Myelomonocytic Skewing In Vitro Discriminates Subgroups of Patients with Myelofibrosis with A Different Phenotype, A Different Mutational Profile and Different Prognosis
Abstract
1. Introduction
2. Results
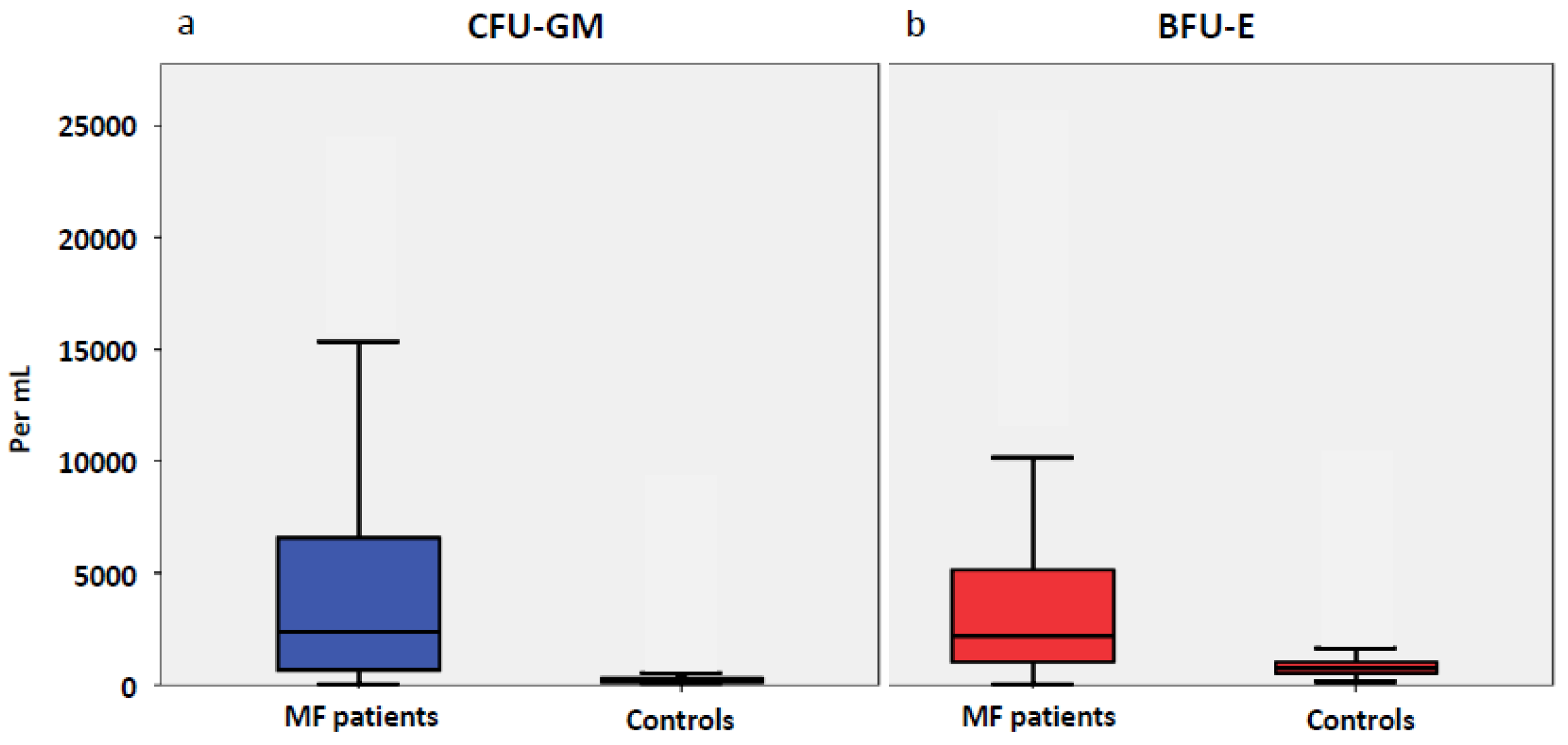
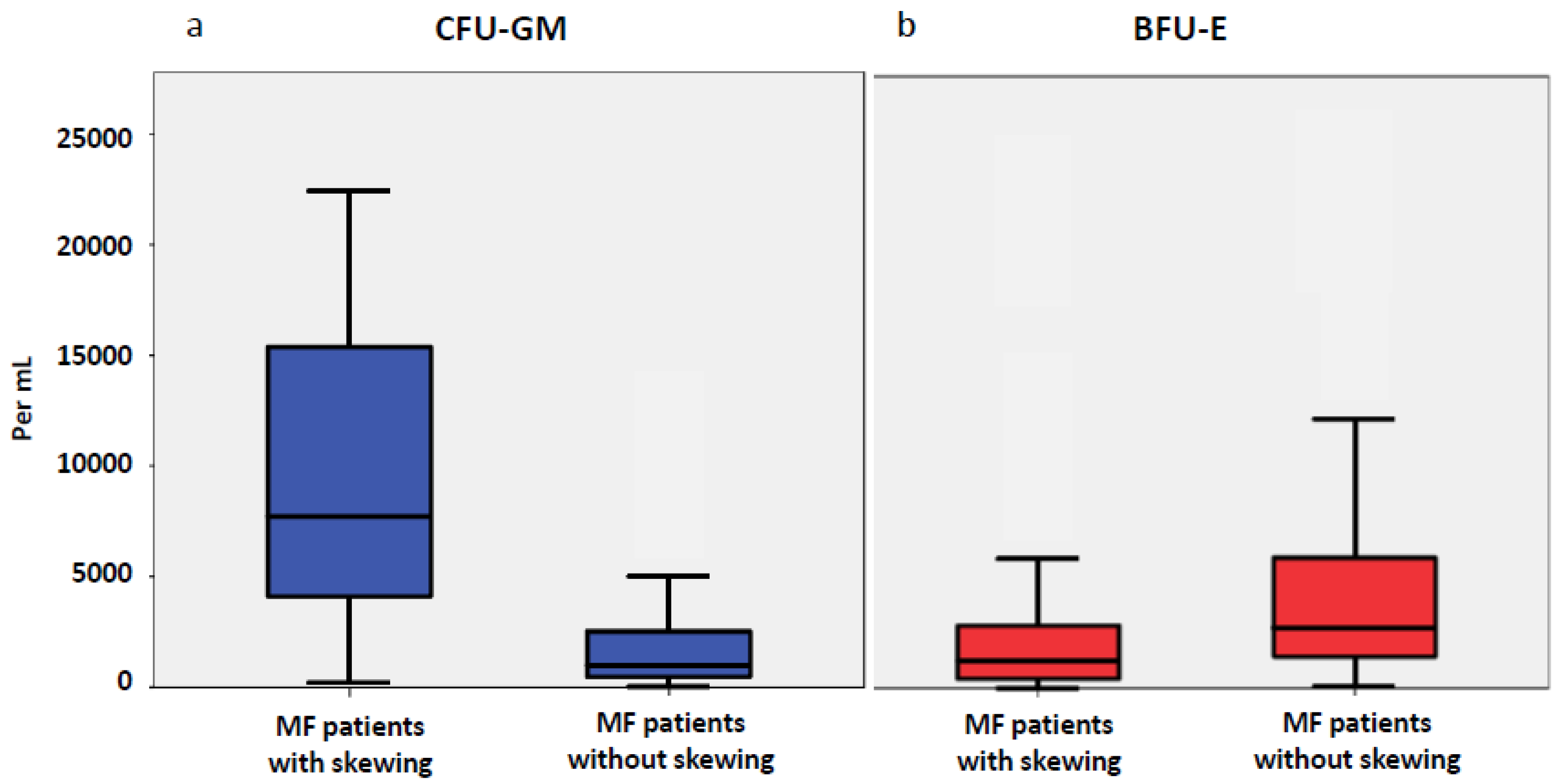
2.1. Progenitor Cells in Patients with MF
2.2. Phenotype of MF Patients with and without Myelomonocytic Skewing
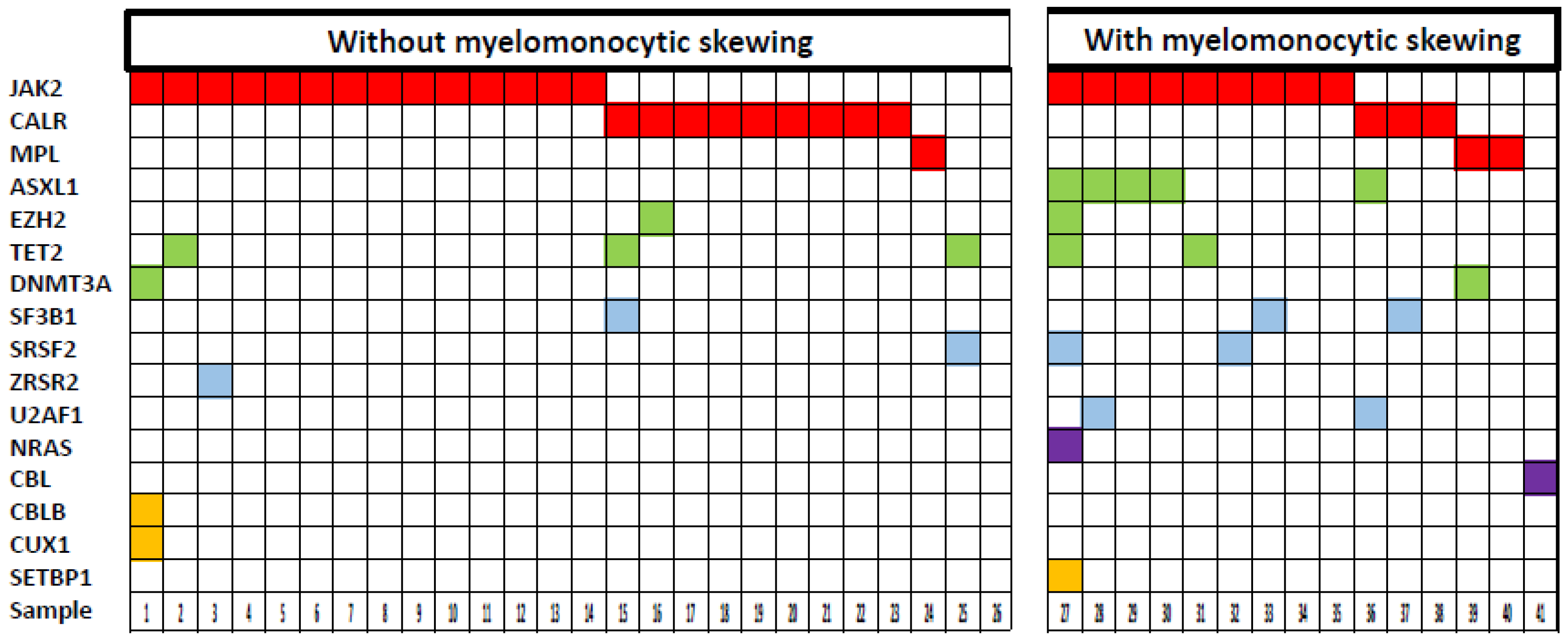
2.3. Mutational Profile of MF Patients with and without Myelomonocytic Skewing
2.4. Survival of MF Patients with and without Myelomonocytic Skewing
3. Discussion
4. Patients and Methods
4.1. Patients
4.2. Colony Assay
4.3. Molecular Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, Z.; Cai, X.; Cai, C.-L.; Wang, J.; Zhang, W.; Petersen, B.E.; Yang, F.-C.; Xu, M. Deletion of Tet2 in Mice Leads to Dysregulated Hematopoietic Stem Cells and Subsequent Development of Myeloid Malignancies. Blood 2011, 118, 4509–4518. [Google Scholar] [CrossRef] [PubMed]
- Pronier, E.; Almire, C.; Mokrani, H.; Vasanthakumar, A.; Simon, A.; da Costa, R.M.M.B.; Massé, A.; Le Couédic, J.-P.; Pendino, F.; Carbonne, B.; et al. Inhibition of TET2-Mediated Conversion of 5-Methylcytosine to 5-Hydroxymethylcytosine Disturbs Erythroid and Granulomonocytic Differentiation of Human Hematopoietic Progenitors. Blood 2011, 118, 2551–2555. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Z.; He, Y.; Pan, F.; Chen, S.; Rhodes, S.; Nguyen, L.; Yuan, J.; Jiang, L.; Yang, X.; et al. Loss of Asxl1 Leads to Myelodysplastic Syndrome-like Disease in Mice. Blood 2014, 123, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Bapat, A.; Keita, N.; Martelly, W.; Kang, P.; Seet, C.; Jacobsen, J.R.; Stoilov, P.; Hu, C.; Crooks, G.M.; Sharma, S. Myeloid Disease Mutations of Splicing Factor SRSF2 Cause G2-M Arrest and Skewed Differentiation of Human Hematopoietic Stem and Progenitor Cells. Stem Cells 2018, 36, 1663–1675. [Google Scholar] [CrossRef]
