The Full Blood Count Blood Test for Colorectal Cancer Detection: A Systematic Review, Meta-Analysis, and Critical Appraisal
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Outcome
2.3. Search Strategy
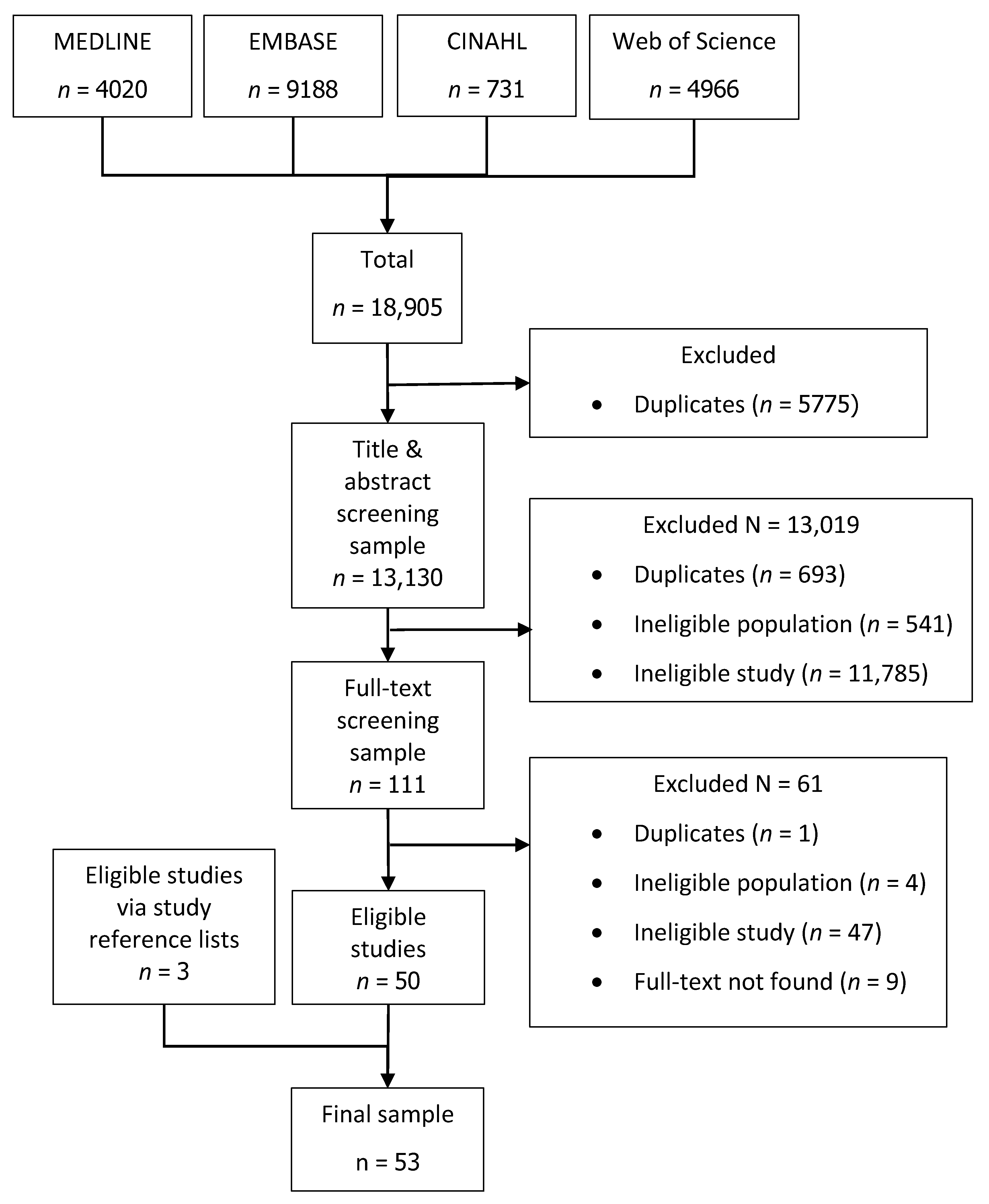
2.4. Study Selection
2.4.1. Screening of Articles
2.4.2. Selection Criteria
2.5. Data Extraction
2.6. Data Analysis and SYNTHESIS
2.6.1. Missing Data
- We estimated the mean difference with associated standard error (SE) in blood levels between those with and without a diagnosis using the methods and formulae provided in the Cochrane Handbook for Systematic Reviews of Interventions [10], if relevant data was available in the article. Where SEs were calculated using non-exact p-values from t-tests, the nearest value was used in the estimation. For example, if an article reported p < 0.001, then the nearest exact value of p = 0.0009 was used.
- If a study did not provide sufficient data for a mean difference to be estimated, we contacted the authors and requested additional data or clarifications.
- If no additional information was obtained from the authors, we approximated the data by measuring the means from graphs in articles. We are aware that this may over- or under-estimate the mean difference and associated SE. However, they were the best estimates we could obtain.
- If none of the above were possible, the mean difference remained missing and was not included in the analysis (but available data was still used in other analyses).
2.6.2. Analysis Methods
2.6.3. Assessment of Bias
3. Results
3.1. Description of Studies
3.1.1. Study Design and Participants
3.1.2. Overview of Analytic Methods
3.1.3. Outcome and Follow-up
3.2. FBC for Colorectal Cancer (Aim 1)
3.2.1. Risk of Bias
3.2.2. Red Blood Cell Count
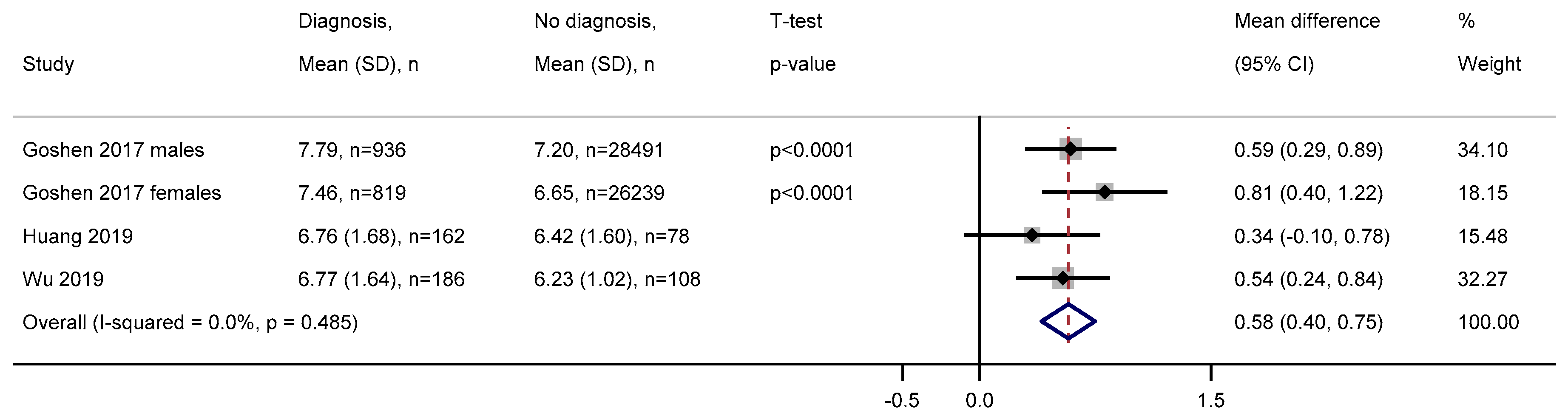
3.2.3. White Blood Cell Count
3.2.4. Haemoglobin
3.2.5. Haematocrit
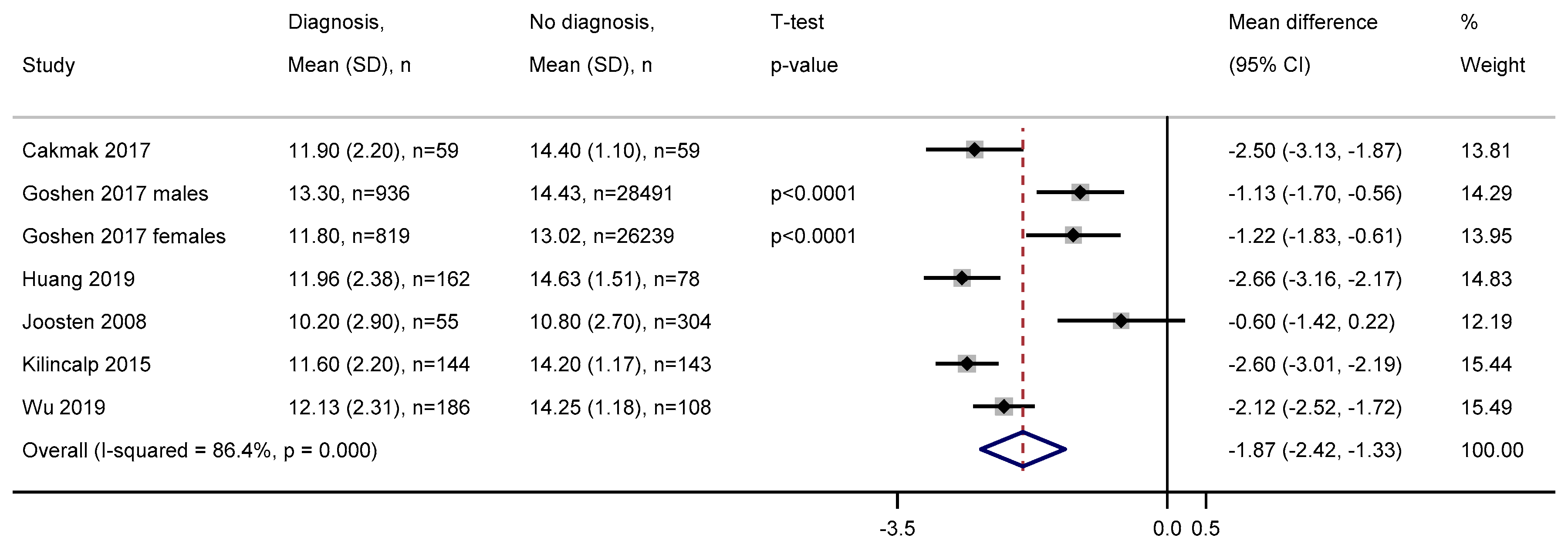
3.2.6. Mean Corpuscular Volume
3.2.7. Mean Corpuscular Haemoglobin
3.2.8. Red Blood Cell Distribution Width
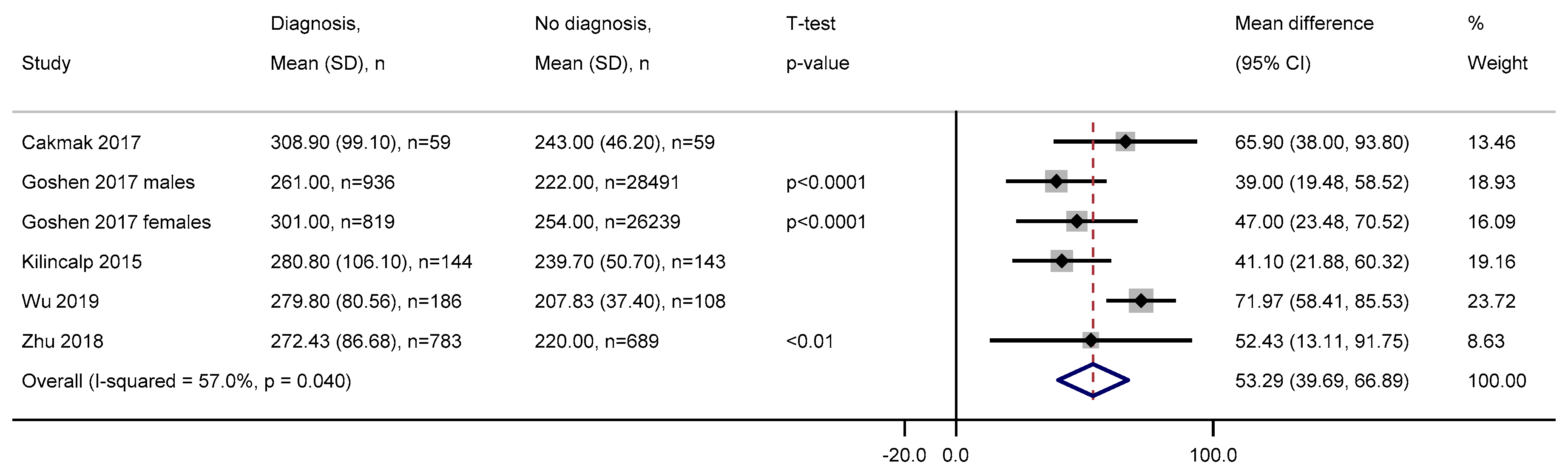
3.2.9. Platelets
3.2.10. Mean Platelet Volume
3.2.11. Differential White Blood Cell Count
3.2.12. Combined Components
3.3. Appraisal of Prediction Models (Aim 2)
