Objective Evaluation of Risk Factors for Radiation Dermatitis in Whole-Breast Irradiation Using the Spectrophotometric L*a*b Color-Space
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Participants
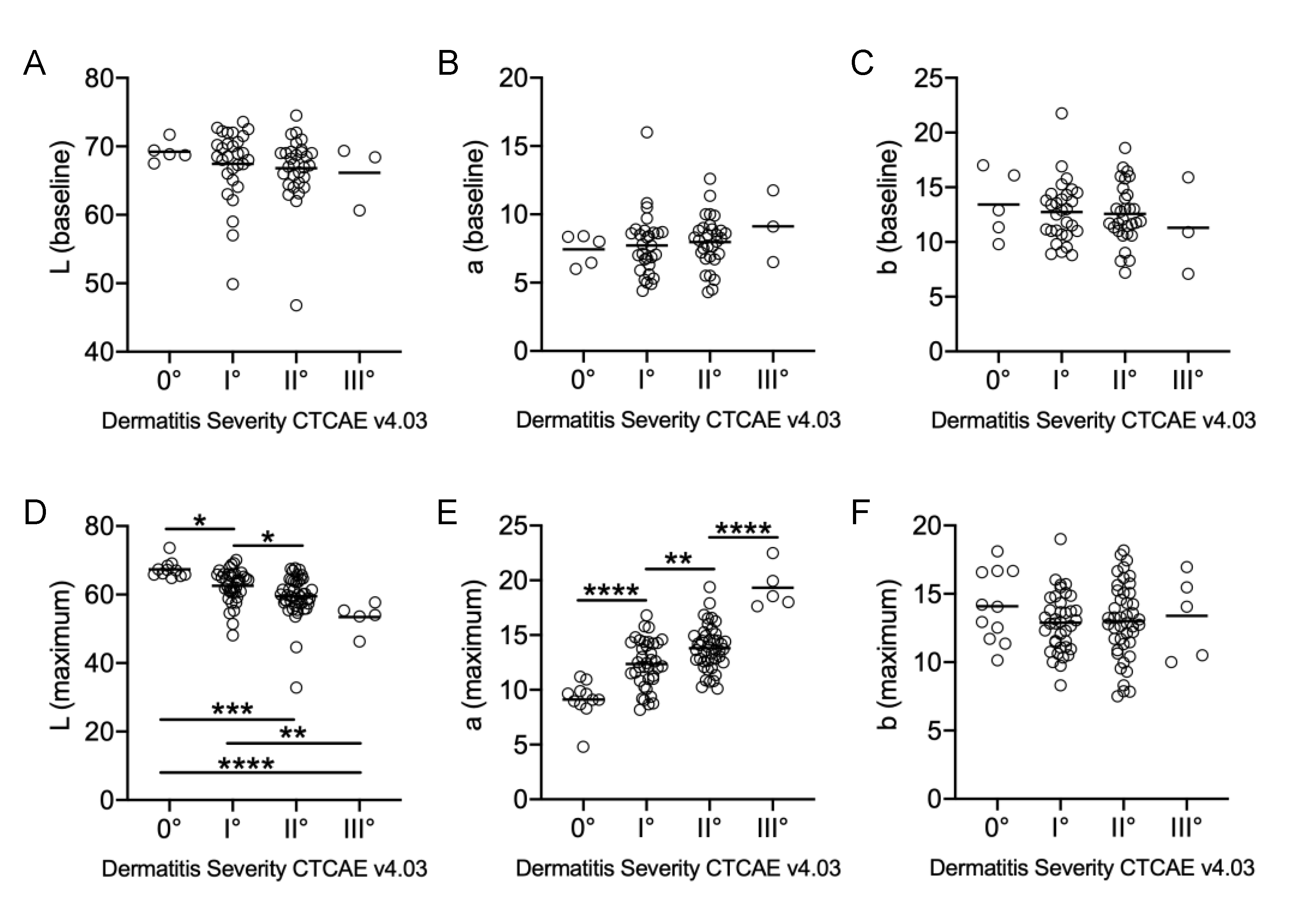
2.2. Visual Examinations and Spectrophotometry Correlate Positively with Maximum a* Values and Inversely for Maximum L* Values
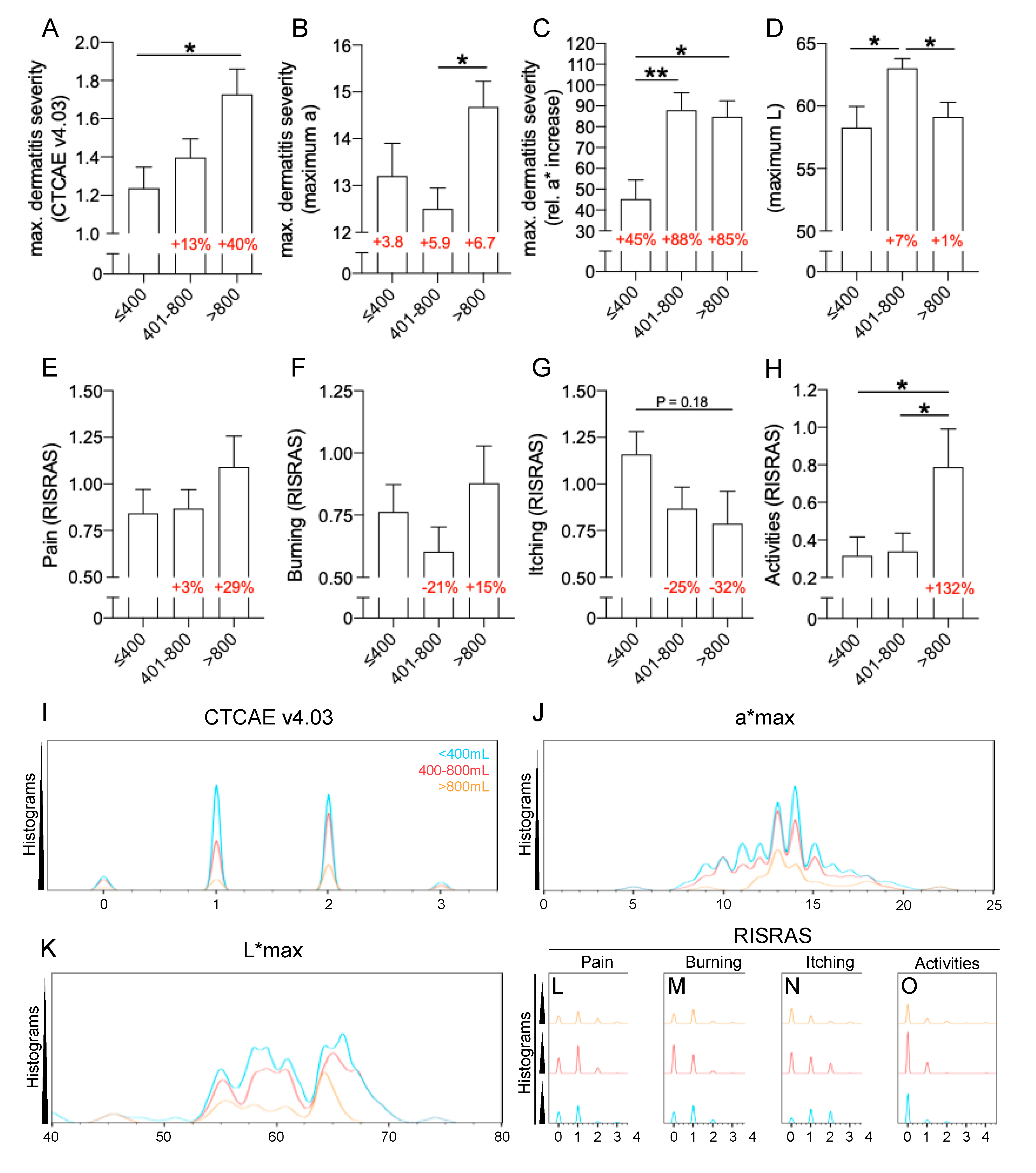
2.3. Patient-Related Risk Factors: Breast Volume and Darker Skin Are Risk Factors for Severe RID
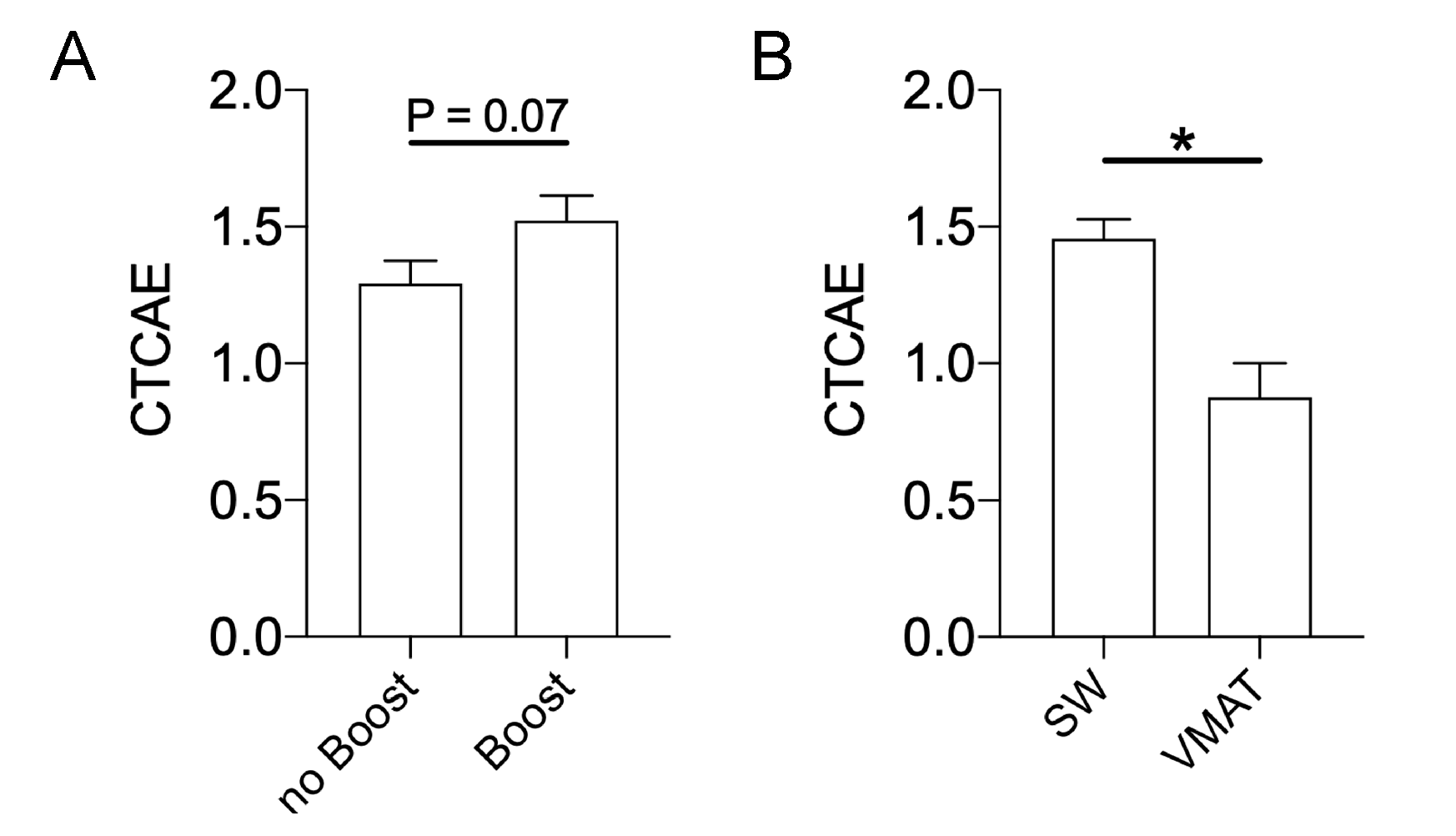
2.4. Impact of a Sequential Boost and Treatment Technique on Maximum RID Severity
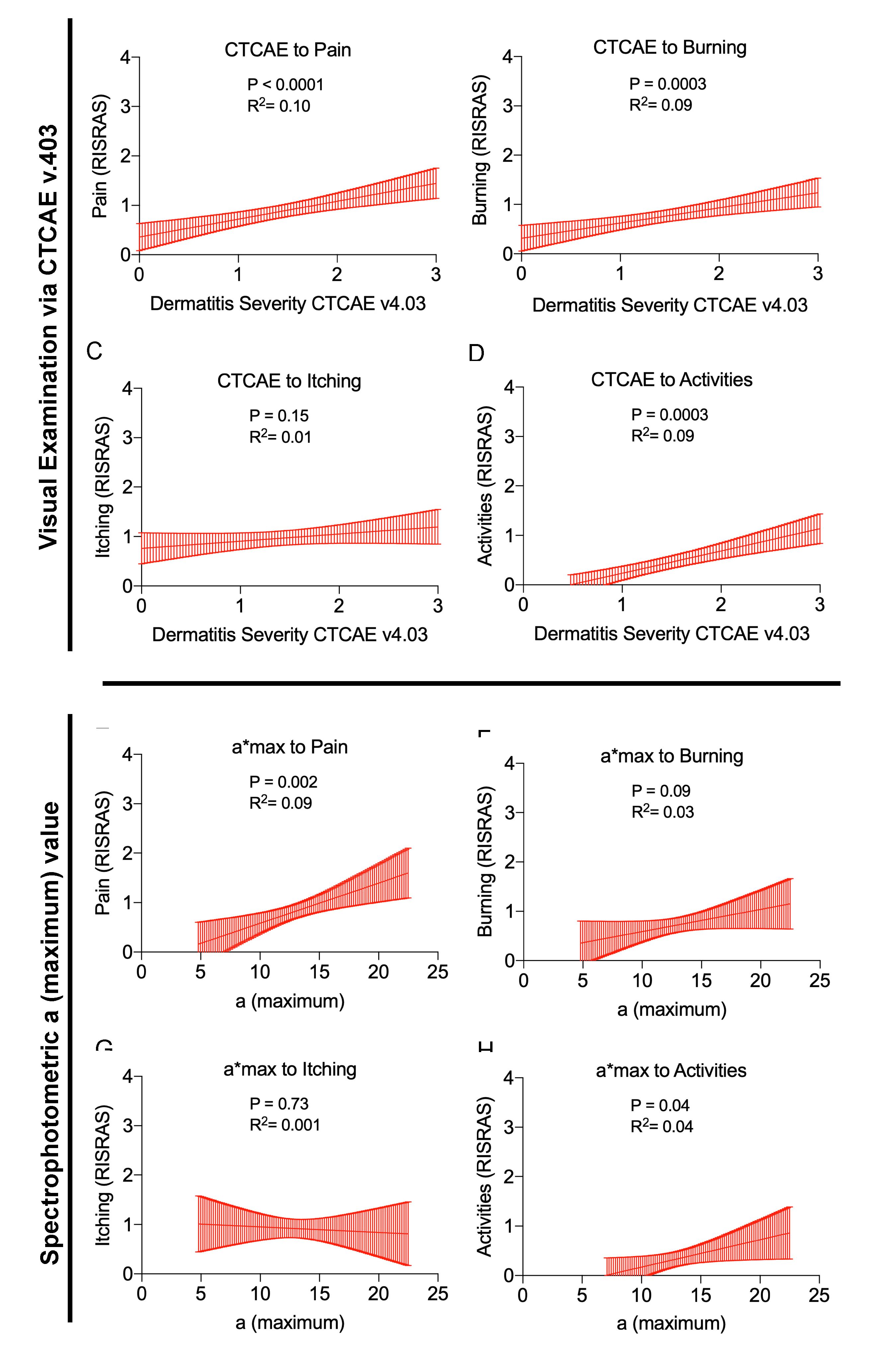
2.5. Correlations between Physician Assessed RID Severity, Objective Skin Color, and Subjective Symptoms
3. Discussion
4. Material and Methods
4.1. Radiation Technique
4.2. Visual Evaluation of Radiation-Induced Dermatitis (RID) and Spectrophotometry (SP)
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| RID | Radiation-Induced Dermatitis |
| RT | Radiotherapy |
| Gy | Gray (Unit) |
| SP | Spectrophotometry |
| CTCAE | Common Terminology Criteria for Adverse Effects |
| RTOG | Radiation Therapy Oncology Group |
| EORTG | European Organization for Research and Treatment of Cancer |
| L* max | maximum L value (spectrophotometry) |
| a* max | maximum a value (spectrophotometry) |
| b* max | maximum b value (spectrophotometry) |
| RISRAS | Radiation-Induced Skin Reaction Assessment Scale |
| fx | Fraction(s) |
| CF | Conventional Fractionation |
| HF | Hypofractionation |
| SD | Standard Deviation |
| WBI | Whole Breast Irradiation |
| BMI | Body Mass Index |
| TNM | TNM Classification of Malignant Tumors |
| DCIS | Ductal Carcinoma In Situ |
| HI(D2%–D98%)/D50% | Homogeneity Index(D2%–D98%)/D50% |
| HI-RTOG | Homogeneity Index (Radiation Therapy Oncology Group) |
| SW | Sliding Window |
| VMAT | Volumetric (partial-) arc therapy |
| IMRT | Intensity-modulated Radiotherapy (sliding-window) |
| Dmean | Mean dose within the target volume |
