The Optimal Duration of Adjuvant Chemotherapy in Colon Cancer
Simple Summary
Abstract
1. Introduction
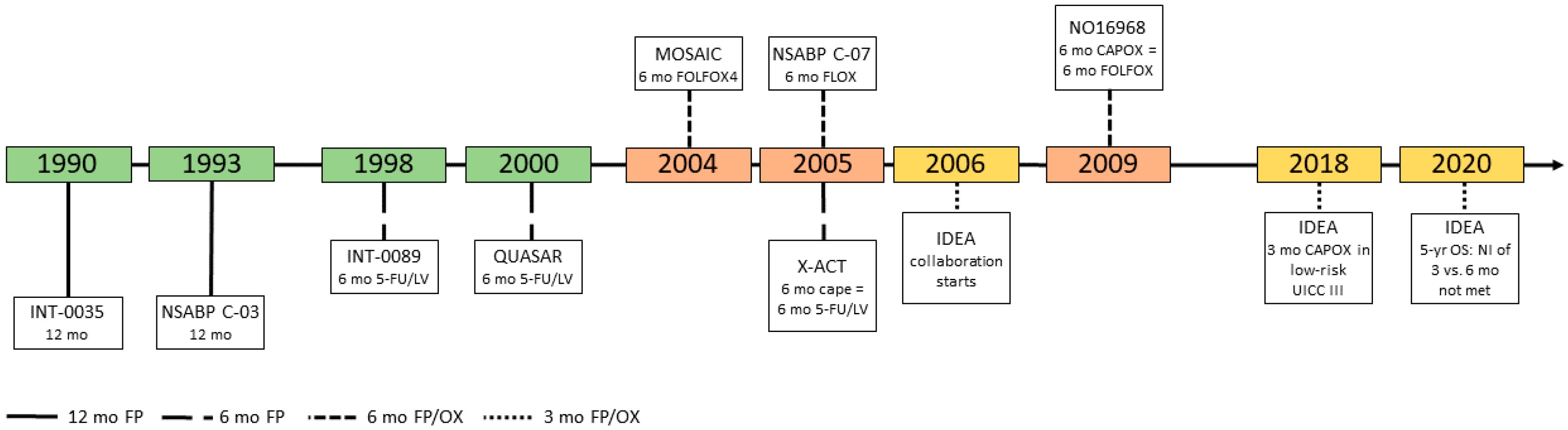
2. Fluoropyrimidines as Single Agent
3. The Addition of Oxaliplatin
4. IDEA Collaboration: Limiting Cumulative Doses of Oxaliplatin Whilst Maintaining Benefit
4.1. Role of Chemotherapy Regimen
4.2. Role of Clinical Prognostic Factors: T- and N-stage
4.3. Combining Clinical Risk Factors and Chemotherapy Regimen
4.4. Newer Parameters: Immunoscore and cfDNA
4.4.1. IDEA: Long-Term Results
4.4.2. IDEA: Shortening Treatment Duration Also in High Risk Stage II Disease?
5. Best Use of Oxaliplatin with FP: What Have We Learned from IDEA?
6. Conclusions: Treatment Duration: Where Are We Today?
- Oxaliplatin treatment duration can be shortened in patients with a (prognostic) low-risk features. Currently, this low risk is seen as pT1-3 with pN1.
- In most prognostic groups, clinical differences are rather small—despite the fact that technically, the statistical non-inferiority of limiting oxaliplatin treatment duration has not been shown. Furthermore, the choice of FP/oxaliplatin regimen seems to be important. However, in all of those clinical risk/choice of FP subgroups (Table 1, yellow fields) the limitation of oxaliplatin treatment duration is individually to consider (Table 1, yellow fields).
- A 6 months treatment duration seems to be mandatory in clinical high-risk patients if FOLFOX is the regimen of choice.
- Six months of a FP alone remains a standard of care in patients with clinical low-risk-features, if a treatment indication exists and/or is considered at all.
- In patients with high-risk stage II disease—with insufficient lymph nodes (<10), perforation and/or T4 stage—the indication for a combination treatment exists. The CAPOX regimen can be considered here with a treatment duration limited to 3 months (with some caution).
- It remains unclear whether for an intermediate risk group (other risk factors than T4 and/or <12 lymph nodes), 3 months of FP/oxaliplatin could substitute a 6 months treatment with a FP as single agent.
