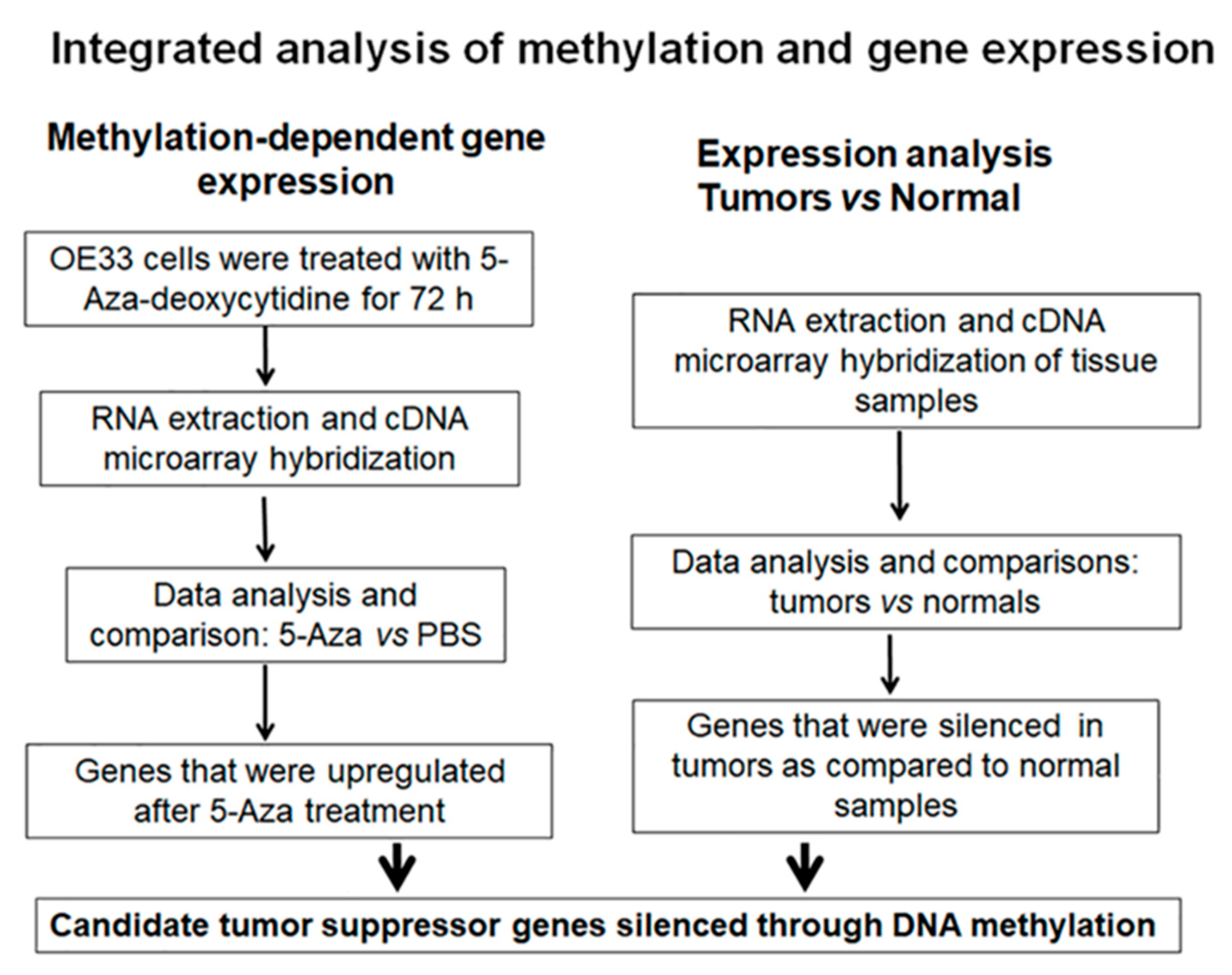
2.1. Integrated Gene Expression Analysis Identified NDRG4 as One of the Top Candidates Silenced by DNA Methylation in EAC
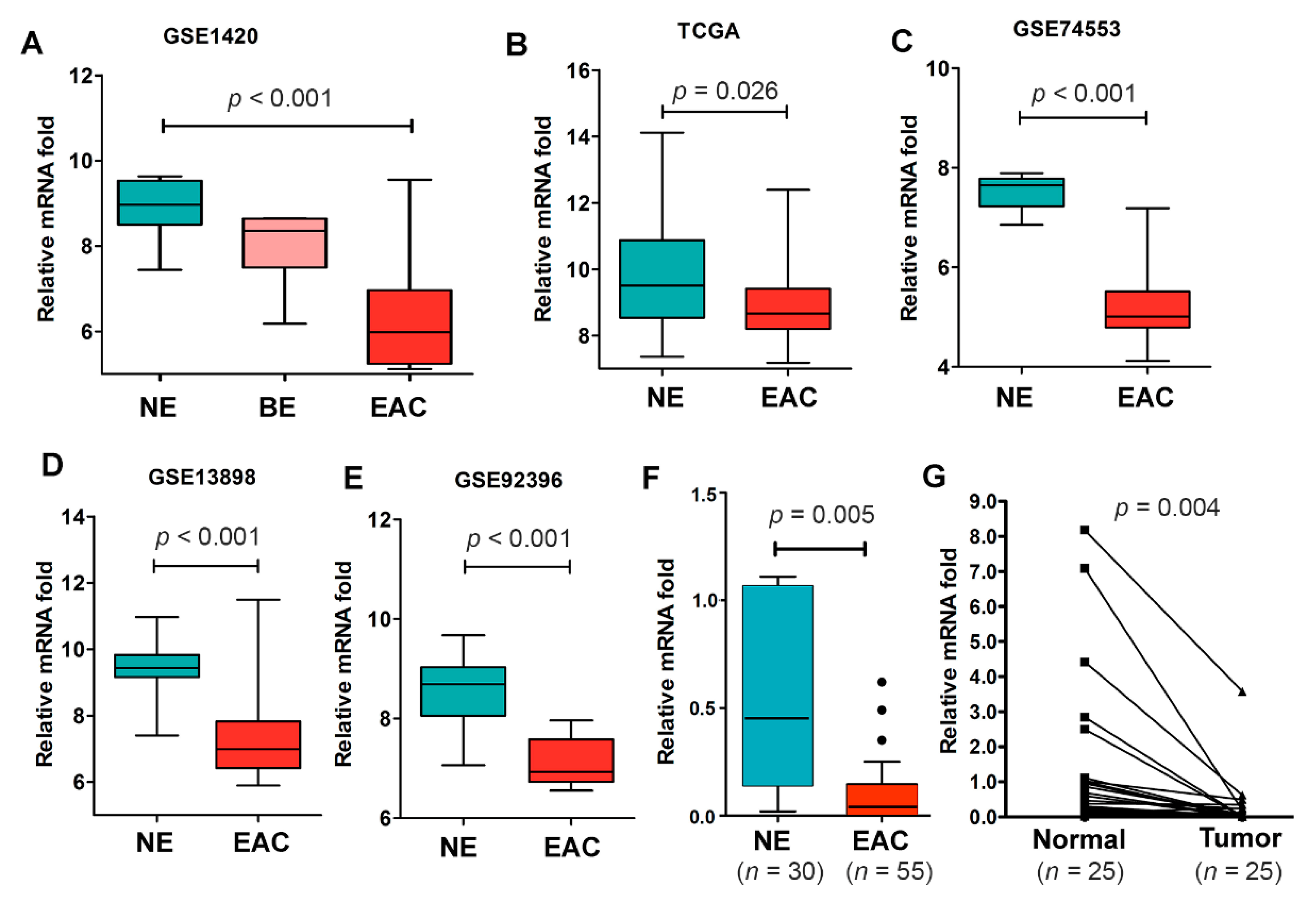
2.2. NDRG4 Gene Expression Is Significantly Downregulated in Barrett’s Esophagus and EACs
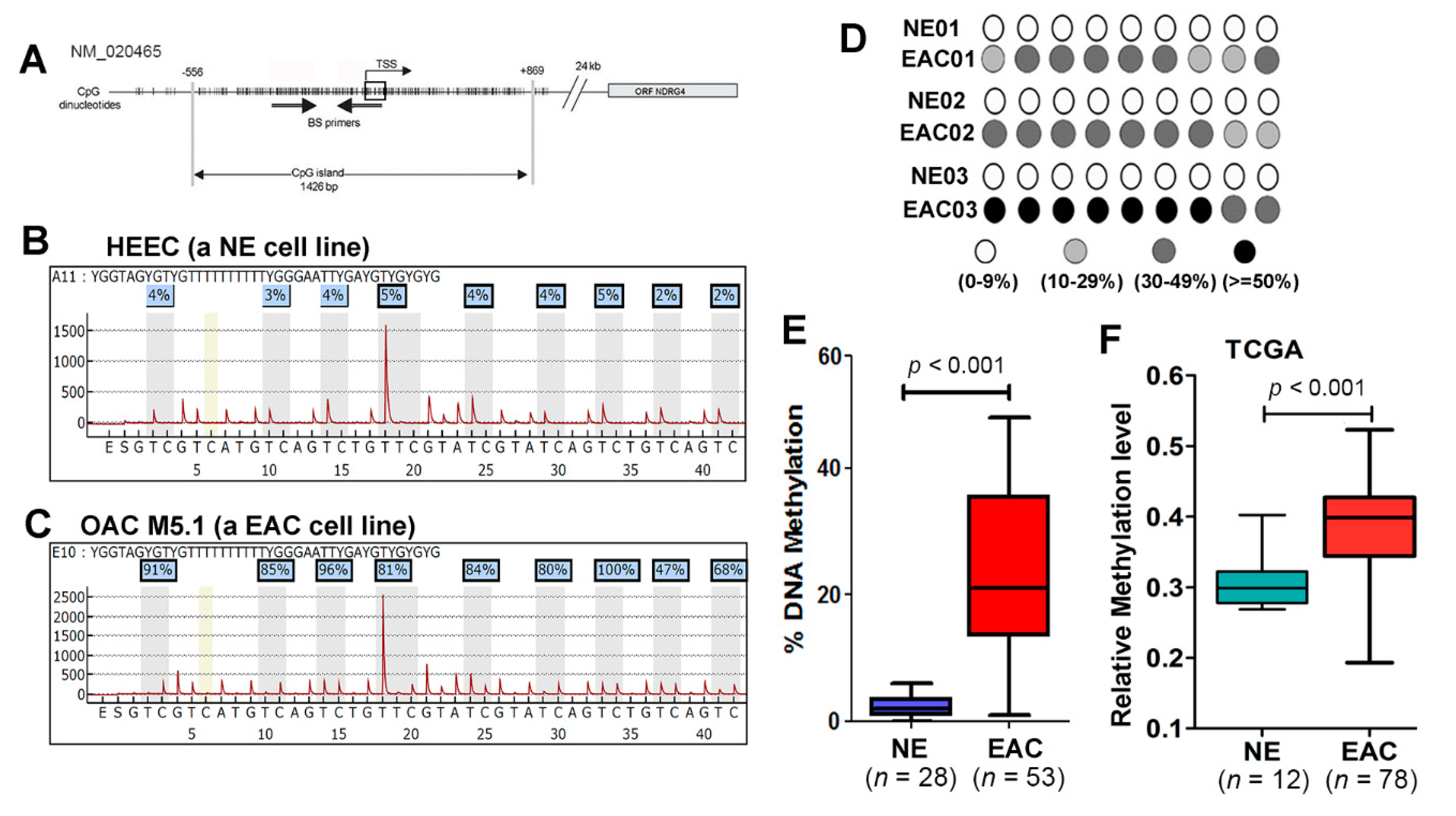
2.3. NDRG4 Promoter Is Hypermethylated in Barrett’s Esophagus and EACs
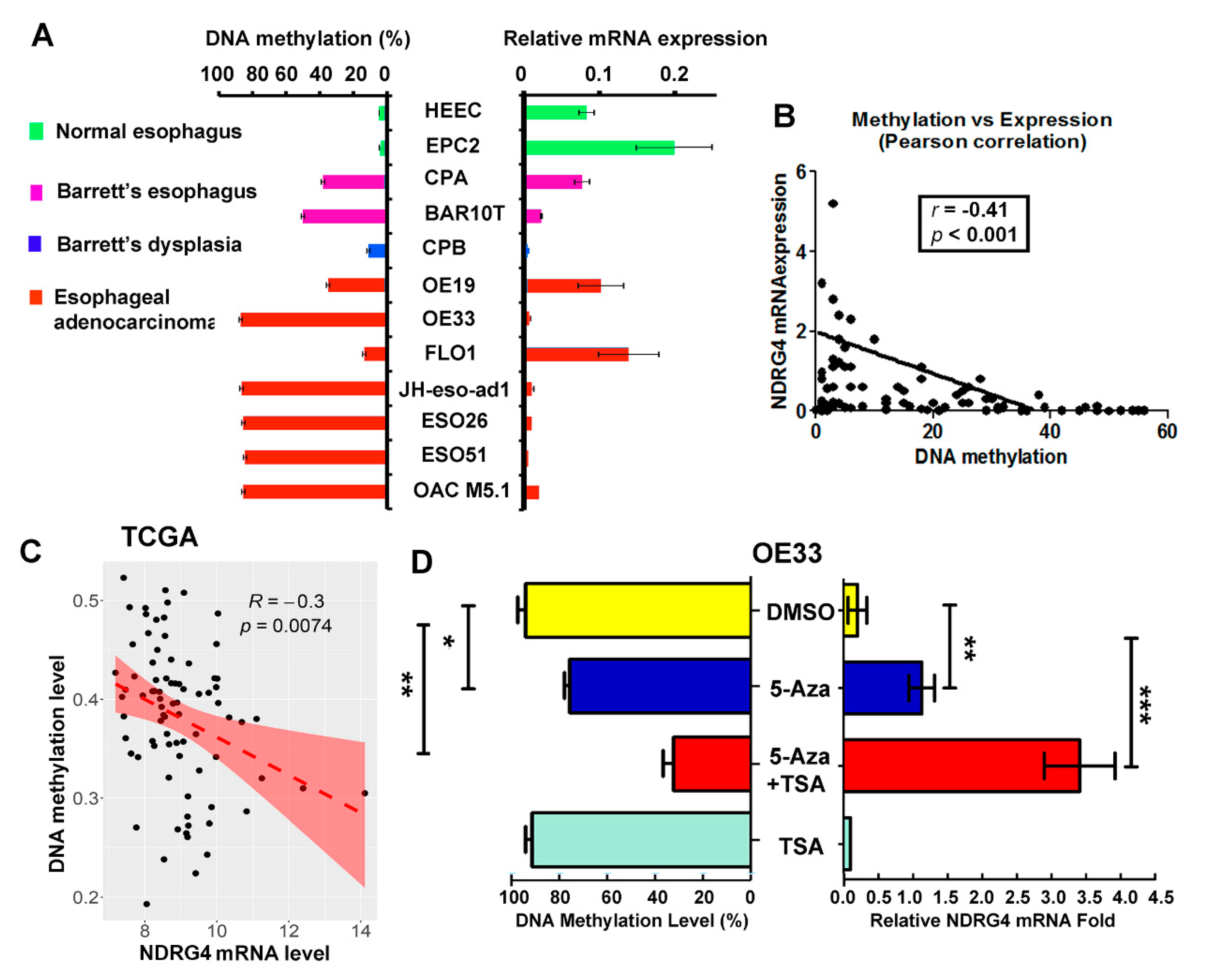
2.4. NDRG4 Promoter Methylation Level Is Inversely Correlated with NDRG4 Gene Expression
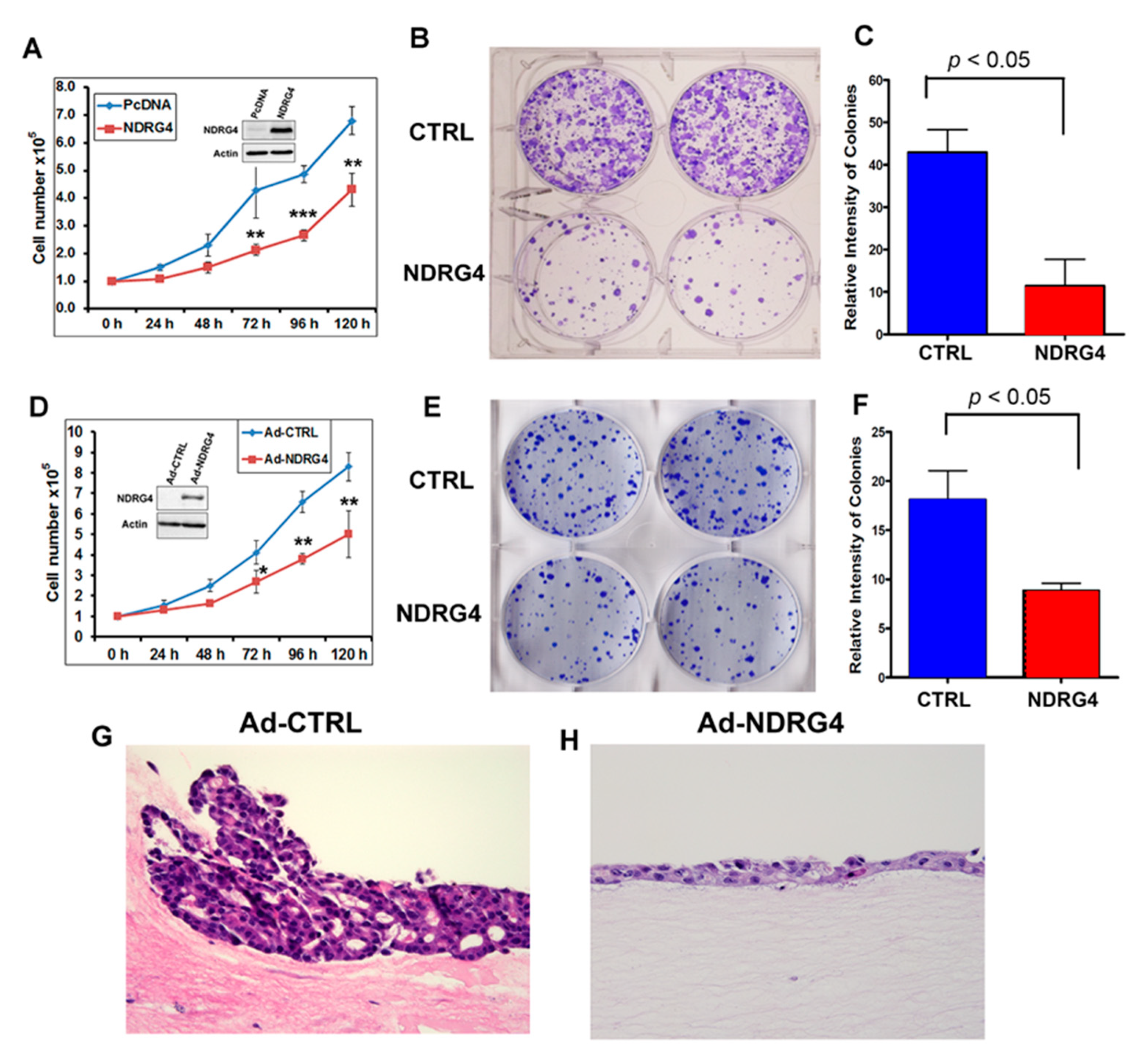
2.5. Reconstitution of NDRG4 Expression Inhibited Tumor Cells Growth
2.6. NDRG4 Is Involved in Cell Cycle Regulation through Downregulating Cyclin D1, CDK4, and CDK6 Expression in EAC Cells
2.7. Reconstitution of NDRG4 Inhibited EAC Cell Proliferation
3. Discussion
4. Methods and Materials
4.1. Cell Lines
4.2. Tissue Samples
4.3. Integrated DNA Methylation-Dependent Gene Expression Analysis
4.4. Quantitative Real Time RT-PCR (qPCR)
4.5. DNA Bisulfite Modification and DNA Methylation Analyses
4.6. 5-Aza-2′ Deoxycytidine (5-Aza) and Trichostatin A (TSA) Treatment
4.7. Cloning and Construction of NDRG4 Expression Plasmids
4.8. Determination of Cell Growth Curve
4.9. Colony Formation Assay
4.10. EdU Cell Proliferation Assay
4.11. Western Blotting Analysis
4.12. 3D Organotypic Cell Culture
4.13. Gene Expression Databases
4.14. Gene Set Enrichment Analysis
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Altorki, N.K.; Skinner, D.B. Adenocarcinoma in Barrett’s esophagus. Semin. Surg. Oncol. 1990, 6, 274–278. [Google Scholar] [CrossRef] [PubMed]