3.3.1. Risk of Bias
3.3.2. Model Building Strategy
3.3.3. Modelling FBC Components
3.3.4. Correlation between FBC Components
3.3.5. Model Reporting
3.3.6. Internal Validation
3.3.7. External Validation
3.3.8. Reliability of Performance
3.4. Repeated FBC Measures
4. Discussion
4.1. FBC Risk Factors
4.2. FBC-Based Prediction Models
4.3. Repeated Measures
4.4. Recommendations
4.4.1. Use Appropriate Methods for FBC Analysis
4.4.2. Account for Missing Data
4.4.3. Assess Change over Time
4.4.4. FBC Levels for Referral
4.4.5. Choice of Outcome Time Window
4.4.6. Adjust Prediction Models for Misfitting
4.4.7. Assess Model Discrimination and Calibration
4.4.8. Critical External Validation of Models
4.4.9. Reporting Results
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cancer Research UK. Bowel Cancer Incidence Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer/incidence#heading-Zero (accessed on 8 May 2020).
- Cancer Research UK. Bowel Cancer Mortality Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer/mortality#heading-Zero (accessed on 8 May 2020).
- Cancer Research UK. Bowel Cancer Survival Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer/survival#heading-Three (accessed on 8 May 2020).
- Hamilton, W.; Lancashire, R.; Sharp, D.; Peters, T.J.; Cheng, K.K.; Marshall, T. The importance of anaemia in diagnosing colorectal cancer: A case–control study using electronic primary care records. Br. J. Cancer 2008, 98, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Spell, D.W.; Jones, D.V., Jr.; Harper, W.F.; David Bessman, J. The value of a complete blood count in predicting cancer of the colon. Cancer Detect. Prev. 2004, 28, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The Prisma Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Virdee, P.S.; Kirtley, S.; Elhussein, L.; Watkinson, P.J.; Holt, T.A.; Birks, J. Components of the full blood count as risk factors for colorectal cancer detection: A systematic review protocol. BMJ Open 2019, 9, e032759. [Google Scholar] [CrossRef]
- EndNote X9. EndNote. Available online: https://endnote.com/ (accessed on 8 May 2020).
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. Available online: www.training.cochrane.org/handbook (accessed on 8 May 2020).
