Evaluation of Prognostic Factors for Survival in Transverse Colon Cancer
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Statistical Analysis
3. Results
3.1. Frequency and Associations of Clinicopathologic Parameters
3.2. Survival Analysis
3.3. Correlations between Tumor Grade and BRAF Status with Clinicopathologic Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Baran, B.; Mert Ozupek, N.; Yerli Tetik, N.; Acar, E.; Bekcioglu, O.; Baskin, Y. Difference Between Left-Sided and Right-Sided Colorectal Cancer: A Focused Review of Literature. Gastroenterol. Res. 2018, 11, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Nawa, T.; Kato, J.; Kawamoto, H.; Okada, H.; Yamamoto, H.; Kohno, H.; Endo, H.; Shiratori, Y. Differences between right- and left-sided colon cancer in patient characteristics, cancer morphology and histology. J. Gastroenterol. Hepatol. 2008, 23, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Benedix, F.; Schmidt, U.; Mroczkowski, P.; Gastinger, I.; Lippert, H.; Kube, R. Colon carcinoma-Classification into right and left sided cancer or according to colonic subsite?-Analysis of 29,568 patients. Eur. J. Surg. Oncol. 2011, 37, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Beale, A.L.; Penney, M.D.; Allison, M.C. The prevalence of iron deficiency among patients presenting with colorectal cancer. Color. Dis. 2005, 7, 398–402. [Google Scholar] [CrossRef]
- Yan Chan, J.C.; Diakos, C.I.; Engel, A.; Hang Chan, D.L.; Pavlakis, N.; Gill, A.; Clarke, S.J. Tumor sidedness is not an independent prognostic marker of colorectal cancer patients undergoing curative resection: A retrospective cohort study. PLoS ONE 2019, 14, e0218207. [Google Scholar] [CrossRef]
- Turner, M.C.; Becerra, D.; Sun, Z.; Watson, J.; Leung, K.; Migaly, J.; Mantyh, C.R.; Blazer, D.G. The side of the primary tumor affects overall survival in colon adenocarcinoma: An analysis of the national cancer database. Tech. Coloproctol. 2019, 23, 537–544. [Google Scholar] [CrossRef]
- Arnold, D.; Lueza, B.; Douillard, J.Y.; Peeters, M.; Lenz, H.J.; Venook, A.; Heinemann, V.; Van Cutsem, E.; Pignon, J.P.; Tabernero, J.; et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann. Oncol. 2017, 28, 1713–1729. [Google Scholar] [CrossRef]
- Snyder, M.; Bottiglieri, S.; Almhanna, K. Impact of Primary Tumor Location on First-line Bevacizumab or Cetuximab in Metastatic Colorectal Cancer. Rev. Recent Clin. Trials 2018, 13, 139–149. [Google Scholar] [CrossRef]