| rel. D2% | Dose received by 2% of the target volume relative to the prescribed dose |
| rel. D50% | Dose received by 50% of the target volume relative to the prescribed dose |
| rel. D98% | Dose received by 98% of the target volume relative to the prescribed dose |
| V≥107% | Volume receiving ≥ 107% of prescribed dose |
| ANOVA | Analysis Of Variance |
References
- Shaitelman, S.F.; Schlembach, P.J.; Arzu, I.; Ballo, M.; Bloom, E.S.; Buchholz, D.; Chronowski, G.M.; Dvorak, T.; Grade, E.; Hoffman, K.E.; et al. Acute and short-term toxic effects of conventionally fractionated vs hypofractionated whole-breast irradiation: A randomized clinical trial. JAMA Oncol. 2015, 1, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Schmeel, L.C.; Koch, D.; Schmeel, F.C.; Röhner, F.; Schoroth, F.; Bücheler, B.M.; Mahlmann, B.; Leitzen, C.; Schüller, H.; Tschirner, S.; et al. Acute radiation-induced skin toxicity in hypofractionated vs. conventional whole-breast irradiation: An objective, randomized multicenter assessment using spectrophotometry. Radiother. Oncol. 2020, 146, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Beşe, N.; Hendry, J.; Jeremic, B. Effects of prolongation of overall treatment time due to unplanned interruptions during radiotherapy of different tumor sites and practical methods for compensation. Int. J. Radiat. Oncol. 2007, 68, 654–661. [Google Scholar] [CrossRef]
- Haviland, J.; Bentzen, S.M.; Bliss, J.M.; Yarnold, J. Prolongation of overall treatment time as a cause of treatment failure in early breast cancer: An analysis of the UK START (Standardisation of Breast Radiotherapy) trials of radiotherapy fractionation. Radiother. Oncol. 2016, 121, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Schmeel, L.C.; Koch, D.; Schmeel, F.C.; Bücheler, B.; Leitzen, C.; Mahlmann, B.; Kunze, D.; Heimann, M.; Brüser, D.; Abramian, A.-V.; et al. Hydrofilm polyurethane films reduce radiation dermatitis severity in Hypofractionated whole-breast irradiation: An objective, intra-patient randomized dual-center assessment. Polymers 2019, 11, 2112. [Google Scholar] [CrossRef]