- DeVault, K.R. Epidemiology and significance of Barrett’s esophagus. Dig. Dis. 2000, 18, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Caygill, C.P.; Reed, P.I.; Johnston, B.J.; Hill, M.J.; Ali, M.H.; Levi, S. A single centre’s 20 years’ experience of columnar-lined (Barrett’s) oesophagus diagnosis. Eur. J. Gastroenterol. Hepatol. 1999, 11, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Pera, M. Trends in incidence and prevalence of specialized intestinal metaplasia, barrett’s esophagus, and adenocarcinoma of the gastroesophageal junction. World J. Surg. 2003, 27, 999–1008. [Google Scholar] [CrossRef]
- Falk, G.W. Barrett’s esophagus. Gastrointest. Endosc. Clin. N. Am. 1994, 4, 773–789. [Google Scholar] [CrossRef]
- Hoff, S.J.; Sawyers, J.L.; Blanke, C.D.; Choy, H.; Stewart, J.R. Prognosis of adenocarcinoma arising in Barrett’s esophagus. Ann. Thorac. Surg. 1998, 65, 176–180. [Google Scholar] [CrossRef]
- Falk, G.W. Gastroesophageal reflux disease and Barrett’s esophagus. Endoscopy 2001, 33, 109–118. [Google Scholar]
- Greenberg, M.V.C.; Bourc’his, D. The diverse roles of DNA methylation in mammalian development and disease. Nat. Rev. Mol. Cell Biol. 2019, 20, 590–607. [Google Scholar] [CrossRef]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef]
- Edwards, J.R.; Yarychkivska, O.; Boulard, M.; Bestor, T.H. DNA methylation and DNA methyltransferases. Epigenetics Chromatin 2017, 10, 23. [Google Scholar] [CrossRef]
- Pfeifer, G.P. Defining Driver DNA methylation changes in human cancer. Int. J. Mol. Sci. 2018, 19, 1166. [Google Scholar] [CrossRef]
- Auerkari, E.I. Methylation of tumor suppressor genes p16(INK4a), p27(Kip1) and E-cadherin in carcinogenesis. Oral. Oncol. 2006, 42, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Kulis, M.; Esteller, M. DNA methylation and cancer. Adv. Genet. 2010, 70, 27–56. [Google Scholar] [CrossRef] [PubMed]
- Kaz, A.M.; Grady, W.M.; Stachler, M.D.; Bass, A.J. Genetic and Epigenetic Alterations in Barrett’s Esophagus and Esophageal Adenocarcinoma. Gastroenterol. Clin. N. Am. 2015, 44, 473–489. [Google Scholar] [CrossRef] [PubMed]
