Classical and Non-Classical Progesterone Signaling in Breast Cancers
Abstract
1. Introduction
2. P4 in Normal Breast Development
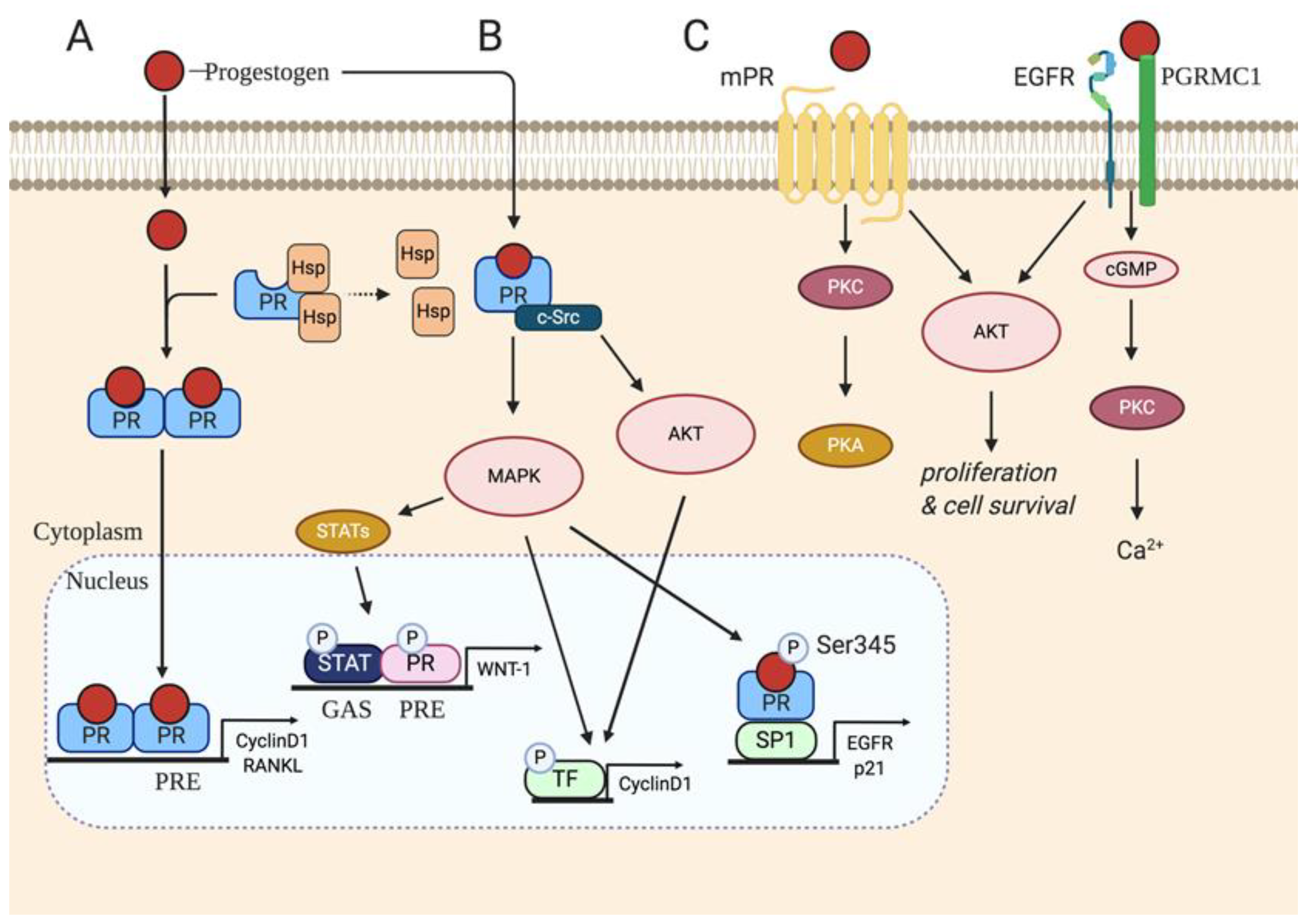
3. P4 Classical Signaling
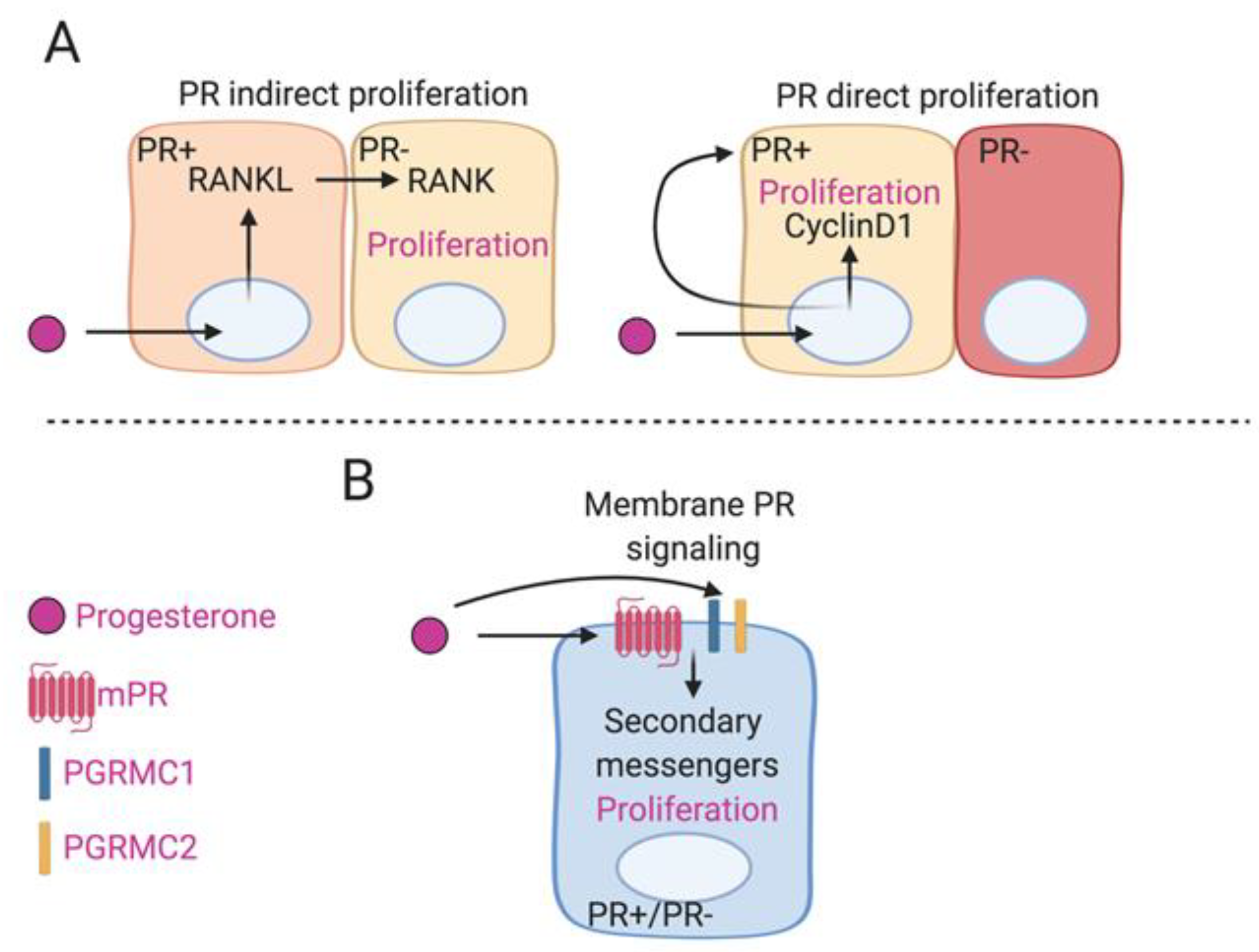
4. Non-Classical P4 Signaling
5. P4 Classical Signaling in Breast Cancer
5.1. Natural P4 vs. Synthetic Progestins
5.2. Targeting PRs for Breast Cancer Treatment
6. Non-Classical P4 Signaling in Breast Cancer
Role of Progesterone Receptor Membrane Component 1 (PGRMC1) in Breast Cancer
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| E2 | Estrogen |
| ER | Estrogen receptor |
| P4 | Progesterone |
| PR | Progesterone receptor |
| TNBC | Triple negative breast cancer |
| WHI | Women’s health initiative |
| HRT | Hormone replacement therapy |
| HER2 | Human epidermal growth factor receptor 2 |
| MPA | Medroxyprogesterone acetate |
| RANKL | Receptor activator of nuclear factor kappa-B ligand |
| RANK | Receptor activator of nuclear factor kappa-B |
| CCND1 | Cyclin D1 |
| HRE | Hormone response element |
| PRE | Progesterone response element |
| EGFR | Epidermal growth factor receptor |
| MAPK | Mitogen activated protein kinase |
| STAT | Signal transducer and activator of transcription |
| mPR | Membrane progesterone receptor |
| GPCR | G protein coupled receptor |
| PAQR | Progestin and adipoQ receptor |
| MAPR | Membrane associated progesterone receptor |
| PGRMC1 | Progesterone receptor membrane component 1 |
| PGRMC2 | Progesterone receptor membrane component 2 |
| GLI-1 | Glioma associated oncogene homolog 1 |
| NF-kB | Nuclear factor kappa B |
| OVX | Ovariectomized |
| ACI | August copenhagen irish |
| SPRM | Selective progesterone receptor modulator |
| TPA | Telapristone acetate |
| NET | Norethisterone |
| CK2 | Casein kinase 2 |
| 5αP | 5α-dihydroprogesterone |
| 3αHP | 3α-dihydroprogesterone |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, P.; Bandlamudi, C.; Cheng, M.L.; Srinivasan, P.; Chavan, S.S.; Friedman, N.D.; Rosen, E.Y.; Richards, A.L.; Bouvier, N.; Selcuklu, S.D.; et al. Tumour lineage shapes BRCA-mediated phenotypes. Nature 2019, 571, 576–579. [Google Scholar] [CrossRef] [PubMed]
- VoPham, T.; DuPré, N.; Tamimi, R.M.; James, P.; Bertrand, K.A.; Vieira, V.; Laden, F.; Hart, J.E. Environmental radon exposure and breast cancer risk in the Nurses’ Health Study II. Environ. Health 2017, 16, 97. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.K.; Choi, D.H.; Park, W.; Cha, H.; Nam, S.J.; Kim, S.W.; Lee, J.E.; Yu, J.; Im, Y.H.; Ahn, J.S.; et al. Effect of Body Mass Index on Survival in Breast Cancer Patients According to Subtype, Metabolic Syndrome, and Treatment. Clin. Breast Cancer 2018, 18, e1141–e1147. [Google Scholar] [CrossRef]
- Apter, D.; Vihko, R. Early menarche, a risk factor for breast cancer, indicates early onset of ovulatory cycles. J. Clin. Endocrinol. Metab. 1983, 57, 82–86. [Google Scholar] [CrossRef]
- Kelsey, J.L.; Gammon, M.D.; John, E.M. Reproductive factors and breast cancer. Epidemiol. Rev. 1993, 15, 36–47. [Google Scholar] [CrossRef]
- Chen, C.L.; Weiss, N.S.; Newcomb, P.; Barlow, W.; White, E. Hormone replacement therapy in relation to breast cancer. JAMA 2002, 287, 734–741. [Google Scholar] [CrossRef]
- Subramani, R.; Lakshmanaswamy, R. Pregnancy and Breast Cancer. Prog. Mol. Biol. Transl. Sci. 2017, 151, 81–111. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Rohan, T.E.; Manson, J.E.; Aragaki, A.K.; Kaunitz, A.; Stefanick, M.L.; Simon, M.S.; Johnson, K.C.; Wactawski-Wende, J.; O’Sullivan, M.J.; et al. Breast Cancer After Use of Estrogen Plus Progestin and Estrogen Alone: Analyses of Data From 2 Women’s Health Initiative Randomized Clinical Trials. JAMA Oncol. 2015, 1, 296–305. [Google Scholar] [CrossRef]
- Olver, I.N. Prevention of breast cancer. Med. J. Aust. 2016, 205, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Perou, C.M. Deconstructing the molecular portraits of breast cancer. Mol. Oncol. 2011, 5, 5–23. [Google Scholar] [CrossRef]
- Godoy-Ortiz, A.; Sanchez-Muñoz, A.; Chica Parrado, M.R.; Álvarez, M.; Ribelles, N.; Rueda Dominguez, A.; Alba, E. Deciphering HER2 Breast Cancer Disease: Biological and Clinical Implications. Front. Oncol. 2019, 9, 1124. [Google Scholar] [CrossRef]
