The Development and Treatment of Lymphatic Dysfunction in Cancer Patients and Survivors
Abstract
1. Introduction
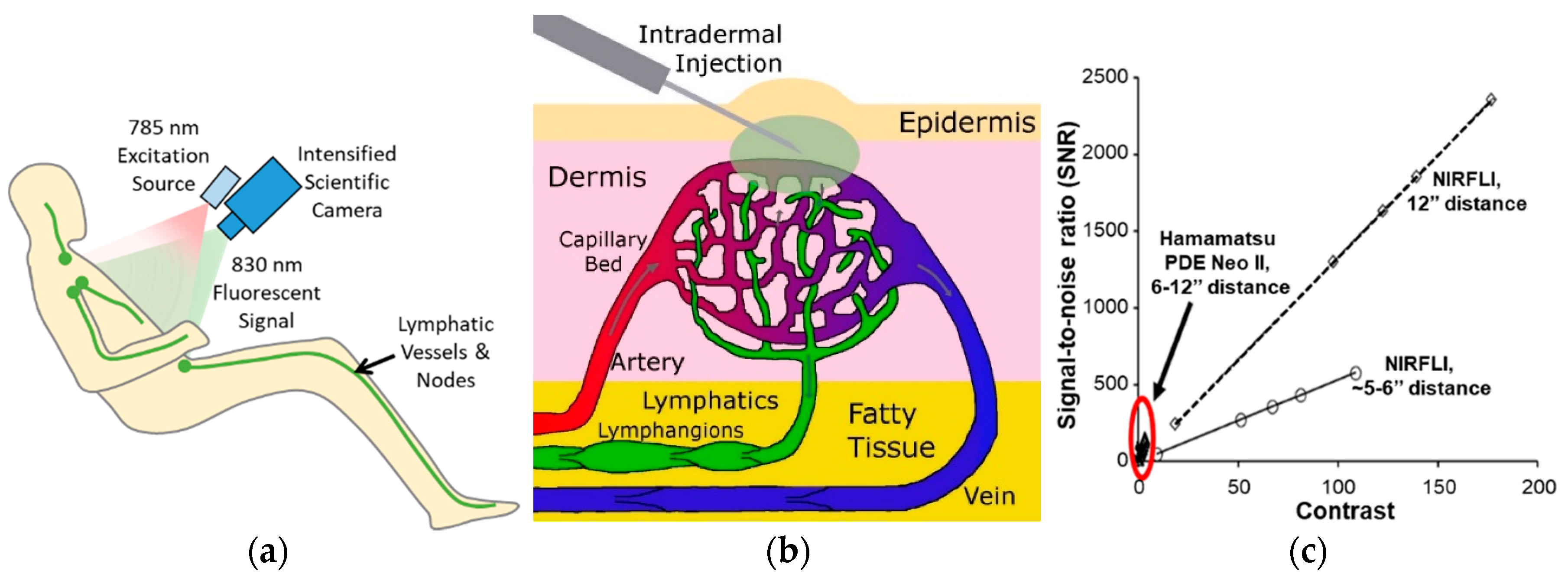
1.1. Near-Infrared Fluorescence Lymphatic Imaging (NIRF-LI)
1.2. Anatomy and Function of Upper and Lower Extremity Lymphatics
1.3. Anatomy and Function of Cranial/Cervical Lymphatics
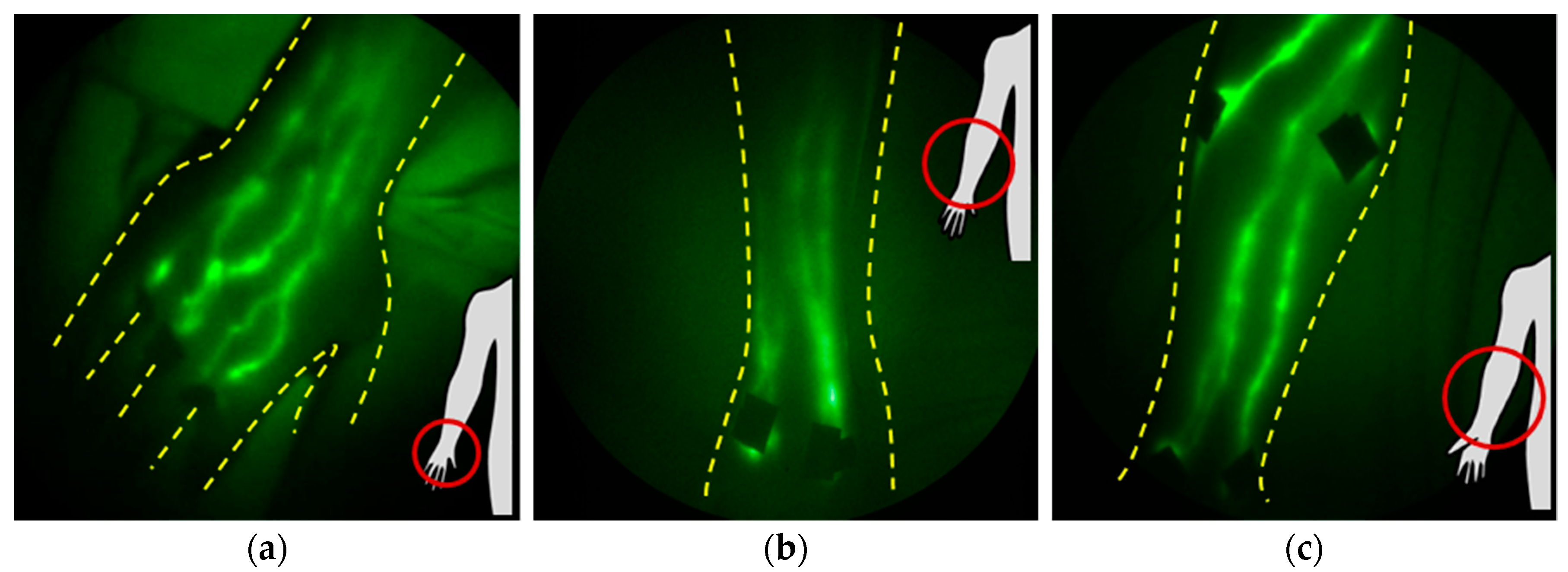
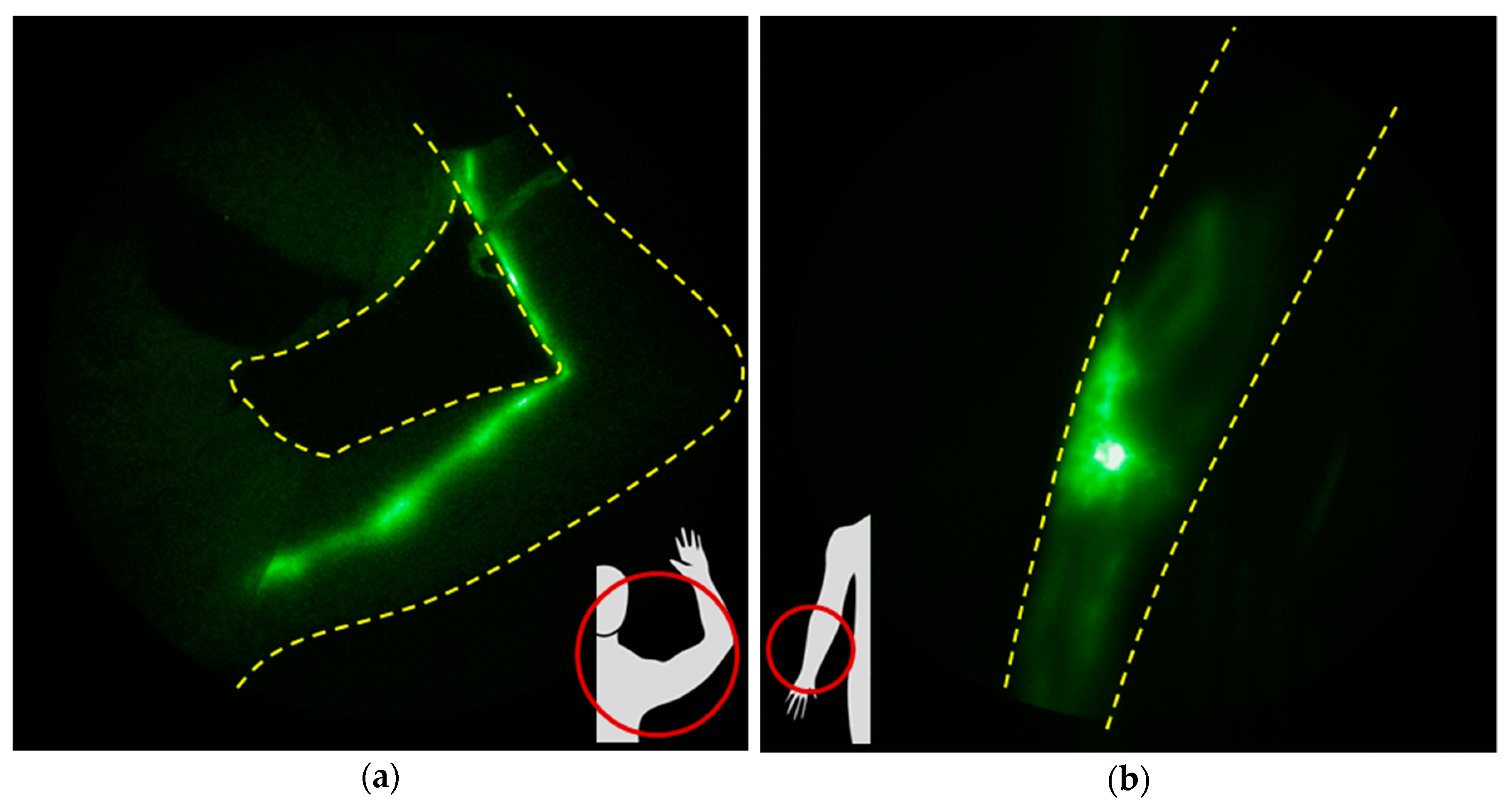
1.4. NIRF-LI Taxonomy of Lymphatic Dysfunction
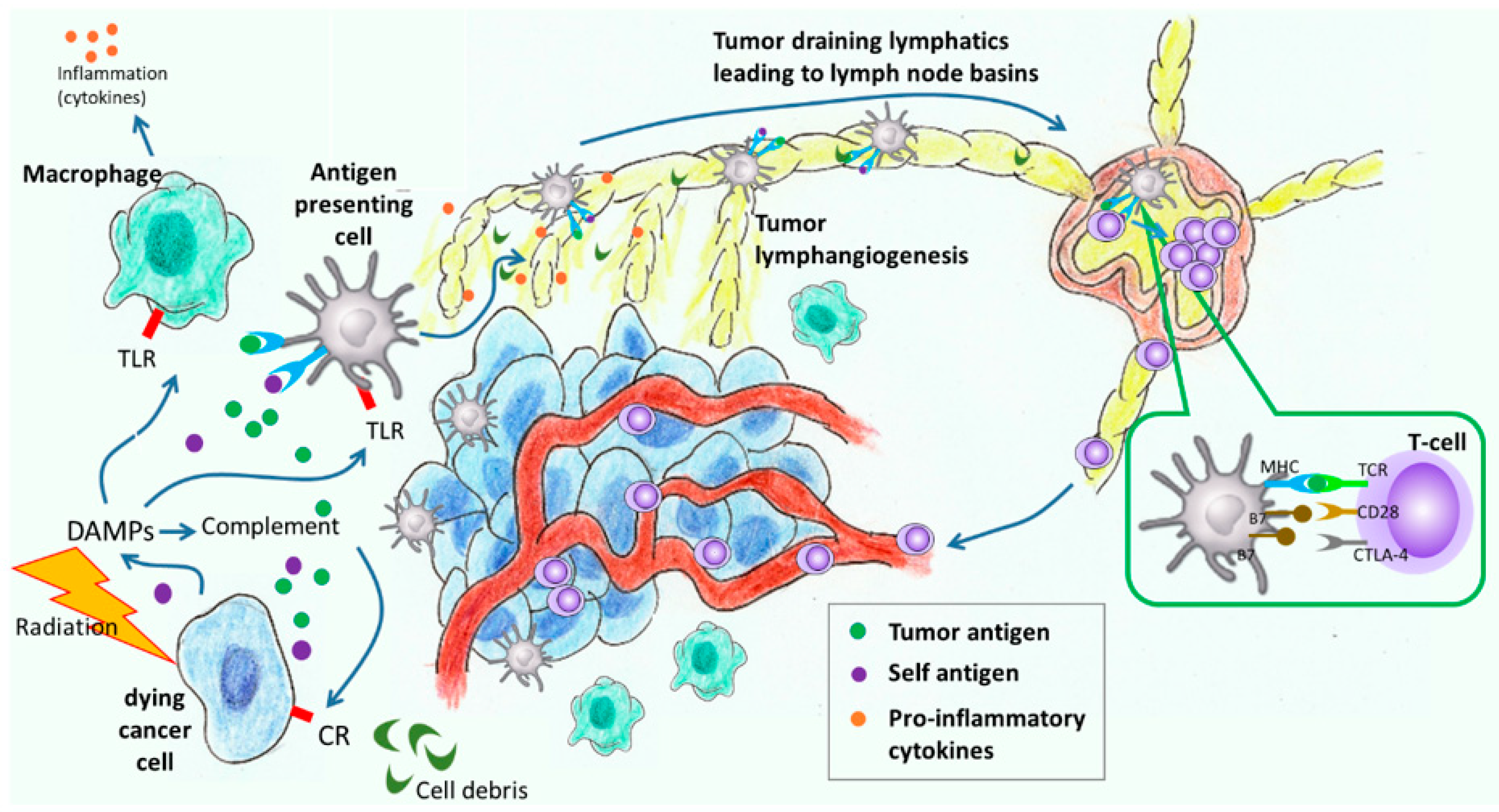
2. Lymphatic Responses to Cancer Progression and Cancer Treatment
2.1. The Effects of Metastasis and Cancer Progression on Lymphatic Function
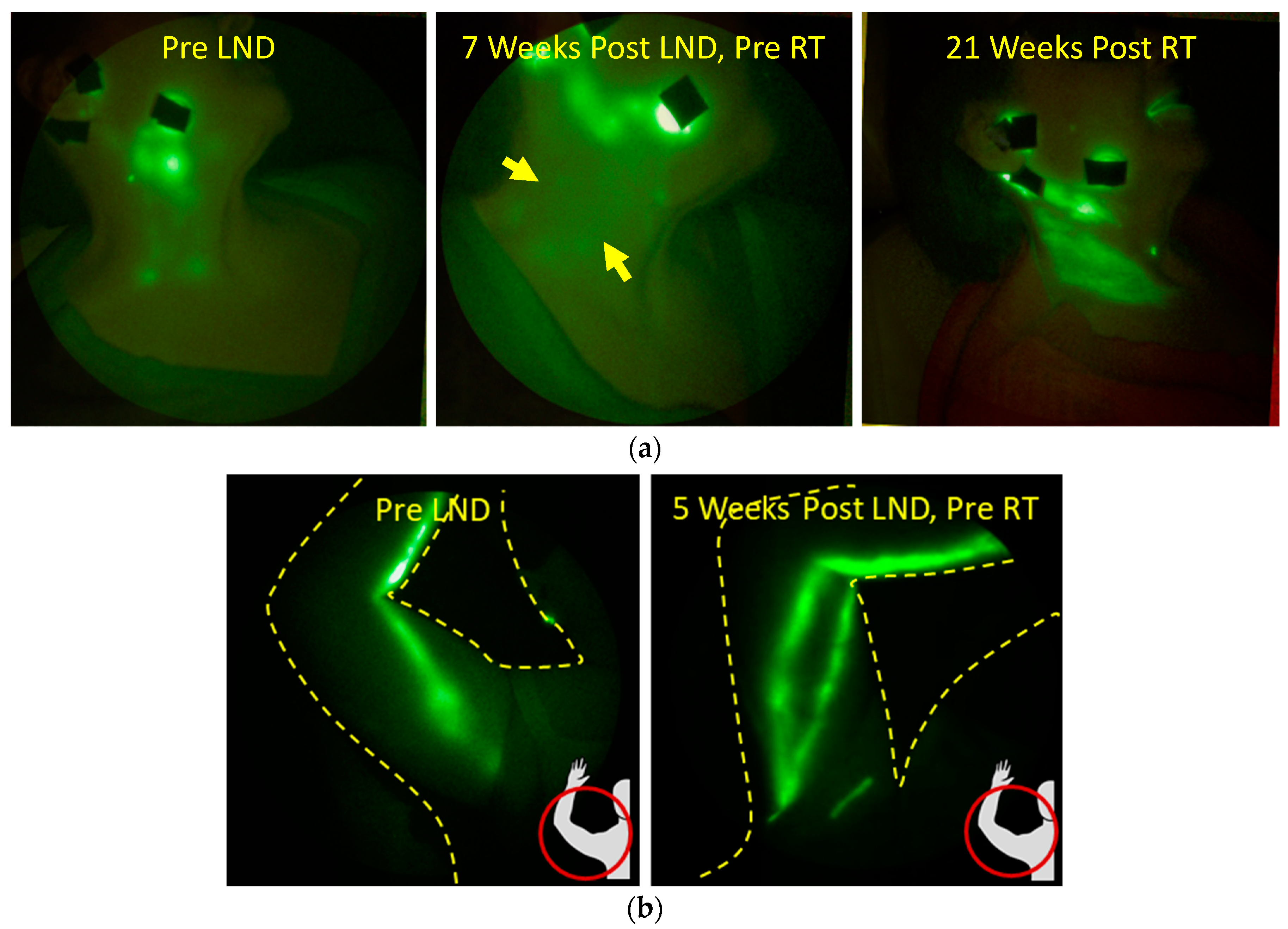
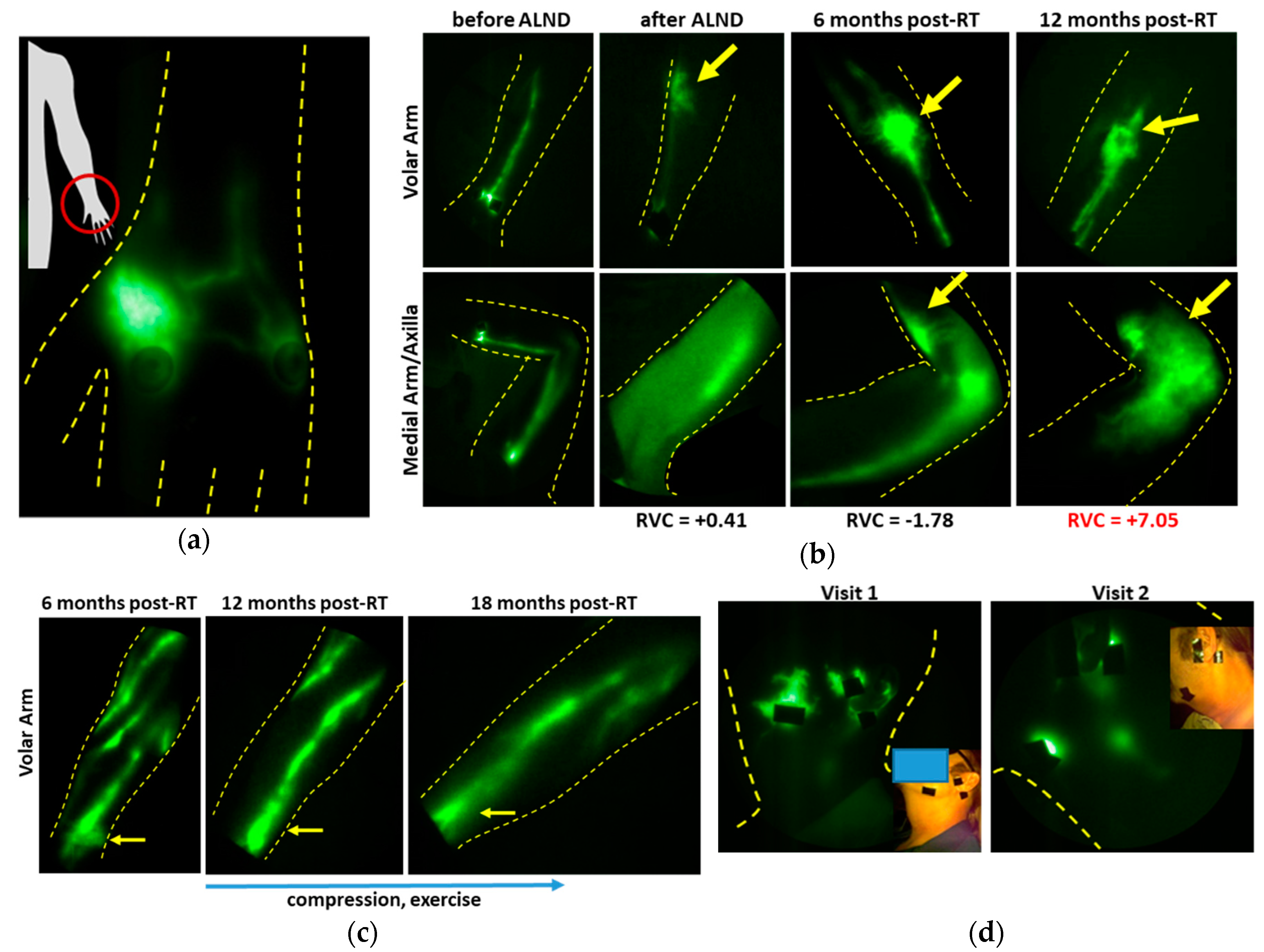
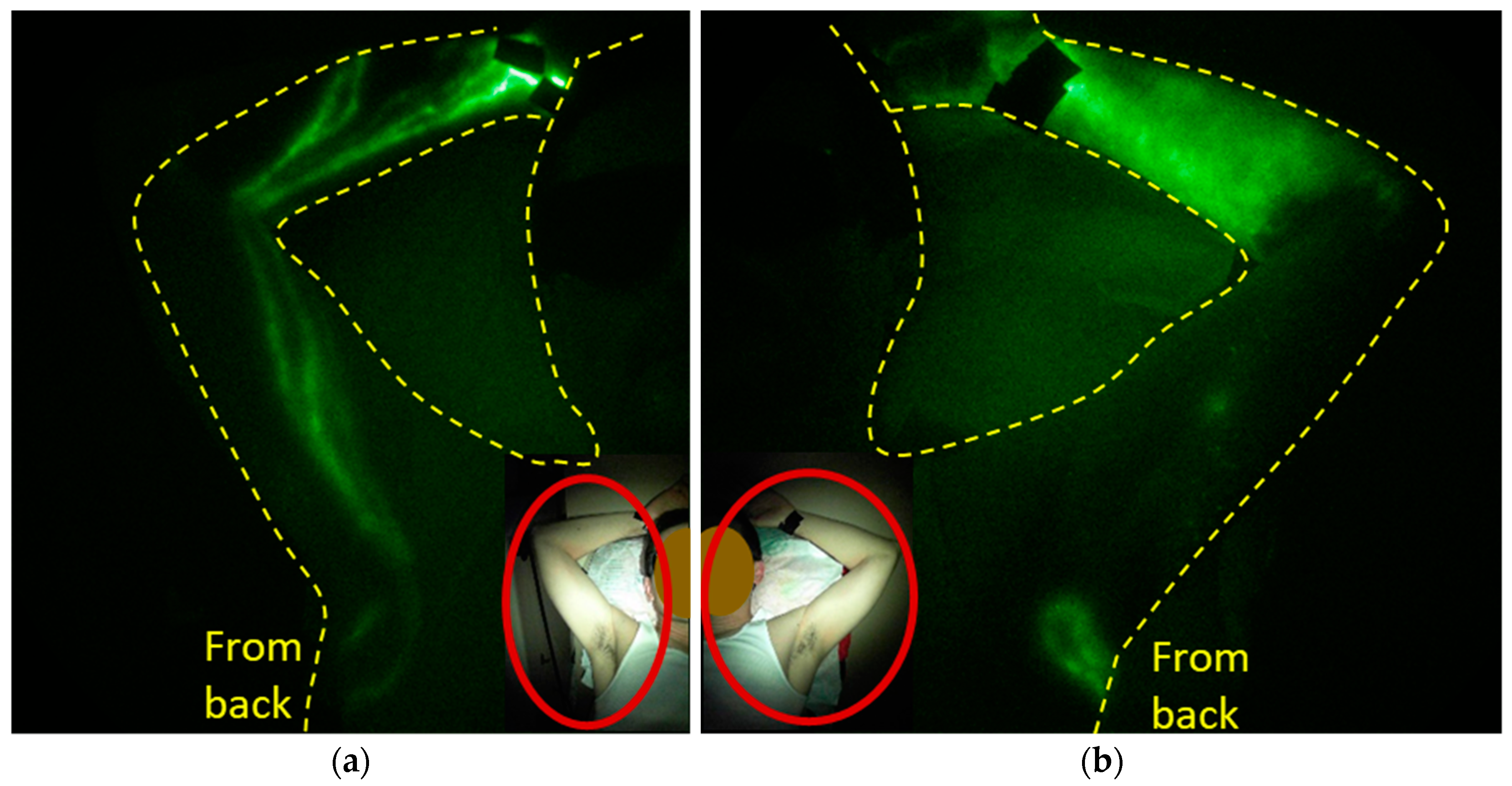
2.2. The Role of Lymph Node Dissection and RT on Lymphatic Function
3. Diagnostic Imaging of Dysfunctional Lymphatics for Staging and Treatment of Lymphedema
3.1. Diagnosis of Lymphatic Dysfunction and Its Treatment in Patients at Risk for Cancer Acquired Lymphedema
3.2. Imaging Lymphatic Response to LE Treatment
4. Conditions and Comorbidities Associated Lymphatic Dysfunction and Lymphedema
4.1. Chronic Venous Disease (CVD)
4.2. Inflammatory/Rheumatological Diseases
4.3. Cellulitis
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jones, D.; Pereira, E.R.; Padera, T.P. Growth and Immune Evasion of Lymph Node Metastasis. Front. Oncol. 2018, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Ludgate, C.M. Optimizing cancer treatments to induce an acute immune response: Radiation Abscopal effects, PAMPs, and DAMPs. Clin. Cancer Res. 2012, 18, 4522–4525. [Google Scholar] [CrossRef] [PubMed]
- Schaue, D.; Micewicz, E.D.; Ratikan, J.A.; Xie, M.W.; Cheng, G.; McBride, W.H. Radiation and inflammation. Semin. Radiat. Oncol. 2015, 25, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, M.B.; Sevick-Muraca, E.M. Cytokines are systemic effectors of lymphatic function in acute inflammation. Cytokine 2013, 64, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Leijte, J.A.P.; van der Ploeg, I.M.C.; Valdes Olmos, R.A.; Nieweg, O.E.; Horenblas, S. Visualization of Tumor Blockage and Rerouting of Lymphatic Drainage in Penile Cancer Patients by Use of SPECT/CT. J. Nucl. Med. 2009, 50, 364–367. [Google Scholar] [CrossRef]
- Kwon, S.; Sevick-Muraca, E.M. Functional lymphatic imaging in tumor-bearing mice. J. Immunol. Methods 2010, 360, 167–172. [Google Scholar] [CrossRef]
- Proulx, S.T.; Luciani, P.; Christiansen, A.; Karaman, S.; Blum, K.S.; Rinderknecht, M.; Leroux, J.-C.; Detmar, M. Use of a PEG-conjugated bright near-infrared dye for functional imaging of rerouting of tumor lymphatic drainage after sentinel lymph node metastasis. Biomaterials 2013, 34, 5128–5137. [Google Scholar] [CrossRef]
- Levick, J.R.; Michel, C.C. Microvascular fluid exchange and the revised Starling principle. Cardiovasc. Res. 2010, 87, 198–210. [Google Scholar] [CrossRef]
- Moore, J.E.; Bertram, C.D. Lymphatic System Flows. Annu. Rev. Fluid Mech. 2018, 50, 459–482. [Google Scholar] [CrossRef]
- O’Donnell, T.F.; Rasmussen, J.C.; Sevick-Muraca, E.M. New diagnostic modalities in the evaluation of lymphedema. J. Vasc. Surg-Venous. L. 2017, 5, 261–273. [Google Scholar] [CrossRef]
- Sevick-Muraca, E.M.; Sharma, R.; Rasmussen, J.C.; Marshall, M.V.; Wendt, J.A.; Pham, H.Q.; Bonefas, E.; Houston, J.P.; Sampath, L.; Adams, K.E.; et al. Imaging of lymph flow in breast cancer patients after microdose administration of a near-infrared fluorophore: Feasibility study. Radiology 2008, 246, 734–741. [Google Scholar] [CrossRef]
- Zhu, B.; Rasmussen, J.C.; Litorja, M.; Sevick-Muraca, E.M. Determining the Performance of Fluorescence Molecular Imaging Devices Using Traceable Working Standards with SI Units of Radiance. IEEE Trans. Med. Imaging 2016, 35, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Kwon, S.; Rasmussen, J.C.; Litorja, M.; Sevick-Muraca, E.M. Comparison of NIR Versus SWIR Fluorescence Image Device Performance Using Working Standards Calibrated with SI Units. IEEE Trans. Med. Imaging 2020, 39, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.C.; Tan, I.C.; Marshall, M.V.; Adams, K.E.; Kwon, S.; Fife, C.E.; Maus, E.A.; Smith, L.; Covington, K.R.; Sevick-Muraca, E.M. Human lymphatic architecture and dynamic transport imaged using near-infrared fluorescence. Transl. Oncol. 2010, 3, 362–372. [Google Scholar] [CrossRef]
- Scallan, J.P.; Zawieja, S.D.; Castorena-Gonzalez, J.A.; Davis, M.J. Lymphatic pumping: Mechanics, mechanisms and malfunction. J. Physiol. 2016, 594, 5749–5768. [Google Scholar] [CrossRef]