Funding
Conflicts of Interest
Abbreviations
| FP | Fluoropyrimidine |
| 5-FU | 5-Fluorouracil |
| IS | Immunoscore® |
| ctDNA | Cell-free tumor DNA |
| LDLV 5-FU | Low-dose LV |
| HDLV 5-FU | High-dose LV |
| LV | Leucovorin |
| LEV | Levasimole |
| NI | Non-inferiority |
| PSN | Peripheral sensory neuropathy |
| UFT/FA | Tegafur/folinic acid |
References
- Laurie, J.A.; Moertel, C.G.; Fleming, T.R.; Wieand, H.S.; Leigh, J.E.; Rubin, J.; McCormack, G.W.; Gerstner, J.B.; Krook, J.E.; Malliard, J.; et al. Surgical adjuvant therapy of large-bowel carcinoma: An evaluation of levamisole and the combination of levamisole and fluorouracil. The North Central Cancer Treatment Group and the Mayo Clinic. J. Clin. Oncol. 1989, 7, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Piedbois, P.; Buyse, M. What can we learn from a meta-analysis of trials testing the modulation of 5-FU by leucovorin? Advanced Colorectal Meta-analysis Project. Ann. Oncol. 1993, 4 (Suppl. 2), 15–19. [Google Scholar] [CrossRef] [PubMed]
- Chau, I.; Cunningham, D. Chemotherapy in colorectal cancer: New options and new challenges. Br. Med. Bull. 2002, 64, 159–180. [Google Scholar] [CrossRef]
- Haller, D.G.; Catalano, P.J.; Macdonald, J.S.; O’Rourke, M.A.; Frontiera, M.S.; Jackson, D.V.; Mayer, R.J. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: Final report of Intergroup 0089. J. Clin. Oncol. 2005, 23, 8671–8678. [Google Scholar] [CrossRef]
- Group, Q.C. Comparison of fluorouracil with additional levamisole, higher-dose folinic acid, or both, as adjuvant chemotherapy for colorectal cancer: A randomised trial. QUASAR Collaborative Group. Lancet 2000, 355, 1588–1596. [Google Scholar]
- Mochizuki, I.; Takiuchi, H.; Ikejiri, K.; Nakamoto, Y.; Kinugasa, Y.; Takagane, A.; Endo, T.; Shinozaki, H.; Takii, Y.; Takahashi, Y.; et al. Safety of UFT/LV and S-1 as adjuvant therapy for stage III colon cancer in phase III trial: ACTS-CC trial. Br. J. Cancer 2012, 106, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Ishiguro, M.; Ikejiri, K.; Mochizuki, I.; Nakamoto, Y.; Kinugasa, Y.; Takagane, A.; Endo, T.; Shinozaki, H.; Takii, Y.; et al. S-1 as adjuvant chemotherapy for stage III colon cancer: A randomized phase III study (ACTS-CC trial). Ann. Oncol. 2014, 25, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, T.; Ishiguro, M.; Nakatani, E.; Yoshida, M.; Inoue, T.; Nakamoto, Y.; Shiomi, A.; Takagane, A.; Sunami, E.; Shinozaki, H.; et al. Updated 5-year survival and exploratory T x N subset analyses of ACTS-CC trial: A randomised controlled trial of S-1 versus tegafur-uracil/leucovorin as adjuvant chemotherapy for stage III colon cancer. ESMO. Open 2018, 3, e000428. [Google Scholar] [CrossRef] [PubMed]
- Twelves, C.; Scheithauer, W.; McKendrick, J.; Seitz, J.F.; Van Hazel, G.; Wong, A.; Díaz-Rubio, E.; Gilberg, F.; Cassidy, J. Capecitabine versus 5-fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: Final results from the X-ACT trial with analysis by age and preliminary evidence of a pharmacodynamic marker of efficacy. Ann. Oncol. 2012, 23, 1190–1197. [Google Scholar] [CrossRef]