- Hayden, J.A.; van der Windt, D.A.; Cartwright, J.L.; Cote, P.; Bombardier, C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef]
- Wolff, R.F.; Moons, K.G.M.; Riley, R.D.; Whiting, P.F.; Westwood, M.; Collins, G.S.; Reitsma, J.B.; Kleijnen, J.; Mallett, S.; Groupdagger, P. PROBAST: A Tool to Assess the Risk of Bias and Applicability of Prediction Model Studies. Ann. Intern. Med. 2019, 170, 51–58. [Google Scholar] [CrossRef]
- Acher, P.L.; Al-Mishlab, T.; Rahman, M.; Bates, T. Iron-deficiency anaemia and delay in the diagnosis of colorectal cancer. Colorectal Dis. 2003, 5, 145–148. [Google Scholar] [CrossRef]
- Ankus, E.; Price, S.J.; Ukoumunne, O.C.; Hamilton, W.; Bailey, S.E.R. Cancer incidence in patients with a high normal platelet count: A cohort study using primary care data. Fam. Pract. 2018, 35, 671–675. [Google Scholar] [CrossRef]
- Ay, S.; Eryilmaz, M.A.; Aksoy, N.; Okus, A.; Unlu, Y.; Sevinc, B. Is early detection of colon cancer possible with red blood cell distribution width? Asian Pac. J. Cancer Prev. 2015, 16, 753–756. [Google Scholar] [CrossRef]
- Ayling, R.M.; Lewis, S.J.; Cotter, F. Potential roles of artificial intelligence learning and faecal immunochemical testing for prioritisation of colonoscopy in anaemia. Br. J. Haematol. 2019, 185, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Bafandeh, Y.; Khoshbaten, M.; Sadat, A.T.E.; Farhang, S. Clinical predictors of colorectal polyps and carcinoma in a low prevalence region: Results of a colonoscopy based study. World J. Gastroenterol. 2008, 14, 1534–1538. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.E.R.; Ukoumunne, O.C.; Shephard, E.A.; Hamilton, W. Clinical relevance of thrombocytosis in primary care: A prospective cohort study of cancer incidence using English electronic medical records and cancer registry data. Br. J. Gen. Pract. 2017, 67, e405–e413. [Google Scholar] [CrossRef] [PubMed]
- Birks, J.; Bankhead, C.; Holt, T.A.; Fuller, A.; Patnick, J. Evaluation of a prediction model for colorectal cancer: Retrospective analysis of 2.5 million patient records. Cancer Med. 2017, 6, 2453–2460. [Google Scholar] [CrossRef] [PubMed]
- Boursi, B.; Mamtani, R.; Hwang, W.T.; Haynes, K.; Yang, Y.X. A Risk Prediction Model for Sporadic CRC Based on Routine Lab Results. Dig. Dis. Sci. 2016, 61, 2076–2086. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, E.; Soylu, S.; Yonem, O.; Yilmaz, A. Neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and red blood cell distribution width as new biomarkers in patients with colorectal cancer. Erciyes Med. J. 2017, 39, 131–136. [Google Scholar] [CrossRef][Green Version]
- Collins, G.S.; Altman, D.G. Identifying patients with undetected colorectal cancer: An independent validation of QCancer (Colorectal). Br. J. Cancer 2012, 107, 260–265. [Google Scholar] [CrossRef]
- Cross, A.J.; Wooldrage, K.; Robbins, E.C.; Pack, K.; Brown, J.P.; Hamilton, W.; Thompson, M.R.; Flashman, K.G.; Halligan, S.; Thomas-Gibson, S.; et al. Whole-colon investigation vs. flexible sigmoidoscopy for suspected colorectal cancer based on presenting symptoms and signs: A multicentre cohort study. Br. J. Cancer 2019, 120, 154–164. [Google Scholar] [CrossRef]
- Cubiella, J.; Vega, P.; Salve, M.; Diaz-Ondina, M.; Alves, M.T.; Quintero, E.; Alvarez-Sanchez, V.; Fernandez-Banares, F.; Boadas, J.; Campo, R.; et al. Development and external validation of a faecal immunochemical test-based prediction model for colorectal cancer detection in symptomatic patients. BMC Med. 2016, 14, 128. [Google Scholar] [CrossRef]
- Fijten, G.H.; Starmans, R.; Muris, J.W.; Schouten, H.J.; Blijham, G.H.; Knottnerus, J.A. Predictive value of signs and symptoms for colorectal cancer in patients with rectal bleeding in general practice. Fam. Pract. 1995, 12, 279–286. [Google Scholar] [CrossRef]
- Firat, F.; Arslan, A.K.; Colak, C.; Harputluoglu, H. Estimation of risk factors associated with colorectal cancer: An application of knowledge discovery in databases. Kuwait J. Sci. 2016, 43, 151–161. [Google Scholar]
- Goldshtein, I.; Neeman, U.; Chodick, G.; Shalev, V. Variations in hemoglobin before colorectal cancer diagnosis. Eur. J. Cancer Prev. 2010, 19, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Goshen, R.; Mizrahi, B.; Akiva, P.; Kinar, Y.; Choman, E.; Shalev, V.; Sopik, V.; Kariv, R.; Narod, S.A. Predicting the presence of colon cancer in members of a health maintenance organisation by evaluating analytes from standard laboratory records. Br. J. Cancer 2017, 116, 944–950. [Google Scholar] [CrossRef]
- Hamilton, W.; Round, A.; Sharp, D.; Peters, T.J. Clinical features of colorectal cancer before diagnosis: A population-based case-control study. Br. J. Cancer 2005, 93, 399–405. [Google Scholar] [CrossRef]
- Hamilton, W.; Lancashire, R.; Sharp, D.; Peters, T.J.; Cheng, K.K.; Marshall, T. The risk of colorectal cancer with symptoms at different ages and between the sexes: A case-control study. BMC Med. 2009, 7, 17. [Google Scholar] [CrossRef]
- Hilsden, R.J.; Heitman, S.J.; Mizrahi, B.; Narod, S.A.; Goshen, R. Prediction of findings at screening colonoscopy using a machine learning algorithm based on complete blood counts (ColonFlag). PLoS ONE 2018, 13, e0207848. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Coupland, C. Identifying patients with suspected colorectal cancer in primary care: Derivation and validation of an algorithm. Br. J. Gen. Pract. 2012, 62, e29–e37. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Coupland, C. Symptoms and risk factors to identify men with suspected cancer in primary care: Derivation and validation of an algorithm. Br. J. Gen. Pract. 2013, 63, e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Coupland, C. Symptoms and risk factors to identify women with suspected cancer in primary care: Derivation and validation of an algorithm. Br. J. Gen. Pract. 2013, 63, e11–e21. [Google Scholar] [CrossRef]
- Hornbrook, M.C.; Goshen, R.; Choman, E.; O’Keeffe-Rosetti, M.; Kinar, Y.; Liles, E.G.; Rust, K.C. Early Colorectal Cancer Detected by Machine Learning Model Using Gender, Age, and Complete Blood Count Data. Dig. Dis. Sci. 2017, 62, 2719–2727. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, Y.; Liao, L.; Liu, S.; Lu, S.; Wu, C.; Wei, C.; Xu, S.; Zhong, H.; Liu, J.; et al. Evaluation of Red Cell Distribution Width to Lymphocyte Ratio as Potential Biomarker for Detection of Colorectal Cancer. Biomed. Res. Int. 2019, 2019, 9852782. [Google Scholar] [CrossRef] [PubMed]
- Hung, N.; Shen, C.C.; Hu, Y.W.; Hu, L.Y.; Yeh, C.M.; Teng, C.J.; Kuan, A.S.; Chen, S.C.; Chen, T.J.; Liu, C.J. Risk of cancer in patients with iron deficiency anemia: A nationwide population-based study. PLoS ONE 2015, 10, e0119647. [Google Scholar] [CrossRef] [PubMed]
- Joosten, E.; Meeuwissen, J.; Vandewinckele, H.; Hiele, M. Iron status and colorectal cancer in symptomatic elderly patients. Am. J. Med. 2008, 121, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Kilincalp, S.; Coban, S.; Akinci, H.; Hamamc, M.; Karaahmet, F.; Coskun, Y.; Ustun, Y.; Simsek, Z.; Erarslan, E.; Yuksel, I. Neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, and mean platelet volume as potential biomarkers for early detection and monitoring of colorectal adenocarcinoma. Eur. J. Cancer Prev. 2015, 24, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Kinar, Y.; Kalkstein, N.; Akiva, P.; Levin, B.; Half, E.E.; Goldshtein, I.; Chodick, G.; Shalev, V. Development and validation of a predictive model for detection of colorectal cancer in primary care by analysis of complete blood counts: A binational retrospective study. J. Am. Med. Inform. Assoc. 2016, 23, 879–890. [Google Scholar] [CrossRef]