- Chen, T.H.-P.; Swarnkar, G.; Mbalaviele, G.; Abu-Amer, Y. Myeloid Lineage Skewing Due to Exacerbated NF-ΚB Signaling Facilitates Osteopenia in Scurfy Mice. Cell Death Dis. 2015, 6, e1723. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Baldwin, A.S.; Friedman, A.D.; Paz-Priel, I. Loss of IKKβ but Not NF-ΚB P65 Skews Differentiation towards Myeloid over Erythroid Commitment and Increases Myeloid Progenitor Self-Renewal and Functional Long-Term Hematopoietic Stem Cells. PLoS ONE 2015, 10, e0130441. [Google Scholar] [CrossRef]
- Oduro, K.A.; Liu, F.; Tan, Q.; Kim, C.-K.; Lubman, O.; Fremont, D.; Mills, J.C.; Choi, K. Myeloid Skewing in Murine Autoimmune Arthritis Occurs in Hematopoietic Stem and Primitive Progenitor Cells. Blood 2012, 120, 2203–2213. [Google Scholar] [CrossRef]
- Liang, Y.; Van Zant, G.; Szilvassy, S.J. Effects of Aging on the Homing and Engraftment of Murine Hematopoietic Stem and Progenitor Cells. Blood 2005, 106, 1479–1487. [Google Scholar] [CrossRef]
- Ohler, L.; Geissler, K.; Hinterberger, W. Diagnostic and Prognostic Value of Colony Formation of Hematopoietic Progenitor Cells in Myeloid Malignancies. Wien. Klin. Wochenschr. 2003, 115, 537–546. [Google Scholar] [CrossRef]
- Tefferi, A. Primary Myelofibrosis: 2019 Update on Diagnosis, Risk-Stratification and Management. Am. J. Hematol. 2018, 93, 1551–1560. [Google Scholar] [CrossRef]
- Vannucchi, A.M.; Lasho, T.L.; Guglielmelli, P.; Biamonte, F.; Pardanani, A.; Pereira, A.; Finke, C.; Score, J.; Gangat, N.; Mannarelli, C.; et al. Mutations and Prognosis in Primary Myelofibrosis. Leukemia 2013, 27, 1861–1869. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Guglielmelli, P.; Nicolosi, M.; Mannelli, F.; Mudireddy, M.; Bartalucci, N.; Finke, C.M.; Lasho, T.L.; Hanson, C.A.; Ketterling, R.P.; et al. GIPSS: Genetically Inspired Prognostic Scoring System for Primary Myelofibrosis. Leukemia 2018, 32, 1631–1642. [Google Scholar] [CrossRef] [PubMed]
- Passamonti, F.; Cervantes, F.; Vannucchi, A.M.; Morra, E.; Rumi, E.; Pereira, A.; Guglielmelli, P.; Pungolino, E.; Caramella, M.; Maffioli, M.; et al. A Dynamic Prognostic Model to Predict Survival in Primary Myelofibrosis: A Study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood 2010, 115, 1703–1708. [Google Scholar] [CrossRef] [PubMed]
- Chervenick, P.A. Increase in Circulating Stem Cells in Patients with Myelofibrosis. Blood 1973, 41, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Chikkappa, G.; Carsten, A.L.; Chanana, A.D.; Chandra, P.; Cronkite, E.P. Increased Granulocytic, Erythrocytic, and Megakaryocytic Progenitors in Myelofibrosis with Myeloid Metaplasia. Am. J. Hematol. 1978, 4, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Croizat, H.; Amato, D.; McLeod, D.L.; Eskinazi, D.; Axelrad, A.A. Differences among Myeloproliferative Disorders in the Behavior of Their Restricted Progenitor Cells in Culture. Blood 1983, 62, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Douer, D.; Fabian, I.; Cline, M.J. Circulating Pluripotent Haemopoietic Cells in Patients with Myeloproliferative Disorders. Br. J. Haematol. 1983, 54, 373–381. [Google Scholar] [CrossRef]
- Sagaster, V.; Jäger, E.; Weltermann, A.; Schwarzinger, I.; Gisslinger, H.; Lechner, K.; Geissler, K.; Oehler, L. Circulating Hematopoietic Progenitor Cells Predict Survival in Patients with Myelofibrosis with Myeloid Metaplasia. Haematologica 2003, 88, 1204–1212. [Google Scholar]