- Zhu, B.; Rasmussen, J.C.; Sevick-Muraca, E.M. A matter of collection and detection for intraoperative and noninvasive near-infrared fluorescence molecular imaging: To see or not to see? Med. Phys. 2014, 41, 022105. [Google Scholar] [CrossRef] [PubMed]
- Itkin, M.; Nadolski, G.J. Modern techniques of lymphangiography and interventions: Current status and future development. Cardiovasc. Interv. Radiol. 2018, 41, 366–376. [Google Scholar] [CrossRef]
- Yoshida, R.Y.; Kariya, S.; Ha-Kawa, S.; Tanigawa, N. Lymphoscintigraphy for imaging of the lymphatic flow disorders. Tech. Vasc. Interv. Radiol. 2016, 19, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Engle, H.; Gazyakan, R.; Rahimi, M.; Hunerbein, M.; Sun, J.; Kneser, U.; Hirche, C. Current techniques for lymphatic imaging: State of the art and future perspectives. Eur. J. Surg. Oncol. 2014, 41, 270–276. [Google Scholar] [CrossRef]
- Sevick-Muraca, E.M.; Kwon, S.; Rasmussen, J.C. Emerging lymphatic imaging technologies for mouse and man. J. Clin. Investig. 2014, 124, 905–914. [Google Scholar] [CrossRef]
- Lillis, A.P.; Krishnamurchy, R. Photoacoustic imaging addresses a long-standing challenge in lymphedema. Radiology 2020, 295, 475–477. [Google Scholar] [CrossRef]
- Niu, G.; Chen, X. Lymphatic imaging: Focus on imaging probes. Theranostics 2015, 5, 686–697. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suami, H.; Scaglioni, M.F. Anatomy of the Lymphatic System and the Lymphosome Concept with Reference to Lymphedema. Semin. Plast. Surg. 2018, 32, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Földi, M.; Földi, E. Foldi’s Textbook of Lymphology for Physicians and Lymphedema Therapists; Elsevier, Urban Fischer: Munich, Germany, 2006; ISBN 978-0-7234-3446-7. [Google Scholar]
- Plog, B.A.; Nedergaard, M. The Glymphatic System in Central Nervous System Health and Disease: Past, Present, and Future. Annu. Rev. Pathol. 2018, 13, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Eide, P.K.; Vatnehol, S.A.S.; Emblem, K.E.; Ringstad, G. Magnetic resonance imaging provides evidence of glymphatic drainage from human brain to cervical lymph nodes. Sci. Rep. 2018, 8, 7194. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.C.; Kwon, S.; Pinal, A.; Bareis, A.; Velasquez, F.C.; Janssen, C.F.; Morrow, J.R.; Fife, C.E.; Karni, R.J.; Sevick-Muraca, E.M. Assessing lymphatic route of CSF outflow and peripheral lymphatic contractile activity during head-down tilt using near-infrared fluorescence imaging. Physiol. Rep. 2020, 8, e14375. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Moon, W.-J. Gadolinium Deposition in the Brain: Current Updates. Korean J. Radiol. 2019, 20, 134–147. [Google Scholar] [CrossRef]
- Rasmussen, J.C.; Tan, I.-C.; Naqvi, S.; Aldrich, M.B.; Maus, E.A.; Blanco, A.I.; Karni, R.J.; Sevick-Muraca, E.M. Longitudinal monitoring of the head and neck lymphatics in response to surgery and radiation. Head Neck 2017, 39, 1177–1188. [Google Scholar] [CrossRef]
- Koroulakis, A.; Agarwal, M. Anatomy, Head and Neck, Lymph Nodes. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Kwon, S.; Janssen, C.F.; Velasquez, F.C.; Sevick-Muraca, E.M. Fluorescence imaging of lymphatic outflow of cerebrospinal fluid in mice. J. Immunol. Methods 2017, 449, 37–43. [Google Scholar] [CrossRef]
- Kwon, S.; Moreno-Gonzalez, I.; Taylor-Presse, K.; Edwards Iii, G.; Gamez, N.; Calderon, O.; Zhu, B.; Velasquez, F.C.; Soto, C.; Sevick-Muraca, E.M. Impaired Peripheral Lymphatic Function and Cerebrospinal Fluid Outflow in a Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 69, 585–593. [Google Scholar] [CrossRef]
- Ma, Q.; Ineichen, B.V.; Detmar, M.; Proulx, S.T. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat. Commun. 2017, 8, 1434. [Google Scholar] [CrossRef]
- Ma, Q.; Schlegel, F.; Bachmann, S.B.; Schneider, H.; Decker, Y.; Rudin, M.; Weller, M.; Proulx, S.T.; Detmar, M. Lymphatic outflow of cerebrospinal fluid is reduced in glioma. Sci. Rep. 2019, 9, 14815. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018, 17, 1016–1024. [Google Scholar] [CrossRef]
- Deng, J.; Ridner, S.H.; Dietrich, M.S.; Wells, N.; Wallston, K.A.; Sinard, R.J.; Cmelak, A.J.; Murphy, B.A. Prevalence of secondary lymphedema in patients with head and neck cancer. J. Pain Symptom Manag. 2012, 43, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.C.; Tan, I.C.; Marshall, M.V.; Fife, C.E.; Sevick-Muraca, E.M. Lymphatic imaging in humans with near-infrared fluorescence. Curr. Opin. Biotechnol. 2009, 20, 74–82. [Google Scholar] [CrossRef]