- Yoshikawa, N.; Inomata, T.; Shimbo, T.; Takahashi, M.; Uesugi, Y.; Juri, H.; Narumi, Y. Appropriate evaluation of and risk factors for radiation dermatitis in breast cancer patients receiving hypofractionated whole-breast irradiation after breast-conserving surgery. Breast Cancer 2012, 21, 170–176. [Google Scholar] [CrossRef]
- Hill-Kayser, C.E.; Vachani, C.; Hampshire, M.K.; Di Lullo, G.A.; Metz, J.M. Cosmetic outcomes and complications reported by patients having undergone breast-conserving treatment. Int. J. Radiat. Oncol. 2012, 83, 839–844. [Google Scholar] [CrossRef]
- Ciammella, P.; Podgornii, A.; Galeandro, M.; Micera, R.; Ramundo, D.; Palmieri, T.; Cagni, E.; Iotti, C. Toxicity and cosmetic outcome of hypofractionated whole-breast radiotherapy: Predictive clinical and dosimetric factors. Radiat. Oncol. 2014, 9, 97. [Google Scholar] [CrossRef]
- Fuzissaki, M.D.A.; Paiva, C.E.; Gozzo, T.D.O.; Maia, M.D.A.; Canto, P.P.L.; Maia, Y. Is there agreement between evaluators that used two scoring systems to measure acute radiation dermatitis? Medicine 2019, 98, e14917. [Google Scholar] [CrossRef] [PubMed]
- Momm, F.; Bartelt, S.; Haigis, K.; Große-Sender, A.; Witucki, G. Spectrophotometric skin measurements correlate with EORTC/RTOG-Common toxicity criteria. Strahlenther. Onkol. 2005, 181, 392–395. [Google Scholar] [CrossRef]
- Partl, R.; Lehner, J.; Winkler, P.; Kapp, K.S. Testing the feasibility of augmented digital skin imaging to objectively compare the efficacy of topical treatments for radiodermatitis. PLoS ONE 2019, 14, e0218018. [Google Scholar] [CrossRef] [PubMed]
- Denekamp, J.; Ball, M.M.; Fowler, J.F. Recovery and repopulation in mouse skin as a function of time after X-Irradiation. Radiat. Res. 1969, 37, 361. [Google Scholar] [CrossRef]
- Fowler, J.; Denekamp, J.; Delapeyre, C.; Harris, S.R.; Sheldon, P. Skin Reactions in Mice after Multifraction X-irradiation. Int. J. Radiat. Boil. Relat. Stud. Phys. Chem. Med. 1974, 25, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Hopewell, J. The Skin: Its Structure and Response to Ionizing Radiation. Int. J. Radiat. Boil. 1990, 57, 751–773. [Google Scholar] [CrossRef] [PubMed]