- Kinar, Y.; Akiva, P.; Choman, E.; Kariv, R.; Shalev, V.; Levin, B.; Narod, S.A.; Goshen, R. Performance analysis of a machine learning flagging system used to identify a group of individuals at a high risk for colorectal cancer. PLoS ONE 2017, 12, e0171759. [Google Scholar] [CrossRef]
- Lawrenson, R.; Logie, J.; Marks, C. Risk of colorectal cancer in general practice patients presenting with rectal bleeding, change in bowel habit or anaemia. Eur. J. Cancer Care 2006, 15, 267–271. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, H.R.; Nam, C.M.; Hwang, U.K.; Jee, S.H. White blood cell count and the risk of colon cancer. Yonsei Med. J. 2006, 47, 646–656. [Google Scholar] [CrossRef]
- Margolis, K.L.; Rodabough, R.J.; Thomson, C.A.; Lopez, A.M.; McTiernan, A. Prospective study of leukocyte count as a predictor of incident breast, colorectal, endometrial, and lung cancer and mortality in postmenopausal women. Arch. Intern. Med. 2007, 167, 1837–1844. [Google Scholar] [CrossRef]
- Marshall, T.; Lancashire, R.; Sharp, D.; Peters, T.J.; Cheng, K.K.; Hamilton, W. The diagnostic performance of scoring systems to identify symptomatic colorectal cancer compared to current referral guidance. Gut 2011, 60, 1242–1248. [Google Scholar] [CrossRef]
- Mashlab, S.; Large, P.; Laing, W.; Ng, O.; D’Auria, M.; Thurston, D.; Thomson, S.; Acheson, A.G.; Humes, D.J.; Banerjea, A.; et al. Anaemia as a risk stratification tool for symptomatic patients referred via the two-week wait pathway for colorectal cancer. Ann. R. Coll. Surg. Engl. 2018, 100, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Naef, M.; Buhlmann, M.; Baer, H.U. Small bowel tumors: Diagnosis, therapy and prognostic factors. Langenbeck’s Arch. Surg. 1999, 384, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Nakama, H.; Zhang, B.; Fattah, A.S.; Zhang, X. Colorectal cancer in iron deficiency anemia with a positive result on immunochemical fecal occult blood. Int. J. Colorectal Dis. 2000, 15, 271–274. [Google Scholar] [PubMed]
- Panagiotopoulou, I.G.; Fitzrol, D.; Parker, R.A.; Kuzhively, J.; Luscombe, N.; Wells, A.D.; Menon, M.; Bajwa, F.M.; Watson, M.A. The yield of colorectal cancer among fast track patients with normocytic and microcytic anaemia. Ann. R. Coll. Surg. Engl. 2014, 96, 289–293. [Google Scholar] [CrossRef][Green Version]
- Panzuto, F.; Chiriatti, A.; Bevilacqua, S.; Giovannetti, P.; Russo, G.; Impinna, S.; Pistilli, F.; Capurso, G.; Annibale, B.; Delle Fave, G.; et al. Symptom-based approach to colorectal cancer: Survey of primary care physicians in Italy. Dig. Liver Dis. 2003, 35, 869–875. [Google Scholar] [CrossRef]
- Pilling, L.C.; Atkins, J.L.; Kuchel, G.A.; Ferrucci, L.; Melzer, D. Red cell distribution width and common disease onsets in 240,477 healthy volunteers followed for up to 9 years. PLoS ONE 2018, 13, e0203504. [Google Scholar] [CrossRef]
- Prizment, A.E.; Anderson, K.E.; Visvanathan, K.; Folsom, A.R. Association of inflammatory markers with colorectal cancer incidence in the atherosclerosis risk in communities study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 297–307. [Google Scholar] [CrossRef]
- Raje, D.; Mukhtar, H.; Oshowo, A.; Ingham Clark, C. What proportion of patients referred to secondary care with iron deficiency anemia have colon cancer? Dis. Colon Rectum 2007, 50, 1211–1214. [Google Scholar] [CrossRef]
- Schneider, C.; Bodmer, M.; Jick, S.S.; Meier, C.R. Colorectal cancer and markers of anemia. Eur. J. Cancer Prev. 2018, 27, 530–538. [Google Scholar] [CrossRef]
- Shi, C.; Xie, M.; Li, L.; Li, K.; Hu, B.L. The association and diagnostic value of red blood cell distribution width in colorectal cancer. Medicine 2019, 98, e15560. [Google Scholar] [CrossRef]
- Song, Y.; Huang, Z.; Kang, Y.; Lin, Z.; Lu, P.; Lin, Q.; Cai, Z.; Cao, Y.; Zhu, X. Clinical Usefulness and Prognostic Value of Red Cell Distribution Width in Colorectal Cancer. Biomed. Res. Int. 2018, 2018, 9858943. [Google Scholar] [CrossRef] [PubMed]
- Stapley, S.; Peters, T.J.; Sharp, D.; Hamilton, W. The mortality of colorectal cancer in relation to the initial symptom at presentation to primary care and to the duration of symptoms: A cohort study using medical records. Br. J. Cancer 2006, 95, 1321–1325. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.R.; O’Leary, D.P.; Flashman, K.; Asiimwe, A.; Ellis, B.G.; Senapati, A. Clinical assessment to determine the risk of bowel cancer using Symptoms, Age, Mass and Iron deficiency anaemia (SAMI). Br. J. Surg. 2017, 104, 1393–1404. [Google Scholar] [CrossRef]
- Van Boxtel-Wilms, S.J.M.; van Boven, K.; Bor, J.H.H.; Bakx, J.C.; Lucassen, P.; Oskam, S.; van Weel, C. The value of reasons for encounter in early detection of colorectal cancer. Eur. J. Gen. Pract. 2016, 22, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Y.; Zhang, X.; Qin, Y.Y.; Qin, J.Q.; Lin, F.Q. Mean platelet volume/platelet count ratio in colorectal cancer: A retrospective clinical study. BMC Cancer 2019, 19, 7. [Google Scholar] [CrossRef]
- Yang, D.; Quan, W.; Wu, J.; Ji, X.; Dai, Y.; Xiao, W.; Chew, H.; Sun, Z.; Li, D. The value of red blood cell distribution width in diagnosis of patients with colorectal cancer. Clin. Chim. Acta 2018, 479, 98–102. [Google Scholar] [CrossRef]
- Zhou, W.W.; Chu, Y.P.; An, G.Y. Significant difference of neutrophil-lymphocyte ratio between colorectal cancer, adenomatous polyp and healthy people. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5386–5391. [Google Scholar]
- Zhu, X.J.; Cao, Y.P.; Lu, P.X.; Kang, Y.L.; Lin, Z.; Hao, T.S.; Song, Y.F. Evaluation of platelet indices as diagnostic biomarkers for colorectal cancer. Sci. Rep. 2018, 8, 7. [Google Scholar] [CrossRef]
- Health Europa. A Closer Look at The Relationship between Inflammation and Bowel Cancer. Available online: https://www.healtheuropa.eu/bowel-cancer/90379/ (accessed on 29 May 2020).
- Terzic, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and colon cancer. Gastroenterology 2010, 138, 2101–2114.e5. [Google Scholar] [CrossRef]
- Hamilton, W. Derivation of a score for identifying colorectal cancer in primary care. Gut 2007, 56 (Suppl. II), A49–A50. [Google Scholar]
- Del Giudice, M.E.; Vella, E.T.; Hey, A.; Simunovic, M.; Harris, W.; Levitt, C. Systematic review of clinical features of suspected colorectal cancer in primary care. Can. Fam. Physician 2014, 60, e405–e415. [Google Scholar]
- Montagnana, M.; Danese, E. Red cell distribution width and cancer. Ann. Transl. Med. 2016, 4, 399. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Anaemia—Iron Deficiency. Available online: https://cks.nice.org.uk/anaemia-iron-deficiency#!topicSummary (accessed on 11 March 2020).
- World Health Organisation (WHO). Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Available online: https://apps.who.int/iris/bitstream/handle/10665/85839/WHO_NMH_NHD_MNM_11.1_eng.pdf?sequence=22&isAllowed=y (accessed on 11 March 2020).