- Prat, A.; Adamo, B.; Cheang, M.C.; Anders, C.K.; Carey, L.A.; Perou, C.M. Molecular characterization of basal-like and non-basal-like triple-negative breast cancer. Oncologist 2013, 18, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Cronin, K.A.; Kurian, A.W.; Andridge, R. Differences in Breast Cancer Survival by Molecular Subtypes in the United States. Cancer Epidemiol. Biomark. Prev. 2018, 27, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Parise, C.A.; Caggiano, V. Risk of mortality of node-negative, ER/PR/HER2 breast cancer subtypes in T1, T2, and T3 tumors. Breast Cancer Res. Treat. 2017, 165, 743–750. [Google Scholar] [CrossRef]
- Ahmed, I.S.; Rohe, H.J.; Twist, K.E.; Craven, R.J. Pgrmc1 (progesterone receptor membrane component 1) associates with epidermal growth factor receptor and regulates erlotinib sensitivity. J. Biol. Chem. 2010, 285, 24775–24782. [Google Scholar] [CrossRef]
- Blackburn, S.A.; Parks, R.M.; Cheung, K.L. Fulvestrant for the treatment of advanced breast cancer. Expert Rev. Anticancer Ther. 2018, 18, 619–628. [Google Scholar] [CrossRef]
- Haricharan, S.; Punturi, N.; Singh, P.; Holloway, K.R.; Anurag, M.; Schmelz, J.; Schmidt, C.; Lei, J.T.; Suman, V.; Hunt, K.; et al. Loss of MutL Disrupts CHK2-Dependent Cell-Cycle Control through CDK4/6 to Promote Intrinsic Endocrine Therapy Resistance in Primary Breast Cancer. Cancer Discov. 2017, 7, 1168–1183. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016, 17, 425–439. [Google Scholar] [CrossRef]
- AlFakeeh, A.; Brezden-Masley, C. Overcoming endocrine resistance in hormone receptor-positive breast cancer. Curr. Oncol. 2018, 25, S18–S27. [Google Scholar] [CrossRef] [PubMed]
- Arpino, G.; Weiss, H.; Lee, A.V.; Schiff, R.; De Placido, S.; Osborne, C.K.; Elledge, R.M. Estrogen receptor-positive, progesterone receptor-negative breast cancer: Association with growth factor receptor expression and tamoxifen resistance. J. Natl. Cancer Inst. 2005, 97, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Purdie, C.A.; Quinlan, P.; Jordan, L.B.; Ashfield, A.; Ogston, S.; Dewar, J.A.; Thompson, A.M. Progesterone receptor expression is an independent prognostic variable in early breast cancer: A population-based study. Br. J. Cancer 2014, 110, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Van Mackelenbergh, M.T.; Denkert, C.; Nekljudova, V.; Karn, T.; Schem, C.; Marmé, F.; Stickeler, E.; Jackisch, C.; Hanusch, C.; Huober, J.; et al. Outcome after neoadjuvant chemotherapy in estrogen receptor-positive and progesterone receptor-negative breast cancer patients: A pooled analysis of individual patient data from ten prospectively randomized controlled neoadjuvant trials. Breast Cancer Res. Treat. 2018, 167, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Creighton, C.J.; Kent Osborne, C.; Van de Vijver, M.J.; Foekens, J.A.; Klijn, J.G.; Horlings, H.M.; Nuyten, D.; Wang, Y.; Zhang, Y.; Chamness, G.C.; et al. Molecular profiles of progesterone receptor loss in human breast tumors. Breast Cancer Res. Treat. 2009, 114, 287–299. [Google Scholar] [CrossRef]
- Viale, G.; Regan, M.M.; Maiorano, E.; Mastropasqua, M.G.; Dell’Orto, P.; Rasmussen, B.B.; Raffoul, J.; Neven, P.; Orosz, Z.; Braye, S.; et al. Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal early breast cancer: BIG 1-98. J. Clin. Oncol. 2007, 25, 3846–3852. [Google Scholar] [CrossRef]
- Thakkar, J.P.; Mehta, D.G. A review of an unfavorable subset of breast cancer: Estrogen receptor positive progesterone receptor negative. Oncologist 2011, 16, 276–285. [Google Scholar] [CrossRef]
- Bae, S.Y.; Kim, S.; Lee, J.H.; Lee, H.C.; Lee, S.K.; Kil, W.H.; Kim, S.W.; Lee, J.E.; Nam, S.J. Poor prognosis of single hormone receptor- positive breast cancer: Similar outcome as triple-negative breast cancer. BMC Cancer 2015, 15, 138. [Google Scholar] [CrossRef]
- Banks, E.; Beral, V.; Reeves, G.; Collaborators, M.W.S. Published results on breast cancer and hormone replacement therapy in the Million Women Study are correct. Climacteric 2004, 7, 415–416. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Anderson, G.L.; Gass, M.; Lane, D.S.; Aragaki, A.K.; Kuller, L.H.; Manson, J.E.; Stefanick, M.L.; Ockene, J.; Sarto, G.E.; et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA 2010, 304, 1684–1692. [Google Scholar] [CrossRef]
- Anderson, G.L.; Chlebowski, R.T.; Aragaki, A.K.; Kuller, L.H.; Manson, J.E.; Gass, M.; Bluhm, E.; Connelly, S.; Hubbell, F.A.; Lane, D.; et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: Extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012, 13, 476–486. [Google Scholar] [CrossRef]
- Inman, J.L.; Robertson, C.; Mott, J.D.; Bissell, M.J. Mammary gland development: Cell fate specification, stem cells and the microenvironment. Development 2015, 142, 1028–1042. [Google Scholar] [CrossRef] [PubMed]