- Hardie, L.J.; Darnton, S.J.; Wallis, Y.L.; Chauhan, A.; Hainaut, P.; Wild, C.P.; Casson, A.G. p16 expression in Barrett’s esophagus and esophageal adenocarcinoma: Association with genetic and epigenetic alterations. Cancer Lett. 2005, 217, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Polineni, R.; Hussein, Z.; Vigoda, I.; Bhagat, T.D.; Bhattacharyya, S.; Maitra, A.; Verma, A. Role of epigenetic alterations in the pathogenesis of Barrett’s esophagus and esophageal adenocarcinoma. Int. J. Clin. Exp. Pathol. 2012, 5, 382–396. [Google Scholar] [PubMed]
- Peng, D.; Hu, T.L.; Jiang, A.; Washington, M.K.; Moskaluk, C.A.; Schneider-Stock, R.; El-Rifai, W. Location-specific epigenetic regulation of the metallothionein 3 gene in esophageal adenocarcinomas. PLoS ONE 2011, 6, e22009. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.F.; Razvi, M.; Chen, H.; Washington, K.; Roessner, A.; Schneider-Stock, R.; El-Rifai, W. DNA hypermethylation regulates the expression of members of the Mu-class glutathione S-transferases and glutathione peroxidases in Barrett’s adenocarcinoma. Gut 2009, 58, 5–15. [Google Scholar] [CrossRef]
- Fitzgerald, R.C. Epigenetic changes wipe out protective mechanisms in Barrett’s oesophagus. Gut 2009, 58, 1–2. [Google Scholar] [CrossRef]
- Melotte, V.; Qu, X.; Ongenaert, M.; van Criekinge, W.; de Bruine, A.P.; Baldwin, H.S.; van Engeland, M. The N-myc downstream regulated gene (NDRG) family: Diverse functions, multiple applications. FASEB J. 2010, 24, 4153–4166. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, S.; Zhang, W.; Liu, X.; Shi, H.; Che, H.; Wang, W.; Li, F.; Yao, L. Human differentiation-related gene NDRG1 is a Myc downstream-regulated gene that is repressed by Myc on the core promoter region. Gene 2008, 417, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Lorentzen, A.; Vogel, L.K.; Lewinsky, R.H.; Saebo, M.; Skjelbred, C.F.; Godiksen, S.; Hoff, G.; Tveit, K.M.; Lothe, I.M.; Ikdahl, T.; et al. Expression of NDRG2 is down-regulated in high-risk adenomas and colorectal carcinoma. BMC Cancer 2007, 7, 192. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Li, N.; Li, S.; Chen, C.; Wang, W.; Xu, C.; Zhang, J.; Jin, H.; Zhang, H.; Zhao, H.; et al. Expression of NDRG2 in esophageal squamous cell carcinoma. Cancer Sci. 2010, 101, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Lorentzen, A.; Lewinsky, R.H.; Bornholdt, J.; Vogel, L.K.; Mitchelmore, C. Expression profile of the N-myc Downstream Regulated Gene 2 (NDRG2) in human cancers with focus on breast cancer. BMC Cancer 2011, 11, 14. [Google Scholar] [CrossRef]
- Kim, Y.J.; Yoon, S.Y.; Kim, J.T.; Song, E.Y.; Lee, H.G.; Son, H.J.; Kim, S.Y.; Cho, D.; Choi, I.; Kim, J.H.; et al. NDRG2 expression decreases with tumor stages and regulates TCF/beta-catenin signaling in human colon carcinoma. Carcinogenesis 2009, 30, 598–605. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Y.; Liu, J.; Li, B.; Xu, Y.; Li, C.; Xu, Q.; Liu, G.; Chen, Y.; Ying, J.; et al. NDRG4 hypermethylation is a potential biomarker for diagnosis and prognosis of gastric cancer in Chinese population. Oncotarget 2017, 8, 8105–8119. [Google Scholar] [CrossRef]
- Melotte, V.; Lentjes, M.H.; van den Bosch, S.M.; Hellebrekers, D.M.; de Hoon, J.P.; Wouters, K.A.; Daenen, K.L.; Partouns-Hendriks, I.E.; Stessels, F.; Louwagie, J.; et al. N-Myc downstream-regulated gene 4 (NDRG4): A candidate tumor suppressor gene and potential biomarker for colorectal cancer. J. Natl. Cancer Inst. 2009, 101, 916–927. [Google Scholar] [CrossRef]
