Electroporation-Based Treatments in Urology
Abstract
1. Introduction
2. The Theoretical Background of EP
3. IRE
3.1. IRE—Renal Cancer
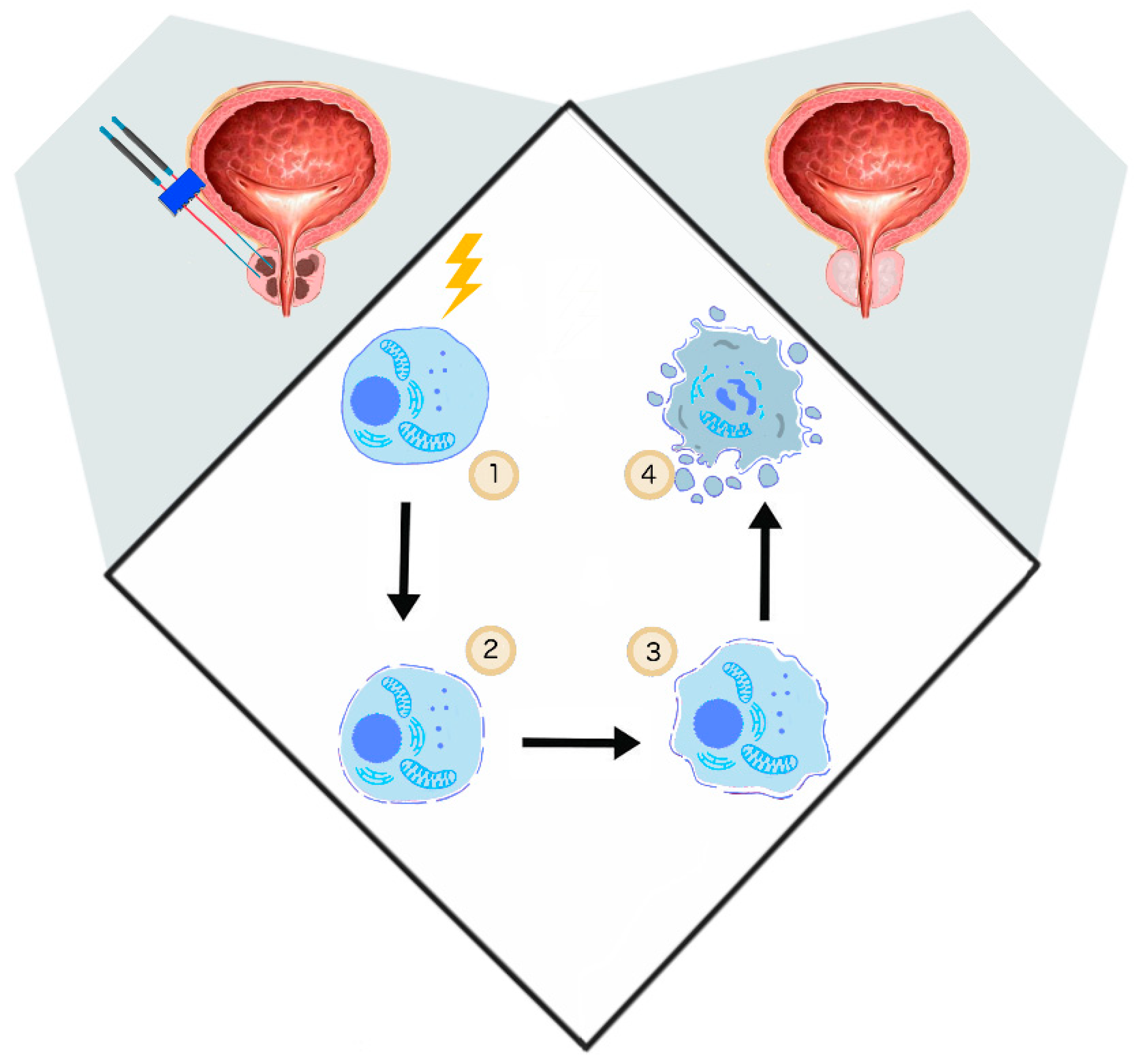
3.2. IRE—Prostate Cancer
3.3. IRE—Urothelial Cancer
3.4. High-Frequency Irreversible Electroporation
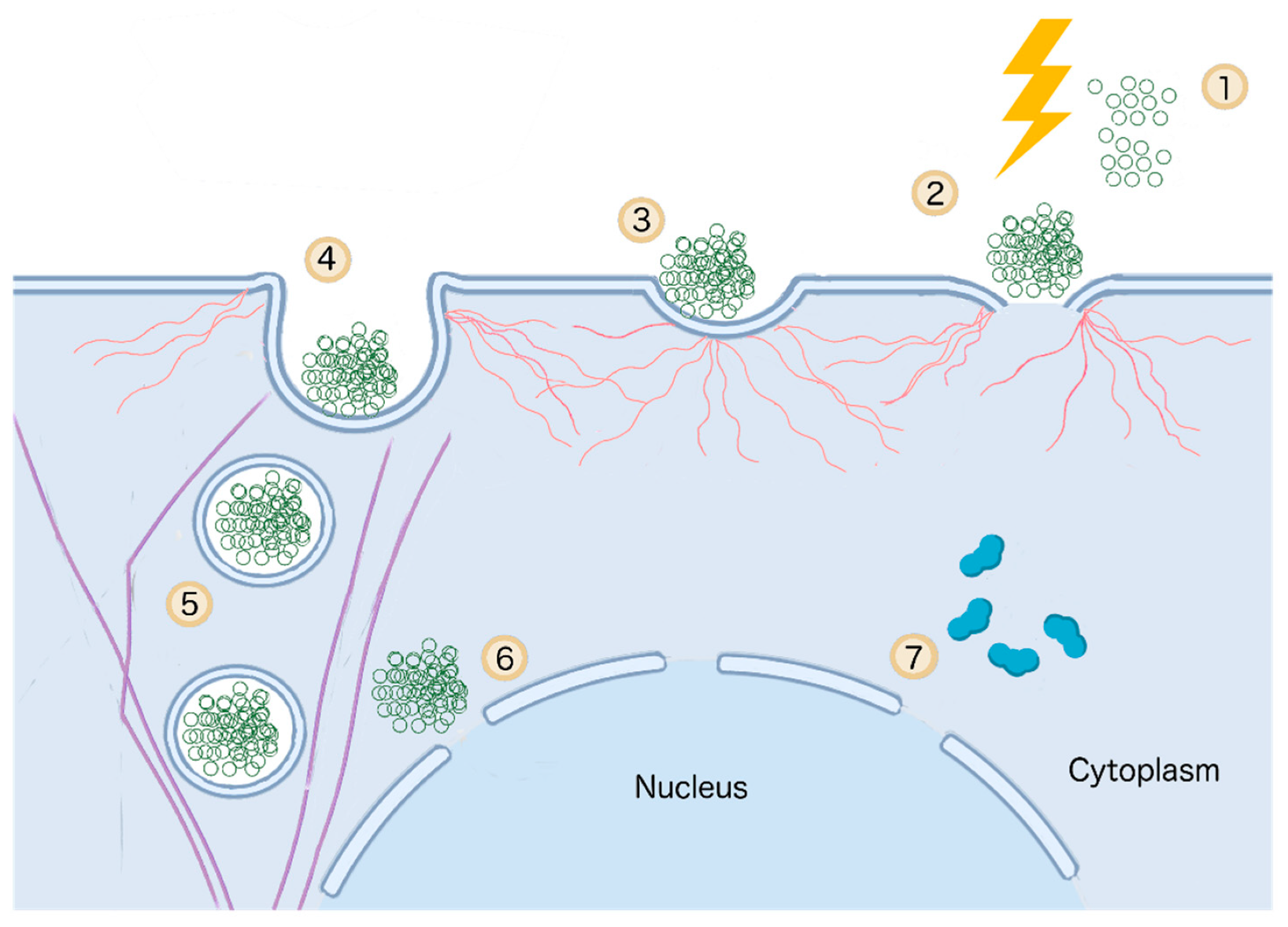
3.5. Immunomodulatory Effect of IRE
4. Electrochemotherapy
4.1. ECT—Prostate Cancer
4.2. ECT—Bladder Cancer
4.3. Gene Electrotransfer
4.4. GET—Prostate Cancer
4.5. GET—Bladder Cancer
4.6. GET—Renal Cancer
4.7. GET—Adoptive Transfer of Autologous T-Cells
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dy, G.W.; Gore, J.L.; Forouzanfar, M.H.; Naghavi, M.; Fitzmaurice, C.; Catto, J. Global Burden of Urologic Cancers, 1990–2013. Eur. Urol. 2016, 71, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Kurhanewicz, J.; Vigneron, D.; Carroll, P.; Coakley, F. Multiparametric magnetic resonance imaging in prostate cancer: Present and future. Curr. Opin. Urol. 2008, 18, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Nassiri, N.; Chang, E.; Lieu, P.; Priester, A.M.; Margolis, D.J.A.; Huang, J.; Reiter, R.E.; Dorey, F.J.; Marks, L.S. Focal therapy eligibility determined by MRI/US fusion biopsy. J. Urol. 2018, 199, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Lino, C.A.; Harper, J.C.; Carney, J.P.; Timlin, J.A. Delivering CRISPR: A review of the challenges and approaches. Drug Deliv. 2018, 25, 1234–1257. [Google Scholar] [CrossRef]