- Lyons, W.R. Hormonal synergism in mammary growth. Proc. R. Soc. Lond. B Biol. Sci. 1958, 149, 303–325. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S. Endocrine control of mammarygland development and function in the C3H/ He Crgl mouse. J. Natl. Cancer Inst. 1958, 21, 1039–1063. [Google Scholar] [PubMed]
- Lange, C.A.; Yee, D. Progesterone and breast cancer. Womens Health 2008, 4, 151–162. [Google Scholar] [CrossRef]
- Okolowsky, N.; Furth, P.A.; Hamel, P.A. Oestrogen receptor-alpha regulates non-canonical Hedgehog-signalling in the mammary gland. Dev. Biol. 2014, 391, 219–229. [Google Scholar] [CrossRef]
- Lydon, J.P.; DeMayo, F.J.; Funk, C.R.; Mani, S.K.; Hughes, A.R.; Montgomery, C.A.; Shyamala, G.; Conneely, O.M.; O’Malley, B.W. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995, 9, 2266–2278. [Google Scholar] [CrossRef]
- Shi, H.Y.; Lydon, J.P.; Zhang, M. Hormonal defect in maspin heterozygous mice reveals a role of progesterone in pubertal ductal development. Mol. Endocrinol. 2004, 18, 2196–2207. [Google Scholar] [CrossRef]
- Atwood, C.S.; Hovey, R.C.; Glover, J.P.; Chepko, G.; Ginsburg, E.; Robison, W.G.; Vonderhaar, B.K. Progesterone induces side-branching of the ductal epithelium in the mammary glands of peripubertal mice. J. Endocrinol. 2000, 167, 39–52. [Google Scholar] [CrossRef]
- Beleut, M.; Rajaram, R.D.; Caikovski, M.; Ayyanan, A.; Germano, D.; Choi, Y.; Schneider, P.; Brisken, C. Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc. Natl. Acad. Sci. USA 2010, 107, 2989–2994. [Google Scholar] [CrossRef]
- Lain, A.R.; Creighton, C.J.; Conneely, O.M. Research resource: Progesterone receptor targetome underlying mammary gland branching morphogenesis. Mol. Endocrinol. 2013, 27, 1743–1761. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.; Shalev, E. Progesterone receptor profile in the decidua and fetal membrane. Front. Biosci. 2007, 12, 634–648. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E. The role of oestrogen and progesterone receptors in human mammary development and tumorigenesis. Breast Cancer Res. 2002, 4, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Vegeto, E.; Shahbaz, M.M.; Wen, D.X.; Goldman, M.E.; O’Malley, B.W.; McDonnell, D.P. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol. Endocrinol. 1993, 7, 1244–1255. [Google Scholar] [CrossRef]
- Taylor, A.H.; McParland, P.C.; Taylor, D.J.; Bell, S.C. The cytoplasmic 60 kDa progesterone receptor isoform predominates in the human amniochorion and placenta at term. Reprod. Biol. Endocrinol. 2009, 7, 22. [Google Scholar] [CrossRef]
- Richer, J.K.; Jacobsen, B.M.; Manning, N.G.; Abel, M.G.; Wolf, D.M.; Horwitz, K.B. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J. Biol. Chem. 2002, 277, 5209–5218. [Google Scholar] [CrossRef]
- Wei, L.L.; Gonzalez-Aller, C.; Wood, W.M.; Miller, L.A.; Horwitz, K.B. 5′-Heterogeneity in human progesterone receptor transcripts predicts a new amino-terminal truncated “C”-receptor and unique A-receptor messages. Mol. Endocrinol. 1990, 4, 1833–1840. [Google Scholar] [CrossRef][Green Version]
- Dai, Q.; Shah, A.A.; Garde, R.V.; Yonish, B.A.; Zhang, L.; Medvitz, N.A.; Miller, S.E.; Hansen, E.L.; Dunn, C.N.; Price, T.M. A truncated progesterone receptor (PR-M) localizes to the mitochondrion and controls cellular respiration. Mol. Endocrinol. 2013, 27, 741–753. [Google Scholar] [CrossRef]
- Conneely, O.M.; Mulac-Jericevic, B.; DeMayo, F.; Lydon, J.P.; O’Malley, B.W. Reproductive functions of progesterone receptors. Recent Prog. Horm. Res. 2002, 57, 339–355. [Google Scholar] [CrossRef]
- Mihm, M.; Gangooly, S.; Muttukrishna, S. The normal menstrual cycle in women. Anim. Reprod. Sci. 2011, 124, 229–236. [Google Scholar] [CrossRef]
- Ferguson, D.J.; Anderson, T.J. Morphological evaluation of cell turnover in relation to the menstrual cycle in the “resting” human breast. Br. J. Cancer 1981, 44, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, H.; Schneider, H.P. Progesterone—Promoter or inhibitor of breast cancer. Climacteric 2013, 16 (Suppl. S1), 54–68. [Google Scholar] [CrossRef] [PubMed]
- Brisken, C.; Park, S.; Vass, T.; Lydon, J.P.; O’Malley, B.W.; Weinberg, R.A. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc. Natl. Acad. Sci. USA 1998, 95, 5076–5081. [Google Scholar] [CrossRef] [PubMed]
- Knutson, T.P.; Lange, C.A. Tracking progesterone receptor-mediated actions in breast cancer. Pharmacol. Ther. 2014, 142, 114–125. [Google Scholar] [CrossRef]
- Tanos, T.; Sflomos, G.; Echeverria, P.C.; Ayyanan, A.; Gutierrez, M.; Delaloye, J.F.; Raffoul, W.; Fiche, M.; Dougall, W.; Schneider, P.; et al. Progesterone/RANKL is a major regulatory axis in the human breast. Sci. Transl. Med. 2013, 5, 182ra155. [Google Scholar] [CrossRef]