- Burrows, P.E.; Gonzalez-Garay, M.L.; Rasmussen, J.C.; Aldrich, M.B.; Guilliod, R.; Maus, E.A.; Fife, C.E.; Kwon, S.; Lapinski, P.E.; King, P.D.; et al. Lymphatic abnormalities are associated with RASA1 gene mutations in mouse and man. Proc. Natl. Acad. Sci. USA 2013, 110, 8621–8626. [Google Scholar] [CrossRef]
- Agollah, G.D.; Gonzalez-Garay, M.L.; Rasmussen, J.C.; Tan, I.C.; Aldrich, M.B.; Darne, C.; Fife, C.E.; Guilliod, R.; Maus, E.A.; King, P.D.; et al. Evidence for SH2 domain-containing 5’-inositol phosphatase-2 (SHIP2) contributing to a lymphatic dysfunction. PLoS ONE 2014, 9, e112548. [Google Scholar] [CrossRef]
- Rasmussen, J.C.; Fife, C.E.; Sevick-Muraca, E.M. Near-Infrared Fluorescence Lymphatic Imaging in Lymphangiomatosis. Lymphat. Res. Biol. 2015, 13, 195–201. [Google Scholar] [CrossRef]
- Gonzalez-Garay, M.L.; Aldrich, M.B.; Rasmussen, J.C.; Guilliod, R.; Lapinski, P.E.; King, P.D.; Sevick-Muraca, E.M. A novel mutation in CELSR1 is associated with hereditary lymphedema. Vasc. Cell 2016, 8, 1. [Google Scholar] [CrossRef]
- Greives, M.R.; Aldrich, M.B.; Sevick-Muraca, E.M.; Rasmussen, J.C. Near-Infrared Fluorescence Lymphatic Imaging of a Toddler with Congenital Lymphedema. Pediatrics 2017, 139. [Google Scholar] [CrossRef]
- Akita, S.; Mitsukawa, N.; Rikihisa, N.; Kubota, Y.; Omori, N.; Mitsuhashi, A.; Tate, S.; Shozu, M.; Satoh, K. Early diagnosis and risk factors for lymphedema following lymph node dissection for gynecologic cancer. Plast. Reconstr. Surg. 2013, 131, 283–290. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yamamoto, N.; Doi, K.; Oshima, A.; Yoshimatsu, H.; Todokoro, T.; Ogata, F.; Mihara, M.; Narushima, M.; Iida, T.; et al. Indocyanine green-enhanced lymphography for upper extremity lymphedema: A novel severity staging system using dermal backflow patterns. Plast. Reconstr. Surg. 2011, 128, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Yamamoto, N.; Fuse, Y.; Narushima, M.; Koshima, I. Optimal sites for supermicrosurgical lymphaticovenular anastomosis: An analysis of lymphatic vessel detection rates on 840 surgical fields in lower extremity lymphedema patients. Plast. Reconstr. Surg. 2018, 142, 924e–930e. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Narushima, M.; Yoshimatsu, H.; Yamamoto, N.; Kikuchi, K.; Todokoro, T.; Iida, T.; Koshima, I. Dynamic indocyanine green (ICG) lymphography for breast cancer-related arm lymphedema. Ann. Plast. Surg. 2014, 73, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.W.; Suami, H.; Skoracki, R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast. Reconstr. Surg. 2013, 132, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Matsuda, N.; Doi, K.; Oshima, A.; Yoshimatsu, H.; Todokoro, T.; Ogata, F.; Mihara, M.; Narushima, M.; Iida, T.; et al. The earliest finding of indocyanine green lymphography in asymptomatic limbs of lower extremity lymphedema patients secondary to cancer tratment: The modified dermal backflow stage and concept of subclinical lymphedema. Plast. Reconstr. Surg. 2011, 128, 314e–321e. [Google Scholar] [CrossRef]
- Unno, N.; Inuzuka, K.; Suzuki, M.; Yamamoto, N.; Sagara, D.; Nishiyama, M.; Konno, H. Preliminary experience with a novel fluorescence lymphography using indocyanine green in patients with secondary lymphedema. J. Vasc. Surg. 2007, 45, 1016–1021. [Google Scholar] [CrossRef]
- Yamamoto, T.; Narushima, M.; Doi, K.; Oshima, A.; Ogata, F.; Mihara, M.; Koshima, I.; Mundinger, G.S. Characteristic indocyanine green lymphography findings in lower extremity lymphedema: The generation of a novel lymphedema severity staging system using dermal backflow patterns. Plast. Reconstr. Surg. 2011, 127, 1979–1986. [Google Scholar] [CrossRef]
- Stacker, S.A.; Williams, S.P.; Karnezis, T.; Shayan, R.; Fox, S.B.; Achen, M.G. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat. Rev. Cancer 2014, 14, 159–172. [Google Scholar] [CrossRef]
- Hurst, N.J.; Dominello, M.; Dyson, G.; Jaratli, H.; Sharma, M.; Ahmed, Y.K.; Melkane, A.E.; Rose, C.; Jacobs, J.; Giorgadze, T.; et al. Intratumoral lymphatic vessel density as a predictor of progression-free and overall survival in locally advanced laryngeal/hypopharyngeal cancer. Head Neck 2016, 38 (Suppl. 1), E417–E420. [Google Scholar] [CrossRef] [PubMed]
- Kataru, R.P.; Ly, C.L.; Shin, J.; Park, H.J.; Baik, J.E.; Rehal, S.; Ortega, S.; Lyden, D.; Mehrara, B.J. Tumor Lymphatic Function Regulates Tumor Inflammatory and Immunosuppressive Microenvironments. Cancer Immunol. Res. 2019, 7, 1345–1358. [Google Scholar] [CrossRef]