- Harvey, L.; Arnold, B.; SLawrence, Z.; Matsudaira, P.; Baltimore, D.; Darnell, J. Molecular Cell Biology, 4th ed.; Freeman & Co.: New York, NY, USA, 2000; ISBN 0-7167-3136-3. [Google Scholar]
- Zimmerman, U. Electric field-medieted fusion and related electrical phenomena. Biochim. Biophys. Acta 1982, 694, 227–277. [Google Scholar] [CrossRef]
- Neumann, E.; Rosenheck, K. Permeability Changes Induced by Electric Impulses in Vesicular Membranes. J. Membr. Biol. 1972, 10, 279–290. [Google Scholar] [CrossRef]
- Kotnik, T.; Pucihar, G.; Miklavcic, D. Induced Transmembrane Voltage and Its Correlation with Electroporation-Mediated Molecular Transport. J. Membr. Biol. 2010, 236, 3–13. [Google Scholar] [CrossRef]
- Teissie, J.; Marie-Pierre, R. An Experimental Evaluation of the Critical Potential Difference. Biophys. J. Vol. 1993, 65, 409–413. [Google Scholar] [CrossRef]
- Teissie, J.; Tsong, T.Y. Electric Field Induced Transient Pores in Phospholipid Bilayer Vesicles. Biochemistry 1981, 20, 1548–1554. [Google Scholar] [CrossRef]
- Cemazar, M.; Jarm, T.; Miklavcic, D. Effect of Electric-Field Intensity on Electropermeabilization and Electrosensitmty of Various Tumor-Cell Lines In Vitro. Electro. Magn. 1998, 17, 263–272. [Google Scholar]
- Gabriel, B.; Teissie, J. Time Courses of Mammalian Cell Electropermeabilization Observed by Millisecond Imaging of Membrane Property Changes during the Pulse. Biophys. J. 1999, 76, 2158–2165. [Google Scholar] [CrossRef]