- Schramek, D.; Leibbrandt, A.; Sigl, V.; Kenner, L.; Pospisilik, J.A.; Lee, H.J.; Hanada, R.; Joshi, P.A.; Aliprantis, A.; Glimcher, L.; et al. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature 2010, 468, 98–102. [Google Scholar] [CrossRef]
- Network, C.G.A. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef]
- Mulac-Jericevic, B.; Lydon, J.P.; DeMayo, F.J.; Conneely, O.M. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc. Natl. Acad. Sci. USA 2003, 100, 9744–9749. [Google Scholar] [CrossRef]
- Faivre, E.J.; Daniel, A.R.; Hillard, C.J.; Lange, C.A. Progesterone receptor rapid signaling mediates serine 345 phosphorylation and tethering to specificity protein 1 transcription factors. Mol. Endocrinol. 2008, 22, 823–837. [Google Scholar] [CrossRef]
- Leehy, K.A.; Truong, T.H.; Mauro, L.J.; Lange, C.A. Progesterone receptors (PR) mediate STAT actions: PR and prolactin receptor signaling crosstalk in breast cancer models. J. Steroid Biochem. Mol. Biol. 2018, 176, 88–93. [Google Scholar] [CrossRef]
- Krietsch, T.; Fernandes, M.S.; Kero, J.; Lösel, R.; Heyens, M.; Lam, E.W.; Huhtaniemi, I.; Brosens, J.J.; Gellersen, B. Human homologs of the putative G protein-coupled membrane progestin receptors (mPRalpha, beta, and gamma) localize to the endoplasmic reticulum and are not activated by progesterone. Mol. Endocrinol. 2006, 20, 3146–3164. [Google Scholar] [CrossRef]
- Valadez-Cosmes, P.; Vázquez-Martínez, E.R.; Cerbón, M.; Camacho-Arroyo, I. Membrane progesterone receptors in reproduction and cancer. Mol. Cell Endocrinol. 2016, 434, 166–175. [Google Scholar] [CrossRef]
- Zhu, Y.; Bond, J.; Thomas, P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc. Natl. Acad. Sci. USA 2003, 100, 2237–2242. [Google Scholar] [CrossRef] [PubMed]
- Tokumoto, M.; Nagahama, Y.; Thomas, P.; Tokumoto, T. Cloning and identification of a membrane progestin receptor in goldfish ovaries and evidence it is an intermediary in oocyte meiotic maturation. Gen. Comp. Endocrinol. 2006, 145, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.S.; Pierron, V.; Michalovich, D.; Astle, S.; Thornton, S.; Peltoketo, H.; Lam, E.W.; Gellersen, B.; Huhtaniemi, I.; Allen, J.; et al. Regulated expression of putative membrane progestin receptor homologues in human endometrium and gestational tissues. J. Endocrinol. 2005, 187, 89–101. [Google Scholar] [CrossRef]
- Tang, Y.T.; Hu, T.; Arterburn, M.; Boyle, B.; Bright, J.M.; Emtage, P.C.; Funk, W.D. PAQR proteins: A novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J. Mol. Evol. 2005, 61, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Ashley, R.L.; Clay, C.M.; Farmerie, T.A.; Niswender, G.D.; Nett, T.M. Cloning and characterization of an ovine intracellular seven transmembrane receptor for progesterone that mediates calcium mobilization. Endocrinology 2006, 147, 4151–4159. [Google Scholar] [CrossRef]
- Hanna, R.; Pang, Y.; Thomas, P.; Zhu, Y. Cell-surface expression, progestin binding, and rapid nongenomic signaling of zebrafish membrane progestin receptors alpha and beta in transfected cells. J. Endocrinol. 2006, 190, 247–260. [Google Scholar] [CrossRef]
- Karteris, E.; Zervou, S.; Pang, Y.; Dong, J.; Hillhouse, E.W.; Randeva, H.S.; Thomas, P. Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors: Potential role in functional progesterone withdrawal at term. Mol. Endocrinol. 2006, 20, 1519–1534. [Google Scholar] [CrossRef]
- Cahill, M.A. Progesterone receptor membrane component 1: An integrative review. J. Steroid Biochem. Mol. Biol. 2007, 105, 16–36. [Google Scholar] [CrossRef]
- Kabe, Y.; Nakane, T.; Koike, I.; Yamamoto, T.; Sugiura, Y.; Harada, E.; Sugase, K.; Shimamura, T.; Ohmura, M.; Muraoka, K.; et al. Haem-dependent dimerization of PGRMC1/Sigma-2 receptor facilitates cancer proliferation and chemoresistance. Nat. Commun. 2016, 7, 11030. [Google Scholar] [CrossRef]
- Hughes, A.L.; Powell, D.W.; Bard, M.; Eckstein, J.; Barbuch, R.; Link, A.J.; Espenshade, P.J. Dap1/PGRMC1 binds and regulates cytochrome P450 enzymes. Cell Metab. 2007, 5, 143–149. [Google Scholar] [CrossRef]
- Wendler, A.; Wehling, M. PGRMC2, a yet uncharacterized protein with potential as tumor suppressor, migration inhibitor, and regulator of cytochrome P450 enzyme activity. Steroids 2013, 78, 555–558. [Google Scholar] [CrossRef]
- Bashour, N.M.; Wray, S. Progesterone directly and rapidly inhibits GnRH neuronal activity via progesterone receptor membrane component 1. Endocrinology 2012, 153, 4457–4469. [Google Scholar] [CrossRef][Green Version]