- Geissler, K.; Hinterberger, W.; Jäger, U.; Bettelheim, P.; Neumann, E.; Haas, O.; Ambros, P.; Chott, A.; Radaszkiewicz, T.; Lechner, K. Deficiency of Pluripotent Hemopoietic Progenitor Cells in Myelodysplastic Syndromes. Blut 1988, 57, 45–49. [Google Scholar] [CrossRef]
- Geissler, K.; Hinterberger, W.; Bettelheim, P.; Haas, O.; Lechner, K. Colony Growth Characteristics in Chronic Myelomonocytic Leukemia. Leuk. Res. 1988, 12, 373–377. [Google Scholar] [CrossRef]
- Haferlach, T.; Nagata, Y.; Grossmann, V.; Okuno, Y.; Bacher, U.; Nagae, G.; Schnittger, S.; Sanada, M.; Kon, A.; Alpermann, T.; et al. Landscape of Genetic Lesions in 944 Patients with Myelodysplastic Syndromes. Leukemia 2014, 28, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, M.M.; Tefferi, A. Cytogenetic and Molecular Abnormalities in Chronic Myelomonocytic Leukemia. Blood Cancer J. 2016, 6, e393. [Google Scholar] [CrossRef]
- Itzykson, R.; Kosmider, O.; Renneville, A.; Morabito, M.; Preudhomme, C.; Berthon, C.; Adès, L.; Fenaux, P.; Platzbecker, U.; Gagey, O.; et al. Clonal Architecture of Chronic Myelomonocytic Leukemias. Blood 2013, 121, 2186–2198. [Google Scholar] [CrossRef] [PubMed]
- Busque, L.; Patel, J.P.; Figueroa, M.E.; Vasanthakumar, A.; Provost, S.; Hamilou, Z.; Mollica, L.; Li, J.; Viale, A.; Heguy, A.; et al. Recurrent Somatic TET2 Mutations in Normal Elderly Individuals with Clonal Hematopoiesis. Nat. Genet. 2012, 44, 1179–1181. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, H.C. Perspectives on Chronic Inflammation in Essential Thrombocythemia, Polycythemia Vera, and Myelofibrosis: Is Chronic Inflammation a Trigger and Driver of Clonal Evolution and Development of Accelerated Atherosclerosis and Second Cancer? Blood 2012, 119, 3219–3225. [Google Scholar] [CrossRef]
