Addition of Radiotherapy to Immunotherapy: Effects on Outcome of Different Subgroups Using a Propensity Score Matching
Abstract
1. Introduction
2. Results
2.1. Patients and Treatment Characteristics
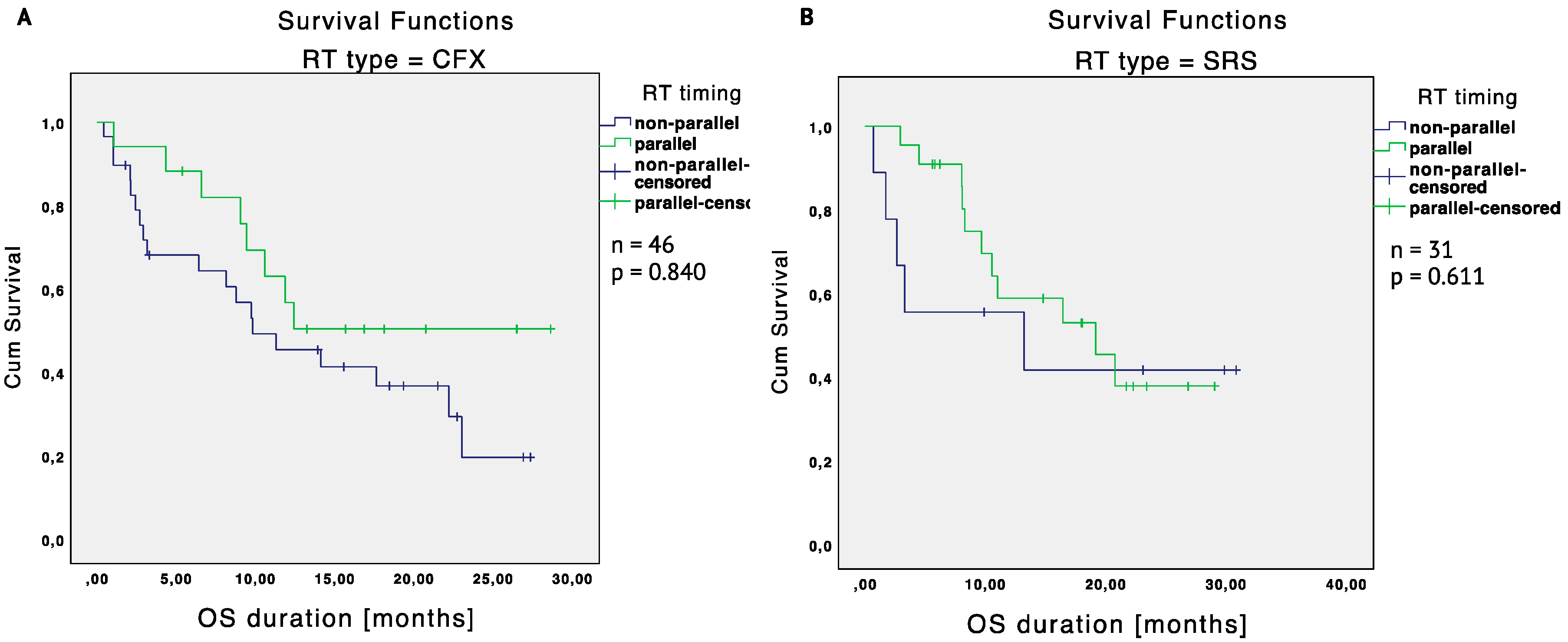
2.2. Immunotherapy and Radiotherapy
2.3. Outcome Evaluation
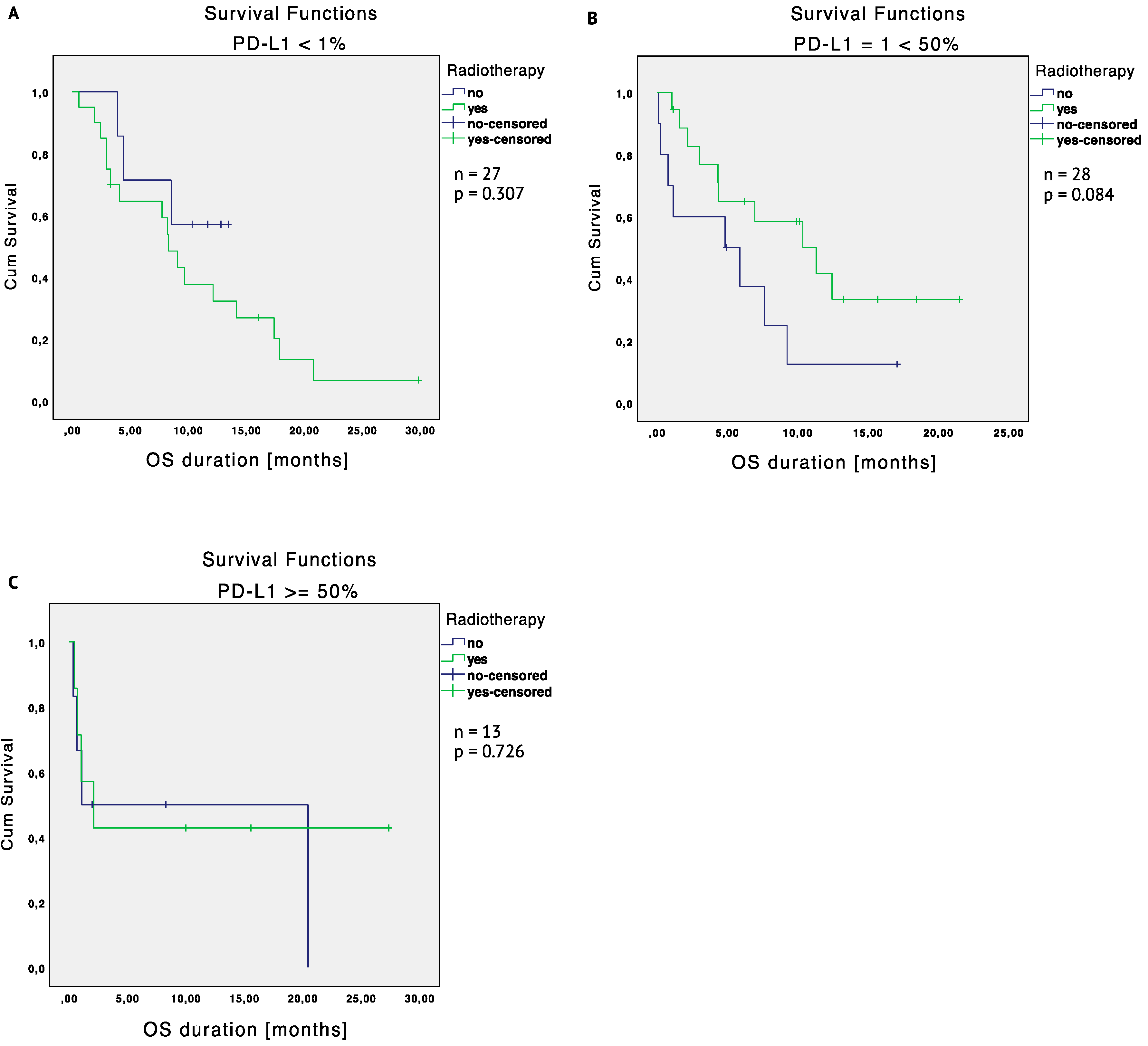
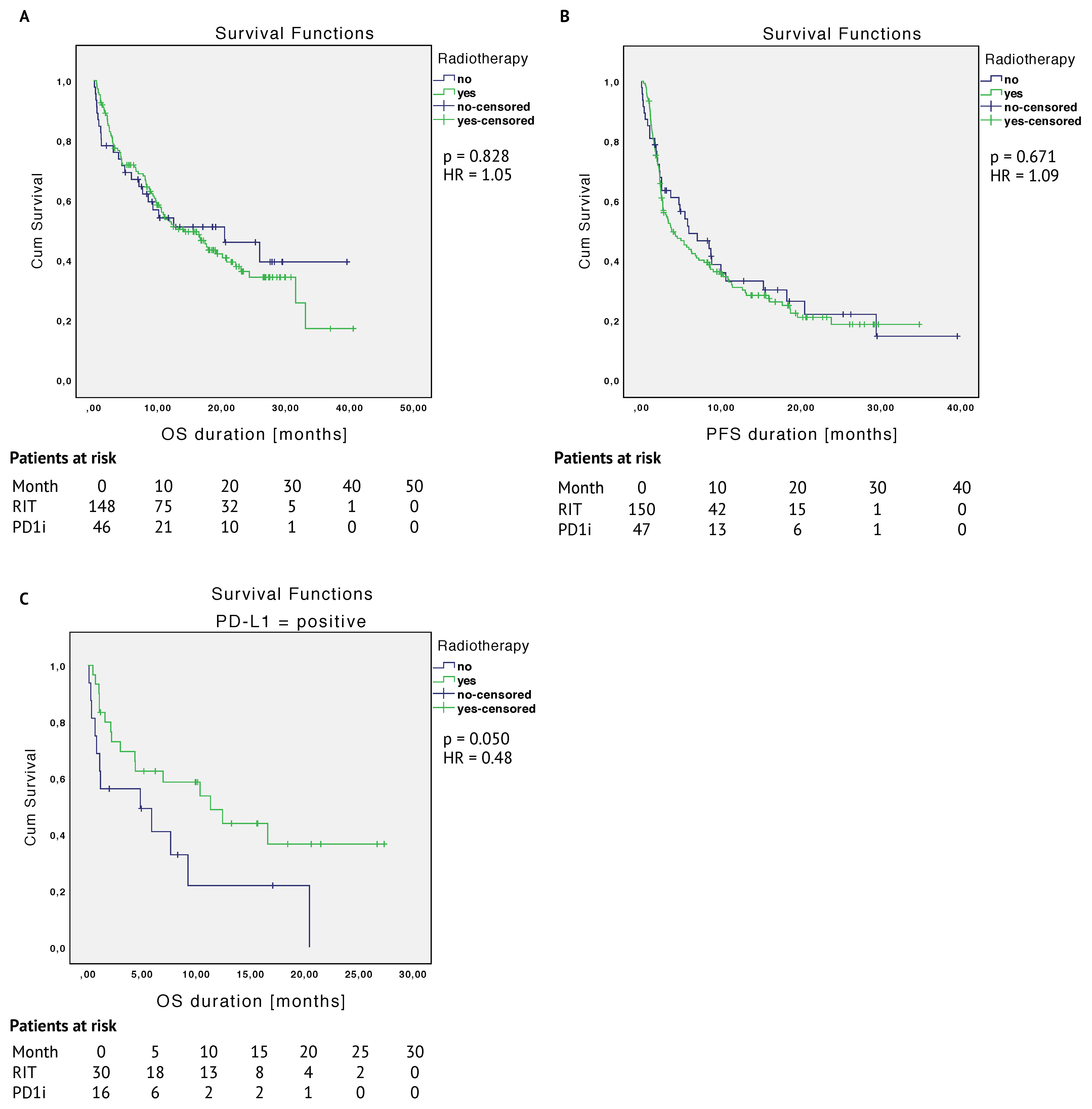
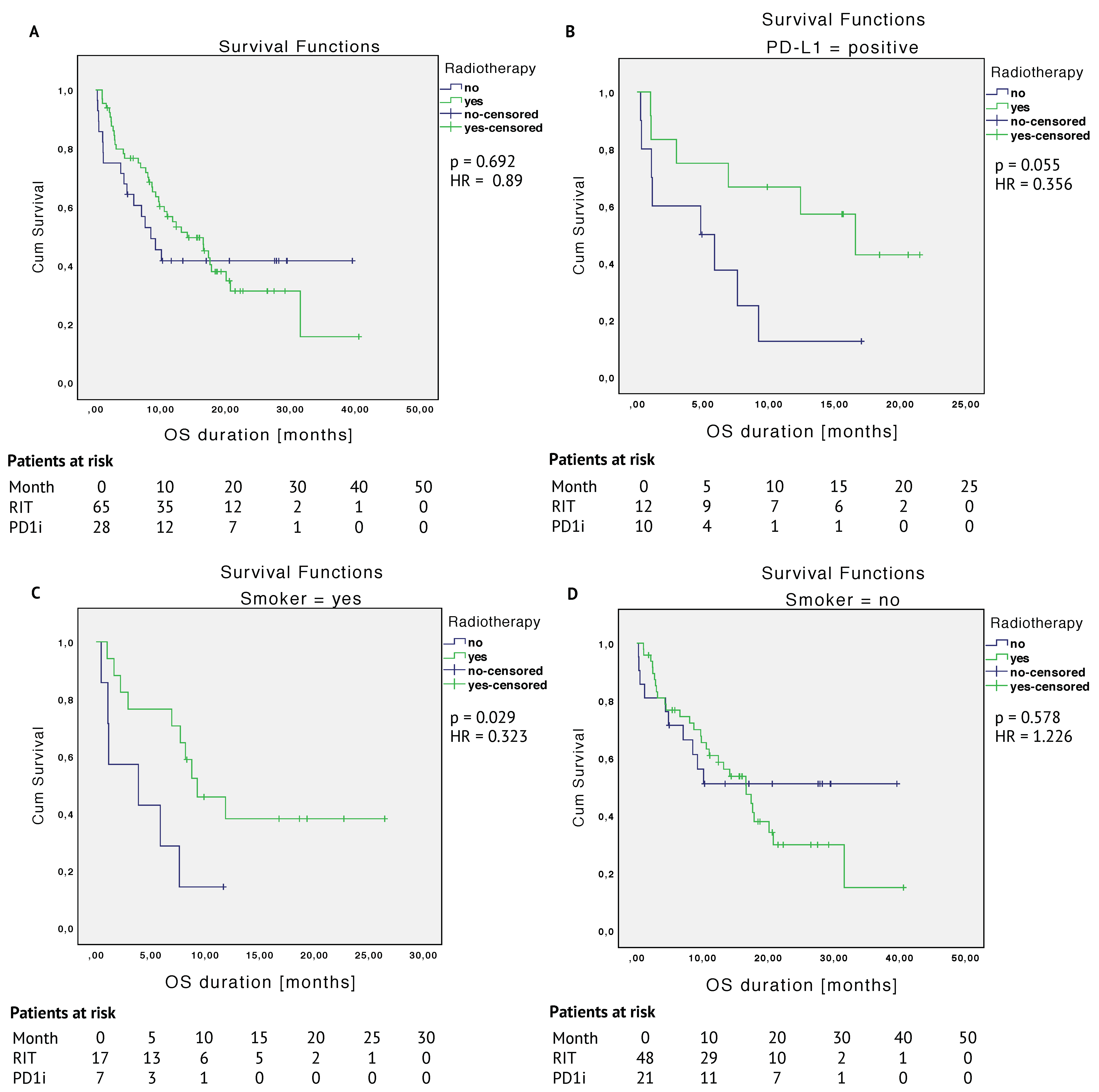
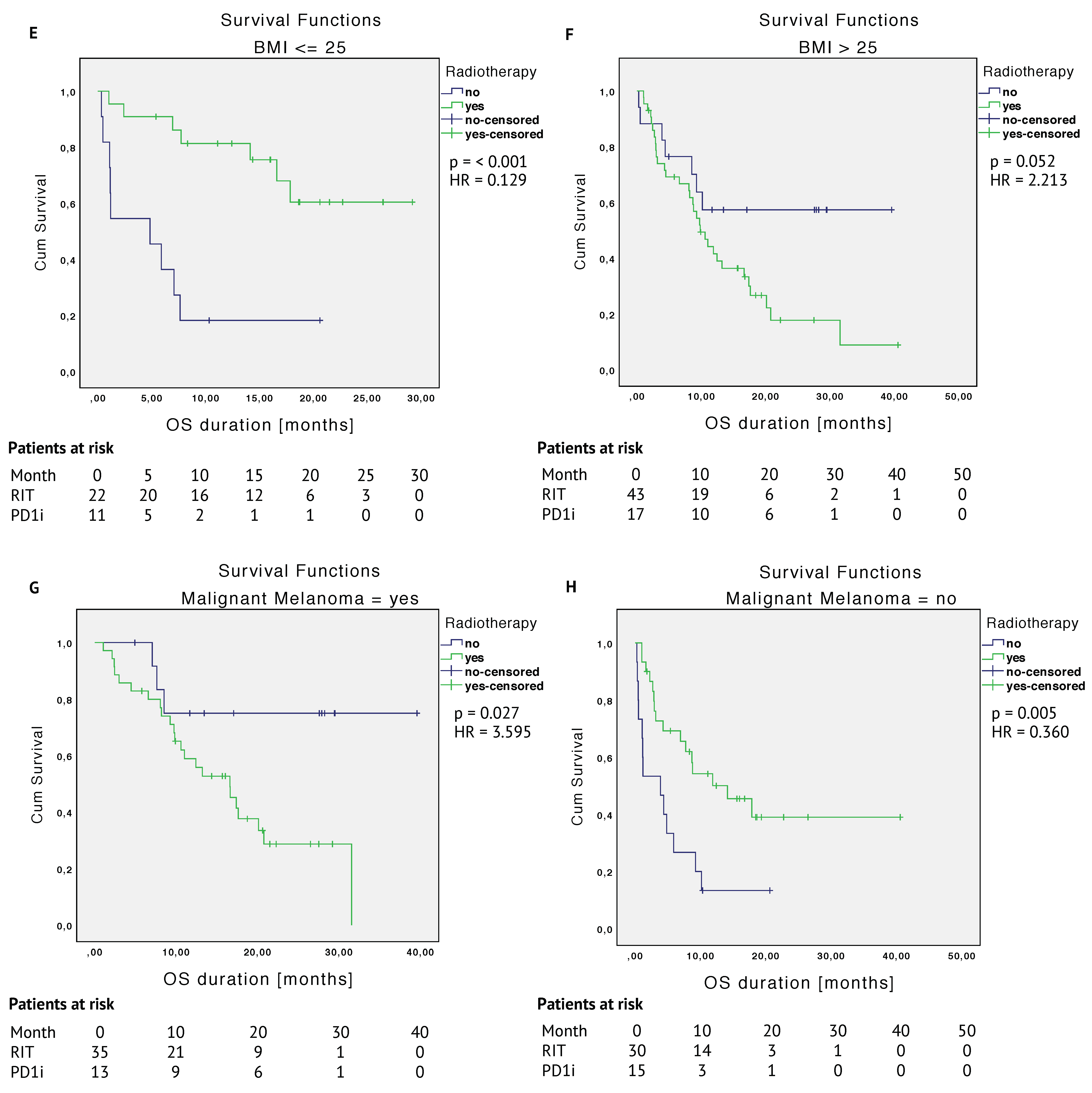
2.3.1. Unmatched Cohort
2.3.2. Matched Cohort
2.4. Safety
3. Discussion
4. Materials and Methods
4.1. Patients and Treatment
4.2. Outcome Evaluation
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
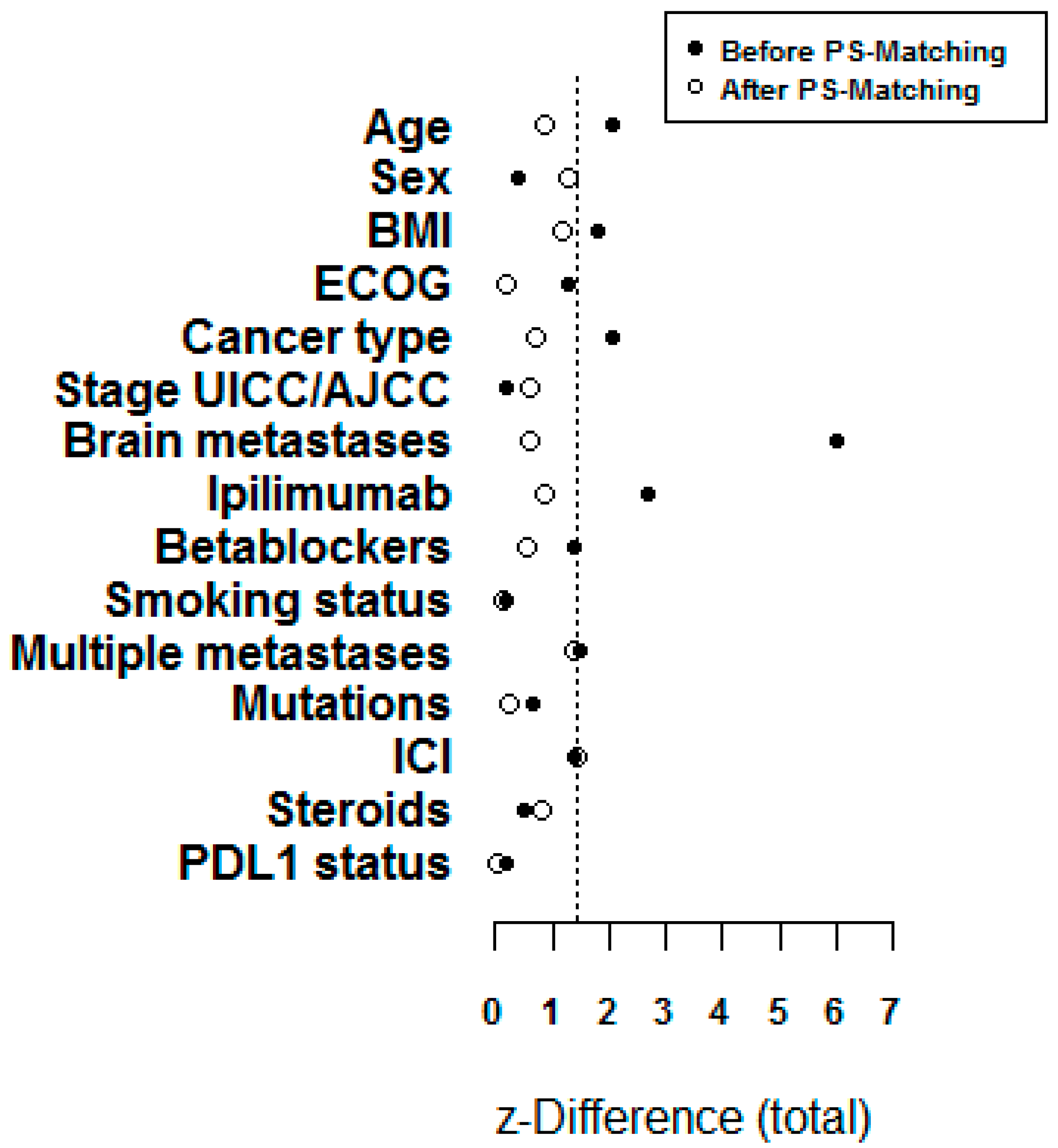
| Characteristic | Prior PSM n = 201 | After PSM n = 96 | ||||
|---|---|---|---|---|---|---|
| Radiotherapy | Radiotherapy | |||||
| yes (RIT) n = 153 | no (PD1i) n = 48 | z-Diff. | yes (RIT) n = 67 | no (PD1i) n = 29 | z-Diff. | |
| Age baseline (years) | 61.4 ± 13.5 | 65.5 ± 11.7 | −2.05 | 64.8 ± 13.6 | 67.1 ± 10.7 | −0.88 |
| Sex (male) | 91 (59.5) | 27 (56.3) | −0.39 | 44 (65.7) | 15 (51.7) | −1.27 |
| ECOG | −1.28 | −0.18 | ||||
| 0 | 50 (33.8) | 22 (48.9) | 27 (41.5) | 13 (46.4) | ||
| 1 | 73 (49.3) | 15 (33.3) | 26 (40.0) | 9 (32.1) | ||
| 2 | 18 (12.2) | 5 (11.1) | 8 (12.3) | 4 (14.3) | ||
| 3 | 5 (3.4) | 2 (4.4) | 4 (6.2) | 2 (7.1) | ||
| 4 | 2 (1.4) | 1 (2.2) | 0 (0.0) | 0 (0.0) | ||
| Cancer type | 2.06 | 0.59 | ||||
| MM | 78 (51.0) | 18 (38.3) | 36 (53.7) | 13 (46.4) | ||
| NSCLC | 53 (34.6) | 15 (31.9) | 17 (25.4) | 8 (28.6) | ||
| SCLC | 2 (1.3) | 0 (0.0) | 2 (3.0) | 0 (0.0) | ||
| RCC | 7 (4.6) | 7 (14.9) | 3 (4.5) | 1 (3.6) | ||
| Bladder CA | 2 (1.3) | 2 (4.3) | 1 (1.5) | 1 (3.6) | ||
| Esophageal CA | 3 (2.0) | 1 (2.1) | 2 (3.0) | 1 (3.6) | ||
| Hodgkin’s lymphoma | 4 (2.6) | 1 (2.1) | 4 (6.0) | 1 (3.6) | ||
| Other | 4 (2.6) | 3 (6.4) | 2 (3.0) | 3(10.7) | ||
| Stage UICC/AJCC | 0.17 | 0.59 | ||||
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| 2 | 2 (1.4) | 1 (2.2) | 2 (3.2) | 1 (3.4) | ||
| 3 | 12 (8.5) | 3 (6.7) | 10 (15.9) | 3 (10.3) | ||
| 4 | 128 (90.1) | 41 (91.1) | 51 (81.0) | 25 (86.2) | ||
| Multiple metastases (yes > 1 site) | 117 (81.8) | 28 (70.0) | 1.49 | 49 (79.0) | 16 (64.0) | 1.38 |
| PD-L1 status (pos.) | 32 (61.5) | 16 (64.0) | 0.21 | 13 (59.1) | 10 (58.8) | −0.02 |
| Brain metastases | 52 (34.0) | 2 (4.2) | 5.99 | 4 (6.0) | 1 (3.4) | 0.58 |
| Prior ipilimumab | 34 (22.5) | 4 (8.5) | 2.69 | 8 (11.9) | 2 (6.9) | 0.84 |
| Betablockers | 58 (39.7) | 11 (28.2) | 1.38 | 27 (40.3) | 10 (34.5) | 0.54 |
| Smoker | 35 (25.0) | 10 (26.3) | −0.16 | 17 (25.4) | 7 (24.1) | 0.13 |
| BMI | 26.8 ± 4.8 | 25.3 ± 4.6 | 1.79 | 27.3 ± 4.6 | 26.1 ± 4.8 | 1.17 |
| 26.08 (15.7–41.6) | 25.7 (15.6–38.6) | 26.1 (15.7–41.6) | 25.7 (15.6–38.6) | |||