- Kwon, S.; Agollah, G.D.; Wu, G.; Chan, W.; Sevick-Muraca, E.M. Direct visualization of changes of lymphatic function and drainage pathways in lymph node metastasis of B16F10 melanoma using near-infrared fluorescence imaging. Biomed. Opt. Express 2013, 4, 967–977. [Google Scholar] [CrossRef]
- Kwon, S.; Velasquez, F.C.; Sevick-Muraca, E.M. Near-infrared fluorescence lymphatic imaging in vascular endothelial growth factor-C overexpressing murine melanoma. Biomed. Opt. Express 2018, 9, 4631–4637. [Google Scholar] [CrossRef]
- Rouhani, S.J.; Eccles, J.D.; Riccardi, P.; Peske, J.D.; Tewalt, E.F.; Cohen, J.N.; Liblau, R.; Mäkinen, T.; Engelhard, V.H. Roles of lymphatic endothelial cells expressing peripheral tissue antigens in CD4 T-cell tolerance induction. Nat. Commun. 2015, 6, 6771. [Google Scholar] [CrossRef] [PubMed]
- Card, C.M.; Yu, S.S.; Swartz, M.A. Emerging roles of lymphatic endothelium in regulating adaptive immunity. J. Clin. Investig. 2014, 124, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Tewalt, E.F.; Cohen, J.N.; Rouhani, S.J.; Engelhard, V.H. Lymphatic endothelial cells—Key players in regulation of tolerance and immunity. Front. Immunol. 2012, 3, 305. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Shi, D.; Liang, Z.; Liu, Y.; Li, Y.; Xing, Y.; Liu, W.; Ai, Z.; Zhuang, J.; Chen, X.; et al. IL-17A secreted from lymphatic endothelial cells promotes tumorigenesis by upregulation of PD-L1 in hepatoma stem cells. J. Hepatol. 2019, 71, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.S.; Femel, J.; Breazeale, A.P.; Loo, C.P.; Thibault, G.; Kaempf, A.; Mori, M.; Tsujikawa, T.; Chang, Y.H.; Lund, A.W. IFNγ-activated dermal lymphatic vessels inhibit cytotoxic T cells in melanoma and inflamed skin. J. Exp. Med. 2018, 215, 3057–3074. [Google Scholar] [CrossRef]
- Humbert, M.; Hugues, S.; Dubrot, J. Shaping of Peripheral T Cell Responses by Lymphatic Endothelial Cells. Front. Immunol. 2016, 7, 684. [Google Scholar] [CrossRef]
- Tewalt, E.F.; Cohen, J.N.; Rouhani, S.J.; Guidi, C.J.; Qiao, H.; Fahl, S.P.; Conaway, M.R.; Bender, T.P.; Tung, K.S.; Vella, A.T.; et al. Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells. Blood 2012, 120, 4772–4782. [Google Scholar] [CrossRef]
- Alitalo, A.; Detmar, M. Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene 2012, 31, 4499–4508. [Google Scholar] [CrossRef]
- Rosenbluth, J.M.; Overmoyer, B.A. Inflammatory Breast Cancer: A Separate Entity. Curr. Oncol. Rep. 2019, 21, 86. [Google Scholar] [CrossRef] [PubMed]
- Agollah, G.D.; Wu, G.; Sevick-Muraca, E.M.; Kwon, S. In vivo lymphatic imaging of a human inflammatory breast cancer model. J. Cancer 2014, 5, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Morfoisse, F.; Tatin, F.; Chaput, B.; Therville, N.; Vaysse, C.; Métivier, R.; Malloizel-Delaunay, J.; Pujol, F.; Godet, A.-C.; De Toni, F.; et al. Lymphatic Vasculature Requires Estrogen Receptor-α Signaling to Protect from Lymphedema. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1346–1357. [Google Scholar] [CrossRef] [PubMed]
- Hidding, J.T.; Beurskens, C.H.G.; van der Wees, P.J.; Bos, W.C.A.M.; van der Nijhuis Sanden, M.W.G.; van Laarhoven, H.W.M. Changes in volume and incidence of lymphedema during and after treatment with docetaxel, doxorubicin, and cyclophosphamide (TAC) in patients with breast cancer. Support. Care Cancer 2018, 26, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Stolarz, A.J.; Sarimollaoglu, M.; Marecki, J.C.; Fletcher, T.W.; Galanzha, E.I.; Rhee, S.W.; Zharov, V.P.; Klimberg, V.S.; Rusch, N.J. Doxorubicin Activates Ryanodine Receptors in Rat Lymphatic Muscle Cells to Attenuate Rhythmic Contractions and Lymph Flow. J. Pharmacol. Exp. Ther. 2019, 371, 278–289. [Google Scholar] [CrossRef]
- Armer, J.M.; Ballman, K.V.; McCall, L.; Ostby, P.L.; Zagar, E.; Kuerer, H.M.; Hunt, K.K.; Boughey, J.C. Factors Associated with Lymphedema in Women with Node-Positive Breast Cancer Treated with Neoadjuvant Chemotherapy and Axillary Dissection. JAMA Surg. 2019, 154, 800–809. [Google Scholar] [CrossRef]
- Ridner, S.H.; Dietrich, M.S.; Spotanski, K.; Doersam, J.K.; Cowher, M.S.; Taback, B.; McLaughlin, S.; Ajkay, N.; Boyages, J.; Koelmeyer, L.; et al. A Prospective Study of L-Dex Values in Breast Cancer Patients Pretreatment and Through 12 Months Postoperatively. Lymphat. Res. Biol. 2018, 16, 435–441. [Google Scholar] [CrossRef]
- Iyigun, Z.E.; Duymaz, T.; Ilgun, A.S.; Alco, G.; Ordu, C.; Sarsenov, D.; Aydin, A.E.; Celebi, F.E.; Izci, F.; Eralp, Y.; et al. Preoperative Lymphedema-Related Risk Factors in Early-Stage Breast Cancer. Lymphat. Res. Biol. 2018, 16, 28–35. [Google Scholar] [CrossRef]