- Schilling, S.H.; Hjelmeland, A.B.; Radiloff, D.R.; Liu, I.M.; Wakeman, T.P.; Fielhauer, J.R.; Foster, E.H.; Lathia, J.D.; Rich, J.N.; Wang, X.F.; et al. NDRG4 is required for cell cycle progression and survival in glioblastoma cells. J. Biol. Chem. 2009, 284, 25160–25169. [Google Scholar] [CrossRef]
- Zhang, Z.; She, J.; Yang, J.; Bu, X.; Ji, G.; Zhu, S.; He, S.; Chu, D. NDRG4 in gastric cancer determines tumor cell proliferation and clinical outcome. Mol. Carcinog. 2018, 57, 762–771. [Google Scholar] [CrossRef]
- Vaes, N.; Schonkeren, S.L.; Brosens, E.; Koch, A.; McCann, C.J.; Thapar, N.; Hofstra, R.M.W.; van Engeland, M.; Melotte, V. A combined literature and in silico analysis enlightens the role of the NDRG family in the gut. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2140–2151. [Google Scholar] [CrossRef]
- Iannone, A.; Losurdo, G.; Pricci, M.; Girardi, B.; Massaro, A.; Principi, M.; Barone, M.; Ierardi, E.; Di Leo, A. Stool Investigations for Colorectal Cancer Screening: From Occult Blood Test to DNA Analysis. J. Gastrointest. Cancer 2016, 47, 143–151. [Google Scholar] [CrossRef]
- Nakagawa, H.; Whelan, K.; Lynch, J.P. Mechanisms of Barrett’s oesophagus: Intestinal differentiation, stem cells, and tissue models. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Thrift, A.P. Barrett’s Esophagus and Esophageal Adenocarcinoma: How Common Are They Really? Dig. Dis. Sci. 2018, 63, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B. The epidemic of esophageal adenocarcinoma. Gastroenterol. Clin. N. Am. 2002, 31, 421–440. [Google Scholar] [CrossRef]
- Yang, X.; Phillips, D.L.; Ferguson, A.T.; Nelson, W.G.; Herman, J.G.; Davidson, N.E. Synergistic activation of functional estrogen receptor (ER)-alpha by DNA methyltransferase and histone deacetylase inhibition in human ER-alpha-negative breast cancer cells. Cancer Res. 2001, 61, 7025–7029. [Google Scholar]