- Lembersky, B.C.; Wieand, H.S.; Petrelli, N.J.; O’Connell, M.J.; Colangelo, L.H.; Smith, R.E.; Seay, T.E.; Giguere, J.K.; Marshall, M.E.; Jacobs, A.D.; et al. Oral uracil and tegafur plus leucovorin compared with intravenous fluorouracil and leucovorin in stage II and III carcinoma of the colon: Results from National Surgical Adjuvant Breast and Bowel Project Protocol C-06. J. Clin. Oncol. 2006, 24, 2059–2064. [Google Scholar] [CrossRef]
- Schmoll, H.-J.; Tabernero, J.; Maroun, J.; Braud, F.D.; Price, T.; Cutsem, E.V.; Hill, M.; Hoersch, S.; Rittweger, K.; Haller, D.G. Capecitabine Plus Oxaliplatin Compared With Fluorouracil/Folinic Acid As Adjuvant Therapy for Stage III Colon Cancer: Final Results of the NO16968 Randomized Controlled Phase III Trial. J. Clin. Oncol. 2015, 33, 3733–3740. [Google Scholar] [CrossRef] [PubMed]
- Kuebler, J.P.; Wieand, H.S.; O’Connell, M.J.; Smith, R.E.; Colangelo, L.H.; Yothers, G.; Petrelli, N.J.; Findlay, M.P.; Seay, T.E.; Atkins, J.N.; et al. Oxaliplatin Combined With Weekly Bolus Fluorouracil and Leucovorin As Surgical Adjuvant Chemotherapy for Stage II and III Colon Cancer: Results From NSABP C-07. J. Clin. Oncol. 2007, 25, 2198–2204. [Google Scholar] [CrossRef] [PubMed]
- André, T.; de Gramont, A.; Vernerey, D.; Chibaudel, B.; Bonnetain, F.; Tijeras-Raballand, A.; Scriva, A.; Hickish, T.; Tabernero, J.; Laethem, J.L.V.; et al. Adjuvant Fluorouracil, Leucovorin, and Oxaliplatin in Stage II to III Colon Cancer: Updated 10-Year Survival and Outcomes According to BRAF Mutation and Mismatch Repair Status of the MOSAIC Study. J. Clin. Oncol. 2015, 33, 4176–4187. [Google Scholar] [CrossRef] [PubMed]
- Yothers, G.; O’Connell, M.J.; Allegra, C.J.; Kuebler, J.P.; Colangelo, L.H.; Petrelli, N.J.; Wolmark, N. Oxaliplatin as adjuvant therapy for colon cancer: Updated results of NSABP C-07 trial, including survival and subset analyses. J. Clin. Oncol. 2011, 29, 3768–3774. [Google Scholar] [CrossRef]
- Schmoll, H.-J.; Cartwright, T.; Tabernero, J.; Nowacki, M.P.; Figer, A.; Maroun, J.; Price, T.; Lim, R.; Cutsem, E.V.; Park, Y.-S.; et al. Phase III Trial of Capecitabine Plus Oxaliplatin As Adjuvant Therapy for Stage III Colon Cancer: A Planned Safety Analysis in 1,864 Patients. J. Clin. Oncol. 2007, 25, 102–109. [Google Scholar] [CrossRef]
- Land, S.R.; Kopec, J.A.; Cecchini, R.S.; Ganz, P.A.; Wieand, H.S.; Colangelo, L.H.; Murphy, K.; Kuebler, J.P.; Seay, T.E.; Needles, B.M.; et al. Neurotoxicity from oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: NSABP C-07. J. Clin. Oncol. 2007, 25, 2205–2211. [Google Scholar] [CrossRef]
- André, T.; Boni, C.; Mounedji-Boudiaf, L.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Zaninelli, M.; Clingan, P.; Bridgewater, J.; et al. Oxaliplatin, Fluorouracil, and Leucovorin as Adjuvant Treatment for Colon Cancer. N. Engl. J. Med. 2004, 350, 2343–2351. [Google Scholar] [CrossRef]
- Grothey, A.; Sobrero, A.F.; Shields, A.F.; Yoshino, T.; Paul, J.; Taieb, J.; Souglakos, J.; Shi, Q.; Kerr, R.; Labianca, R.; et al. Duration of Adjuvant Chemotherapy for Stage III Colon Cancer. N. Engl. J. Med. 2018, 378, 1177–1188. [Google Scholar] [CrossRef]
- Labianca, R.; Lonardi, S.; Rosati, G.; Di Bartolomeo, M.; Ronzoni, M.; Pella, N.; Scartozzi, M.; Banzi, M.; Zampino, M.G.; Pasini, F.; et al. FOLFOX4/XELOX in stage II–III colon cancer: Efficacy and safety results of the Italian Three Or Six Colon Adjuvant (TOSCA) trial. Ann. Oncol. 2017, 28, v614. [Google Scholar] [CrossRef]