- Finegold, D.N.; Schacht, V.; Kimak, M.A.; Lawrence, E.C.; Foeldi, E.; Karlsson, J.M.; Baty, C.J.; Ferrell, R.E. HGF and MET mutations in primary and secondary lymphedema. Lymphat. Res. Biol. 2008, 6, 65–68. [Google Scholar] [CrossRef]
- Newman, B.; Lose, F.; Kedda, M.-A.; Francois, M.; Ferguson, K.; Janda, M.; Yates, P.; Spurdle, A.B.; Hayes, S.C. Possible genetic predisposition to lymphedema after breast cancer. Lymphat. Res. Biol. 2012, 10, 2–13. [Google Scholar] [CrossRef]
- Hadizadeh, M.; Mohaddes Ardebili, S.M.; Salehi, M.; Young, C.; Mokarian, F.; McClellan, J.; Xu, Q.; Kazemi, M.; Moazam, E.; Mahaki, B.; et al. GJA4/Connexin 37 Mutations Correlate with Secondary Lymphedema Following Surgery in Breast Cancer Patients. Biomedicines 2018, 6, 23. [Google Scholar] [CrossRef]
- Kwon, S.; Price, R.E. Characterization of internodal collecting lymphatic vessel function after surgical removal of an axillary lymph node in mice. Biomed. Opt. Express 2016, 7, 1100–1115. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Agollah, G.D.; Wu, G.; Sevick-Muraca, E.M. Spatio-temporal changes of lymphatic contractility and drainage patterns following lymphadenectomy in mice. PLoS ONE 2014, 9, e106034. [Google Scholar] [CrossRef]
- Blum, K.S.; Proulx, S.T.; Luciani, P.; Leroux, J.-C.; Detmar, M. Dynamics of lymphatic regeneration and flow patterns after lymph node dissection. Breast Cancer Res. Treat. 2013, 139, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, Y.; Akita, S.; Akita, H.; Miura, N.; Gomi, M.; Manabe, I.; Kubota, Y.; Mitsukawa, N. Development of a mouse model for the visual and quantitative assessment of lymphatic trafficking and function by in vivo imaging. Sci. Rep. 2018, 8, 5921. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Janssen, C.F.; Velasquez, F.C.; Zhang, S.; Aldrich, M.B.; Shaitelman, S.F.; DeSnyder, S.M.; Sevick-Muraca, E.M. Radiation Dose-Dependent Changes in Lymphatic Remodeling. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 852–860. [Google Scholar] [CrossRef]
- Olson, L.E.; Wilson, J.F.; Cox, J.D. Cutaneous lymphoid hyperplasia: Results of radiation therapy. Radiology 1985, 155, 507–509. [Google Scholar] [CrossRef]
- Narayanan, S.A.; Ford, J.; Zawieja, D.C. Impairment of lymphatic endothelial barrier function by X-ray irradiation. Int. J. Radiat. Biol. 2019, 95, 562–570. [Google Scholar] [CrossRef]
- Avraham, T.; Yan, A.; Zampell, J.C.; Daluvoy, S.V.; Haimovitz-Friedman, A.; Cordeiro, A.P.; Mehrara, B.J. Radiation therapy causes loss of dermal lymphatic vessels and interferes with lymphatic function by TGF-beta1-mediated tissue fibrosis. Am. J. Physiol. Cell Physiol. 2010, 299, C589–C605. [Google Scholar] [CrossRef]
- Jayaraj, A.; Raju, S.; May, C.; Pace, N. The diagnostic unreliability of classic physical signs of lymphedema. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 890–897. [Google Scholar] [CrossRef]
- Armer, J.M.; Ballman, K.V.; McCall, L.; Armer, N.C.; Sun, Y.; Udmuangpia, T.; Hunt, K.K.; Mittendorf, E.A.; Byrd, D.R.; Julian, T.B.; et al. Lymphedema symptoms and limb measurement changes in breast cancer survivors treated with neoadjuvant chemotherapy and axillary dissection: Results of American College of Surgeons Oncology Group (ACOSOG) Z1071 (Alliance) substudy. Support. Care Cancer 2019, 27, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Qin, E.S.; Bowen, M.J.; Chen, W.F. Diagnostic accuracy of bioimpedance spectroscopy in patients with lymphedema: A retrospective cohort analysis. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Spitz, J.A.; Chao, A.; Peterson, D.M.; Subramaniam, V.; Prakash, S.; Skoracki, R.J. Bioimpedance spectroscopy is not associated with a clinical diagnosis of breast cancer-related lymphedema. Lymphology 2019, 52, 134–142. [Google Scholar] [PubMed]
- Yamamoto, T.; Yamamoto, N.; Yoshimatsu, H.; Narushima, M.; Koshima, I. Factors Associated with Lower Extremity Dysmorphia Caused by Lower Extremity Lymphoedema. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 69–77. [Google Scholar] [CrossRef]
- Cheng, M.-H.; Pappalardo, M.; Lin, C.; Kuo, C.-F.; Lin, C.-Y.; Chung, K.C. Validity of the Novel Taiwan Lymphoscintigraphy Staging and Correlation of Cheng Lymphedema Grading for Unilateral Extremity Lymphedema. Ann. Surg. 2018, 268, 513–525. [Google Scholar] [CrossRef]
- Douglass, J.; Kelly-Hope, L. Comparison of Staging Systems to Assess Lymphedema Caused by Cancer Therapies, Lymphatic Filariasis, and Podoconiosis. Lymphat. Res. Biol. 2019, 17, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Campisi, C.; Campisi, C.; Accogli, S.; Campisi, C.; Boccardo, F. Lymphedema staging and surgical indications in geriatric age. BMC Geriatr. 2010, 10, A50. [Google Scholar] [CrossRef]