- Elliott, M.A.; Verstovsek, S.; Dingli, D.; Schwager, S.M.; Mesa, R.A.; Li, C.Y.; Tefferi, A. Monocytosis Is an Adverse Prognostic Factor for Survival in Younger Patients with Primary Myelofibrosis. Leuk. Res. 2007, 31, 1503–1509. [Google Scholar] [CrossRef]
- Tefferi, A.; Shah, S.; Mudireddy, M.; Lasho, T.L.; Barraco, D.; Hanson, C.A.; Ketterling, R.P.; Elliott, M.A.; Patnaik, M.S.; Pardanani, A.; et al. Monocytosis Is a Powerful and Independent Predictor of Inferior Survival in Primary Myelofibrosis. Br. J. Haematol. 2018, 183, 835–838. [Google Scholar] [CrossRef]
- Vardiman, J.W.; Thiele, J.; Arber, D.A.; Brunning, R.D.; Borowitz, M.J.; Porwit, A.; Harris, N.L.; Beau, M.M.L.; Hellstrom-Lindberg, E.; Tefferi, A.; et al. The 2008 Revision of the World Health Organization (WHO) Classification of Myeloid Neoplasms and Acute Leukemia: Rationale and Important Changes. Blood 2009, 114, 937–951. [Google Scholar] [CrossRef]
- Barosi, G.; Mesa, R.A.; Thiele, J.; Cervantes, F.; Campbell, P.J.; Verstovsek, S.; Dupriez, B.; Levine, R.L.; Passamonti, F.; Gotlib, J.; et al. International Working Group for Myelofibrosis Research and Treatment (IWG-MRT). Proposed Criteria for the Diagnosis of Post-Polycythemia Vera and Post-Essential Thrombocythemia Myelofibrosis: A Consensus Statement from the International Working Group for Myelofibrosis Research and Treatment. Leukemia 2008, 22, 437–438. [Google Scholar] [CrossRef]
- Geissler, K.; Peschel, C.; Niederwieser, D.; Strobl, H.; Goldschmitt, J.; Ohler, L.; Bettelheim, P.; Kahls, P.; Huber, C.; Lechner, K.; et al. Potentiation of Granulocyte Colony-Stimulating Factor-Induced Mobilization of Circulating Progenitor Cells by Seven-Day Pretreatment with Interleukin-3. Blood 1996, 87, 2732–2739. [Google Scholar] [CrossRef]