- Glebov, O.K.; Rodriguez, L.M.; Nakahara, K.; Jenkins, J.; Cliatt, J.; Humbyrd, C.J.; DeNobile, J.; Soballe, P.; Simon, R.; Wright, G.; et al. Distinguishing right from left colon by the pattern of gene expression. Cancer Epidemiol. Biomarkers Prev. 2003, 12, 755–762. [Google Scholar]
- Cremolini, C.; Benelli, M.; Fontana, E.; Pagani, F.; Rossini, D.; Fucà, G.; Busico, A.; Conca, E.; Di Donato, S.; Loupakis, F.; et al. Benefit from anti-EGFRs in RAS and BRAF wild-type metastatic transverse colon cancer: A clinical and molecular proof of concept study. ESMO Open 2019, 4, e000489. [Google Scholar] [CrossRef]
- Missiaglia, E.; Jacobs, B.; D’Ario, G.; Di Narzo, A.F.; Soneson, C.; Budinska, E.; Popovici, V.; Vecchione, L.; Gerster, S.; Yan, P.; et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann. Oncol. 2014, 25, 1995–2001. [Google Scholar] [CrossRef]
- Venook, A.P.; Niedzwiecki, D.; Innocenti, F.; Fruth, B.; Greene, C.; O’Neil, B.H.; Shaw, J.E.; Atkins, J.N.; Horvath, L.E.; Polite, B.N.; et al. Impact of primary (1o) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): Analysis of CALGB/SWOG 80405 (Alliance). J. Clin. Oncol. 2016, 34, 15. [Google Scholar] [CrossRef]
- Kim, K.; Kim, Y.W.; Shim, H.; Kim, B.R.; Kwon, H.Y. Differences in clinical features and oncologic outcomes between metastatic right and left colon cancer. J. B.U.ON. 2018, 23, 11–18. [Google Scholar]
- Loree, J.M.; Pereira, A.A.L.; Lam, M.; Willauer, A.N.; Raghav, K.; Dasari, A.; Van Morris, K.; Advani, S.; Menter, D.G.; Eng, C.; et al. Classifying colorectal cancer by tumor location rather than sidedness highlights a continuum in mutation profiles and consensus molecular subtypes. Clin. Cancer Res. 2018, 24, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Yuefen, P.; Wei, W.; Quan, Q.; Jing, Z.; Jiamin, X.; Shuwen, H. Analysis of prognosis, genome, microbiome, and microbial metabolome in different sites of colorectal cancer. J. Transl. Med. 2019, 17, 353. [Google Scholar] [CrossRef]
- Song, G.A.; Deng, G.; Bell, I.; Kakar, S.; Sleisenger, M.H.; Kim, Y.S. Mucinous carcinomas of the colorectum have distinct molecular genetic characteristics. Int. J. Oncol. 2005, 26, 745–750. [Google Scholar] [CrossRef]
- Ghazi, S.; Lindforss, U.; Lindberg, G.; Berg, E.; Lindblom, A.; Papadogiannakis, N. Analysis of colorectal cancer morphology in relation to sex, age, location, and family history. J. Gastroenterol. 2012, 47, 619–634. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; De Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Dienstmann, R.; Vermeulen, L.; Guinney, J.; Kopetz, S.; Tejpar, S.; Tabernero, J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat. Rev. Cancer 2017, 17, 79–92. [Google Scholar] [CrossRef]