- Parker, C.G.; Galmozzi, A.; Wang, Y.; Correia, B.E.; Sasaki, K.; Joslyn, C.M.; Kim, A.S.; Cavallaro, C.L.; Lawrence, R.M.; Johnson, S.R.; et al. Ligand and Target Discovery by Fragment-Based Screening in Human Cells. Cell 2017, 168, 527–541.e529. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, D.; Wehling, M.; Leube, B.; Falkenstein, E. Cloning and tissue expression of two putative steroid membrane receptors. Biol. Chem. 1998, 379, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Jühlen, R.; Landgraf, D.; Huebner, A.; Koehler, K. Identification of a novel putative interaction partner of the nucleoporin ALADIN. Biol. Open 2016, 5, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, C.; Huck, V.; Wehling, M.; Wendler, A. In vitro inhibition of SKOV-3 cell migration as a distinctive feature of progesterone receptor membrane component type 2 versus type 1. Steroids 2012, 77, 1543–1550. [Google Scholar] [CrossRef]
- Galmozzi, A.; Kok, B.P.; Kim, A.S.; Montenegro-Burke, J.R.; Lee, J.Y.; Spreafico, R.; Mosure, S.; Albert, V.; Cintron-Colon, R.; Godio, C.; et al. PGRMC2 is an intracellular haem chaperone critical for adipocyte function. Nature 2019, 576, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Scarpin, K.M.; Graham, J.D.; Mote, P.A.; Clarke, C.L. Progesterone action in human tissues: Regulation by progesterone receptor (PR) isoform expression, nuclear positioning and coregulator expression. Nucl. Recept. Signal. 2009, 7, e009. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.D.; Yager, M.L.; Hill, H.D.; Byth, K.; O’Neill, G.M.; Clarke, C.L. Altered progesterone receptor isoform expression remodels progestin responsiveness of breast cancer cells. Mol. Endocrinol. 2005, 19, 2713–2735. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, B.M.; Schittone, S.A.; Richer, J.K.; Horwitz, K.B. Progesterone-independent effects of human progesterone receptors (PRs) in estrogen receptor-positive breast cancer: PR isoform-specific gene regulation and tumor biology. Mol. Endocrinol. 2005, 19, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.A.; Bellance, C.; Guiochon-Mantel, A.; Lombès, M.; Loosfelt, H. Differential regulation of breast cancer-associated genes by progesterone receptor isoforms PRA and PRB in a new bi-inducible breast cancer cell line. PLoS ONE 2012, 7, e45993. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Suarez, E.; Branstetter, D.; Armstrong, A.; Dinh, H.; Blumberg, H.; Dougall, W.C. RANK overexpression in transgenic mice with mouse mammary tumor virus promoter-controlled RANK increases proliferation and impairs alveolar differentiation in the mammary epithelia and disrupts lumen formation in cultured epithelial acini. Mol. Cell Biol. 2007, 27, 1442–1454. [Google Scholar] [CrossRef]
- Grimm, S.L.; Hartig, S.M.; Edwards, D.P. Progesterone Receptor Signaling Mechanisms. J. Mol. Biol. 2016, 428, 3831–3849. [Google Scholar] [CrossRef]
- Jones, D.H.; Nakashima, T.; Sanchez, O.H.; Kozieradzki, I.; Komarova, S.V.; Sarosi, I.; Morony, S.; Rubin, E.; Sarao, R.; Hojilla, C.V.; et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature 2006, 440, 692–696. [Google Scholar] [CrossRef]
- Boopalan, T.; Arumugam, A.; Parada, J.; Saltzstein, E.; Lakshmanaswamy, R. Receptor activator for nuclear factor-κB ligand signaling promotes progesterone-mediated estrogen-induced mammary carcinogenesis. Cancer Sci. 2015, 106, 25–33. [Google Scholar] [CrossRef]
- Tang, Z.N.; Zhang, F.; Tang, P.; Qi, X.W.; Jiang, J. Hypoxia induces RANK and RANKL expression by activating HIF-1α in breast cancer cells. Biochem. Biophys. Res. Commun. 2011, 408, 411–416. [Google Scholar] [CrossRef]
- Van Dam, P.A.; Verhoeven, Y.; Trinh, X.B.; Wouters, A.; Lardon, F.; Prenen, H.; Smits, E.; Baldewijns, M.; Lammens, M. RANK/RANKL signaling inhibition may improve the effectiveness of checkpoint blockade in cancer treatment. Crit. Rev. Oncol. Hematol. 2019, 133, 85–91. [Google Scholar] [CrossRef]
- González-Suárez, E. RANKL inhibition: A promising novel strategy for breast cancer treatment. Clin. Transl. Oncol. 2011, 13, 222–228. [Google Scholar] [CrossRef]
- Roy, P.G.; Pratt, N.; Purdie, C.A.; Baker, L.; Ashfield, A.; Quinlan, P.; Thompson, A.M. High CCND1 amplification identifies a group of poor prognosis women with estrogen receptor positive breast cancer. Int. J. Cancer 2010, 127, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Brisken, C. Progesterone signalling in breast cancer: A neglected hormone coming into the limelight. Nat. Rev. Cancer 2013, 13, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Diep, C.H.; Knutson, T.P.; Lange, C.A. Active FOXO1 Is a Key Determinant of Isoform-Specific Progesterone Receptor Transactivation and Senescence Programming. Mol. Cancer Res. 2016, 14, 141–162. [Google Scholar] [CrossRef] [PubMed]