| Pembrolizumab | 75 (49.0) | 18 (37.5) | 1.39 | 36 (53.7) | 11 (37.9) | 1.41 |
| Nivolumab | 78 (51.0) | 30 (62.5) | 31 (46.3) | 18 (62.1) | ||
| Steroids | 34 (23.3) | 9 (20.0) | 0.48 | 11 (16.7) | 7 (24.1) | −0.82 |
| Mutated genes | 0.68 | 0.23 | ||||
| BRAF | 35 (47.9) | 6 (35.3) | 11 (37.9) | 4 (28.6) | ||
| NRAS | 19 (26.0) | 7 (41.2) | 9 (31.0) | 6 (42.9) | ||
| TP53 | 4 (5.5) | 0 (0.0) | 2 (6.9) | 0 (0.0) | ||
| KRAS | 5 (6.8) | 2 (11.8) | 2 (6.9) | 2 (14.3) | ||
| Other | 9 (12.3) | 2 (11.8) | 5 (17.2) | 2 (14.3) | ||
| None | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Type of Treatment | Characteristic | Patients n | Mild Tox. (Grade 1 + 2) n (%) | Severe Tox. (Grade 3 + 4) n (%) | Not Specified | p Value |
|---|---|---|---|---|---|---|
| PD1i (n = 48) | Skin | 38 | 5 (13.2) | 2 (5.3) | 0 | 0.002 |
| Fatigue | 34 | 5 (14.7) | 0 | 1 (2.9) | 0.695 | |
| Vertigo | 33 | 0 | 1 (3.0) | 0 | 0.019 | |
| Headache | 44 | 2 (4.5) | 1 (2.3) | 0 | 0.362 | |
| Lung | 46 | 1 (2.2) | 7 (15.2) | 1 (2.2) | 0.115 | |
| GI | 43 | 6 (14.0) | 1 (2.3) | 0 | 0.950 | |
| Thyroid | 44 | 3 (6.8) | 2 (4.5) | 0 | 0.035 | |
| Liver | 43 | 1 (2.3) | 0 | 0 | 0.646 | |
| Fever | 32 | 1 (3.1) | 0 | 1 (3.1) | 0.556 | |
| RIT (n = 153) | Skin | 145 | 65 (44.8) | 1 (0.7) | 0 | |
| Fatigue | 144 | 59 (41.0) | 7 (4.9) | 2 (1.4) | ||
| Vertigo | 139 | 23 (16.5) | 2 (1.4) | 0 | ||
| Headache | 145 | 13 (9.0) | 2 (1.4) | 0 | ||
| Lung | 147 | 17 (11.6) | 15 (10.2) | 0 | ||
| GI | 147 | 40 (27.2) | 8 (5.4) | 1 (0.7) | ||
| Thyroid | 143 | 15 (10.5) | 0 | 1 (0.7) | ||
| Liver | 147 | 3 (2.0) | 3 (2.0) | 0 | ||
| Fever | 129 | 15 (11.6) | 3 (2.3) | 2 (1.6) |
| Characteristic | Type of Treatment | Total n = 201 n (%) | p Value | |
|---|---|---|---|---|
| PD1i n = 48 n (%) | RIT n = 153 n (%) | |||
| Skin | 7 (18.4) | 66 (45.5) | 73 (36.3) | 0.002 |
| Fatigue | 6 (17.6) | 68 (47.2) | 74 (36.8) | 0.002 |
| Nausea | 1 (3.0) | 25 (18.0) | 26 (12.9) | 0.031 |
| Headache | 3 (6.8) | 15 (10.3) | 18 (8.9) | 0.485 |
| Lung | 9 (19.6) | 32 (21.8) | 41 (20.3) | 0.750 |
| Abdominal | 7 (16.3) | 49 (33.3) | 56 (27.8) | 0.031 |
| Thyroid | 5 (11.4) | 16 (11.2) | 21 (10.4) | 0.974 |
| Liver | 1 (2.3) | 6 (4.1) | 7 (3.4) | 0.581 |
| Fever | 2 (6.3) | 20 (15.5) | 22 (10.9) | 0.172 |
References
- Robert, C.; Ribas, A.; Schachter, J.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.M.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019, 20, 1239–1251. [Google Scholar] [CrossRef]
- Leighl, N.B.; Hellmann, M.D.; Hui, R.; Carcereny, E.; Felip, E.; Ahn, M.-J.; Eder, J.P.; Balmanoukian, A.S.; Aggarwal, C.; Horn, L.; et al. Pembrolizumab in patients with advanced non-small-cell lung cancer (KEYNOTE-001): 3-year results from an open-label, phase 1 study. Lancet Respir. Med. 2019, 7, 347–357. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harringtong, K.J.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018, 81, 45–51. [Google Scholar] [CrossRef]