- Hopewell, J.W.; Nyman, J.; Turesson, I. Time factor for acute tissue reactions following fractionated irradiation: A balance between repopulation and enhanced radiosensitivity. Int. J. Radiat. Boil. 2003, 79, 513–524. [Google Scholar] [CrossRef]
- Yamazaki, H.; Takenaka, T.; Aibe, N.; Suzuki, G.; Yoshida, K.; Nakamura, S.; Masui, K.; Kimoto, T.; Sasaki, N.; Nishimura, T.; et al. Comparison of radiation dermatitis between hypofractionated and conventionally fractionated postoperative radiotherapy: Objective, longitudinal assessment of skin color. Sci. Rep. 2018, 8, 12306. [Google Scholar] [CrossRef]
- Kitajima, M.; Mikami, K.; Noto, Y.; Itaki, C.; Fukushi, Y.; Hirota, Y.; Mariya, Y.; Tsushima, M.; Kattou, K.; Osanai, T. Quantitative assessment of radiodermatitis through a non-invasive objective procedure in patients with breast cancer. Mol. Clin. Oncol. 2019, 12, 89–93. [Google Scholar] [CrossRef]
- Russell, N.; Knaken, H.; Bruinvis, I.; Hart, A.; Begg, A.; Lebesque, J. Quantification of patient to patient variation of skin erythema developing as a response to radiotherapy. Radiother. Oncol. 1994, 30, 213–221. [Google Scholar] [CrossRef]
- Tucker, S.L.; Turesson, I.; Thames, H.D. Evidence for individual differences in the radiosensitivity of human skin. Eur. J. Cancer 1992, 28, 1783–1791. [Google Scholar] [CrossRef]
- Turesson, I.; Nyman, J.; Holmberg, E.; Odén, A. Prognostic factors for acute and late skin reactions in radiotheraphy patients. Int. J. Radiat. Oncol. 1996, 36, 1065–1075. [Google Scholar] [CrossRef]
- Turesson, I.; Notter, G.; Wickström, I.; Johansson, K.-A.; Eklund, S. The influence of irradiation time per treatment session on acute and late skin reactions: A study on human skin. Radiother. Oncol. 1984, 2, 235–245. [Google Scholar] [CrossRef]
- Turesson, I.; Notter, G. Skin reaction as a biologic parameter for control of different dose schedules and gap correction. Acta Radiol. Ther. Phys. Boil. 1976, 15, 162–176. [Google Scholar] [CrossRef]