- Dressing, G.E.; Knutson, T.P.; Schiewer, M.J.; Daniel, A.R.; Hagan, C.R.; Diep, C.H.; Knudsen, K.E.; Lange, C.A. Progesterone receptor-cyclin D1 complexes induce cell cycle-dependent transcriptional programs in breast cancer cells. Mol. Endocrinol. 2014, 28, 442–457. [Google Scholar] [CrossRef] [PubMed]
- Hopp, T.A.; Weiss, H.L.; Hilsenbeck, S.G.; Cui, Y.; Allred, D.C.; Horwitz, K.B.; Fuqua, S.A. Breast cancer patients with progesterone receptor PR-A-rich tumors have poorer disease-free survival rates. Clin. Cancer Res. 2004, 10, 2751–2760. [Google Scholar] [CrossRef]
- Mote, P.A.; Gompel, A.; Howe, C.; Hilton, H.N.; Sestak, I.; Cuzick, J.; Dowsett, M.; Hugol, D.; Forgez, P.; Byth, K.; et al. Progesterone receptor A predominance is a discriminator of benefit from endocrine therapy in the ATAC trial. Breast Cancer Res. Treat. 2015, 151, 309–318. [Google Scholar] [CrossRef]
- Rojas, P.A.; May, M.; Sequeira, G.R.; Elia, A.; Alvarez, M.; Martínez, P.; Gonzalez, P.; Hewitt, S.; He, X.; Perou, C.M.; et al. Progesterone Receptor Isoform Ratio: A Breast Cancer Prognostic and Predictive Factor for Antiprogestin Responsiveness. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef]
- McGowan, E.M.; Clarke, C.L. Effect of overexpression of progesterone receptor A on endogenous progestin-sensitive endpoints in breast cancer cells. Mol. Endocrinol. 1999, 13, 1657–1671. [Google Scholar] [CrossRef]
- McGowan, E.M.; Saad, S.; Bendall, L.J.; Bradstock, K.F.; Clarke, C.L. Effect of progesterone receptor a predominance on breast cancer cell migration into bone marrow fibroblasts. Breast Cancer Res. Treat. 2004, 83, 211–220. [Google Scholar] [CrossRef]
- Shyamala, G.; Yang, X.; Silberstein, G.; Barcellos-Hoff, M.H.; Dale, E. Transgenic mice carrying an imbalance in the native ratio of A to B forms of progesterone receptor exhibit developmental abnormalities in mammary glands. Proc. Natl. Acad. Sci. USA 1998, 95, 696–701. [Google Scholar] [CrossRef]
- National Institutes of Health. National Institutes of Health State-of-the-Science Conference statement: Management of menopause-related symptoms. Ann. Intern. Med. 2005, 142, 1003–1013. [Google Scholar] [CrossRef]
- Santen, R.J. Risk of breast cancer with progestins: Critical assessment of current data. Steroids 2003, 68, 953–964. [Google Scholar] [CrossRef]
- Asi, N.; Mohammed, K.; Haydour, Q.; Gionfriddo, M.R.; Vargas, O.L.; Prokop, L.J.; Faubion, S.S.; Murad, M.H. Progesterone vs. synthetic progestins and the risk of breast cancer: A systematic review and meta-analysis. Syst. Rev. 2016, 5, 121. [Google Scholar] [CrossRef] [PubMed]
- Fournier, A.; Berrino, F.; Riboli, E.; Avenel, V.; Clavel-Chapelon, F. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. Int. J. Cancer 2005, 114, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Blank, E.W.; Wong, P.Y.; Lakshmanaswamy, R.; Guzman, R.; Nandi, S. Both ovarian hormones estrogen and progesterone are necessary for hormonal mammary carcinogenesis in ovariectomized ACI rats. Proc. Natl. Acad. Sci. USA 2008, 105, 3527–3532. [Google Scholar] [CrossRef]
- Shull, J.D.; Spady, T.J.; Snyder, M.C.; Johansson, S.L.; Pennington, K.L. Ovary-intact, but not ovariectomized female ACI rats treated with 17beta-estradiol rapidly develop mammary carcinoma. Carcinogenesis 1997, 18, 1595–1601. [Google Scholar] [CrossRef][Green Version]
- Chlebowski, R.T.; Manson, J.E.; Anderson, G.L.; Cauley, J.A.; Aragaki, A.K.; Stefanick, M.L.; Lane, D.S.; Johnson, K.C.; Wactawski-Wende, J.; Chen, C.; et al. Estrogen plus progestin and breast cancer incidence and mortality in the Women’s Health Initiative Observational Study. J. Natl. Cancer Inst. 2013, 105, 526–535. [Google Scholar] [CrossRef]
- Hilton, H.N.; Graham, J.D.; Kantimm, S.; Santucci, N.; Cloosterman, D.; Huschtscha, L.I.; Mote, P.A.; Clarke, C.L. Progesterone and estrogen receptors segregate into different cell subpopulations in the normal human breast. Mol. Cell Endocrinol. 2012, 361, 191–201. [Google Scholar] [CrossRef]
- Gaddy, V.T.; Barrett, J.T.; Delk, J.N.; Kallab, A.M.; Porter, A.G.; Schoenlein, P.V. Mifepristone induces growth arrest, caspase activation, and apoptosis of estrogen receptor-expressing, antiestrogen-resistant breast cancer cells. Clin. Cancer Res. 2004, 10, 5215–5225. [Google Scholar] [CrossRef][Green Version]
