Elderly Hepatocellular Carcinoma Patients: Open or Laparoscopic Approach?
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
2.2. Perioperative and Pathologic Characteristics
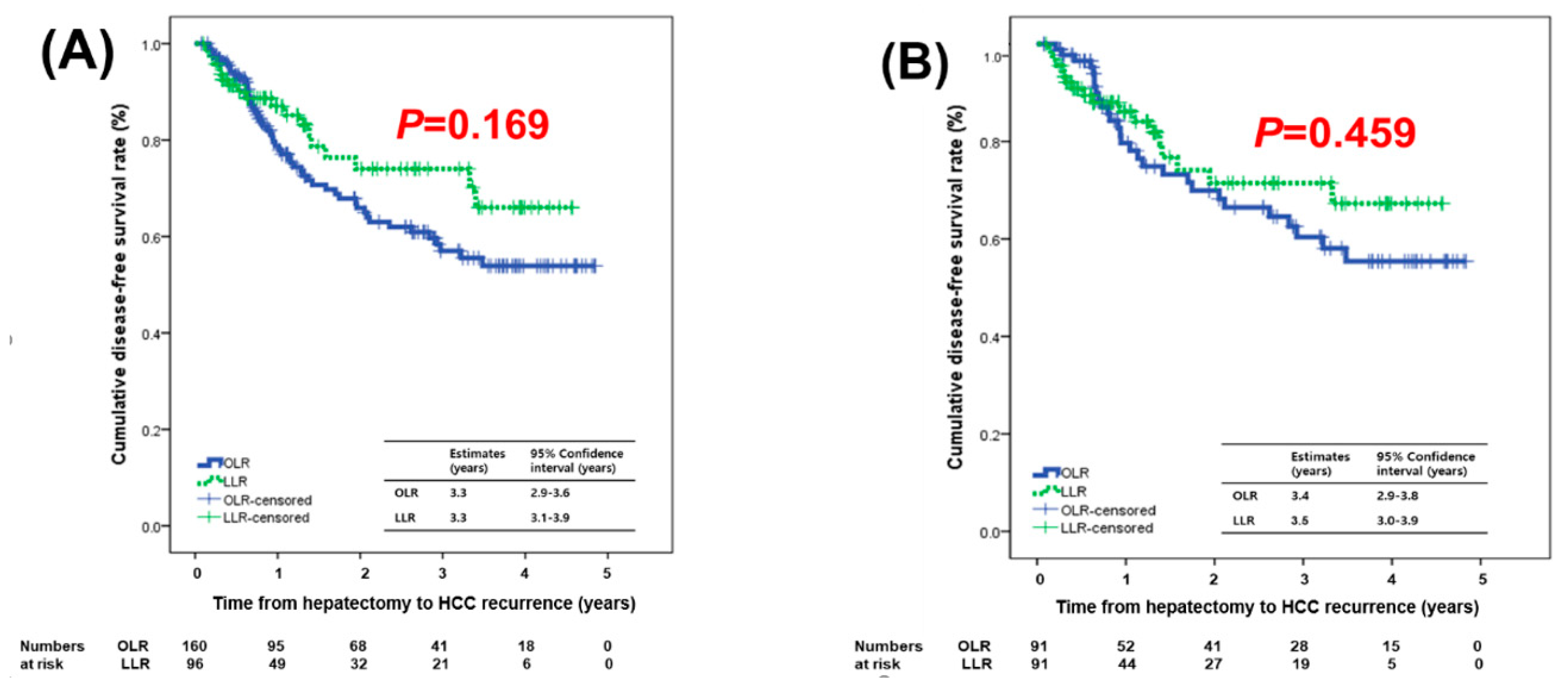
2.3. Tumor Recurrence and Survival
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Clinical Data
4.3. Surgical Techniques in Liver Resection
4.4. Perioperative Management
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Availability of Data and Material
Abbreviations
| LLR | laparoscopic liver resection |
| OLR | open liver resection |
| HCC | hepatocellular carcinoma |
| PVTT | portal vein tumor thrombosis |
| CD | Clavien–Dindo |
| CUSA | Cavitron Ultrasonic Surgical Aspirator |
| DFS | disease-free survival |
| PS | patient survival |
| NBNC | non-B non-C |
| NASH | non-alcoholic steatohepatitis |
References
- Korc-Grodzicki, B.; Downey, R.J.; Shahrokni, A.; Kingham, T.P.; Patel, S.G.; Audisio, R.A. Surgical considerations in older adults with cancer. J. Clin. Oncol. 2014, 32, 2647–2653. [Google Scholar] [CrossRef] [PubMed]
- Asahina, Y.; Tsuchiya, K.; Tamaki, N.; Hirayama, I.; Tanaka, T.; Sato, M.; Yasui, Y.; Hosokawa, T.; Ueda, K.; Kuzuya, T.; et al. Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infection. Hepatology 2010, 52, 518–527. [Google Scholar] [CrossRef]
- Smith, B.D.; Smith, G.L.; Hurria, A.; Hortobagyi, G.N.; Buchholz, T.A. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J. Clin. Oncol. 2009, 27, 2758–2765. [Google Scholar] [CrossRef] [PubMed]
- Nomi, T.; Fuks, D.; Kawaguchi, Y.; Mal, F.; Nakajima, Y.; Gayet, B. Laparoscopic major hepatectomy for colorectal liver metastases in elderly patients: A single-center, case-matched study. Surg. Endosc. 2015, 29, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Cucchetti, A.; Sposito, C.; Pinna, A.D.; Citterio, D.; Ercolani, G.; Flores, M.; Cescon, M.; Mazzaferro, V. Effect of age on survival in patients undergoing resection of hepatocellular carcinoma. Br. J. Surg. 2016, 103, e93–e99. [Google Scholar] [CrossRef] [PubMed]
- Faber, W.; Stockmann, M.; Schirmer, C.; Mollerarnd, A.; Denecke, T.; Bahra, M.; Klein, F.; Schott, E.; Neuhaus, P.; Seehofer, D. Significant impact of patient age on outcome after liver resection for HCC in cirrhosis. Eur. J. Surg. Oncol. 2014, 40, 208–213. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, J.M.; Lee, S.; Choi, J.Y.; Cho, W.; Choi, G.S.; Park, J.B.; Kwon, C.H.; Kim, S.J.; Joh, J.W. The prognosis in cases of hepatocellular carcinoma after hepatectomy: Young patients versus older patients. Korean J. Hepatobiliary Pancreat. Surg. 2015, 19, 154–160. [Google Scholar] [CrossRef]