- Lee, M.S.; Menter, D.G.; Kopetz, S. Right versus left colon cancer biology: Integrating the consensus molecular subtypes. JNCCN J. Natl. Compr. Cancer Netw. 2017, 15, 411–419. [Google Scholar] [CrossRef]
- Cheng, L.; Eng, C.; Nieman, L.Z.; Kapadia, A.S.; Du, X.L. Trends in colorectal cancer incidence by anatomic site and disease stage in the United States from 1976 to 2005. Am. J. Clin. Oncol. Cancer Clin. Trials 2011, 34, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, B. Are there two sides to colorectal cancer? Int. J. Cancer 2002, 101, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Compton, C.C.; Fielding, L.P.; Burgart, L.J.; Conley, B.; Cooper, H.S.; Hamilton, S.R.; Hammond, M.E.H.; Henson, D.E.; Hutter, R.V.P.; Nagle, R.B.; et al. Prognostic factors in colorectal cancer: College of American Pathologists consensus statement 1999. Arch. Pathol. Lab. Med. 2000, 124, 979–994. [Google Scholar]
- Stewart, S.L.; Wike, J.M.; Kato, I.; Lewis, D.R.; Michaud, F. A population-based study of colorectal cancer histology in the United States, 1998-2001. Cancer 2006, 107, 1128–1141. [Google Scholar] [CrossRef]
- Kim, H.; Jen, J.; Vogelstein, B.; Hamilton, S.R. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am. J. Pathol. 1994, 145, 148–156. [Google Scholar]
- Forster, S.; Sattler, H.P.; Hack, M.; Romanakis, K.; Rohde, V.; Seitz, G.; Wullich, B. Microsatellite instability in sporadic carcinomas of the proximal colon: Association with diploid DNA content, negative protein expression of p53, and distinct histomorphologic features. Surgery 1998, 123, 13–18. [Google Scholar] [CrossRef]
- Ribic, C.M.; Sargent, D.J.; Moore, M.J.; Thibodeau, S.N.; French, A.J.; Goldberg, R.M.; Hamilton, S.R.; Laurent-Puig, P.; Gryfe, R.; Shepherd, L.E.; et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N. Engl. J. Med. 2003, 349, 247–257. [Google Scholar] [CrossRef]
- Passardi, A.; Canale, M.; Valgiusti, M.; Ulivi, P. Immune checkpoints as a target for colorectal cancer treatment. Int. J. Mol. Sci. 2017, 18, 1324. [Google Scholar] [CrossRef]
- Van Eeghen, E.E.; Bakker, S.D.; van Bochove, A.; Loffeld, R.J.L.F. Impact of age and comorbidity on survival in colorectal cancer. J. Gastrointest. Oncol. 2015, 6, 605–612. [Google Scholar] [CrossRef]
- Sarfati, D.; Hill, S.; Blakely, T.; Robson, B.; Purdie, G.; Dennett, E.; Cormack, D.; Dew, K. The effect of comorbidity on the use of adjuvant chemotherapy and survival from colon cancer: A retrospective cohort study. BMC Cancer 2009, 9, 116. [Google Scholar] [CrossRef]