- Poole, A.J.; Li, Y.; Kim, Y.; Lin, S.C.; Lee, W.H.; Lee, E.Y. Prevention of Brca1-mediated mammary tumorigenesis in mice by a progesterone antagonist. Science 2006, 314, 1467–1470. [Google Scholar] [CrossRef]
- Michna, H.; Schneider, M.R.; Nishino, Y.; el Etreby, M.F. Antitumor activity of the antiprogestins ZK 98.299 and RU 38.486 in hormone dependent rat and mouse mammary tumors: Mechanistic studies. Breast Cancer Res. Treat. 1989, 14, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.F.; Willsher, P.C.; Winterbottom, L.; Blamey, R.W.; Thorpe, S. Onapristone, a progesterone receptor antagonist, as first-line therapy in primary breast cancer. Eur. J. Cancer 1999, 35, 214–218. [Google Scholar] [CrossRef]
- Attardi, B.J.; Burgenson, J.; Hild, S.A.; Reel, J.R.; Blye, R.P. CDB-4124 and its putative monodemethylated metabolite, CDB-4453, are potent antiprogestins with reduced antiglucocorticoid activity: In vitro comparison to mifepristone and CDB-2914. Mol. Cell Endocrinol. 2002, 188, 111–123. [Google Scholar] [CrossRef]
- Attardi, B.J.; Burgenson, J.; Hild, S.A.; Reel, J.R. In vitro antiprogestational/antiglucocorticoid activity and progestin and glucocorticoid receptor binding of the putative metabolites and synthetic derivatives of CDB-2914, CDB-4124, and mifepristone. J. Steroid Biochem. Mol. Biol. 2004, 88, 277–288. [Google Scholar] [CrossRef]
- Wiehle, R.D.; Christov, K.; Mehta, R. Anti-progestins suppress the growth of established tumors induced by 7,12-dimethylbenz(a)anthracene: Comparison between RU486 and a new 21-substituted-19-nor-progestin. Oncol. Rep. 2007, 18, 167–174. [Google Scholar] [CrossRef][Green Version]
- Davaadelger, B.; Murphy, A.R.; Clare, S.E.; Lee, O.; Khan, S.A.; Kim, J.J. Mechanism of Telapristone Acetate (CDB4124) on Progesterone Receptor Action in Breast Cancer Cells. Endocrinology 2018, 159, 3581–3595. [Google Scholar] [CrossRef]
- Peluso, J.J. Non-genomic actions of progesterone in the normal and neoplastic mammalian ovary. Semin. Reprod. Med. 2007, 25, 198–207. [Google Scholar] [CrossRef]
- Dressing, G.E.; Alyea, R.; Pang, Y.; Thomas, P. Membrane progesterone receptors (mPRs) mediate progestin induced antimorbidity in breast cancer cells and are expressed in human breast tumors. Horm. Cancer 2012, 3, 101–112. [Google Scholar] [CrossRef]
- Ruan, X.; Zhang, Y.; Mueck, A.O.; Willibald, M.; Seeger, H.; Fehm, T.; Brucker, S.; Neubauer, H. Increased expression of progesterone receptor membrane component 1 is associated with aggressive phenotype and poor prognosis in ER-positive and negative breast cancer. Menopause 2017, 24, 203–209. [Google Scholar] [CrossRef]
- Wu, X.; Sun, L.; Wang, X.; Su, P.; Li, Z.; Zhang, C.; Wang, Y.; Gao, P.; Ma, R. Breast Cancer Invasion and Metastasis by mPRα Through the PI3K/Akt Signaling Pathway. Pathol. Oncol. Res. 2016, 22, 471–476. [Google Scholar] [CrossRef]
- Pang, Y.; Thomas, P. Progesterone signals through membrane progesterone receptors (mPRs) in MDA-MB-468 and mPR-transfected MDA-MB-231 breast cancer cells which lack full-length and N-terminally truncated isoforms of the nuclear progesterone receptor. Steroids 2011, 76, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhou, W.; Zhang, H.; Hu, Y.; Yu, L.; Zhang, Y.; Wang, S.; Wang, P.; Xia, W. Progesterone suppresses triple-negative breast cancer growth and metastasis to the brain via membrane progesterone receptor α. Int. J. Mol. Med. 2017, 40, 755–761. [Google Scholar] [CrossRef]
- Xie, M.; Zhou, L.; Chen, X.; Gainey, L.O.; Xiao, J.; Nanes, M.S.; Hou, A.; You, S.; Chen, Q. Progesterone and Src family inhibitor PP1 synergistically inhibit cell migration and invasion of human basal phenotype breast cancer cells. Biomed. Res. Int. 2015, 2015, 426429. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Zhu, X.; Liu, Z.; Shrubsole, M.; Varma, V.; Mayer, I.A.; Dai, Q.; Chen, Q.; You, S. Membrane progesterone receptor alpha as a potential prognostic biomarker for breast cancer survival: A retrospective study. PLoS ONE 2012, 7, e35198. [Google Scholar] [CrossRef] [PubMed]
- Salazar, M.; Lerma-Ortiz, A.; Hooks, G.M.; Ashley, A.K.; Ashley, R.L. Progestin-mediated activation of MAPK and AKT in nuclear progesterone receptor negative breast epithelial cells: The role of membrane progesterone receptors. Gene 2016, 591, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Cahill, M.A.; Jazayeri, J.A.; Catalano, S.M.; Toyokuni, S.; Kovacevic, Z.; Richardson, D.R. The emerging role of progesterone receptor membrane component 1 (PGRMC1) in cancer biology. Biochim. Biophys. Acta 2016, 1866, 339–349. [Google Scholar] [CrossRef]