- Kim, J.M.; Cho, B.I.; Kwon, C.H.; Joh, J.W.; Park, J.B.; Lee, J.H.; Kim, S.J.; Paik, S.W.; Park, C.K. Hepatectomy is a reasonable option for older patients with hepatocellular carcinoma. Am. J. Surg. 2015, 209, 391–397. [Google Scholar] [CrossRef]
- Kim, J.M.; Kwon, C.H.D.; Yoo, H.; Kim, K.S.; Lee, J.; Kim, K.; Choi, G.S.; Joh, J.W. Which approach is preferred in left hepatocellular carcinoma? Laparoscopic versus open hepatectomy using propensity score matching. BMC Cancer 2018, 18, 668. [Google Scholar] [CrossRef]
- Yoon, Y.I.; Kim, K.H.; Kang, S.H.; Kim, W.J.; Shin, M.H.; Lee, S.K.; Jung, D.H.; Park, G.C.; Ahn, C.S.; Moon, D.B.; et al. Pure Laparoscopic Versus Open Right Hepatectomy for Hepatocellular Carcinoma in Patients with Cirrhosis: A Propensity Score Matched Analysis. Ann. Surg. 2017, 265, 856–863. [Google Scholar] [CrossRef]
- Ciria, R.; Cherqui, D.; Geller, D.A.; Briceno, J.; Wakabayashi, G. Comparative Short-term Benefits of Laparoscopic Liver Resection: 9000 Cases and Climbing. Ann. Surg. 2016, 263, 761–777. [Google Scholar] [CrossRef]
- Rhu, J.; Choi, G.S.; Kwon, C.H.D.; Kim, J.M.; Joh, J.W. Learning curve of laparoscopic living donor right hepatectomy. Br. J. Surg. 2020, 107, 278–288. [Google Scholar] [CrossRef]
- Korean Liver Cancer, A.; National Cancer, C. 2018 Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Gut. Liver 2019, 13, 227–299. [Google Scholar] [CrossRef] [PubMed]
- Tee, M.C.; Chen, L.; Peightal, D.; Franko, J.; Kim, P.T.; Brahmbhatt, R.D.; Raman, S.; Scudamore, C.H.; Chung, S.W.; Segedi, M. Minimally invasive hepatectomy is associated with decreased morbidity and resource utilization in the elderly. Surg. Endosc. 2019. [Google Scholar] [CrossRef]
- Chen, K.; Pan, Y.; Maher, H.; Zhang, B.; Zheng, X.Y. Laparoscopic hepatectomy for elderly patients: Major findings based on a systematic review and meta-analysis. Medicine 2018, 97, e11703. [Google Scholar] [CrossRef] [PubMed]
- Mirici-Cappa, F.; Gramenzi, A.; Santi, V.; Zambruni, A.; Di Micoli, A.; Frigerio, M.; Maraldi, F.; Di Nolfo, M.A.; Del Poggio, P.; Benvegnu, L.; et al. Treatments for hepatocellular carcinoma in elderly patients are as effective as in younger patients: A 20-year multicentre experience. Gut 2010, 59, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.; Kwon, C.H.D.; Choi, J.Y.; Lee, S.H.; Kim, J.M.; Choi, G.S.; Joh, J.W.; Kim, S.J.; Kim, G.S.; Koh, K.C. Impact of technical innovation on surgical outcome of laparoscopic major liver resection: 10 years’ experience at a large-volume center. Ann. Surg. Treat. Res. 2019, 96, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Ihm, S.H.; Kim, G.H.; Kim, J.H.; Kim, K.I.; Lee, H.Y.; Lee, J.H.; Park, J.M.; Park, S.; Pyun, W.B.; et al. 2018 Korean Society of Hypertension guidelines for the management of hypertension: Part I-epidemiology of hypertension. Clin. Hypertens. 2019, 25, 16. [Google Scholar] [CrossRef]