- Petrelli, F.; Labianca, R.; Zaniboni, A.; Lonardi, S.; Galli, F.; Rulli, E.; Rosati, G.; Corallo, S.; Ronzoni, M.; Cardellino, G.G.; et al. Assessment of Duration and Effects of 3 vs 6 Months of Adjuvant Chemotherapy in High-Risk Stage II Colorectal Cancer: A Subgroup Analysis of the TOSCA Randomized Clinical Trial. JAMA Oncol. 2020, 6, 547–551. [Google Scholar] [CrossRef]
- Iveson, T.; Boyd, K.A.; Kerr, R.S.; Robles-Zurita, J.; Saunders, M.P.; Briggs, A.H.; Cassidy, J.; Hollander, N.H.; Tabernero, J.; Haydon, A.; et al. 3-month versus 6-month adjuvant chemotherapy for patients with high-risk stage II and III colorectal cancer: 3-year follow-up of the SCOT non-inferiority RCT. HTA 2019, 23, 1–88. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Vernerey, D.; Mineur, L.; Bennouna, J.; Desrame, J.; Faroux, R.; Fratte, S.; Hug de Larauze, M.; Paget-Bailly, S.; Chibaudel, B.; et al. Three Versus 6 Months of Oxaliplatin-Based Adjuvant Chemotherapy for Patients With Stage III Colon Cancer: Disease-Free Survival Results From a Randomized, Open-Label, International Duration Evaluation of Adjuvant (IDEA) France, Phase III Trial. J. Clin. Oncol. 2018, 36, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Souglakos, J.; Boukovinas, I.; Kakolyris, S.; Xynogalos, S.; Ziras, N.; Athanasiadis, A.; Androulakis, N.; Christopoulou, A.; Vaslamatzis, M.; Ardavanis, A.; et al. Three- versus six-month adjuvant FOLFOX or CAPOX for high-risk stage II and stage III colon cancer patients: The efficacy results of Hellenic Oncology Research Group (HORG) participation to the International Duration Evaluation of Adjuvant Chemotherapy (IDEA) project. Ann. Oncol. 2019, 30, 1304–1310. [Google Scholar] [PubMed]
- Yoshino, T.; Yamanaka, T.; Kotaka, M.; Manaka, D.; Eto, T.; Hasegawa, J.; Takagane, A.; Nakamura, M.; Kato, T.; Munemoto, Y.; et al. Efficacy of 3 versus 6 months of oxaliplatin-based adjuvant chemotherapy for stage III colon cancer (CC): Results from phase III ACHIEVE trial as part of the International Duration Evaluation of Adjuvant therapy (IDEA) Collaboration. Ann. Oncol. 2017, 28, v614. [Google Scholar] [CrossRef]
- Yoshino, T.; Yamanaka, T.; Oki, E.; Kotaka, M.; Manaka, D.; Eto, T.; Hasegawa, J.; Takagane, A.; Nakamura, M.; Kato, T.; et al. Efficacy and Long-term Peripheral Sensory Neuropathy of 3 vs 6 Months of Oxaliplatin-Based Adjuvant Chemotherapy for Colon Cancer: The ACHIEVE Phase 3 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1574–1581. [Google Scholar] [CrossRef]
- André, T.; Iveson, T.; Labianca, R.; Meyerhardt, J.A.; Souglakos, I.; Yoshino, T.; Paul, J.; Sobrero, A.; Taieb, J.; Shields, A.F.; et al. The IDEA (International Duration Evaluation of Adjuvant Chemotherapy) Collaboration: Prospective Combined Analysis of Phase III Trials Investigating Duration of Adjuvant Therapy with the FOLFOX (FOLFOX4 or Modified FOLFOX6) or XELOX (3 versus 6 months) Regimen for Patients with Stage III Colon Cancer: Trial Design and Current Status. Curr. Colorectal Cancer Rep. 2013, 9, 261–269. [Google Scholar]
- Iveson, T.; Sobrero, A.F.; Yoshino, T.; Sougklakos, I.; Ou, F.-S.; Meyers, J.P.; Shi, Q.; Saunders, M.P.; Labianca, R.; Yamanaka, T.; et al. Prospective pooled analysis of four randomized trials investigating duration of adjuvant (adj) oxaliplatin-based therapy (3 vs 6 months {m}) for patients (pts) with high-risk stage II colorectal cancer (CC). J. Clin. Oncol. 2019, 37, 3501. [Google Scholar] [CrossRef]