- Parés-Badell, O.; Banqué, M.; Macià, F.; Castells, X.; Sala, M. Impact of comorbidity on survival by tumour location: Breast, colorectal and lung cancer (2000–2014). Cancer Epidemiol. 2017, 49, 66–74. [Google Scholar] [CrossRef]
- Boakye, D.; Rillmann, B.; Walter, V.; Jansen, L.; Hoffmeister, M.; Brenner, H. Impact of comorbidity and frailty on prognosis in colorectal cancer patients: A systematic review and meta-analysis. Cancer Treat. Rev. 2018, 64, 30–39. [Google Scholar] [CrossRef]
- Yamano, T.; Yamauchi, S.; Kimura, K.; Babaya, A.; Hamanaka, M.; Kobayashi, M.; Fukumoto, M.; Tsukamoto, K.; Noda, M.; Tomita, N.; et al. Influence of age and comorbidity on prognosis and application of adjuvant chemotherapy in elderly Japanese patients with colorectal cancer: A retrospective multicentre study. Eur. J. Cancer 2017, 81, 90–101. [Google Scholar] [CrossRef]
- Elliot, A.H.; Martling, A.; Glimelius, B.; Johansson, H.; Nilsson, P.J. Impact of pre-treatment patient-related selection parameters on outcome in rectal cancer. Eur. J. Surg. Oncol. 2016, 42, 1667–1673. [Google Scholar] [CrossRef]
- Sabel, M.S.; Terjimanian, M.; Conlon, A.S.C.; Griffith, K.A.; Morris, A.M.; Mulholland, M.W.; Englesbe, M.J.; Holcombe, S.; Wang, S.C. Analytic morphometric assessment of patients undergoing colectomy for colon cancer. J. Surg. Oncol. 2013, 108, 169–175. [Google Scholar] [CrossRef]
- Ho, Y.H.; Siu, S.K.K.; Buttner, P.; Stevenson, A.; Lumley, J.; Stitz, R. The effect of obstruction and perforation on colorectal cancer disease-free survival. World J. Surg. 2010, 34, 1091–1101. [Google Scholar] [CrossRef]
- Chen, T.M.; Huang, Y.T.; Wang, G.C. Outcome of colon cancer initially presenting as colon perforation and obstruction. World J. Surg. Oncol. 2017, 15, 164. [Google Scholar] [CrossRef]
- Warschkow, R.; Tarantino, I.; Huttner, F.J.; Schmied, B.M.; Guller, U.; Diener, M.K.; Ulrich, A. Predictive value of mucinous histology in colon cancer: A population-based, propensity score matched analysis. Br. J. Cancer 2016, 114, 1027–1032. [Google Scholar] [CrossRef]
- Catalano, V.; Loupakis, F.; Graziano, F.; Bisonni, R.; Torresi, U.; Vincenzi, B.; Mari, D.; Giordani, P.; Alessandroni, P.; Salvatore, L.; et al. Prognosis of mucinous histology for patients with radically resected stage II and III colon cancer. Ann. Oncol. 2012, 23, 135–141. [Google Scholar] [CrossRef]