- Schmeel, L.C.; Koch, D.; Stumpf, S.; Leitzen, C.; Simon, B.; Schüller, H.; Vornholt, S.; Schoroth, F.; Müdder, T.; Röhner, F.; et al. Prophylactically applied Hydrofilm polyurethane film dressings reduce radiation dermatitis in adjuvant radiation therapy of breast cancer patients. Acta Oncol. 2018, 57, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Pignol, J.-P.; Olivotto, I.; Rakovitch, E.; Gardner, S.; Sixel, K.; Beckham, W.; Vu, T.T.T.; Truong, P.; Ackerman, I.; Paszat, L. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J. Clin. Oncol. 2008, 26, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- De Santis, M.C.; Bonfantini, F.; Di Salvo, F.; Dispinzieri, M.; Mantero, E.; Soncini, F.; Baili, P.; Sant, M.; Bianchi, G.V.; Maggi, C.; et al. Factors influencing acute and late toxicity in the era of adjuvant hypofractionated breast radiotherapy. Breast 2016, 29, 90–95. [Google Scholar] [CrossRef]
- Porock, D.; Kristjanson, L.; Nikoletti, S.; Cameron, F.; Pedler, P. Predicting the severity of radiation skin reactions in women with breast cancer. Oncol. Nurs. Forum 1998, 25, 1019–1029. [Google Scholar]
- Badakhshi, H.; Kaul, D.; Nadobny, J.; Wille, B.; Sehouli, J.; Budach, V. Image-guided volumetric modulated arc therapy for breast cancer: A feasibility study and plan comparison with three-dimensional conformal and intensity-modulated radiotherapy. Br. J. Radiol. 2013, 86, 20130515. [Google Scholar] [CrossRef]
- Virén, T.; Heikkilä, J.; Myllyoja, K.; Koskela, K.; Lahtinen, T.; Seppälä, J. Tangential volumetric modulated arc therapy technique for left-sided breast cancer radiotherapy. Radiat. Oncol. 2015, 10, 79. [Google Scholar] [CrossRef]
- Qiu, J.-J.; Chang, Z.; Wu, Q.J.; Yoo, S.; Horton, J.; Yin, F.-F. Impact of volumetric modulated Arc therapy technique on treatment with partial breast irradiation. Int. J. Radiat. Oncol. 2010, 78, 288–296. [Google Scholar] [CrossRef]
- Osman, S.O.S.; Hol, S.; Poortmans, P.M.; Essers, M. Volumetric modulated arc therapy and breath-hold in image-guided locoregional left-sided breast irradiation. Radiother. Oncol. 2014, 112, 17–22. [Google Scholar] [CrossRef]