- Koelmeyer, L.A.; Borotkanics, R.J.; Alcorso, J.; Prah, P.; Winch, C.J.; Nakhel, K.; Dean, C.M.; Boyages, J. Early surveillance is associated with less incidence and severity of breast cancer-related lymphedema compared with a traditional referral model of care. Cancer 2019, 125, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Stout Gergich, N.L.; Pfalzer, L.A.; McGarvey, C.; Springer, B.; Gerber, L.H.; Soballe, P. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer 2008, 112, 2809–2819. [Google Scholar] [CrossRef]
- Gutierrez, C.; Karni, R.J.; Naqvi, S.; Aldrich, M.B.; Zhu, B.; Morrow, J.R.; Sevick-Muraca, E.M.; Rasmussen, J.C. Head and Neck Lymphedema: Treatment Response to Single and Multiple Sessions of Advanced Pneumatic Compression Therapy. Otolaryngol. Head Neck Surg. 2019, 160, 622–626. [Google Scholar] [CrossRef]
- Aldrich, M.; Guilliod, R.; Fife, C.E.; Maus, E.A.; Smith, L.A.; Rasmussen, J.C.; Sevick-Muraca, E.M. Lymphatic abnormalities in the normal contralateral arms of subjects with breast cancer-related lymphedema as assessed by near-infrared fluorescent imaging. Biomed. Opt. Express 2012, 3, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Pain, S.J.; Purushotham, A.D.; Barber, R.W.; Ballinger, J.R.; Solanki, C.K.; Mortimer, P.S.; Peters, A.M. Variation in lymphatic function may predispose to development of breast cancer-related lymphoedema. Eur. J. Surg. Oncol. 2004, 30, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Burnand, K.M.; Glass, D.M.; Mortimer, P.S.; Peters, A.M. Lymphatic dysfunction in the apparently clinically normal contralateral limbs of patients with unilateral lower limb swelling. Clin. Nucl. Med. 2012, 37, 9–13. [Google Scholar] [CrossRef]
- de Almeida, C.A.; Lins, E.M.; Brandão, S.C.S.; Ferraz, Á.A.B.; Pinto, F.C.M.; de Barros Marques, S.R. Lymphoscintigraphic abnormalities in the contralateral lower limbs of patients with unilateral lymphedema. J. Vasc. Surg. Venous Lymphat. Disord. 2017, 5, 363–369. [Google Scholar] [CrossRef]
- Tan, I.C.; Maus, E.A.; Rasmussen, J.C.; Marshall, M.V.; Adams, K.E.; Fife, C.E.; Smith, L.A.; Chan, W.; Sevick-Muraca, E.M. Assessment of Lymphatic Contractile Function After Manual Lymphatic Drainage Using Near-Infrared Fluorescence Imaging. Arch. Phys. Med. Rehabil. 2011, 92, 756–764.e1. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.E.; Rasmussen, J.C.; Darne, C.; Tan, I.-C.; Aldrich, M.B.; Marshall, M.V.; Fife, C.E.; Maus, E.A.; Smith, L.A.; Guilloid, R.; et al. Direct evidence of lymphatic function improvement after advanced pneumatic compression device treatment of lymphedema. Biomed. Opt. Express 2010, 1, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Maus, E.A.; Tan, I.C.; Rasmussen, J.C.; Marshall, M.V.; Fife, C.E.; Smith, L.A.; Guilliod, R.; Sevick-Muraca, E.M. Near-infrared fluorescence imaging of lymphatics in head and neck lymphedema. Head Neck 2012, 34, 448–453. [Google Scholar] [CrossRef]
- Boccardo, F.; Casabona, F.; De Cian, F.; Friedman, D.; Villa, G.; Bogliolo, S.; Ferrero, S.; Murelli, F.; Campisi, C. Lymphedema microsurgical preventive healing approach: A new technique for primary prevention of arm lymphedema after mastectomy. Ann. Surg. Oncol. 2009, 16, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Inbal, A.; Teven, C.M.; Chang, D.W. Latissimus dorsi flap with vascularized lymph node transfer for lymphedema treatment: Technique, outcomes, indications and review of literature. J. Surg. Oncol. 2017, 115, 72–77. [Google Scholar] [CrossRef]
- Carl, H.M.; Walia, G.; Bello, R.; Clarke-Pearson, E.; Hassanein, A.H.; Cho, B.; Pedreira, R.; Sacks, J.M. Systematic Review of the Surgical Treatment of Extremity Lymphedema. J. Reconstr. Microsurg. 2017, 33, 412–425. [Google Scholar] [CrossRef]
- Gould, D.J.; Mehrara, B.J.; Neligan, P.; Cheng, M.-H.; Patel, K.M. Lymph node transplantation for the treatment of lymphedema. J. Surg. Oncol. 2018, 118, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Gratzon, A.; Schultz, J.; Secrest, K.; Lee, K.; Feiner, J.; Klein, R.D. Clinical and Psychosocial Outcomes of Vascularized Lymph Node Transfer for the Treatment of Upper Extremity Lymphedema after Breast Cancer Therapy. Ann. Surg. Oncol. 2017, 24, 1475–1481. [Google Scholar] [CrossRef]