- Wang, Z.X.; Yang, L.P.; Wu, H.X.; Yang, D.D.; Ding, P.R.; Xie, D.; Chen, G.; Li, Y.H.; Wang, F.; Xu, R.H. Appraisal of prognostic interaction between sidedness and mucinous histology in colon cancer: A population-based study using inverse probability propensity score weighting. J. Cancer 2019, 10, 388–396. [Google Scholar] [CrossRef]
- Barresi, V.; Bonetti, L.R.; Leni, A.; Caruso, R.A.; Tuccari, G. Histological grading in colorectal cancer: New insights and perspectives. Histol. Histopathol. 2015, 30, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.Z.; Pan, W.T.; Lin, J.Z.; Wang, Z.X.; Pan, Z.Z.; Wang, F.H.; Yang, D.J.; Xu, R.H. Comparison of survival between right-sided and left-sided colon cancer in different situations. Cancer Med. 2018, 7, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Loupakis, F.; Yang, D.; Yau, L.; Feng, S.; Cremolini, C.; Zhang, W.; Maus, M.K.H.; Antoniotti, C.; Langer, C.; Scherer, S.J.; et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef] [PubMed]
- Roberto, M.; Marchetti, P.; Arrivi, G.; Di Pietro, F.R.; Cascinu, S.; Gelsomino, F.; Caputo, F.; Cerma, K.; Ghidini, M.; Ratti, M.; et al. The treatment paradigm of right-sided metastatic colon cancer: Harboring BRAF mutation makes the difference. Int. J. Colorectal Dis. 2020, 35, 1513–1527. [Google Scholar] [CrossRef]
- Lee, G.H.; Malietzis, G.; Askari, A.; Bernardo, D.; Al-Hassi, H.O.; Clark, S.K. Is right-sided colon cancer different to left-sided colorectal cancer?—A systematic review. Eur. J. Surg. Oncol. 2015, 41, 300–308. [Google Scholar] [CrossRef]
- Clancy, C.; Burke, J.P.; Kalady, M.F.; Coffey, J.C. BRAF mutation is associated with distinct clinicopathological characteristics in colorectal cancer: A systematic review and meta-analysis. Color. Dis. 2013, 15, e711–e718. [Google Scholar] [CrossRef]
- Roma, C.; Rachiglio, A.M.; Pasquale, R.; Fenizia, F.; Iannaccone, A.; Tatangelo, F.; Antinolfi, G.; Parrella, P.; Graziano, P.; Sabatino, L.; et al. BRAF V600E mutation in metastatic colorectal cancer: Methods of detection and correlation with clinical and pathologic features. Cancer Biol. Ther. 2016, 17, 840–848. [Google Scholar] [CrossRef]
- Yokota, T.; Ura, T.; Shibata, N.; Takahari, D.; Shitara, K.; Nomura, M.; Kondo, C.; Mizota, A.; Utsunomiya, S.; Muro, K.; et al. BRAF mutation is a powerful prognostic factor in advanced and recurrent colorectal cancer. Br. J. Cancer 2011, 104, 856–862. [Google Scholar] [CrossRef]
- Fariña-Sarasqueta, A.; van Lijnschoten, G.; Moerland, E.; Creemers, G.J.; Lemmens, V.E.P.P.; Rutten, H.J.T.; van den Brule, A.J.C. The BRAF V600E mutation is an independent prognostic factor for survival in stage II and stage III colon cancer patients. Ann. Oncol. 2010, 21, 2396–2402. [Google Scholar] [CrossRef]