- Sharma, P.; Callahan, M.K.; Bono, P.; Kim, J.W.; Spiliopoulou, P.; Calvo, E.; Pillai, R.N.; Ott, P.A.; De Braud, F.G.; Morse, M.; et al. Nivolumab monotherapy in metastatic urothelial carcinoma: Longer-term efficacy and safety results from the CheckMate 032 study. J. Clin. Oncol. 2018, 36 (Suppl. 6), 414. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Postow, M.A.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.; McDermott, D.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 2015, 372, 2006–2017. [Google Scholar] [CrossRef]
- Dahl, O.; Dale, J.E.; Brydøy, M. Rationale for combination of radiation therapy and immune checkpoint blockers to improve cancer treatment. Acta Oncol. 2019, 58, 9–20. [Google Scholar] [CrossRef]
- Rückert, M.; Deloch, L.; Fietkau, R.; Frey, B.; Hecht, M.; Gaipl, U.S. Immune modulatory effects of radiotherapy as basis for well-reasoned radioimmunotherapies. Strahlenther. Onkol. 2018, 194, 509–519. [Google Scholar] [CrossRef]
- Hwang, W.L.; Pike, L.R.G.; Royce, T.J.; Mahal, B.A.; Loeffler, J.S. Safety of combining radiotherapy with immune-checkpoint inhibition. Nat. Rev. Clin. Oncol. 2018, 15, 477–494. [Google Scholar] [CrossRef]
- Demaria, S.; Formenti, S.C. Role of T lymphocytes in tumor response to radiotherapy. Front. Oncol. 2012, 2, 95. [Google Scholar] [CrossRef]
- Derer, A.; Deloch, L.; Rubner, Y.; Fietkau, R.; Frey, B.; Gaipl, U.S. Radio-Immunotherapy-Induced Immunogenic Cancer Cells as Basis for Induction of Systemic Anti-Tumor Immune Responses-Pre-Clinical Evidence and Ongoing Clinical Applications. Front. Immunol. 2015, 6, 505. [Google Scholar] [CrossRef]
- Formenti, S.C.; Demaria, S. Radiation therapy to convert the tumor into an in situ vaccine. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 879–880. [Google Scholar] [CrossRef] [PubMed]
- Formenti, S.C.; Demaria, S. Systemic effects of local radiotherapy. Lancet Oncol. 2009, 10, 718–726. [Google Scholar] [CrossRef]
- Abuodeh, Y.; Venkat, P.; Kim, S. Systematic review of case reports on the abscopal effect. Curr. Probl. Cancer 2016, 40, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Ngwa, W.; Irabor, O.C.; Schoenfeld, J.D.; Hesser, J.; Demaria, S.; Formenti, S.C. Using immunotherapy to boost the abscopal effect. Nat. Rev. Cancer 2018, 18, 313–322. [Google Scholar] [CrossRef]
- Reynders, K.; Illidge, T.; Siva, S.; Chang, J.Y.; De Ruysscher, D. The abscopal effect of local radiotherapy: Using immunotherapy to make a rare event clinically relevant. Cancer Treat. Rev. 2015, 41, 503–510. [Google Scholar] [CrossRef]