- Ou, J.N.; Torrisani, J.; Unterberger, A.; Provencal, N.; Shikimi, K.; Karimi, M.; Ekstrom, T.J.; Szyf, M. Histone deacetylase inhibitor Trichostatin A induces global and gene-specific DNA demethylation in human cancer cell lines. Biochem. Pharmacol. 2007, 73, 1297–1307. [Google Scholar] [CrossRef]
- Wu, L.P.; Wang, X.; Li, L.; Zhao, Y.; Lu, S.; Yu, Y.; Zhou, W.; Liu, X.; Yang, J.; Zheng, Z.; et al. Histone deacetylase inhibitor depsipeptide activates silenced genes through decreasing both CpG and H3K9 methylation on the promoter. Mol. Cell Biol. 2008, 28, 3219–3235. [Google Scholar] [CrossRef]
- Xiong, Y.; Dowdy, S.C.; Podratz, K.C.; Jin, F.; Attewell, J.R.; Eberhardt, N.L.; Jiang, S.W. Histone deacetylase inhibitors decrease DNA methyltransferase-3B messenger RNA stability and down-regulate de novo DNA methyltransferase activity in human endometrial cells. Cancer Res. 2005, 65, 2684–2689. [Google Scholar] [CrossRef]
- Schonkeren, S.L.; Massen, M.; van der Horst, R.; Koch, A.; Vaes, N.; Melotte, V. Nervous NDRGs: The N-myc downstream-regulated gene family in the central and peripheral nervous system. Neurogenetics 2019, 20, 173–186. [Google Scholar] [CrossRef]
- Kotipatruni, R.P.; Ren, X.; Thotala, D.; Jaboin, J.J. NDRG4 is a novel oncogenic protein and p53 associated regulator of apoptosis in malignant meningioma cells. Oncotarget 2015, 6, 17594–17604. [Google Scholar] [CrossRef]
- Chu, D.; Zhang, Z.; Zhou, Y.; Li, Y.; Zhu, S.; Zhang, J.; Zhao, Q.; Ji, G.; Wang, W.; Zheng, J. NDRG4, a novel candidate tumor suppressor, is a predictor of overall survival of colorectal cancer patients. Oncotarget 2015, 6, 7584–7596. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, G.; Zhou, F.; Su, B.; Li, Y. DNA methylation profiles in cancer diagnosis and therapeutics. Clin. Exp. Med. 2018, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Locke, W.J.; Guanzon, D.; Ma, C.; Liew, Y.J.; Duesing, K.R.; Fung, K.Y.C.; Ross, J.P. DNA Methylation Cancer Biomarkers: Translation to the Clinic. Front. Genet. 