- Roth, A.D.; Tejpar, S.; Delorenzi, M.; Yan, P.; Fiocca, R.; Klingbiel, D.; Dietrich, D.; Biesmans, B.; Bodoky, G.; Barone, C.; et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: Results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J. Clin. Oncol. 2010, 28, 466–474. [Google Scholar] [CrossRef]


| Total | N. | % | |
|---|---|---|---|
| 97 | 100 | ||
| Age | 68 (36–90) | ||
| Median (range) | |||
| ≤70 years | 60 | 62 | |
| >70 years | 37 | 38 | |
| Sex | |||
| Male | 59 | 61 | |
| Female | 38 | 39 | |
| Transverse locations | |||
| 2/3 proximal | 40 | 41 | |
| 1/3 distal | 57 | 59 | |
| ECOG PS | |||
| 0 | 66 | 68 | |
| ≥1 | 31 | 32 | |
| Charlson Comorbidity Index (n = 94) | |||
| ≤8 | 48 | 51 | |
| >8 | 46 | 49 | |
| Tumor onset (n = 73) | |||
| Anemia | 28 | 32 | |
| Obstruction/Perforation | 22 | 30 | |
| Pain/fever/weight loss | 23 | 38 | |
| Surgery of primary tumor | |||
| Yes | 83 | 86 | |
| Not | 14 | 14 | |
| AJCC TNM stage | |||
| I | 3 | 3 | |
| II | 24 | 25 | |
| III | 22 | 23 | |
| IV | 48 | 49 | |
| Pathological Tumour size (n = 75) | |||
| T1 | 3 | 4 | |
| T2 | 1 | 1 | |
| T3 | 55 | 73 | |
| T4 | 16 | 21 | |
| Pathological Node status (n = 75) | |||
| N0 | 28 | 37 | |
| N1 | 24 | 32 | |
| N2 | 23 | 31 | |
| Mucinous Histology (n = 85) | 28 | 30 | |
| Lymphovascular/Perineural invasion (n = 62) | |||
| Not | 23 | 37 | |
| Yes | 39 | 63 | |
| Tumour differentiation (n = 84) | |||
| G1 | 3 | 3 | |
| G2 | 41 | 49 | |
| G3 | 40 | 48 | |
| Microsatellite Instability (n = 31) | |||
| MSS | 23 | 74 | |
| MSI-H | 8 | 26 | |
| KRAS status (n = 72) | |||
| Wild-type | 45 | 63 | |
| Mutant | 27 | 37 | |
| BRAF status (n = 58) | |||
| Wild-type | 44 | 76 | |
| Mutant | 14 | 24 | |
| Adjuvant chemotherapy (n = 49) | |||
| Yes | 30 | 61 | |
| Not | 19 | 39 | |
| Variables | Disease Free Survival | Overall Survival | ||||
|---|---|---|---|---|---|---|
| Univariate | Univariate | Multivariate | ||||
| HR (95%CI) | p * | HR (95%CI) | p * | HR (95%CI) | p * | |
| Age | ||||||
| <70 | Ref | 0.210 | Ref | Ref | ||
| ≥70 | 1.63 (0.75–3.50) | 2.27 (1.29–3.98) | 0.004 | 1.49 (0.50–4.44) | 0.466 | |
| Sex | ||||||
| Female | Ref | 0.386 | Ref | |||
| Male | 0.71 (0.32–1.54) | 0.93 (0.52–1.22) | 0.828 | |||
| Transverse location | ||||||
| 2/3 proximal | Ref | Ref | ||||
| 1/3 distal | 0.56 (0.26–1.20) | 0.138 | 0.85 (0.49–1.49) | 0.587 | ||
| ECOG PS | ||||||
| 0 | Ref | 0.863 | Ref | Ref | ||
| ≥1 | 1.07 (0.47–2.64) | 2.36 (1.34–4.13) | 0.003 | 1.2 (0.31–2.26) | 0.707 | |
| CCI | ||||||