- Schnur, J.B.; Ouellette, S.C.; DiLorenzo, T.A.; Green, S.; Montgomery, G.H. A qualitative analysis of acute skin toxicity among breast cancer radiotherapy patients. Psycho-Oncol. 2011, 20, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Drobin, K.; Marczyk, M.; Halle, M.; Danielsson, D.; Papiez, A.; Sangsuwan, T.; Bendes, A.; Hong, M.-G.; Qundos, U.; Harms-Ringdahl, M.; et al. Molecular profiling for predictors of radiosensitivity in patients with breast or head-and-neck cancer. Cancers 2020, 12, 753. [Google Scholar] [CrossRef] [PubMed]
- Mbah, C.; De Ruyck, K.; De Schrijver, S.; De Sutter, C.; Schiettecatte, K.; Monten, C.; Paelinck, L.; De Neve, W.; Thierens, H.; West, C.M.; et al. A new approach for modeling patient overall radiosensitivity and predicting multiple toxicity endpoints for breast cancer patients. Acta Oncol. 2018, 57, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Grossberg, A.J.; Lei, X.; Xu, T.; Shaitelman, S.F.; Hoffman, K.E.; Bloom, E.S.; Stauder, M.C.; Tereffe, W.; Schlembach, P.J.; Woodward, W.A.; et al. Association of transforming growth factor β Polymorphism C-509T with radiation-induced fibrosis among patients with early-stage breast cancer: A secondary analysis of a randomized clinical trial. JAMA Oncol. 2018, 4, 1751–1757. [Google Scholar] [CrossRef]

| Characteristic | Parameter | Value |
|---|---|---|
| Gender | ||
| Female | 142 | |
| Age [a] | ||
| Mean ± SD | 59.7 ± 11.0 | |
| Range | 19–84 | |
| BMI | ||
| Mean ± SD | 26.8 ± 4.5 | |
| Range | 18–45.7 | |
| BMI < 20 | 7 | |
| BMI 20–25 | 30 | |
| BMI 25–30 | 80 | |
| BMI 30–35 | 21 | |
| BMI > 35 | 4 | |
| Breast volume | ||
| [mL] | Mean ± SD | 623.6 ± 347.6 |
| Range | 124–1771 | |
| Vol. > 400 mL | 30.65% | |
| Vol. 400–800 mL | 42.74% | |
| Vol. > 800 mL | 26.61% | |
| Ethnicity | ||
| Caucasian | 142 | |
| Initial L* values | ||
| Mean ± SD | 67.2 ± 4.81 | |
| Range | 46.8–76.5 | |
| Initial L* < 65 | 22.40% | |
| Initial L* 65–70 | 52.20% | |