2019, 10, 1150. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Cheng, J.; Cao, X.; Surowy, H.; Burwinkel, B. Blood-based DNA methylation as biomarker for breast cancer: A systematic review. Clin. Epigenetics 2016, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.; Pan, K.; Linnekamp, J.F.; Medema, J.P.; Kandimalla, R. DNA methylation based biomarkers in colorectal cancer: A systematic review. Biochim. Biophys. Acta 2016, 1866, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Ren, H. Blood-based DNA Methylation Biomarkers for Early Detection of Colorectal Cancer. J. Proteom. Bioinform. 2018, 11, 120–126. [Google Scholar] [CrossRef]
- Chen, J.; Sun, H.; Tang, W.; Zhou, L.; Xie, X.; Qu, Z.; Chen, M.; Wang, S.; Yang, T.; Dai, Y.; et al. DNA methylation biomarkers in stool for early screening of colorectal cancer. J. Cancer 2019, 10, 5264–5271. [Google Scholar] [CrossRef]
- Lu, H.; Huang, S.; Zhang, X.; Wang, D.; Zhang, X.; Yuan, X.; Zhang, Q.; Huang, Z. DNA methylation analysis of SFRP2, GATA4/5, NDRG4 and VIM for the detection of colorectal cancer in fecal DNA. Oncol. Lett. 2014, 8, 1751–1756. [Google Scholar] [CrossRef]
- Dong, J.; Buas, M.F.; Gharahkhani, P.; Kendall, B.J.; Onstad, L.; Zhao, S.; Anderson, L.A.; Wu, A.H.; Ye, W.; Bird, N.C.; et al. Determining Risk of Barrett’s Esophagus and Esophageal Adenocarcinoma Based on Epidemiologic Factors and Genetic Variants. Gastroenterology 2018, 154, 1273–1281.e1273. [Google Scholar] [CrossRef]
- Peng, D.; Belkhiri, A.; Hu, T.; Chaturvedi, R.; Asim, M.; Wilson, K.T.; Zaika, A.; El-Rifai, W. Glutathione peroxidase 7 protects against oxidative DNA damage in oesophageal cells. Gut 2011, 61, 1250–1260. [Google Scholar] [CrossRef]