- Schaverien, M.V.; Aldrich, M.B. New and Emerging Treatments for Lymphedema. Semin. Plast. Surg. 2018, 32, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, A.R.; King, S.W.; Ramsden, A.J.; Furniss, D. Do surgical interventions for limb lymphoedema reduce cellulitis attack frequency? Microsurgery 2017, 37, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Suami, H.; Hanasono, M.M.; Womack, V.A.; Wong, F.C.; Chang, E.I. Long-term outcomes of the minimally invasive free vascularized omental lymphatic flap for the treatment of lymphedema. J. Surg. Oncol. 2017, 115, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Hara, H.; Tange, S.; Zhou, H.P.; Kawahara, M.; Shimizu, Y.; Murai, N. Multisite Lymphaticovenular Bypass Using Supermicrosurgery Technique for Lymphedema Management in Lower Lymphedema Cases. Plast. Reconstr. Surg. 2016, 138, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Gennaro, P.; Gabriele, G.; Salini, C.; Chisci, G.; Cascino, F.; Xu, J.-F.; Ungari, C. Our supramicrosurgical experience of lymphaticovenular anastomosis in lymphoedema patients to prevent cellulitis. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 674–679. [Google Scholar]
- Mihara, M.; Hara, H.; Furniss, D.; Narushima, M.; Iida, T.; Kikuchi, K.; Ohtsu, H.; Gennaro, P.; Gabriele, G.; Murai, N. Lymphaticovenular anastomosis to prevent cellulitis associated with lymphoedema. Br. J. Surg. 2014, 101, 1391–1396. [Google Scholar] [CrossRef]
- Rasmussen, J.C.; Aldrich, M.B.; Tan, I.-C.; Darne, C.; Zhu, B.; O’Donnell, T.F.; Fife, C.E.; Sevick-Muraca, E.M. Lymphatic transport in patients with chronic venous insufficiency and venous leg ulcers following sequential pneumatic compression. J. Vasc. Surg. Venous Lymphat. Disord. 2016, 4, 9–17. [Google Scholar] [CrossRef]
- Son, A.; O’Donnell, T.F.; Izhakoff, J.; Gaebler, J.A.; Niecko, T.; Iafrati, M.A. Lymphedema-associated comorbidities and treatment gap. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 724–730. [Google Scholar] [CrossRef]
- Dean, S.M.; Valenti, E.; Hock, K.; Leffler, J.; Compston, A.; Abraham, W.T. The clinical characteristics of lower extremity lymphedema in 440 patients. J. Vasc. Surg. Venous Lymphat. Disord. 2020. [Google Scholar] [CrossRef]
- Rasmussen, J.C.; Zhu, B.; Morrow, J.R.; Aldrich, M.B.; Sahihi, A.; Harlin, A.; Fife, C.E.; O’Donnell, T.F., Jr.; Sevick-Muraca, E.M. Degradation of Lymphatic Anatomy and Function in Early Venous Insufficiency. J. Vasc. Surg. Venous Lymphat. Disord. 2020, in press. [Google Scholar]
- Bouta, E.M.; Wood, R.W.; Perry, S.W.; Brown, E.B.; Ritchlin, C.T.; Xing, L.; Schwarz, E.M. Measuring intranodal pressure and lymph viscosity to elucidate mechanisms of arthritic flare and therapeutic outcomes. Ann. N. Y. Acad. Sci. 2011, 1240, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Benaglio, F.; Vitolo, B.; Scarabelli, M.; Binda, E.; Bugatti, S.; Caporali, R.; Montecucco, C.; Manzo, A. The draining lymph node in rheumatoid arthritis: Current concepts and research perspectives. BioMed Res. Int. 2015, 2015, 420251. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, M.B.; Velasquez, F.C.; Kwon, S.; Azhdarinia, A.; Pinkston, K.; Harvey, B.R.; Chan, W.; Rasmussen, J.C.; Ross, R.F.; Fife, C.E.; et al. Lymphatic delivery of etanercept via nanotopography improves response to collagen-induced arthritis. Arthritis Res. Ther. 2017, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, H.; Bell, R.; Bouta, E.M.; Wood, R.W.; Xing, L.; Ritchlin, C.T.; Schwarz, E.M. Lymphatic imaging to assess rheumatoid flare: Mechanistic insights and biomarker potential. Arthritis Res. Ther. 2016, 18, 194. [Google Scholar] [CrossRef] [PubMed]
- Joos, E.; Bourgeois, P.; Famaey, J.P. Lymphatic disorders in rheumatoid arthritis. Semin. Arthritis Rheum. 1993, 22, 392–398. [Google Scholar] [CrossRef]
- Grillet, B.; Dequeker, J. Rheumatoid lymphedema. J. Rheumatol. 1987, 14, 1095–1097. [Google Scholar] [PubMed]
- Mulherin, D.M.; FitzGerald, O.; Bresnihan, B. Lymphedema of the upper limb in patients with psoriatic arthritis. Semin. Arthritis Rheum. 1993, 22, 350–356. [Google Scholar] [CrossRef]
- Rajasekhar, L.; Habibi, S.; Sudhakar, P.; Gumdal, N. Lymphatic obstruction as a cause of extremity edema in systemic lupus erythematosus. Clin. Rheumatol. 2013, 32 (Suppl. 1), S11–S13. [Google Scholar] [CrossRef]
- Hampton, H.R.; Bailey, J.; Tomura, M.; Brink, R.; Chtanova, T. Microbe-dependent lymphatic migration of neutrophils modulates lymphocyte proliferation in lymph nodes. Nat. Commun. 2015, 6, 7139. [Google Scholar] [CrossRef] [PubMed]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.S.; Friday, S.; Li, S.; Patel, R.M.; Subramanian, V.; et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 2013, 5, 178ra40. [Google Scholar] [CrossRef] [PubMed]