- Gallagher, A.M.; Dedman, D.; Padmanabhan, S.; Leufkens, H.G.M.; de Vries, F. The accuracy of date of death recording in the Clinical Practice Research Datalink GOLD database in England compared with the Office for National Statistics death registrations. Pharm. Drug Saf. 2019, 28, 563–569. [Google Scholar] [CrossRef]
- Lo Re, V., 3rd; Haynes, K.; Forde, K.A.; Localio, A.R.; Schinnar, R.; Lewis, J.D. Validity of The Health Improvement Network (THIN) for epidemiologic studies of hepatitis C virus infection. Pharm. Drug Saf. 2009, 18, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Mathur, R.; Bhaskaran, K.; Chaturvedi, N.; Leon, D.A.; vanStaa, T.; Grundy, E.; Smeeth, L. Completeness and usability of ethnicity data in UK-based primary care and hospital databases. J. Public Health 2014, 36, 684–692. [Google Scholar] [CrossRef]
- Muthee, V.; Bochner, A.F.; Osterman, A.; Liku, N.; Akhwale, W.; Kwach, J.; Prachi, M.; Wamicwe, J.; Odhiambo, J.; Onyango, F.; et al. The impact of routine data quality assessments on electronic medical record data quality in Kenya. PLoS ONE 2018, 13, e0195362. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007, 4, e296. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, E.I.; Smeeth, L.; Guttmann, A.; Harron, K.; Moher, D.; Petersen, I.; Sorensen, H.T.; von Elm, E.; Langan, S.M.; Committee, R.W. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015, 12, e1001885. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. BMJ 2015, 350, g7594. [Google Scholar] [CrossRef]
- Altman, D.G.; Royston, P. The cost of dichotomising continuous variables. BMJ 2006, 332, 1080. [Google Scholar] [CrossRef]
- Riley, R.D.; Windt, D.A.v.d.; Croft, P.; Moons, K.G.M. Prognosis Research in Healthcare: Concepts, Methods, and Impact; Oxford University: Oxford, UK, 2019. [Google Scholar]
- Steyerberg, E.W.; Vickers, A.J.; Cook, N.R.; Gerds, T.; Gonen, M.; Obuchowski, N.; Pencina, M.J.; Kattan, M.W. Assessing the performance of prediction models: A framework for traditional and novel measures. Epidemiology 2010, 21, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Speights, V.O.; Johnson, M.W.; Stoltenberg, P.H.; Rappaport, E.S.; Helbert, B.; Riggs, M.W. Complete blood count indices in colorectal carcinoma. Arch. Pathol. Lab. Med. 1992, 116, 258–260. [Google Scholar] [PubMed]
| Article | Study Type | Study Design | Geographic Location | Patient Setting | Patient Type | Patient Population | Average Age | % Male |
|---|---|---|---|---|---|---|---|---|
| Acher 2003 [13] | Retrospective | Cohort | UK | Unclear | Anyone | Inclusion: men aged >50 years and women > 55 years with histologically proven CRC in 1996–1999. Exclusion: patients with recurrent CRC. | ||
| Ankus 2018 [14] | Retrospective | Cohort | UK | Primary care | Anyone | Inclusion: random 10,000 from CPRD with first platelet count from 2000–2013 of 150–399 109/L, aged ≥ 40 years at the time of the platelet count with no prior cancer diagnosis. Exclusion: diagnosed with non-melanoma skin cancer after index date. | 22.6 | |
| Ay 2015 [15] | Retrospective | Case-control | Turkey | Unclear | Anyone | Inclusion: FBC within one week of diagnosis. Exclusion: patients with anaemia, haematological disorders, active infection, blood transfusion made < 3 months, venous thrombosis <6 months, receiving iron deficiency treatment, hypertension, cardiac failure, inflammatory intestinal disease and rheumatoid arthritis. | 60.3 | |
| Ayling 2019 [16] 1 | Prospective | Cohort | UK | Secondary care | Symptomatic | Inclusion: patients in the Gastroenterology Clinic in Derriford Hospital, Plymouth, between March 2014 and March 2017, referred with a low haemoglobin on a 2-week wait cancer pathway. Additionally, a cohort of consecutive patients who attended the Royal London Hospital for colonoscopy during 2017. | 48.1 | |
| Bafandeh 2008 [17] | Prospective | Cohort | Iran | Unclear | Symptomatic | Inclusion: 480 consecutive patients with unexplained lower gastrointestinal tract symptoms for > 3 months who underwent total colonoscopy between May 2005-April 2007. Exclusion: failure to reach the caecum or referred for polypectomy. | 42.7 | 56 |
| Bailey 2017 [18] | Retrospective | Cohort | UK | Primary care | Anyone | Inclusion: patients who had had a primary care FBC taken. | 30.7 | |
| Birks 2017 [19] 1 | Retrospective | Cohort | UK | Primary care | Anyone | Inclusion: all patients with ≥ 1 FBC present in their record. Exclusion: <12 months registered with the general practice, < 2 years of follow-up data following the index date, history of CRC before the index date, CRC precursors, haemoglobin gene defects. | 54.2 | 44.1 |
| Boursi 2016 [20] 2 | Retrospective | Case-control | UK | Primary care | Anyone | Inclusion: all those in 1995–2013 from THIN. Exclusion: those with a diagnosis of CRC syndromes, familial history of CRC, IBD, or unacceptable medical records. | 69.7 | 47.4 |
| Cakmak 2017 [21] | Retrospective | Case-control | Turkey | Unclear | Anyone | Inclusion: patients who underwent colonoscopy screening and diagnosed with colon adenocarcinoma from biopsy. Exclusion: patients with co-existing infections, hematologic diseases, renal diseases, vascular diseases, or other cancer types. | 65.4 | 53.4 |
| Collins 2012 [22] 1 | Retrospective | Cohort | UK | Primary care | Anyone | The same entry criteria as the original model development study were used (Hippisley-Cox 2012). | 48 | 49.6 |
| Cross 2019 [23] | Retrospective | Cohort | UK | Secondary care | Symptomatic | Inclusion: patients from the SIGGAR trials, who were ≥ 55 years and judged to be in need of and fit enough for a whole colon investigation with full bowel preparation. Exclusion: if they were in follow-up for CRC, had undergone whole colon investigation < 6 months, familial adenomatous polyposis or Lynch syndrome, previously diagnosed with irritable bowel disease. | 69 | 41 |
| Cubiella 2016 [24] 1,3 | Prospective | Cohort | Spain | Other | Symptomatic | Inclusion: the derivation cohort consisted of consecutive patients with gastrointestinal symptoms referred for colonoscopy from primary and secondary health care to Complexo Hospitalario Universitario de Ourense, Spain. The validation cohort included a prospective cohort of patients with gastrointestinal symptoms referred for colonoscopy in 11 hospitals in Spain. Exclusion: age < 18 years, pregnant, asymptomatic individuals undergoing colonoscopy for CRC screening, previous history of colonic disease who underwent a surveillance colonoscopy, requiring hospital admission, symptoms ceased < 3 months before evaluation, and declined to participate after reading the informed consent form. | 66 | 50.1 |
| Fijten 1995 [25] | Prospective | Cohort | Netherlands | Primary care | Symptomatic | Inclusion: overt rectal bleeding was the reason for encounter or < 3 months visible rectal blood loss. Exclusion: age < 18 or > 75 years, pregnant, urgent admission to a hospital, and no follow-up data available. | 42 | 44 |
| Firat 2016 [26] 2 | Retrospective | Case-control | Turkey | Unclear | Anyone | Inclusion: CRC cases and controls between 1 January 2010 and 1 March 2014 from Inonu University Turgut Ozal Center of Medicine, Department of Oncology. | 58.6 | 56.3 |
| Goldshtein 2010 [27] | Retrospective | Case-control | Israel | Primary care | Anyone | Inclusion: MHS members aged 45–75 years diagnosed with CRC between 1/1/2004 and 14/1/2009. Exclusion: haemoglobin values below 11.7 g/dl for women and 12.6 g/dl for men at any point during the first year of follow-up. Controls had no documented history of cancer. | ||
| Goshen 2017 [28] 3 | Retrospective | Case-control | Israel | Primary care | Anyone | Inclusion: MHS enrolees with and without a CRC diagnosis between 40 and 75 years of age in 2002–2011, ≥ 1 blood test recorded before diagnosis. Exclusion: individuals with any form of cancer before 2002. | 52.1 | |