- Bae, J.C. Trends of Diabetes Epidemic in Korea. Diabetes Metab. J. 2018, 42, 377–379. [Google Scholar] [CrossRef]
- Dokmak, S.; Fteriche, F.S.; Borscheid, R.; Cauchy, F.; Farges, O.; Belghiti, J. 2012 Liver resections in the 21st century: We are far from zero mortality. HPB 2013, 15, 908–915. [Google Scholar] [CrossRef]
- Tranchart, H.; Gaillard, M.; Chirica, M.; Ferretti, S.; Perlemuter, G.; Naveau, S.; Dagher, I. Multivariate analysis of risk factors for postoperative complications after laparoscopic liver resection. Surg. Endosc. 2015, 29, 2538–2544. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Eguchi, H.; Nagano, H.; Kubo, S.; Nakai, T.; Kaibori, M.; Hayashi, M.; Takemura, S.; Tanaka, S.; Nakata, Y.; et al. Perioperative allogenic blood transfusion is a poor prognostic factor after hepatocellular carcinoma surgery: A multi-center analysis. Surg. Today 2018, 48, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Bagante, F.; Spolverato, G.; Strasberg, S.M.; Gani, F.; Thompson, V.; Hall, B.L.; Bentrem, D.J.; Pitt, H.A.; Pawlik, T.M. Minimally Invasive vs. Open Hepatectomy: A Comparative Analysis of the National Surgical Quality Improvement Program Database. J. Gastrointest. Surg. 2016, 20, 1608–1617. [Google Scholar] [CrossRef] [PubMed]
- Nanashima, A.; Abo, T.; Nonaka, T.; Fukuoka, H.; Hidaka, S.; Takeshita, H.; Ichikawa, T.; Sawai, T.; Yasutake, T.; Nakao, K.; et al. Prognosis of patients with hepatocellular carcinoma after hepatic resection: Are elderly patients suitable for surgery? J. Surg. Oncol. 2011, 104, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, S.; Brustia, R.; Goumard, C.; Sepulveda, A.; Perdigao, F.; Soubrane, O.; Scatton, O. Clinical impact of laparoscopic hepatectomy: Technical and oncological viewpoints. Surg. Endosc. 2017, 31, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibanes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Kim, J.M.; Kwon, C.H.; Joh, J.W.; Park, J.B.; Ko, J.S.; Lee, J.H.; Kim, S.J.; Park, C.K. The effect of alkaline phosphatase and intrahepatic metastases in large hepatocellular carcinoma. World J. Surg. Oncol. 2013, 11, 40. [Google Scholar] [CrossRef]
- Lee, N.; Cho, C.W.; Kim, J.M.; Choi, G.S.; Kwon, C.H.D.; Joh, J.W. Application of temporary inflow control of the Glissonean pedicle method provides a safe and easy technique for totally laparoscopic hemihepatectomy by Glissonean approach. Ann. Surg. Treat Res. 2017, 92, 383–386. [Google Scholar] [CrossRef]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef]