- Martinod, K.; Wagner, D.D. Thrombosis: Tangled up in NETs. Blood 2014, 123, 2768–2776. [Google Scholar] [CrossRef] [PubMed]
- Teerachaisakul, M.; Ekataksin, W.; Durongwatana, S.; Taneepanichskul, S. Risk factors for cellulitis in patients with lymphedema: A case-controlled study. Lymphology 2013, 46, 150–156. [Google Scholar] [PubMed]
- Ohkuma, M.; Okada, E. Bradykinin, PGE2, and Interleukin-6 (IL-6) involved in pathogenesis of acute cellulitis in lymphedema. Lymphology 1998, 31, s231–s232. [Google Scholar]
- Ellis Simonsen, S.M.; van Orman, E.R.; Hatch, B.E.; Jones, S.S.; Gren, L.H.; Hegmann, K.T.; Lyon, J.L. Cellulitis incidence in a defined population. Epidemiol. Infect. 2006, 134, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Hersh, A.L.; Chambers, H.F.; Maselli, J.H.; Gonzales, R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch. Intern. Med. 2008, 168, 1585–1591. [Google Scholar] [CrossRef]
- Solucient (Firm). DRG Handbook: Comparative Clinical and Financial Benchmarks 2006; Solucient: Evanston, IL, USA, 2006; ISBN 978-1-57372-356-5. [Google Scholar]
- Gunderson, C.G.; Martinello, R.A. A systematic review of bacteremias in cellulitis and erysipelas. J. Infect. 2012, 64, 148–155. [Google Scholar] [CrossRef]
- Weng, Q.Y.; Raff, A.B.; Cohen, J.M.; Gunasekera, N.; Okhovat, J.-P.; Vedak, P.; Joyce, C.; Kroshinsky, D.; Mostaghimi, A. Costs and Consequences Associated with Misdiagnosed Lower Extremity Cellulitis. JAMA Dermatol. 2017, 153, 141–146. [Google Scholar] [CrossRef]
- Connor, M.P.; Gamelli, R. Challenges of cellulitis in a lymphedematous extremity: A case report. Cases J. 2009, 2, 9377. [Google Scholar] [CrossRef]
- Kasai-Sakamoto, A.; Yokoyama, Y.; Mizunuma, H. A case of cellulitis that complicated lymphedema of the lower limb and produced systemic inflammatory response syndrome (SIRS). Eur. J. Gynaecol. Oncol. 2006, 27, 419–421. [Google Scholar] [PubMed]
- Swartz, M.N. Clinical practice. Cellulitis. N. Engl. J. Med. 2004, 350, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Chira, S.; Miller, L.G. Staphylococcus aureus is the most common identified cause of cellulitis: A systematic review. Epidemiol. Infect. 2010, 138, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Maclellan, R.A.; Greene, A.K. Lymphedema. Semin. Pediatr. Surg. 2014, 23, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Crisp, J.G.; Takhar, S.S.; Moran, G.J.; Krishnadasan, A.; Dowd, S.E.; Finegold, S.M.; Summanen, P.H.; Talan, D.A.; Emergency ID Net Study Group. Inability of polymerase chain reaction, pyrosequencing, and culture of infected and uninfected site skin biopsy specimens to identify the cause of cellulitis. Clin. Infect. Dis. 2015, 61, 1679–1687. [Google Scholar] [CrossRef]
- Karaca-Mandic, P.; Hirsch, A.T.; Rockson, S.G.; Ridner, S.H. The Cutaneous, Net Clinical, and Health Economic Benefits of Advanced Pneumatic Compression Devices in Patients with Lymphedema. JAMA Dermatol. 2015, 151, 1187–1193. [Google Scholar] [CrossRef]
- Blumberg, S.N.; Berland, T.; Rockman, C.; Mussa, F.; Brooks, A.; Cayne, N.; Maldonado, T. Pneumatic Compression Improves Quality of Life in Patients with Lower-Extremity Lymphedema. Ann. Vasc. Surg. 2016, 30, 40–44. [Google Scholar] [CrossRef]
- Kwon, S.; Velasquez, F.C.; Rasmussen, J.C.; Greives, M.R.; Turner, K.D.; Morrow, J.R.; Hwu, W.-J.; Ross, R.F.; Zhang, S.; Sevick-Muraca, E.M. Nanotopography-based lymphatic delivery for improved anti-tumor responses to checkpoint blockade immunotherapy. Theranostics 2019, 22, 8332–8343. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldrich, M.B.; Rasmussen, J.C.; Fife, C.E.; Shaitelman, S.F.; Sevick-Muraca, E.M. The Development and Treatment of Lymphatic Dysfunction in Cancer Patients and Survivors. Cancers 2020, 12, 2280. https://doi.org/10.3390/cancers12082280
Aldrich MB, Rasmussen JC, Fife CE, Shaitelman SF, Sevick-Muraca EM. The Development and Treatment of Lymphatic Dysfunction in Cancer Patients and Survivors. Cancers. 2020; 12(8):2280. https://doi.org/10.3390/cancers12082280
Chicago/Turabian StyleAldrich, Melissa B., John C. Rasmussen, Caroline E. Fife, Simona F. Shaitelman, and Eva M. Sevick-Muraca. 2020. "The Development and Treatment of Lymphatic Dysfunction in Cancer Patients and Survivors" Cancers 12, no. 8: 2280. https://doi.org/10.3390/cancers12082280
APA StyleAldrich, M. B., Rasmussen, J. C., Fife, C. E., Shaitelman, S. F., & Sevick-Muraca, E. M. (2020). The Development and Treatment of Lymphatic Dysfunction in Cancer Patients and Survivors. Cancers, 12(8), 2280. https://doi.org/10.3390/cancers12082280