| Hamilton 2005 [29] | Retrospective | Case-control | UK | Primary care | Anyone | Inclusion: patients aged ≥ 40 years with a primary CRC diagnosed in 1998–2002 at the Royal Devon and Exeter Hospital. Cases without positive histology were included if the records contained a specialist diagnosis of cancer based on strong clinical evidence. Controls were alive at the time of diagnosis of their case. Exclusion: unobtainable records, no consultations < 2 years before diagnosis, previous CRC, or residence outside Exeter at the time of diagnosis. | 50.7 | |
| Hamilton 2008 [4] | Retrospective | Case-control | UK | Primary care | Anyone | Inclusion: patients with CRC aged ≥ 30 years and diagnosed between January 2000 and July 2006. Controls were free from CRC. All participants had ≥ 2 years of electronic records prior to the date of diagnosis of the case. | 53.1 | |
| Hamilton 2009 [30] | Retrospective | Case-control | UK | Primary care | Anyone | Inclusion: patients aged ≥ 30 years between January 2001 and July 2006 and ≥ 2 years of full electronic records before diagnosis. Cases had CRC diagnosis and controls did not. | 53.1 | |
| Hilsden 2018 [31] 1 | Retrospective | Cohort | Canada | Secondary care | Symptomatic | Inclusion: individuals aged 50–75 years who underwent a successful colonoscopy between January 2013 and June 2015 with bowel preparation rated by the endoscopist as adequate to detect polyps > 5 mm, at average risk for CRC, with a personal or family history of polyps or CRC. Exclusion: positive guaiac or immunochemical fecal occult blood test, history of CRC, known or suspected genetic predisposition to cancer or no FBC result < 1 year prior to their colonoscopy. | 45.3 | |
| Hippisley-Cox 2012 [32] 2 | Retrospective | Cohort | UK | Primary care | Symptomatic | Inclusion: patients aged 30–84 years registered from practices between 1 January 2000 and 30 September 2010. Exclusion: no postcode-related Townsend score, history of CRC at baseline, and recorded red flag symptom ≤ 12 months to the study entry date that might indicate CRC. | 50.1 | |
| Hippisley-Cox 2013 [33] 2 | Retrospective | Cohort | UK | Primary care | Symptomatic | Inclusion: males aged 25–89 years from practices between 1 January 2000 and 1 April 2012. Exclusion: no postcode-related Townsend score or recorded red flag symptom ≤12 months before the study entry date were excluded. | 48 | 100 |
| Hippisley-Cox 2013 [34] 2 | Retrospective | Cohort | UK | Primary care | Symptomatic | Inclusion: females aged 25–89 years from practices between 1 January 2000 and 1 April 2012. Exclusion: no postcode-related Townsend score or recorded red flag symptom ≤ 12 months before the study entry date were excluded. | 50.2 | 0 |
| Hornbrook 2017 [35] 1 | Retrospective | Case-control | USA | Unclear | Anyone | Inclusion: CRC from the Kaiser Permanente Tumor Registry diagnosed with CRC, had multiple FBCs ≤ 6 months of CRC diagnosis, and ≥180 days of continuous enrolment prior to CRC diagnosis. Controls received at least one outpatient FBC between 2000 and 2013, were aged 40–89 years at time of at least one FBC, had no history of cancer diagnoses in the database, were continuously enrolled from 180 days prior to FBC date through 24 months after the FBC date. Exclusion: CRC patients with any cancer diagnosis prior to the CRC diagnosis date. | 58 | 44.2 |
| Huang 2019 [36] | Retrospective | Case-control | China | Unclear | Anyone | Inclusion: patients newly diagnosed with CRC at the first affiliated Hospital of Guangxi Medical University (Nanning, China) from June 2017 to October 2018. Controls had benign colorectal polyps or were healthy. Exclusion: haematological disorders, kidney disease, acute/chronic infections, coronary artery disease, hypertension, diabetes mellitus, medical treatment with anticoagulant, undergone transfusions ≤ 3 months, received neoadjuvant therapy, or had other cancers. | 53.4 | 62 |
| Hung 2015 [37] | Retrospective | Cohort | Taiwan | Unclear | Symptomatic | Inclusion: patients newly diagnosed with iron deficiency anaemia between January 1, 2000 and December 31, 2010, aged ≥ 20 years at the time of IDA, and with no prior malignancies. | 24 | |
| Joosten 2008 [38] | Retrospective | Case-control | Belgium | Secondary care | Symptomatic | Inclusion: patients admitted to the acute geriatric ward and the geriatric day care centre of the University Hospitals Leuven, referred for colonoscopy during January 2002 to June 2007. Exclusion: patients with a history of CRC, incomplete colonoscopy, polyp surveillance, previous colon surgery, red cell transfusion, or iron therapy ≤2 months. | 82.3 | 61.6 |
| Kilincalp 2015 [39] | Retrospective | Case-control | Turkey | Unclear | Anyone | Inclusion: CRC cases diagnosed by colonoscopy with colorectal resection thereafter and those with histological confirmation of adenocarcinomas. Exclusion: coexistent haematological disorders, renal disease, chronic infection, coronary artery or cerebrovascular disease, other types of cancers, received preoperative chemoradiotherapy and postoperative infections including wound infections. | 60.7 | 67.9 |
| Kinar 2016 [40] 1, 2 | Retrospective | Cohort | Israel | Primary care | Anyone | Inclusion: all insured individuals above age 40 years. | 57.8 | 46.8 |
| Kinar 2017 [41] 1 | Retrospective | Cohort | Israel | Primary care | Anyone | Inclusion: the development set was aged 50–75 on January 1, 2008 with ≥ 1 FBC recorded in the MHS during the six-month testing period. Exclusion: a diagnosis of any cancer recorded in the National Cancer Registry prior to January 1, 2008, or no blood test taken during the testing period. | 60.9 | 44 |
| Lawrenson 2006 [42] | Retrospective | Cohort | UK | Primary care | Symptomatic | Inclusion: patients aged 40–89, registered in practices from England and Wales contributing to the GPRD at any time between 1 January 1992 to 31 December 1999, and with at least 1 year of data. | ||
| Lee 2006 [43] | Retrospective | Cohort | Korea | Unclear | Anyone | Inclusion: government employees, teachers, and their dependents insured by the Korean Medical Insurance Corporation in 1993 and 1995. Exclusion: no white blood cell record in their examinations, history of any cancer at enrolment, cancer-related death before the start of follow-up and missing data on any covariate. | 56.7 | 25.7 |
| Margolis 2007 [44] | Retrospective | Cohort | USA | Unclear | Anyone | Inclusion: postmenopausal women aged 50–79 years recruited at 40 clinical centres throughout the United States between September 1, 1993 and December 31, 1998 from a hormone trial, dietary modification trial, and calcium/vitamin D supplementation trial. The observational study included women screened but ineligible for the trials or recruited through a direct invitation for screening into the observational study. Exclusion: history of cancer except non-melanoma skin cancer at baseline, missing baseline white blood cell count, missing data regarding cancer history at baseline, white blood cell count > 15.0 × 109/L or <2.5 × 109/L. | 63 | 0 |
| Marshall 2011 [45] 1, 3 | Retrospective | Case-control | UK | Primary care | Anyone | Inclusion: The development set had patients aged ≥ 30 years with or without a diagnosis of CRC between January 2001 and July 2006 and ≥2 years of records before diagnosis. The validation was a case-control study in a single primary care trust in Exeter UK, aged > 40 years between 1998 and 2002. | 53 | |
| Mashlab 2018 [46] | Retrospective | Cohort | UK | Secondary care | Symptomatic | Inclusion: patients referred under the 2-week wait pathway for suspected CRC from the referral database created by specialist nurses at the colorectal service. Exclusion: duplicate and rejected referrals, cases with no FBC on referral, no investigations or an unknown outcome. | 45.4 | |
| Naef 1999 [47] | Retrospective | Cohort | Switzerland | Unclear | Anyone | Inclusion: primary and secondary small-bowel tumours treated in the department between January 1984 and December 1993, as well as associated syndromes. Exclusion: ileocecal valve and peri-ampullary duodenal tumours. | 61.4 | 55.6 |