- Peng, D.; Hu, T.; Soutto, M.; Belkhiri, A.; Zaika, A.; El-Rifai, W. Glutathione peroxidase 7 has potential tumour suppressor functions that are silenced by location-specific methylation in oesophageal adenocarcinoma. Gut 2013, 63, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hu, T.; Zhu, S.; Mukaisho, K.; El-Rifai, W.; Peng, D.F. Glutathione peroxidase 7 suppresses cancer cell growth and is hypermethylated in gastric cancer. Oncotarget 2017, 8, 54345–54356. [Google Scholar] [CrossRef] [PubMed]
- Kalabis, J.; Wong, G.S.; Vega, M.E.; Natsuizaka, M.; Robertson, E.S.; Herlyn, M.; Nakagawa, H.; Rustgi, A.K. Isolation and characterization of mouse and human esophageal epithelial cells in 3D organotypic culture. Nat. Protoc. 2012, 7, 235–246. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, L.; Hu, T.; Lu, H.; Peng, D. N-MYC Downstream Regulated Gene 4 (NDRG4), a Frequent Downregulated Gene through DNA Hypermethylation, plays a Tumor Suppressive Role in Esophageal Adenocarcinoma. Cancers 2020, 12, 2573. https://doi.org/10.3390/cancers12092573
Cao L, Hu T, Lu H, Peng D. N-MYC Downstream Regulated Gene 4 (NDRG4), a Frequent Downregulated Gene through DNA Hypermethylation, plays a Tumor Suppressive Role in Esophageal Adenocarcinoma. Cancers. 2020; 12(9):2573. https://doi.org/10.3390/cancers12092573
Chicago/Turabian StyleCao, Longlong, Tianling Hu, Heng Lu, and Dunfa Peng. 2020. "N-MYC Downstream Regulated Gene 4 (NDRG4), a Frequent Downregulated Gene through DNA Hypermethylation, plays a Tumor Suppressive Role in Esophageal Adenocarcinoma" Cancers 12, no. 9: 2573. https://doi.org/10.3390/cancers12092573
APA StyleCao, L., Hu, T., Lu, H., & Peng, D. (2020). N-MYC Downstream Regulated Gene 4 (NDRG4), a Frequent Downregulated Gene through DNA Hypermethylation, plays a Tumor Suppressive Role in Esophageal Adenocarcinoma. Cancers, 12(9), 2573. https://doi.org/10.3390/cancers12092573