- Neubauer, H.; Adam, G.; Seeger, H.; Mueck, A.O.; Solomayer, E.; Wallwiener, D.; Cahill, M.A.; Fehm, T. Membrane-initiated effects of progesterone on proliferation and activation of VEGF in breast cancer cells. Climacteric 2009, 12, 230–239. [Google Scholar] [CrossRef]
- Willibald, M.; Bayer, G.; Stahlhut, V.; Poschmann, G.; Stühler, K.; Gierke, B.; Pawlak, M.; Seeger, H.; Mueck, A.O.; Niederacher, D.; et al. Progesterone receptor membrane component 1 is phosphorylated upon progestin treatment in breast cancer cells. Oncotarget 2017, 8, 72480–72493. [Google Scholar] [CrossRef]
- Cai, G.; Ruan, X.; Gu, M.; Zhao, Y.; Wang, Y.; Mueck, A.O. PGRMC1 in animal breast cancer tissue and blood is associated with increased tumor growth with norethisterone in contrast to progesterone and dydrogesterone: Four-arm randomized placebo-controlled xenograft study. Gynecol. Endocrinol. 2020. [Google Scholar] [CrossRef]
- Faber, A.C.; Li, D.; Song, Y.; Liang, M.C.; Yeap, B.Y.; Bronson, R.T.; Lifshits, E.; Chen, Z.; Maira, S.M.; García-Echeverría, C.; et al. Differential induction of apoptosis in HER2 and EGFR addicted cancers following PI3K inhibition. Proc. Natl. Acad. Sci. USA 2009, 106, 19503–19508. [Google Scholar] [CrossRef]
- Grapa, C.M.; Mocan, T.; Gonciar, D.; Zdrehus, C.; Mosteanu, O.; Pop, T.; Mocan, L. Epidermal Growth Factor Receptor and Its Role in Pancreatic Cancer Treatment Mediated by Nanoparticles. Int. J. Nanomed. 2019, 14, 9693–9706. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Zhang, D.; Bartholomeusz, C.; Doihara, H.; Hortobagyi, G.N.; Ueno, N.T. Role of epidermal growth factor receptor in breast cancer. Breast Cancer Res. Treat. 2012, 136, 331–345. [Google Scholar] [CrossRef]
- Wang, Z. ErbB Receptors and Cancer. Methods Mol. Biol. 2017, 1652, 3–35. [Google Scholar] [CrossRef]
- Ritter, C.A.; Arteaga, C.L. The epidermal growth factor receptor-tyrosine kinase: A promising therapeutic target in solid tumors. Semin. Oncol. 2003, 30, 3–11. [Google Scholar] [CrossRef]
- Yoshitani, N.; Satou, K.; Saito, K.; Suzuki, S.; Hatanaka, H.; Seki, M.; Shinozaki, K.; Hirota, H.; Yokoyama, S. A structure-based strategy for discovery of small ligands binding to functionally unknown proteins: Combination of in silico screening and surface plasmon resonance measurements. Proteomics 2005, 5, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.S.; Rohe, H.J.; Twist, K.E.; Mattingly, M.N.; Craven, R.J. Progesterone receptor membrane component 1 (Pgrmc1): A heme-1 domain protein that promotes tumorigenesis and is inhibited by a small molecule. J. Pharmacol. Exp. Ther. 2010, 333, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Pedroza, D.A.; Rajamanickam, V.; Subramani, R.; Bencomo, A.; Galvez, A.; Lakshmanaswamy, R. Progesterone receptor membrane component 1 promotes the growth of breast cancers by altering the phosphoproteome and augmenting EGFR/PI3K/AKT signalling. Br. J. Cancer 2020. [Google Scholar] [CrossRef]
- Zhang, Y.; Ruan, X.; Mi, X.; Mueck, A.O. Expression of PGRMC1 in paraffin-embedded tissues of breast cancer. Int. J. Clin. Exp. Pathol. 2017, 10, 9639–9643. [Google Scholar]
- Di, L.J.; Byun, J.S.; Wong, M.M.; Wakano, C.; Taylor, T.; Bilke, S.; Baek, S.; Hunter, K.; Yang, H.; Lee, M.; et al. Genome-wide profiles of CtBP link metabolism with genome stability and epithelial reprogramming in breast cancer. Nat. Commun. 2013, 4, 1449. [Google Scholar] [CrossRef]
- Gross, P.; Honnorat, N.; Varol, E.; Wallner, M.; Trappanese, D.M.; Sharp, T.E.; Starosta, T.; Duran, J.M.; Koller, S.; Davatzikos, C.; et al. Nuquantus: Machine learning software for the characterization and quantification of cell nuclei in complex immunofluorescent tissue images. Sci. Rep. 2016, 6, 23431. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedroza, D.A.; Subramani, R.; Lakshmanaswamy, R. Classical and Non-Classical Progesterone Signaling in Breast Cancers. Cancers 2020, 12, 2440. https://doi.org/10.3390/cancers12092440
Pedroza DA, Subramani R, Lakshmanaswamy R. Classical and Non-Classical Progesterone Signaling in Breast Cancers. Cancers. 2020; 12(9):2440. https://doi.org/10.3390/cancers12092440
Chicago/Turabian StylePedroza, Diego A., Ramadevi Subramani, and Rajkumar Lakshmanaswamy. 2020. "Classical and Non-Classical Progesterone Signaling in Breast Cancers" Cancers 12, no. 9: 2440. https://doi.org/10.3390/cancers12092440
APA StylePedroza, D. A., Subramani, R., & Lakshmanaswamy, R. (2020). Classical and Non-Classical Progesterone Signaling in Breast Cancers. Cancers, 12(9), 2440. https://doi.org/10.3390/cancers12092440