| Variables | Before PS Matching | After PS Matching | ||||
|---|---|---|---|---|---|---|
| OLR (n = 160) | LLR (n = 96) | p-Value | OLR (n = 91) | LLR (n = 91) | p-Value | |
| Sex (male) | 136 (85.0%) | 70 (72.9%) | 0.023 | 70 (76.9%) | 68 (74.7%) | 0.863 |
| Age > 75 | 25 (15.6%) | 12 (12.5%) | 0.583 | 12 (13.2%) | 11 (12.1%) | 0.823 |
| Diabetes | 52 (32.5%) | 38 (39.6%) | 0.280 | 30 (33.0%) | 37 (40.7%) | 0.357 |
| Hypertension (HTN) | 84 (52.5%) | 53 (55.2%) | 0.699 | 50 (54.9%) | 50 (54.9%) | 1.000 |
| Heart disease except HTN | 0.921 | 0.391 | ||||
| None | 149 (93.1%) | 89 (92.7%) | 84 (92.3%) | 85 (93.4%) | ||
| Myocardial infarction | 1 (0.6%) | 1 (1.0%) | 0 (0%) | 1 (1.1%) | ||
| Angina pectoris | 7 (4.4%) | 5 (5.2%) | 5 (5.5%) | 5 (5.5%) | ||
| Arrhythmia | 3 (1.9%) | 1 (1.0%) | 2 (2.2%) | 0 (0%) | ||
| Cerebrovascular accident | 3 (1.9%) | 1 (1.0%) | 0.603 | 0 (0%) | 1 (1.1%) | 0.316 |
| Pulmonary disease | 0.257 | 0.180 | ||||
| None | 147 (91.9%) | 91 (94.8%) | 81 (89.0%) | 86 (94.5%) | ||
| Asthma | 6 (3.8%) | 0 (0%) | 5 (5.5%) | 0 (0%) | ||
| COPD | 2 (1.3%) | 3 (3.1%) | 1 (1.1%) | 3 (3.3%) | ||
| Lung cancer | 2 (1.3%) | 0 (0%) | 1 (1.1%) | 0 (0%) | ||
| Pneumonia | 1 (0.6%) | 0 (0%) | 1 (1.1%) | 0 (0%) | ||
| Pneumoconiosis | 1 (0.6%) | 0 (0%) | 1 (1.1%) | 0 (0%) | ||
| Pneumothorax | 0 (0%) | 1 (1.0%) | 0 (0%) | 1 (1.1%) | ||
| Tuberculosis | 1 (0.6%) | 1 (1.0%) | 1 (1.1%) | 1 (1.1%) | ||
| Etiology | 0.097 | 0.764 | ||||
| NBNC | 84 (52.5%) | 36 (37.5%) | 36 (39.6%) | 34 (37.4%) | ||
| HBV | 48 (30.0%) | 45 (46.9%) | 35 (38.5%) | 42 (46.2%) | ||
| HCV | 21 (13.1%) | 12 (12.5%) | 14 (15.4%) | 12 (13.2%) | ||
| HBV, HCV | 2 (1.3%) | 1 (1.0%) | 2 (2.2%) | 1 (1.1%) | ||
| NASH | 5 (3.1%) | 2 (2.1%) | 4 (4.4%) | 2 (2.2%) | ||
| WBC | 5800 (1730–10,300) | 5500 (2370–12,800) | 0.219 | 5640 (1730–9790) | 5560 (2370–12,800) | 0.875 |
| NLR | 0.58 (0.14–2.91) | 0.53 (0.09–1.86) | 0.415 | 0.60 (0.15–1.87) | 0.52 (0.09–1.86) | 0.179 |
| Hemoglobin | 13.5 (8.4–16.8) | 13.7 (9.6–17.0) | 0.418 | 13.5 (9.7–16.4) | 13.7 (9.6–17.0) | 0.475 |
| Platelet | 174,000 (12,000–710,000) | 171,000 (14,000–385,000) | 0.401 | 158,000 (12,000–710,000) | 174,000 (14,000–385,000) | 0.287 |
| Total bilirubin | 0.6 (0.2–1.7) | 0.6 (0.2–1.8) | 0.713 | 0.6 (0.2–1.7) | 0.6 (0.2–1.9) | 0.543 |
| AST | 29 (13–147) | 25 (14–140) | 0.010 | 29 (15–88) | 25 (14–140) | 0.061 |
| ALT | 26 (5–268) | 23 (5–297) | 0.051 | 24 (5–81) | 23 (5–297) | 0.405 |
| ALP | 79 (33–259) | 72 (35–393) | 0.007 | 78 (33–259) | 71 (35–393) | 0.026 |