- Michot, J.-M.; Mazeron, R.; Dercle, L.; Ammari, S.; Canova, C.; Marabelle, A.; Rose, S.; Rubin, E.; Deutsch, E.; Soria, J.-C.; et al. Abscopal effect in a Hodgkin lymphoma patient treated by an anti-programmed death 1 antibody. Eur. J. Cancer 2016, 66, 91–94. [Google Scholar] [CrossRef]
- Chandra, R.A.; Wilhite, T.J.; Balboni, T.A.; Alexander, B.M.; Spektor, A.; Ott, P.A.; Ng, A.K.; Hodi, F.S.; Schoenfeld, J.D. A systematic evaluation of abscopal responses following radiotherapy in patients with metastatic melanoma treated with ipilimumab. Oncoimmunology 2015, 4, e1046028. [Google Scholar] [CrossRef]
- Trommer, M.; Yeo, S.Y.; Persigehl, T.; Bunck, A.; Grüll, H.; Schlaak, M.; Theurich, S.; von Bergwelt-Baildon, M.; Morgenthaler, J.; Herter, J.M.; et al. Abscopal Effects in Radio-Immunotherapy-Response Analysis of Metastatic Cancer Patients with Progressive Disease Under Anti-PD-1 Immune Checkpoint Inhibition. Front. Pharmacol. 2019, 10, 511. [Google Scholar] [CrossRef]
- Deng, L.; Liang, H.; Burnette, B.; Beckett, M.; Darga, T.; Weichselbaum, R.R.; Fu, Y.-X. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014, 124, 687–695. [Google Scholar] [CrossRef]
- Sharabi, A.B.; Lim, M.; DeWeese, T.L.; Drake, C.G. Radiation and checkpoint blockade immunotherapy: Radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015, 16, e498–e509. [Google Scholar] [CrossRef]
- Dovedi, S.J.; Adlard, A.L.; Lipowska-Bhalla, G.; McKenna, C.; Jones, S.; Cheadle, E.J.; Stratford, I.J.; Poon, E.; Morrow, M.; Stewart, R.; et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014, 74, 5458–5468. [Google Scholar] [CrossRef]
- Frey, B.; Rubner, Y.; Kulzer, L.; Werthmöller, N.; Weiss, E.-M.; Fietkau, R.; Gaipl, U.S. Antitumor immune responses induced by ionizing irradiation and further immune stimulation. Cancer Immunol. Immunother. 2014, 63, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef] [PubMed]
- Samstein, R.M.; Lee, C.-H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013, 499, 214–218. [Google Scholar] [CrossRef]
- Norum, J.; Nieder, C. Tobacco smoking and cessation and PD-L1 inhibitors in non-small cell lung cancer (NSCLC): A review of the literature. ESMO Open 2018, 3, e000406. [Google Scholar] [CrossRef]
- Vajaitu, C.; Draghici, C.C.; Solomon, I.; Lisievici, C.V.; Popa, A.V.; Lupu, M.; Caruntu, C.; Constantin, M.M.; Voiculescu, V.M. The Central Role of Inflammation Associated with Checkpoint Inhibitor Treatments. J. Immunol. Res. 2018, 2018, 4625472. [Google Scholar] [CrossRef]
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Annu. Rev. Pathol. 2016, 11, 421–449. [Google Scholar] [CrossRef]