| <5 | Ref | ** Ref | Ref | |||
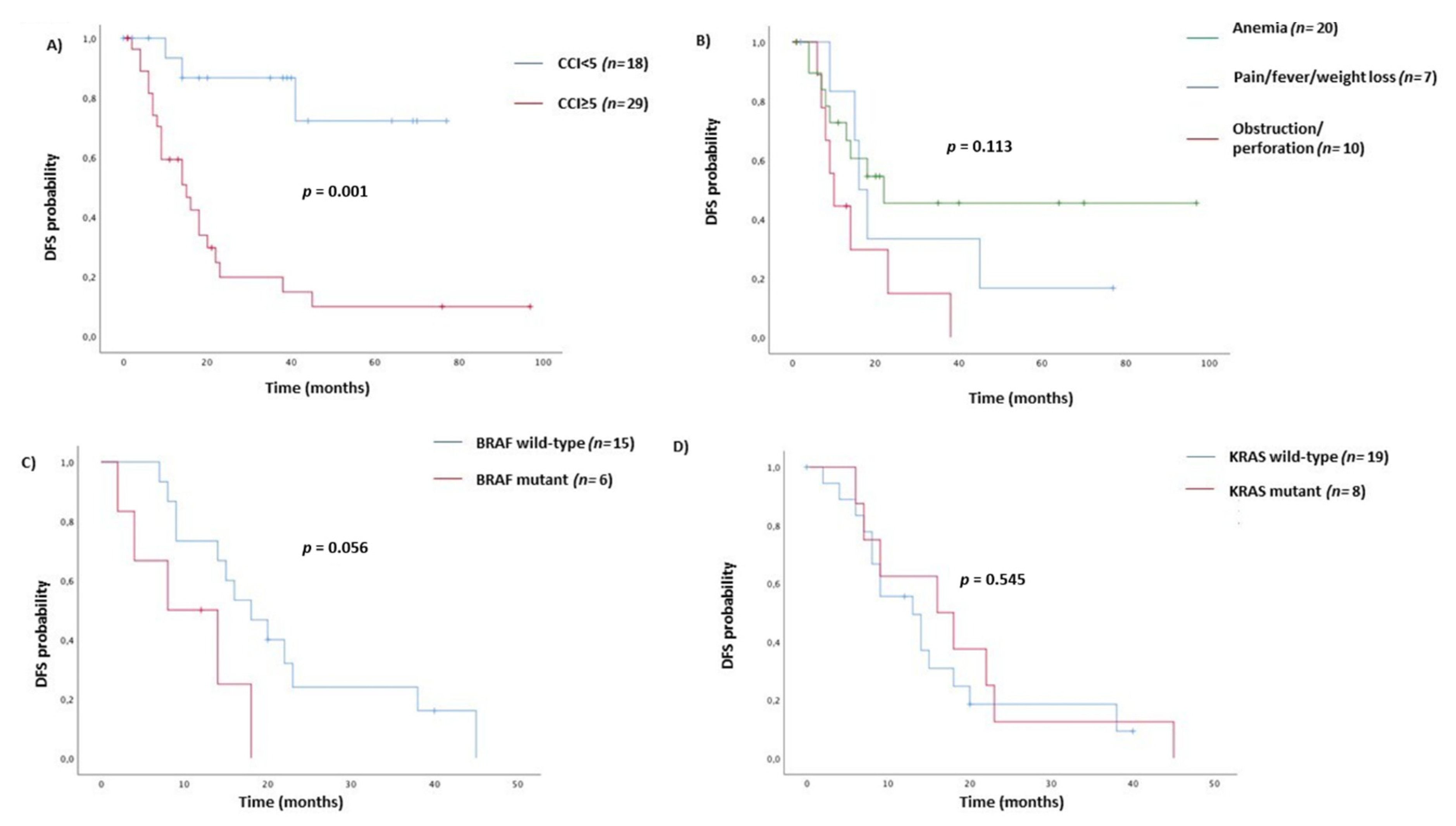
| ≥5 | 7.67 (2.27–25.92) | 0.001 | 2.11 (1.18–3.80) | 0.012 | 1.56 (0.25–1.63) | 0.348 |
| Tumor Onset | ||||||
| Anemia | Ref | Ref | ||||
| Pain/fever/weight loss | 1.35 (0.45–4.07) | 0.584 | 1.23 (0.55–2.76) | 0.602 | ||
| Obstruction or perforation | 2.65 (1.00–7.01) | 0.050 | 1.96(0.87–4.38) | 0.101 | ||
| Primary tumor resected | ||||||
| No | NA | Ref | Ref | |||
| Yes | 0.22 (0.10–0.47) | <0.001 | 0.25 (0.05–1.27) | 0.097 | ||
| AJCC 7th Stage | ||||||
| I–II | Ref | Ref | Ref | |||
| III | 1.13 (0.52–2.45) | 0.757 | 6.57 (2.26–8.48) | 0.010 | 1.04 (0.32–3.27) | 0.957 |
| IV | NA | 7.38 (4.31–8.80) | 0.001 | 2.73 (0.95–7.63) | 0.061 | |
| pT | ||||||
| 1–3 | Ref | 0.553 | Ref | |||
| 4 | 1.38 (0.47–3.99) | 1.64 (0.73–3.68) | 0.229 | |||
| pN | ||||||
| 0 | Ref | Ref | ||||
| 1 | 1.02 (0.37–2.71) | 0.229 | 1.54 (0.59–4.04) | 0.377 | ||
| 2 | 1.25 (0.42–3.80) | 0.632 | 1.65 (1.07–2.54) | 0.022 | ||
| Mucinous Histology | ||||||
| Not | Ref | 0.095 | Ref | |||
| Yes | 0.43 (0.16–1.15) | 1.17 (0.64–2.13) | 0.603 | |||
| Grade | ||||||
| G1–G2 | Ref | 0.671 | Ref | Ref | ||
| G3 | 1.20 (0.52–2.79) | 2.29 (1.24–4.22) | 0.008 | 5.26 (1.98–14.01) | 0.001 | |
| LV/Pn invasion | ||||||
| Not | Ref | 0.925 | Ref | |||
| Yes | 1.04 (0.41–2.66) | 2.27 (1.01–5.14) | 0.050 | |||
| KRAS status | ||||||
| Wild-type | Ref | 0.545 | Ref | |||
| Mutant | 1.32 (0.53–3.28) | 1.16 (0.64–2.12) | 0.611 | |||
| BRAF status | ||||||
| Wild-type | Ref | Ref | Ref | |||
| Mutant | 3.03 (0.97–9.50) | 0.056 | 5.16 (2.20–12.10) | <0.0001 | 3.71 (1.07–12.89) | 0.039 |
| MMR status | ||||||
| MSS | Ref | Ref | ||||
| MSI | 1.80 (0.44–7.32) | 0.410 | 1.44 (0.38–5.51) | 0.592 | ||
| Chemotherapy | ||||||
| Yes | Ref | Ref | ||||
| No | 1.28 (0.58–2.86) | 0.532 | 1.43 (0.52–3.97) | 0.484 | ||
| Variables | Tumor Grade | BRAF | ||||
|---|---|---|---|---|---|---|
| G1–G2 (n = 44) | G3 (n = 40) | p | Wilde-type (n = 44) | Mutant (n = 14) | p | |
| n(%) | n(%) | n(%) | n(%) | |||
| Age | ||||||
| ≤70 | 32 (73) | 17 (42) | 31 (70) | 5 (36) | ||
| >70 | 12 (27) | 23 (58) | 0.005 | 13 (30) | 9 (64) | 0.020 |
| Sex | ||||||
| Female | 19 (43) | 15 (37) | 18 (41) | 7 (50) | ||
| Male | 25 (57) | 25 (63) | 0.596 | 26 (59) | 7 (50) | 0.550 |
| ECOG PS | ||||||
| 0 | 36 (82) | 21 (52) | 34 (77) | 5 (36) | ||
| ≥1 | 8 (18) | 19 (48) | 0.004 | 10 (26) | 9 (64) | 0.004 |
| Tumor Onset Pain/fever/weight loss | 8 (25) | 10 (32) | 0.712 | 13 (38) | 3 (27) | 0.798 |