- Blair, H.A. Immunoscore®: A Diagnostic Assay for Clinical Management of Colon Cancer. Mol. Diagn. Ther. 2020, 24, 365–370. [Google Scholar] [CrossRef]
- Pagès, F.; André, T.; Taieb, J.; Vernerey, D.; Henriques, J.; Borg, C.; Marliot, F.; Ben Jannet, R.; Louvet, C.; Mineur, L.; et al. Prognostic and predictive value of the Immunoscore in stage III colon cancer patients treated with oxaliplatin in the prospective IDEA France PRODIGE-GERCOR cohort study. Ann. Oncol. 2020, 31, 921–929. [Google Scholar] [CrossRef]
- Pagès, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.-S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. The Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef]
- Taieb, J.; Taly, V.; Vernerey, D.; Bourreau, C.; Bennouna, J.; Faroux, R.; Desrame, J.; Bouche, O.; Borg, C.; Egreteau, J.; et al. Analysis of circulating tumour DNA (ctDNA) from patients enrolled in the IDEA-FRANCE phase III trial: Prognostic and predictive value for adjuvant treatment duration. Ann. Oncol. 2019, 30, v867. [Google Scholar] [CrossRef]
- Sobrero, A.F.; Meyerhardt, J.A.; Grothey, A.; Iveson, T.; Yoshino, T.; Sougklakos, I.; Meyers, J.P.; Labianca, R.; Saunders, M.P.; Vernerey, D.; et al. Overall survival (OS) and long-term disease-free survival (DFS) of three versus six months of adjuvant (adj) oxaliplatin and fluoropyrimidine-based therapy for patients (pts) with stage III colon cancer (CC): Final results from the IDEA (International Duration Evaluation of Adj chemotherapy) collaboration. J. Clin. Oncol. 2020, 38, 4004. [Google Scholar] [CrossRef]
- Yoshino, T.; Yamanaka, T.; Shiozawa, M.; Manaka, D.; Kotaka, M.; Gamoh, M.; Shiomi, A.; Makiyama, A.; Munemoto, Y.; Rikiyama, T.; et al. ACHIEVE-2 trial: A randomized phase III trial investigating duration of adjuvant (adj) oxaliplatin-based therapy (3 vs 6 months) for patients (pts) with high-risk stage II colon cancer (CC). Ann. Oncol. 2019, 30, v199. [Google Scholar] [CrossRef]
- Sobrero, A.; Lonardi, S.; Rosati, G.; Di Bartolomeo, M.; Ronzoni, M.; Pella, N.; Scartozzi, M.; Banzi, M.; Zampino, M.G.; Pasini, F.; et al. FOLFOX or CAPOX in Stage II to III Colon Cancer: Efficacy Results of the Italian Three or Six Colon Adjuvant Trial. J. Clin. Oncol. 2018, 36, 1478–1485. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Cederquist, L.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; et al. NCCN Guidelines Insights: Colon Cancer, Version 2.2018. J. Natl. Compr. Canc. Netw. 2018, 16, 359–369. [Google Scholar] [CrossRef]
- Argiles, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised Colon Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up. Ann. Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Labianca, R.; Nordlinger, B.; Beretta, G.D.; Mosconi, S.; Mandalà, M.; Cervantes, A.; Arnold, D. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24 (Suppl. 6), vi64–vi72. [Google Scholar] [CrossRef]
- Yoshino, T. New IDEA Collaboration ‘Circulate IDEA’. Available online: https://oncologypro.esmo.org/meeting-resources/esmo-2019-congress/New-IDEA-collaboration-CIRCULATE-IDEA (accessed on 6 August 2020).