| Nakama 2000 [48] | Unclear | Cohort | Japan | Unclear | Asymptomatic | Inclusion: asymptomatic persons aged 40–60 years who participated in a medical check-up for CRC as recommended by the companies with which they were employed. | ||
| Panagiotopoulou 2014 [49] | Retrospective | Unclear | UK | Unclear | Symptomatic | Inclusion: consecutive referrals for suspected CRC received at Centre A between November 2008 and June 2009 and Centre B between April 2010 and March 2011 using the cancer services prospectively maintained database. Exclusion: no blood tests available, previous history of CRC/panproctocolectomy and diagnosis of CRC in another hospital. | 46.2 | |
| Panzuto 2003 [50] | Prospective | Cohort | Italy | Primary care | Symptomatic | Inclusion: outpatients with symptoms considered suspicious for the presence of a colon disease to rule out CRC. Exclusion: previous diagnoses of colorectal disorders or a recent large bowel examination. | 61 | 42.9 |
| Pilling 2018 [51] | Retrospective | Cohort | UK | Other | Anyone | Inclusion: volunteers aged 40–70 years recruited by postal invitation from the UK Biobank study, living ≤ 25 miles of assessment centres in Great Britain, seen between 2006 and 2010. Exclusion: those with anaemia, coronary artery disease, cancer, type-2 diabetes, stroke, chronic obstructive pulmonary disease, or hypertension. | 55 | 51.8 |
| Prizment 2011 [52] | Prospective | Cohort | USA | Other | Anyone | Inclusion: patients aged 45–69 years from the ARIC study of atherosclerosis in 1987–1989, from suburban Minneapolis, Forsyth County, Jackson, and Washington County. Exclusion: prevalent cancer at the start of follow-up, did not consent to participate, or had missing biomarker information. | 53.9 | 46 |
| Raje 2007 [53] | Retrospective | Cohort | UK | Secondary care | Asymptomatic | Inclusion: females > 50 and males > 40 years with iron deficiency anaemia referred to one district general hospital during 2003. Exclusion: patients with haemoglobinopathy. | 40.1 | |
| Schneider 2018 [54] | Retrospective | Case-control | UK | Primary care | Anyone | Inclusion: patients in CPRD aged 18–89 years with a read-coded CRC diagnosis and matched control. Exclusion: history of any cancer before the index date except non-melanoma skin cancer. | 55.5 | |
| Shi 2019 [55] | Retrospective | Case-control | China | Unclear | Anyone | Inclusion: patients with CRC from historical biopsy undergoing radical surgery at the People’s Hospital of Liuzhou or those with colon polyps, with blood test data from 2 weeks before surgery. Exclusion: previous neoadjuvant therapy, presence of infection, and age of > 85 years. | 61.7 | 54.5 |
| Song 2018 [56] | Retrospective | Case-control | China | Unclear | Anyone | Inclusion: patients with CRC diagnosed at Fujian Medical University Union Hospital (China) from June 2015 to November 2017, or controls with colorectal adenomas patients or healthy participants. Exclusion: patients with anaemia, hematologic disorders, blood transfusion made ≤ 3 months, receiving iron deficiency treatment and with active inflammation. | 59.7 | |
| Spell 2004 [5] | Retrospective | Case-control | USA | Other | Anyone | Inclusion: CRC cases aged ≥ 18 years with ≥ 1 FBC recorded prior to surgery, performed at University of Texas Medical Branch. Controls were without CRC during the same time with a routine flexible sigmoidoscopy and ≥ 1 FBC < 6 months of the procedure. Exclusion: other colon malignancies (cases only), no FBC data prior iron therapy or red blood cell transfusion, chemotherapy or radiation therapy < 1 year of diagnosis, documented vitamin B12/folate deficiencies, or rectal cancer. | 47.5 | |
| Stapley 2006 [57] | Retrospective | Cohort | UK | Primary care | Symptomatic | Inclusion: primary CRC in patients aged ≥ 40 years from Exeter Primary Care Trust, diagnosed between 1998 and 2002. | 73 | 51 |
| Thompson 2017 [58] 2 | Retrospective | Cohort | UK | Secondary care | Symptomatic | Inclusion: newly referred to a colorectal surgical clinic undergoing sigmoidoscopy and/or whole colon investigation. Exclusion: previous bowel cancer diagnosis and subsequent referral to the colorectal clinic, or no sigmoidoscopy/WCI performed. | 60.1 | 43.8 |
| van Boxtel-Wilms 2016 [59] | Retrospective | Case-control | Netherlands | Primary care | Symptomatic | Inclusion: patients with or without CRC between 1 January 1992 and 31 December 2011 with ≥ 2 years of record before index date, and controls with a GP encounter < 1 month of the index date. | 56.5 | |
| Wu 2019 [60] | Retrospective | Case-control | China | Unclear | Anyone | Inclusion: patients who underwent surgical resection after CRC diagnosis but did not receive pharmacological treatment. Exclusion: pregnancy or lactation, other malignancies, thyroid disease, diabetes, cardiovascular disease, autoimmune diseases, kidney disease, haematological disease, or blood transfusion < 3 months before admission. | 53.6 | 59.9 |
| Yang 2018 [61] | Retrospective | Case-control | China | Unclear | Anyone | Inclusion: newly diagnosed and pathologically proven patients with CRC or benign colon polyps admitted to Shanghai Tongji Hospital between July 2014 and June 2017. Exclusion: patients with cardiovascular, kidney, blood, or other malignant diseases, or blood transfusion < 3 months of admission. | ||
| Zhou 2017 [62] | Retrospective | Case-control | China | Unclear | Anyone | Inclusion: patients with CRC or adenomatous polyp histologically confirmed whose families provided written informed consent. Healthy people had no symptoms and cancer history. Exclusion: acute infective disease and haematological disorders. | ||
| Zhu 2018 [63] | Retrospective | Case-control | China | Unclear | Anyone | Inclusion: CRC at Fujian Medical University Union Hospital (China) from June 2015 to October 2017 with no prior treatment, or controls with colorectal adenomas or healthy volunteers. Exclusion: haematological disorders, coronary artery disease, hypertension, diabetes mellitus, medical treatment with anticoagulant, and acetylic salicylic acid. | 60 | 60.1 |
| Article | Participation | Attrition | Prognostic Factor | Outcome | Confounders | Analysis & Reporting |
|---|---|---|---|---|---|---|
| Acher 2003 [13] | High | Low | Moderate | Low | High | High |
| Ankus 2018 [14] | Low | Low | Low | Moderate | High | Low |
| Ay 2015 [15] | Moderate | Low | Low | Moderate | High | High |
| Bafandeh 2008 [17] | Low | High | High | Moderate | High | High |
| Bailey 2017 [18] | Low | Low | Low | Low | High | Low |
| Boursi 2016 [20] | Low | Low | Moderate | Low | Moderate | Moderate |
| Cakmak 2017 [21] | Low | Low | Moderate | Low | High | Moderate |
| Cross 2019 [23] | Moderate | Low | Low | High | High | Low |
| Cubiella 2016 [24] | Low | Moderate | Low | Moderate | Moderate | Low |
| Fijten 1995 [25] | Low | Moderate | Moderate | High | High | Moderate |
| Firat 2016 [26] | Moderate | Low | High | Moderate | Moderate | High |
| Goldshtein 2010 [27] | Moderate | Low | Moderate | High | High | High |
| Goshen 2017 [28] | Moderate | Low | High | Moderate | Moderate | High |
| Hamilton 2005 [29] | Moderate | Low | Low | Low | Low | Moderate |
| Hamilton 2008 [4] | Low | Low | Moderate | Low | Low | Moderate |
| Hamilton 2009 [30] | Low | Low | Low | Low | Low | High |
| Hippisley-Cox 2012 [32] | Low | Low | Moderate | Low | Low | Low |
| Hippisley-Cox 2013 [33] | Low | Low | Moderate | Low | Low | Low |
| Hippisley-Cox 2013 [34] | Low | Low | Moderate | Low | Low | Low |
| Huang 2019 [36] | Low | Low | Low | Low | Moderate | Moderate |
| Hung 2015 [37] | Low | Low | High | Moderate | Moderate | Low |
| Joosten 2008 [38] | Low | Low | Low | Low | Moderate | Moderate |
| Kilincalp 2015 [39] | Low | Low | Low | Low | Moderate | Low |
| Kinar 2016 [40] | Low | Low | Moderate | Low | Moderate | High |
| Lawrenson 2006 [42] | Moderate | Low | High | Moderate | Moderate | Moderate |
| Lee 2006 [43] | Low | Low | Moderate | Moderate | Moderate | Low |