| INR | 1.04 (0.89–1.47) | 1.04 (0.90–1.41) | 0.408 | 1.04 (0.90–1.27) | 1.04 (0.90–1.41) | 0.966 |
| Albumin | 4.3 (3.1–5.5) | 4.3 (3.2–5.2) | 0.395 | 4.3 (3.1–55) | 4.3 (3.2–5.2) | 0.639 |
| Creatinine | 0.90 (0.48–1.77) | 0.91 (0.51–4.21) | 0.723 | 0.89 (0.52–1.77) | 0.91 (0.51–4.21) | 0.430 |
| CRP | 0.12 (0.03–8.38) | 0.07 (0.03–4.98) | 0.524 | 0.07 (0.03–3.98) | 0.07 (0.03–4.98) | 0.919 |
| AFP > 40 | 46 (30.1%) | 35 (36.8%) | 0.330 | 21 (23.1%) | 33 (36.3%) | 0.074 |
| PIVKA-II > 70 | 76 (49.4%) | 37 (40.7%) | 0.233 | 29 (31.9%) | 37 (40.7%) | 0.280 |
| ICG-R15 | 11.3 (2.7–24.5) | 10.8 (3.4–44.6) | 0.752 | 11.8 (2.7–24.5) | 10.6 (3.4–44.6) | 0.431 |
| Variables | Before PS Matching | After PS Matching | ||||
|---|---|---|---|---|---|---|
| OLR (n = 160) | LLR (n = 96) | p-Value | OLR (n = 91) | LLR (n = 91) | p-Value | |
| ASA classification | 0.949 | 0.648 | ||||
| 1 | 10 (6.3%) | 3 (3.1%) | 3 (3.3%) | 2 (2.2%) | ||
| 2 | 134 (83.8%) | 86 (89.6%) | 78 (85.7%) | 82 (90.1%) | ||
| 3 | 16 (10.0%) | 7 (7.3%) | 10 (11.0%) | 7 (7.7%) | ||
| Previous abdominal operation | 42 (26.3%) | 18 (18.8%) | 0.223 | 23 (25.3%) | 16 (17.6%) | 0.278 |
| Extent of resection (major) | 79 (49.4%) | 46 (48.4%) | 0.898 | 40 (44.0%) | 44 (48.9%) | 0.552 |
| Operation time (min) | 222 (71–599) | 239 (43–590) | 0.345 | 204 (71–408) | 240 (43–590) | 0.040 |
| Blood loss during operation (mL) | 300 (30–3000) | 250 (30–2500) | 0.750 | 200 (50–1500) | 250 (50–2500) | 0.172 |
| RBC transfusion during operation | 10 (6.3%) | 4 (4.2%) | 0.579 | 1 (1.1%) | 4 (4.4%) | 0.368 |
| Tumor size (cm) | 3.9 (0.3–17.0) | 2.6 (0.9–14.0) | <0.001 | 2.9 (0.3–13.2) | 2.6 (0.9–14.0) | 0.445 |
| Tumor size > 10 cm | 12 (7.5%) | 4 (4.2%) | 0.425 | 3 (3.3%) | 4 (4.4%) | 0.700 |
| Tumor grade 3 or 4 | 21 (13.3%) | 5 (5.2%) | 0.053 | 11 (12.4%) | 4 (4.4%) | 0.063 |
| Tumor necrosis | 67 (43.5%) | 35 (38.0%) | 0.425 | 33 (37.9%) | 34 (39.1%) | 0.876 |
| Complete encapsulation | 121 (77.6%) | 68 (70.8%) | 0.189 | 63 (72.4%) | 64 (70.3%) | 0.843 |
| Microvascular invasion | 110 (68.8%) | 58 (60.4%) | 0.178 | 51 (56.0%) | 58 (63.7%) | 0.364 |
| Portal vein tumor thrombosis | 5 (3.1%) | 7 (7.3%) | 0.139 | 0 (0%) | 7 (7.7%) | 0.014 |
| Intrahepatic metastasis | 6 (3.8%) | 2 (2.1%) | 0.714 | 3 (3.3%) | 2 (2.2%) | 0.650 |
| Cirrhosis | 50 (31.3%) | 46 (47.9%) | 0.011 | 40 (44.0%) | 43 (47.3%) | 0.766 |
| Free resection margin (mm) | 10 (2–55) | 10 (2–65) | 0.428 | 10 (2–55) | 10 (2–65) | 0.865 |
| Hospitalization (day) | 10 (5–177) | 7 (4–41) | <0.001 | 9 (5–177) | 7 (4–41) | <0.001 |
| Variables | OLR (n = 160) | LLR (n = 96) | p-Value |