- Klampfl, T.; Gisslinger, H.; Harutyunyan, A.S.; Nivarthi, H.; Rumi, E.; Milosevic, J.D.; Them, N.C.C.; Berg, T.; Gisslinger, B.; Pietra, D.; et al. Somatic Mutations of Calreticulin in Myeloproliferative Neoplasms. N. Engl. J. Med. 2013, 369, 2379–2390. [Google Scholar] [CrossRef] [PubMed]
- Schischlik, F.; Jäger, R.; Rosebrock, F.; Hug, E.; Schuster, M.; Holly, R.; Fuchs, E.; Feenstra, J.D.M.; Bogner, E.; Gisslinger, B.; et al. Mutational Landscape of the Transcriptome Offers Putative Targets for Immunotherapy of Myeloproliferative Neoplasms. Blood 2019, 134, 199–210. [Google Scholar] [CrossRef] [PubMed]


| Variables | All MF Patients (n = 81) | MF Patients with Myelomonocytic Skewing (n = 26) | MF Patients without Myelomonocytic Skewing (n = 55) | p-Value |
|---|---|---|---|---|
| Age in years; median (range) | 66.0 (25–88) | 69 (42–86) | 64 (25–88) | 0.116 |
| Sex (Male); n (%) | 45/81 (56%) | 18/26 (69%) | 27/55 (49%) | 0.089 |
| WBC × 10⁹/L; median (range) | 8.4 (2.4–96) | 12.1 (3.1–96) | 8.2 (2.4–44.7) | 0.036 |
| Hemoglobin g/dL, median (range) | 11.9 (6.6–16.3) | 9.6 (6.6–14.3) | 13.0 (8.3–16.3) | <0.001 |
| Platelets × 10⁹/L; median (range) | 424 (15–1584) | 244 (15–1450) | 516 (18–1584) | 0.002 |
| PB blasts %; median (range) | 0 (0–10) | 0.5 (0–10) | 0 (0–2) | 0.018 |
| Leukoerythroblastic change (%) | 22/81 (27%) | 11/26 (42%) | 11/55 (20%) | 0.035 |
| Monocytes × 10⁹/L; median (range) | 0.50 (0–16.3) | 0.51 (0–16.3) | 0.50 (0–1.6) | 0.704 |
| Intermediate-2 and high-risk category according to DIPSS (%) | 24/81 (30%) | 15/26 (58%) | 9/55 (16%) | <0.001 |
| Post PV/ET MF (%) | 6/81 (7%) | 2/26 (8%) | 4/55 (7%) | 0.946 |
| Factors | Factor Present Md OS (mo) | Factor Absent Md OS (mo) | Hazard Ratio | p-Value (Log-Rank) |
|---|---|---|---|---|
| Skewing present | 46 | 138 | 3.98 | <0.001 |
| WBC > 25 × 109/L | 5 | 83 | 3.66 | 0.025 |
| Hb < 10 g/dL | 46 | 139 | 5.39 | <0.001 |