| Occlusion or perforation | 11 (34) | 8 (26) | 10 (29) | 4 (36) | ||
| Anemia | 13 (41) | 13 (42) | 11 (32) | 4 (36) | ||
| AJCC 7th Stage | ||||||
| I–II | 17 (39) | 9 (22) | 0.174 | 8 (18) | 1 (7) | 0.226 |
| III | 8 (18) | 13 (32) | 7 (16) | 5 (36) | ||
| IV | 19 (43) | 18 (45) | 29 (66) | 8 (57) | ||
| Mucinous H | ||||||
| Not | 34 (79) | 25 (62) | 0.096 | 29 (69) | 9 (64) | 0.741 |
| Yes | 9 (21) | 15 (37) | 13 (31) | 5 (36) | ||
| pT | ||||||
| 1–3 | 30 (77) | 26 (79) | 0.850 | 24 (77) | 7 (78) | 0.982 |
| 4 | 9 (23) | 7 (21) | 7 (23) | 2 (22) | ||
| pN | ||||||
| 0 | 17 (44) | 10 (30) | 0.294 | 8 (26) | 1 (11) | 0.323 |
| 1 | 13 (33) | 10 (30) | 11 (35) | 2 (22) | ||
| 2 | 9 (23) | 13 (40) | 12 (39) | 6 (67) | ||
| LV/Pn invasion | ||||||
| Not | 15 (50) | 8 (25) | 7 (26) | 3 (30) | ||
| Yes | 15 (50) | 24 (75) | 0.042 | 20 (74) | 7 (70) | 0.804 |
| KRAS status | ||||||
| Wild-type | 20 (64) | 19 (68) | 0.787 | 23 (52) | 14 (100) | |
| Mutant | 11 (36) | 9 (32) | 21 (48) | 0 | 0.001 | |
| BRAF status | ||||||
| Wild-type | 24 (92) | 12 (55) | ||||
| Mutant | 2 (8) | 10 (45) | 0.003 | |||
| MMR status | ||||||
| MSS | 13 (87) | 9 (69) | 0.262 | 14 (87) | 3 (75) | 0.531 |
| MSI | 2 (13) | 4 (31) | 2 (13) | 1 (25) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roberto, M.; Arrivi, G.; Lo Bianco, F.; Cascinu, S.; Gelsomino, F.; Caputo, F.; Cerma, K.; Ghidini, M.; Ratti, M.; Pizzo, C.; et al. Evaluation of Prognostic Factors for Survival in Transverse Colon Cancer. Cancers 2020, 12, 2457. https://doi.org/10.3390/cancers12092457
Roberto M, Arrivi G, Lo Bianco F, Cascinu S, Gelsomino F, Caputo F, Cerma K, Ghidini M, Ratti M, Pizzo C, et al. Evaluation of Prognostic Factors for Survival in Transverse Colon Cancer. Cancers. 2020; 12(9):2457. https://doi.org/10.3390/cancers12092457
Chicago/Turabian StyleRoberto, Michela, Giulia Arrivi, Francesca Lo Bianco, Stefano Cascinu, Fabio Gelsomino, Francesco Caputo, Krisida Cerma, Michele Ghidini, Margherita Ratti, Claudio Pizzo, and et al. 2020. "Evaluation of Prognostic Factors for Survival in Transverse Colon Cancer" Cancers 12, no. 9: 2457. https://doi.org/10.3390/cancers12092457
APA StyleRoberto, M., Arrivi, G., Lo Bianco, F., Cascinu, S., Gelsomino, F., Caputo, F., Cerma, K., Ghidini, M., Ratti, M., Pizzo, C., Ficorella, C., Parisi, A., Cortellini, A., Urbano, F., Calandrella, M. L., Dell’Aquila, E., Minelli, A., Fulgenzi, C. A. M., Gariazzo, L., ... Mazzuca, F. (2020). Evaluation of Prognostic Factors for Survival in Transverse Colon Cancer. Cancers, 12(9), 2457. https://doi.org/10.3390/cancers12092457