- Pitt, J.M.; Vétizou, M.; Daillère, R.; Roberti, M.P.; Yamazaki, T.; Routy, B.; Lepage, P.; Boneca, I.G.; Chamaillard, M.; Kroemer, G.; et al. Resistance Mechanisms to Immune-Checkpoint Blockade in Cancer: Tumor-Intrinsic and -Extrinsic Factors. Immunity 2016, 44, 1255–1269. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.S.; Long, G.V.; Scolyer, R.A.; Teng, M.W.L.; Smyth, M.J. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat. Rev. 2017, 52, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.K.; Wu, Y.-L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Huang, X.; Fu, L. Impact of smoking on efficacy of PD-1/PD-L1 inhibitors in non-small cell lung cancer patients: A meta-analysis. Onco Targets Ther. 2018, 11, 3691–3696. [Google Scholar] [CrossRef]
- Cortellini, A.; Bersanelli, M.; Buti, S.; Cannita, K.; Santini, D.; Perrone, F.; Giusti, R.; Tiseo, M.; Michiara, M.; Di Marino, P.; et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: When overweight becomes favorable. J. Immunother. Cancer 2019, 7, 1–11. [Google Scholar] [CrossRef]
- McQuade, J.L.; Daniel, C.R.; Hess, K.R.; Mak, C.; Wang, D.Y.; Rai, R.R.; Park, J.J.; Haydu, L.E.; Spencer, C.; Wongchenko, M.; et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: A retrospective, multicohort analysis. Lancet Oncol. 2018, 19, 310–322. [Google Scholar] [CrossRef]
- Knispel, S.; Stang, A.; Zimmer, L.; Lax, H.; Gutzmer, R.; Heinzerling, L.; Weishaupt, C.; Pföhler, C.; Gesierich, A.; Herbst, R.; et al. Impact of a preceding radiotherapy on the outcome of immune checkpoint inhibition in metastatic melanoma: A multicenter retrospective cohort study of the DeCOG. J. Immunother. Cancer 2020, 8, e000395. [Google Scholar] [CrossRef]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.-F.; Testori, A.; Grob, J.-J.; et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef]
- Theurich, S.; Rothschild, S.I.; Hoffmann, M.; Fabri, M.; Sommer, A.; Garcia-Marquez, M.; Thelen, M.; Schill, C.; Merki, R.; Schmid, T.; et al. Local Tumor Treatment in Combination with Systemic Ipilimumab Immunotherapy Prolongs Overall Survival in Patients with Advanced Malignant Melanoma. Cancer Immunol. Res. 2016, 4, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Barker, C.A.; Postow, M.A.; Khan, S.A.; Beal, K.; Parhar, P.K.; Yamada, Y.; Lee, N.Y.; Wolchok, J.D. Concurrent radiotherapy and ipilimumab immunotherapy for patients with melanoma. Cancer Immunol. Res. 2013, 1, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E.; et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Shaverdian, N.; Lisberg, A.E.; Bornazyan, K.; Veruttipong, D.; Goldman, J.W.; Formenti, S.C.; Garon, E.B.; Lee, P. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: A secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017, 18, 895–903. [Google Scholar] [CrossRef]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef]