| PLT < 100 × 109/L | 21 | 83 | 2.95 | 0.007 |
| PB Blasts present | 47 | 123 | 1.95 | 0.103 |
| Age > 65 years | 53 | 139 | 3.66 | 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geissler, K.; Gisslinger, B.; Jäger, E.; Jäger, R.; Schiefer, A.-I.; Bogner, E.; Fuchs, E.; Schischlik, F.; Alpar, D.; Simonitsch-Klupp, I.; et al. Myelomonocytic Skewing In Vitro Discriminates Subgroups of Patients with Myelofibrosis with A Different Phenotype, A Different Mutational Profile and Different Prognosis. Cancers 2020, 12, 2291. https://doi.org/10.3390/cancers12082291
Geissler K, Gisslinger B, Jäger E, Jäger R, Schiefer A-I, Bogner E, Fuchs E, Schischlik F, Alpar D, Simonitsch-Klupp I, et al. Myelomonocytic Skewing In Vitro Discriminates Subgroups of Patients with Myelofibrosis with A Different Phenotype, A Different Mutational Profile and Different Prognosis. Cancers. 2020; 12(8):2291. https://doi.org/10.3390/cancers12082291
Chicago/Turabian StyleGeissler, Klaus, Bettina Gisslinger, Eva Jäger, Roland Jäger, Ana-Iris Schiefer, Edith Bogner, Elisabeth Fuchs, Fiorella Schischlik, Donat Alpar, Ingrid Simonitsch-Klupp, and et al. 2020. "Myelomonocytic Skewing In Vitro Discriminates Subgroups of Patients with Myelofibrosis with A Different Phenotype, A Different Mutational Profile and Different Prognosis" Cancers 12, no. 8: 2291. https://doi.org/10.3390/cancers12082291
APA StyleGeissler, K., Gisslinger, B., Jäger, E., Jäger, R., Schiefer, A.-I., Bogner, E., Fuchs, E., Schischlik, F., Alpar, D., Simonitsch-Klupp, I., Kralovics, R., & Gisslinger, H. (2020). Myelomonocytic Skewing In Vitro Discriminates Subgroups of Patients with Myelofibrosis with A Different Phenotype, A Different Mutational Profile and Different Prognosis. Cancers, 12(8), 2291. https://doi.org/10.3390/cancers12082291