| Margolis 2007 [44] | Low | Low | Moderate | Low | Moderate | Moderate |
| Marshall 2011 [45] | Low | Low | Low | Low | Low | Low |
| Mashlab 2018 [46] | Low | Low | Low | Moderate | High | Low |
| Naef 1999 [47] | High | Low | Low | High | High | Moderate |
| Nakama 2000 [48] | High | Low | Low | Moderate | High | Low |
| Panagiotopoulou 2014 [49] | Moderate | Low | Low | High | Moderate | Moderate |
| Panzuto 2003 [50] | Moderate | Low | Low | Low | Moderate | Moderate |
| Pilling 2018 [51] | Low | Low | Moderate | High | Low | Moderate |
| Prizment 2011 [52] | Low | Low | Low | Moderate | Low | Moderate |
| Raje 2007 [53] | Moderate | Low | Low | Low | High | Moderate |
| Schneider 2018 [54] | Low | Low | Low | Low | Low | Moderate |
| Shi 2019 [55] | Low | Low | Low | Moderate | High | Low |
| Song 2018 [56] | Low | Low | Low | High | High | Moderate |
| Spell 2004 [5] | Moderate | Low | Low | Low | Moderate | Moderate |
| Stapley 2006 [57] | Low | Low | Low | Low | Moderate | High |
| Thompson 2017 [58] | Moderate | Low | Low | Low | Moderate | Low |
| van Boxtel-Wilms 2016 [59] | Low | Low | Moderate | Low | Moderate | Low |
| Wu 2019 [60] | Low | Low | Low | High | High | Low |
| Yang 2018 [61] | Moderate | Low | Low | High | High | Low |
| Zhou 2017 [62] | Low | Low | Low | High | High | Low |
| Zhu 2018 [63] | Low | Low | Low | High | High | Low |
| Total low (%) | 31 (66%) | 44 (94%) | 31 (66%) | 23 (49%) | 10 (21%) | 20 (43%) |
| Total moderate (%) | 13 (28%) | 2 (4%) | 13 (28%) | 13 (28%) | 18 (38%) | 18 (38%) |
| Total high (%) | 3 (6%) | 1 (2%) | 5 (11%) | 11 (23%) | 19 (41%) | 9 (19%) |
| Article | Model Name/Description | Participants | Predictors | Outcome | Analysis |
|---|---|---|---|---|---|
| Development: | |||||
| Boursi 2016 [20] | Laboratory model | Low | Low | Low | High |
| Boursi 2016 [20] | Combined model | Low | Low | Low | High |
| Cubiella 2016 [24] | COLONPREDICT | Low | Low | Unclear | High |
| Firat 2016 [26] | High | Unclear | Unclear | High | |
| Goshen 2017 [28] | Model for males 1 | High | High | High | High |
| Goshen 2017 [28] | Model for females 1 | High | High | High | High |
| Hippisley-Cox 2012 [32] | QCancer Colorectal males | Low | Low | Low | High |
| Hippisley-Cox 2012 [32] | QCancer Colorectal females | Low | Low | Low | High |
| Hippisley-Cox 2013 [33] | QCancer males | Low | Low | Low | High |
| Hippisley-Cox 2013 [34] | QCancer females | Low | Low | Low | High |
| Kinar 2016 [40] | ColonFlag | Low | Low | Low | High |
| Marshall 2011 [45] | Bristol-Birmingham | Low | Low | Low | High |
| Thompson 2017 [58] | Low | Low | Unclear | High | |
| Total low | 10 | 10 | 6 | 0 | |
| Total high | 3 | 2 | 2 | 13 | |
| Total unclear | 0 | 1 | 5 | 0 | |
| External validation: | |||||
| Ayling 2019 [16] | ColonFlag | Low | Unclear | Unclear | High |
| Birks 2017 [19] | ColonFlag | Low | Low | Low | Low |
| Collins 2012 [22] | QCancer Colorectal males | Low | Low | Low | Low |
| Collins 2012 [22] | QCancer Colorectal females | Low | Low | Low | Low |
| Cubiella 2016 [24] | COLONPREDICT | Low | Low | Unclear | High |
| Hilsden 2018 [31] | ColonFlag | Low | Low | Unclear | High |
| Hornbrook 2017 [35] | ColonFlag | Low | Low | Low | High |
| Kinar 2016 [40] | ColonFlag | Low | Low | Low | High |
| Kinar 2017 [41] | ColonFlag | Low | Low | Low | High |
| Marshall 2011 [45] | Bristol-Birmingham | Low | Low | Low | Low |
| Marshall 2011 [45] | CAPER 2 | Low | Low | Low | Low |
| Total low | 11 | 10 | 8 | 5 | |
| Total high | 0 | 1 | 0 | 6 | |
| Total unclear | 0 | 0 | 3 | 0 | |
| Article | Model Name or Description | Outcome Window | No. Cases/Controls | Model Building Method | Predictors in the Final Model |
|---|---|---|---|---|---|
| Boursi 2016 [20] | Laboratory model | 1 year | 4929/11311 | Logistic regression | Haematocrit, mean corpuscular volume, lymphocyte count, neutrophil-lymphocyte ratio |
| Boursi 2016 [20] | Combined model | 1 year | 3375/8560 | Logistic regression | Haemoglobin, mean corpuscular volume, white blood cell count, neutrophil-lymphocyte ratio, platelets, sex, previous metformin prescriptions, previous prescriptions for oral hypoglycemic drugs other than metformin |
| Cubiella 2016 [24] | COLONPREDICT | 1 week | 214/1358 | Logistic regression | Change in bowel habit, rectal bleeding, benign anorrectal lesion, rectal mass, serum CEA, haemoglobin, faecal haemoglobin, previous colonoscopy, aspirin use, sex, age |
| Firat 2016 [26] | At diagnosis | Machine-learning | Platelets, haemoglobin, sodium, total bilirubin, creatinine, calcium | ||
| Goshen 2017 [28] | Model for males | 1–6 months | 936/28491 | Logistic regression | Haemoglobin, mean corpuscular volume, monocyte count, platelets, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, iron, ferritin |
| Goshen 2017 [28] | Model for females | 1–6 months | 819/26239 | Logistic regression | Haemoglobin, mean corpuscular volume, neutrophil count, platelets, red blood cell distribution width, alanine aminotransferase, protein, iron, ferritin |
| Hippisley-Cox 2012 [32] | QCancer Colorectal males | 2 years | Cox regression | Alcohol status, family history of gastrointestinal cancer, haemoglobin, rectal bleeding, abdominal pain, appetite loss, weight loss, change in bowel habit in previous year | |
| Hippisley-Cox 2012 [32] | QCancer Colorectal females | 2 years | Cox regression | Family history of gastrointestinal cancer, haemoglobin, rectal bleeding, abdominal pain, appetite loss, weight loss | |
| Hippisley-Cox 2013 [33] | QCancer males | 2 years | 2607/1217648 | Logistic regression | Haemoglobin, family history of gastrointestinal cancer, alcohol status, abdominal distension, abdominal pain, appetite loss, rectal bleeding, venous thromboembolism, weight loss, change in bowel habit, constipation |
| Hippisley-Cox 2013 [34] | QCancer females | 2 years | 3250/1240550 | Logistic regression | Haemoglobin, family history of gastrointestinal cancer, alcohol status, abdominal distension, abdominal pain, appetite loss, rectal bleeding, weight loss, change in bowel habit, constipation |
| Kinar 2016 [40] | ColonFlag | 3–6 months | 2437 | Machine-learning | Age, sex, all 20 FBC components |
| Marshall 2011 [45] | Bristol-Birmingham | 2 years | 5477/38314 | Logistic regression | Constipation, diarrhoea, change in bowel habit, flatulence, irritable bowel syndrome, abdominal pain/antispasmodic, rectal bleeding, haemoglobin, mean corpuscular volume, weight loss, deep venous thrombosis/pulmonary embolism, diabetes, obesity |
| Thompson 2017 [58] | 3 years | 990/16413 | Logistic regression | Age, sex, symptom combinations, physical signs, iron-deficiency anaemia, rectal bleeding, change in bowel habit, other characteristics of colorectal cancer |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virdee, P.S.; Marian, I.R.; Mansouri, A.; Elhussein, L.; Kirtley, S.; Holt, T.; Birks, J. The Full Blood Count Blood Test for Colorectal Cancer Detection: A Systematic Review, Meta-Analysis, and Critical Appraisal. Cancers 2020, 12, 2348. https://doi.org/10.3390/cancers12092348
Virdee PS, Marian IR, Mansouri A, Elhussein L, Kirtley S, Holt T, Birks J. The Full Blood Count Blood Test for Colorectal Cancer Detection: A Systematic Review, Meta-Analysis, and Critical Appraisal. Cancers. 2020; 12(9):2348. https://doi.org/10.3390/cancers12092348
Chicago/Turabian StyleVirdee, Pradeep S., Ioana R. Marian, Anita Mansouri, Leena Elhussein, Shona Kirtley, Tim Holt, and Jacqueline Birks. 2020. "The Full Blood Count Blood Test for Colorectal Cancer Detection: A Systematic Review, Meta-Analysis, and Critical Appraisal" Cancers 12, no. 9: 2348. https://doi.org/10.3390/cancers12092348
APA StyleVirdee, P. S., Marian, I. R., Mansouri, A., Elhussein, L., Kirtley, S., Holt, T., & Birks, J. (2020). The Full Blood Count Blood Test for Colorectal Cancer Detection: A Systematic Review, Meta-Analysis, and Critical Appraisal. Cancers, 12(9), 2348. https://doi.org/10.3390/cancers12092348