- Taube, J.M.; Anders, R.A.; Young, G.D.; Xu, H.; Sharma, R.; McMiller, T.L.; Chen, S.; Klein, A.P.; Pardoll, D.M.; Topalian, S.L.; et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl. Med. 2012, 4, 127ra37. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Nowicki, T.S.; Hu-Lieskovan, S.; Ribas, A. Mechanisms of Resistance to PD-1 and PD-L1 Blockade. Cancer J. 2018, 24, 47–53. [Google Scholar] [CrossRef]
- Derer, A.; Frey, B.; Fietkau, R.; Gaipl, U.S. Immune-modulating properties of ionizing radiation: Rationale for the treatment of cancer by combination radiotherapy and immune checkpoint inhibitors. Cancer Immunol. Immunother. 2016, 65, 779–786. [Google Scholar] [CrossRef]
- Barker, H.E.; Paget, J.T.E.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef]
- Demaria, S.; Formenti, S.C. Sensors of ionizing radiation effects on the immunological microenvironment of cancer. Int. J. Radiat. Biol. 2009, 83, 819–825. [Google Scholar] [CrossRef]
- Lauber, K.; Ernst, A.; Orth, M.; Herrmann, M.; Belka, C. Dying cell clearance and its impact on the outcome of tumor radiotherapy. Front. Oncol. 2012, 2, 116. [Google Scholar] [CrossRef] [PubMed]
- Condeelis, J.; Pollard, J.W. Macrophages: Obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006, 124, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Borregaard, N.; Wynn, T.A. Phenotypic and functional plasticity of cells of innate immunity: Macrophages, mast cells and neutrophils. Nat. Immunol. 2011, 12, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Teresa Pinto, A.; Laranjeiro Pinto, M.; Patrícia Cardoso, A.; Monteiro, C.; Teixeira Pinto, M.; Filipe Maia, A.; Castro, P.; Figueira, R.; Monteiro, A.; Marques, M.; et al. Ionizing radiation modulates human macrophages towards a pro-inflammatory phenotype preserving their pro-invasive and pro-angiogenic capacities. Sci. Rep. 2016, 6, 18765. [Google Scholar] [CrossRef]
- Frey, B.; Hehlgans, S.; Rödel, F.; Gaipl, U.S. Modulation of inflammation by low and high doses of ionizing radiation: Implications for benign and malign diseases. Cancer Lett. 2015, 368, 230–237. [Google Scholar] [CrossRef]
- Wunderlich, R.; Rühle, P.F.; Deloch, L.; Rödel, F.; Fietkau, R.; Gaipl, U.S.; Frey, B. Ionizing radiation reduces the capacity of activated macrophages to induce T-cell proliferation, but does not trigger dendritic cell-mediated non-targeted effects. Int. J. Radiat. Biol. 2019, 95, 33–43. [Google Scholar] [CrossRef]
- Lee, Y.; Auh, S.L.; Wang, Y.; Burnette, B.; Wang, Y.; Meng, Y.; Beckett, M.; Sharma, R.; Chin, R.; Tu, T.; et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: Changing strategies for cancer treatment. Blood 2009, 114, 589–595. [Google Scholar] [CrossRef]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, N.; Formenti, S.C.; Demaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef]