| Initial L* > 70 | 25.40% | |
| T Classification (TNM) | ||
| DCIS | 18 | |
| T1 | 92 | |
| T2 | 32 | |
| Radiation therapy | ||
| 50 Gy in 25 fx | 71 | |
| 40.05 Gy in 15 fx | 71 | |
| Seq. boost of 16 | ||
| Gy in 8 fx | 67 | |
| Boost vol. [mL] | ||
| Mean ± SD | 200.4 ± 107 | |
| Range | 40–505 | |
| HI(D2%–D98%)/D50% | 0.144 ± 0.04 | |
| HIRTOG (max. isodose in | ||
| target/reference isodose) | 1.07 ± 0.03 | |
| rel. D2% [%] | 94.29 ± 14.43 | |
| rel. D50% [%] | 90.56 ± 13.75 | |
| rel. D98% [%] | 81.24 ± 12.88 | |
| Treatment technique | ||
| Sliding-window | 115 | |
| VMAT | 7 |
| Characteristic | Parameter | L* Max | a* Max |
|---|---|---|---|
| CTCAE 0° | Mean ± SD | 67.35 ± 2.44 | 9.11 ± 1.68 |
| Median | 67.0 | 9.1 | |
| 95% CI | 65.9–68.8 | 8.1–10.1 | |
| Range | 64.8–73.6 | 4.8–11.2 | |
| CTCAE I° | Mean ± SD | 62.55 ± 4.78 | 10.95 ± 2.18 |
| Median | 64.0 | 11.0 | |
| 95% CI | 61.0–64.1 | 10.3–11.6 | |
| Range | 48.1–70.1 | 8.18–16.8 | |
| CTCAE II° | Mean ± SD | 59.47 ± 6.08 | 12.68 ± 2.01 |
| Median | 59.6 | 12.84 | |
| 95% CI | 57.7–61.3 | 12.1–13.3 | |
| Range | 32.9–67.7 | 10.1–19.4 | |
| CTCAE III° | Mean ± SD | 53.4 ± 4.29 | 19.32 ± 1.97 |
| Median | 54.0 | 18.56 | |
| 95% CI | 49.6–57.2 | 17.6–21.1 | |
| Range | 46.3–57.7 | 17.6–22.5 |
| Parameter | CTCAE | L* Max. | a* Max. |
|---|---|---|---|
| Age [a] | 0.1289 (−) | 0.0825 (+) | 0.5491 (−) |
| BMI [kg/m2] | 0.2282 (+) | 0.9428 (−) | 0.1212 (+) |
| Breast volume [mL] | 0.0047 (+) | 0.9379 (+) | 0.0509 (+) |
| Boost (y/n) | 0.0635 (+) | 0.5084 (−) | 0.9629 (+) |
| Boost vol. [mL] | 0.6119 (+) | 0.6456 (−) | 0.4453 (+) |
| Mean Dose to PTV [%] | 0.4435 (−) | 0.9059 (−) | 0.9889 (+) |
| rel. D2% | 0.5635 (−) | 0.1062 (−) | 0.1147 (+) |
| rel. D50% | 0.5532 (−) | 0.1087 (−) | 0.1210 (+) |
| rel. D98% | 0.5595 (−) | 0.1011 (−) | 0.1178 (+) |
| V≥107% [mL] | 0.8424 (+) | 0.5715 (−) | 0.9127 (+) |
| HIRTOG | 0.8209 (+) | 0.8924 (+) | 0.3468 (+) |
| HI(D2%–D98%)/D50% | 0.7125 (+) | 0.7217 (+) | 0.7711 (+) |
| L* baseline | 0.2848 (−) | <0.0001 (+) | 0.0155 (−) |