- Sinicrope, F.A.; Ou, F.-S.; Zemla, T.; Nixon, A.B.; Mody, K.; Levasseur, A.; Dueck, A.C.; Dhanarajan, A.R.; Lieu, C.H.; Cohen, D.J.; et al. Randomized trial of standard chemotherapy alone or combined with atezolizumab as adjuvant therapy for patients with stage III colon cancer and deficient mismatch repair (ATOMIC, Alliance A021502). J. Clin. Oncol. 2019, 37, e15169. [Google Scholar] [CrossRef]

| Regimen | All-Patient Cohort | ||||||||||
| CAPOX | FOLFOX | ||||||||||
| 3 yr DFS (%) (95% CI) | HR (95% CI) | 3 yr DFS (%) (95% CI) | HR (95% CI) | 3 yr DFS (%) (95% CI) | HR (95% CI) | ||||||
| 3 m | 6 m | 3 m | 6 m | 3 m | 6 m | ||||||
| UICC III | Risk Group | low-risk (T1–3 N1) | 85.0 (83.1–86.9) | 83.1 (81.1–85.2) | 0.85 (0.71–1.01) | 81.9 (80.2–83.6) | 83.5 (81.9–85.1) | 1.10 (0.96–1.26) | 83.1 (81.8–84.4) | 83.3 (82.1–84.6) | 1.01 (0.9–1.12) |
| high-risk (T4 and/or N2) | 64.1 (61.3–67.1) | 64.0 (61.2–67.0) | 1.02 (0.89–1.17) | 61.5 (58.9–64.1) | 64.7 (62.2–67.3) | 1.20 (1.07–1.35) | 62.7 (60.8–64.4) | 64.4 (62.6–66.4) | 1.12 (1.03–1.23) | ||
| Regimen | All-Patient Cohort | ||||||||||
| CAPOX | FOLFOX | ||||||||||
| 5 yr OS (%) (95% CI) | HR (95% CI) | 5 yr OS (%) (95% CI) | HR (95% CI) | 5 yr OS (%) (95% CI) | HR (95% CI) | ||||||
| 3 m | 6 m | 3 m | 6 m | 3 m | 6 m | ||||||
| UICC III | Risk Group | low-risk (T1–3 N1) | 90.4 (88.9–92.0) | 88.1 (86.3–89.8) | 0.85 (0.69–1.04) | 89.1 (87.8–90.5) | 89.4 (88.1–90.7) | 1.02 (0.87–1.19) | 89.6 (88.6–90.7) | 88.9 (87.8–90.0) | 0.95 (0.84–1.08) |
| high-risk (T4 and/or N2) | 71.4 (68.7–74.2) | 72.4 (69.7–75.2) | 1.03 (0.89–1.20) | 72.5 (70.2–74.9) | 75.3 (73.1–77.6) | 1.12 (0.98–1.27) | 72.0 (70.3–73.8) | 74.1 (72.4–75.9) | 1.08 (0.98–1.19) | ||
| Regimen | All-Patient Cohort | ||||||||||
| CAPOX | FOLFOX | ||||||||||
| 5 yr DFS (%) (80% CI) | HR (80% CI) | 5 yr DFS (%) (80% CI) | HR (80% CI) | 5 yr DFS (%) (80% CI) | HR (80 % CI) | ||||||
| 3 m | 6 m | 3 m | 6 m | 3 m | 6 m | ||||||
| UICC II | Risk Group | high-risk | 81.7 (n/a) | 82.0 (n/a) | 1.02 (0.88–1.17) | 79.2 (n/a) | 86.5 (n/a) | 1.42 (1.19–1.70) | 80.7 | 83.9 | 1.18 (1.05–1.31) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collienne, M.; Arnold, D. The Optimal Duration of Adjuvant Chemotherapy in Colon Cancer. Cancers 2020, 12, 2509. https://doi.org/10.3390/cancers12092509
Collienne M, Arnold D. The Optimal Duration of Adjuvant Chemotherapy in Colon Cancer. Cancers. 2020; 12(9):2509. https://doi.org/10.3390/cancers12092509
Chicago/Turabian StyleCollienne, Maike, and Dirk Arnold. 2020. "The Optimal Duration of Adjuvant Chemotherapy in Colon Cancer" Cancers 12, no. 9: 2509. https://doi.org/10.3390/cancers12092509
APA StyleCollienne, M., & Arnold, D. (2020). The Optimal Duration of Adjuvant Chemotherapy in Colon Cancer. Cancers, 12(9), 2509. https://doi.org/10.3390/cancers12092509