- Schemper, M.; Smith, T.L. A note on quantifying follow-up in studies of failure time. Control. Clin. Trials 1996, 17, 343–346. [Google Scholar] [CrossRef]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, U.N.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, 143–152. [Google Scholar] [CrossRef]
- Kuss, O. The z-difference can be used to measure covariate balance in matched propensity score analyses. J. Clin. Epidemiol. 2013, 66, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | All Patients n = 201 |
|---|---|
| Age (years) | 62.4 ± 13.2 |
| Sex | |
| Female | 83 (41.3) |
| Male | 118 (58.7) |
| ECOG | |
| 0 | 72 (37.3) |
| 1 | 88 (45.6) |
| 2 | 23 (11.9) |
| 3 | 7 (3.6) |
| 4 | 3 (1.6) |
| Cancer type | |
| MM | 96 (48.0) |
| NSCLC | 68 (34.0) |
| RCC | 14 (7.0) |
| Other | 22 (11.0) |
| Stage UICC/AJCC | |
| 2 | 3 (1.6) |
| 3 | 15 (8.0) |
| 4 | 169 (90.4) |
| Multiple metastases (yes > 1 site) | |
| Yes | 145 (79.2) |
| No | 38 (20.8) |
| PD-L1 status | |
| Positive | 48 (62.3) |
| Negative | 29 (37.7) |
| Brain metastases | |
| Yes | 54 (26.9) |
| No | 147 (73.1) |
| Prior ipilimumab | |
| Yes | 38 (19.2) |
| No | 160 (80.8) |
| Smoker | |
| Yes | 45 (25.3) |
| No | 133 (74.7) |
| BMI | |
| ≤25 | 73 (36.3) |
| >25 | 109 (54.2) |
| Characteristic | Unmatched | Matched | ||||
|---|---|---|---|---|---|---|
| Radiotherapy | Radiotherapy | |||||
| yes (RIT) n = 153 | no (PD1i) n = 48 | p Value | yes (RIT) n = 67 | no (PD1i) n = 29 | p Value | |
| Age baseline (years) | 61.4 ± 13.5 | 65.5 ± 11.7 | 0.038 | 64.8 ± 13.6 | 67.1 ± 10.7 | 0.441 |
| Sex | 0.692 | 0.197 | ||||
| Female | 62 (40.5) | 21 (43.8) | 23 (34.3) | 14 (48.3) | ||
| Male | 91 (59.5) | 27 (56.3) | 44 (65.7) | 15 (51.7) | ||
| ECOG | 0.360 | 0.915 | ||||
| 0 | 50 (33.8) | 22 (48.9) | 27 (41.5) | 13 (46.4) | ||
| 1 | 73 (49.3) | 15 (33.3) | 26 (40.0) | 9 (32.1) | ||
| 2 | 18 (12.2) | 5 (11.1) | 8 (12.3) | 4 (14.3) | ||
| 3 | 5 (3.4) | 2 (4.4) | 4 (6.2) | 2 (7.1) | ||
| 4 | 2 (1.4) | 1 (2.2) | 0 (0.0) | 0 (0.0) | ||
| Cancer type | 0.166 | 0.775 | ||||
| MM | 78 (51.0) | 18 (38.3) | 36 (53.7) | 13 (46.4) | ||
| NSCLC | 53 (34.6) | 15 (31.9) | 17 (25.4) | 8 (28.6) | ||
| RCC | 7 (4.6) | 7 (14.9) | 3 (4.5) | 1 (3.6) | ||
| Other | 15 (9.8) | 7 (14.9) | 11 (16.5) | 6 (21.5) | ||
| Stage UICC/AJCC | 0.869 | 0.779 | ||||
| 2 | 2 (1.4) | 1 (2.2) | 2 (3.2) | 1 (3.4) | ||
| 3 | 12 (8.5) | 3 (6.7) | 10 (15.9) | 3 (10.3) | ||
| 4 | 128 (90.1) | 41 (91.1) | 51 (81.0) | 25 (86.2) | ||
| Multiple Metastases (yes > 1 site) | 0.103 | 0.144 | ||||
| Yes | 117 (81.8) | 28 (70.0) | 49 (79.0) | 16 (64.0) | ||
| No | 26 (18.2) | 12 (30.0) | 13 (21.0) | 9 (36.0) | ||
| PD-L1 status | 0.835 | 0.987 | ||||
| Positive | 32 (61.5) | 16 (64.0) | 13 (59.1) | 10 (58.8) | ||
| Negative | 20 (38.5) | 9 (36.0) | 9 (40.9) | 7 (41.2) | ||
| Brain metastases | <0.001 | 0.610 | ||||
| Yes | 52 (34.0) | 2 (4.2) | 4 (6.0) | 1 (3.4) | ||