| a* baseline | 0.2892 (+) | <0.0001 (−) | 0.0006 (+) |
| b* baseline | 0.3702 (−) | 0.0002 (−) | 0.5846 (+) |
| CTCAE | <0.0001 (−) | <0.0001 (+) | |
| L* max. | <0.0001 (−) | <0.0001 (−) | |
| a* max. | <0.0001 (+) | <0.0001 (−) | |
| b* max. | 0.5229 (−) | 0.1258 (−) | 0.4628 (+) |
| Pain (RISRAS) | <0.0001 (+) | 0.0115 (−) | 0.002 (+) |
| Burning (RISRAS) | 0.0003 (+) | 0.025 (−) | 0.0856 (+) |
| Itching (RISRAS) | 0.1517 (+) | 0.8369 (−) | 0.7335 (−) |
| Activities (RISRAS) | <0.0001 (+) | 0.0011 (−) | 0.0409 (+) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Böhner, A.M.C.; Koch, D.; Schmeel, F.C.; Röhner, F.; Schoroth, F.; Sarria, G.R.; Abramian, A.-V.; Baumert, B.G.; Giordano, F.A.; Schmeel, L.C. Objective Evaluation of Risk Factors for Radiation Dermatitis in Whole-Breast Irradiation Using the Spectrophotometric L*a*b Color-Space. Cancers 2020, 12, 2444. https://doi.org/10.3390/cancers12092444
Böhner AMC, Koch D, Schmeel FC, Röhner F, Schoroth F, Sarria GR, Abramian A-V, Baumert BG, Giordano FA, Schmeel LC. Objective Evaluation of Risk Factors for Radiation Dermatitis in Whole-Breast Irradiation Using the Spectrophotometric L*a*b Color-Space. Cancers. 2020; 12(9):2444. https://doi.org/10.3390/cancers12092444
Chicago/Turabian StyleBöhner, Alexander M. C., David Koch, Frederic Carsten Schmeel, Fred Röhner, Felix Schoroth, Gustavo R. Sarria, Alina-Valik Abramian, Brigitta Gertrud Baumert, Frank Anton Giordano, and Leonard Christopher Schmeel. 2020. "Objective Evaluation of Risk Factors for Radiation Dermatitis in Whole-Breast Irradiation Using the Spectrophotometric L*a*b Color-Space" Cancers 12, no. 9: 2444. https://doi.org/10.3390/cancers12092444
APA StyleBöhner, A. M. C., Koch, D., Schmeel, F. C., Röhner, F., Schoroth, F., Sarria, G. R., Abramian, A.-V., Baumert, B. G., Giordano, F. A., & Schmeel, L. C. (2020). Objective Evaluation of Risk Factors for Radiation Dermatitis in Whole-Breast Irradiation Using the Spectrophotometric L*a*b Color-Space. Cancers, 12(9), 2444. https://doi.org/10.3390/cancers12092444