|---|---|---|---|
| Complication | 27 (16.9%) | 10 (10.4%) | 0.199 |
| Clavien–Dindo grade | 0.986 | ||
| 1 | 7 | 3 | |
| 2 | 10 | 3 | |
| 3 | 8 | 3 | |
| 4 | 2 | 1 | |
| Surgical complications | 19 (11.9%) | 8 (8.3%) | 0.372 |
| Wound | 3 | 0 | |
| Bleeding | 2 | 0 | |
| Biloma | 3 | 2 | |
| Ascites | 0 | 3 | |
| Hematuria | 1 | 0 | |
| Nausea/vomiting | 1 | 0 | |
| Atrial fibrillation | 1 | 0 | |
| Hematemesis | 1 | 0 | |
| Renal dysfunction | 2 | 1 | |
| Delirium | 4 | 0 | |
| Hyperbilirubinemia | 1 | 1 | |
| Cerebrovascular accidents | 0 | 1 | |
| Pulmonary complications | 8 (5.0%) | 2 (2.1%) | 0.329 |
| Pneumonia | 2 | 1 | |
| Atelectasis | 2 | 1 | |
| Pleural effusion | 2 | 0 | |
| Acute respiratory distress syndrome | 1 | 0 | |
| Pulmonary artery embolization | 1 | 0 |
| Variables | Before PS Matching | After PS Matching | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | ||
| HCC recurrence | |||||||
| NLR | 2.973 | 1.436–6.154 | 0.003 | RBC transfusion during operation | 3.920 | 1.030–14.923 | 0.045 |
| ICG-R15 | 1.056 | 1.013–1.101 | 0.009 | ||||
| Portal vein tumor thrombosis | 3.353 | 1.271–8.847 | 0.015 | ||||
| Intrahepatic metastasis | 4.830 | 1.750–13.330 | 0.002 | ||||
| Mortality | |||||||
| Age > 75 | 3.426 | 1.250–9.391 | 0.017 | Age > 75 | 11.333 | 2.471–51.982 | 0.002 |
| Intrahepatic metastasis | 16.463 | 5.948–45.566 | <0.001 | Microvascular invasion | 7.502 | 1.193–47.167 | 0.032 |
| Hospitalization | 1.030 | 1.019–1.042 | <0.001 | Intrahepatic metastasis | 29.092 | 6.627–127.716 | <0.001 |
| AFP > 40 | 3.912 | 1.472–10.395 | 0.006 | RBC transfusion during operation | 13.341 | 2.466–73.266 | 0.003 |
| Hospitalization | 1.044 | 1.026–1.062 | <0.001 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.M.; Kim, S.; Rhu, J.; Choi, G.-S.; Kwon, C.H.D.; Joh, J.-W. Elderly Hepatocellular Carcinoma Patients: Open or Laparoscopic Approach? Cancers 2020, 12, 2281. https://doi.org/10.3390/cancers12082281
Kim JM, Kim S, Rhu J, Choi G-S, Kwon CHD, Joh J-W. Elderly Hepatocellular Carcinoma Patients: Open or Laparoscopic Approach? Cancers. 2020; 12(8):2281. https://doi.org/10.3390/cancers12082281
Chicago/Turabian StyleKim, Jong Man, Sangjin Kim, Jinsoo Rhu, Gyu-Seong Choi, Choon Hyuck David Kwon, and Jae-Won Joh. 2020. "Elderly Hepatocellular Carcinoma Patients: Open or Laparoscopic Approach?" Cancers 12, no. 8: 2281. https://doi.org/10.3390/cancers12082281
APA StyleKim, J. M., Kim, S., Rhu, J., Choi, G.-S., Kwon, C. H. D., & Joh, J.-W. (2020). Elderly Hepatocellular Carcinoma Patients: Open or Laparoscopic Approach? Cancers, 12(8), 2281. https://doi.org/10.3390/cancers12082281