| No | 101 (66.0) | 46 (95.8) | 63 (94.0) | 28 (96.6) | ||
| Prior ipilimumab | 0.033 | 0.458 | ||||
| Yes | 34 (22.5) | 4 (8.5) | 8 (11.9) | 2 (6.9) | ||
| No | 117 (77.5) | 43 (91.5) | 59 (88.1) | 27 (93.1) | ||
| Smoker | 0.869 | 0.898 | ||||
| Yes | 35 (25.0) | 10 (26.3) | 17 (25.4) | 7 (24.1) | ||
| No | 105 (75.0) | 28 (73.7) | 50 (74.6) | 22 (75.9) | ||
| BMI | 0.430 | 0.600 | ||||
| <18.5 | 4 (2.6) | 2 (4.2) | 1 (1.5) | 1 (3.4) | ||
| 18.5–24.9 | 50 (32.7) | 17 (35.4) | 21 (31.3) | 10 (34.5) | ||
| 25.0–29.9 | 56 (36.6) | 19 (39.6) | 28 (41.8) | 14 (48.3) | ||
| >30 | 30 (19.6) | 4 (8.3) | 17 (25.4) | 4 (13.8) | ||
| Pembrolizumab | 75 (49.0) | 18 (37.5) | 0.163 | 36 (53.7) | 11 (37.9) | 0.155 |
| Nivolumab | 78 (51.0) | 30 (62.5) | 31 (46.3) | 18 (62.1) | ||
| Cycles | 7.0 (3.0–16.0) | 10.0 (1.0–19.0) | 0.982 | 7.0 (3.0–16.0) | 3.0 (1.0–17.0) | 0.081 |
| RT single dose | 3.0 (2.0–9.0) | - | – | 3.0 (2.0–3.0) | – | – |
| RT total dose | 35.0 (20.0–45.0) | – | – | 37.5 (28.5–50.0) | – | – |
| RT localization | – | – | ||||
| Brain | 51 (34.0) | 0 (0.0) | 14 (21.2) | 0 (0.0) | ||
| Lung | 15 (10.0) | 0 (0.0) | 9 (13.6) | 0 (0.0) | ||
| Bone/soft tissue | 49 (32.7) | 0 (0.0) | 22 (33.3) | 0 (0.0) | ||
| Lymph node | 31 (20.7) | 0 (0.0) | 20 (30.3) | 0 (0.0) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trommer, M.; Kinsky, J.; Adams, A.; Hellmich, M.; Schlaak, M.; von Bergwelt-Baildon, M.; Celik, E.; Rosenbrock, J.; Morgenthaler, J.; Herter, J.M.; et al. Addition of Radiotherapy to Immunotherapy: Effects on Outcome of Different Subgroups Using a Propensity Score Matching. Cancers 2020, 12, 2429. https://doi.org/10.3390/cancers12092429
Trommer M, Kinsky J, Adams A, Hellmich M, Schlaak M, von Bergwelt-Baildon M, Celik E, Rosenbrock J, Morgenthaler J, Herter JM, et al. Addition of Radiotherapy to Immunotherapy: Effects on Outcome of Different Subgroups Using a Propensity Score Matching. Cancers. 2020; 12(9):2429. https://doi.org/10.3390/cancers12092429
Chicago/Turabian StyleTrommer, Maike, Jaika Kinsky, Anne Adams, Martin Hellmich, Max Schlaak, Michael von Bergwelt-Baildon, Eren Celik, Johannes Rosenbrock, Janis Morgenthaler, Jan M. Herter, and et al. 2020. "Addition of Radiotherapy to Immunotherapy: Effects on Outcome of Different Subgroups Using a Propensity Score Matching" Cancers 12, no. 9: 2429. https://doi.org/10.3390/cancers12092429
APA StyleTrommer, M., Kinsky, J., Adams, A., Hellmich, M., Schlaak, M., von Bergwelt-Baildon, M., Celik, E., Rosenbrock, J., Morgenthaler, J., Herter, J. M., Linde, P., Mauch, C., Theurich, S., Marnitz, S., & Baues, C. (2020). Addition of Radiotherapy to Immunotherapy: Effects on Outcome of Different Subgroups Using a Propensity Score Matching. Cancers, 12(9), 2429. https://doi.org/10.3390/cancers12092429