- Weavera, J.; Smith, K.; Essera, A.T.; Sona, R.; Gowrishankar, R.T. A brief overview of electroporation pulse strength-duration space: A region where additional intracellular effects are expected. Bioelectrochemistry 2012, 87, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Silve, A.; Guimerà Brunet, A.; Al-Sakere, B.; Ivorra, A.; Mir, L.M. Comparison of the effects of the repetition rate between microsecond and nanosecond pulses: Electropermeabilization-induced electro-desensitization? Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 2139–2151. [Google Scholar] [CrossRef]
- Onik, G.; Mikus, P.; Rubinsky, B. Irreversible Electroporation: Implications for Prostate Ablation. Technol. Cancer Res. Treat. 2007, 6, 295–300. [Google Scholar] [CrossRef]
- Donthula, V.; Camps-Raga, B.; Islam, N.E.; Ślusarz, A.; Lubahn, D.B.; Ganjam, V. Effects of nanosecond pulsed electric fields on the human prostate cancer cell line lncap. IEEE Trans. Dielectr. Electr. Insul. 2009, 16, 1311–1316. [Google Scholar] [CrossRef]
- Gimsa, J.; Wachner, D. Analytical Description of the Transmembrane Voltage Induced on Arbitrarily Oriented Ellipsoidal and Cylindrical Cells. Biophys. J. 2001, 81, 1888–1896. [Google Scholar] [CrossRef]
- Rols, M.; Teissie, J. Electropermeabilization of Mammalian Cells to Macromolecules: Control by Pulse Duration. Biophys. J. 1998, 75, 1415–1423. [Google Scholar] [CrossRef]
- Winterhalter, M.; Helfrich, W. Deformation of Spherical Vesicles by Electric Fields. J. Colloid Interface Sci. 1988, 122, 583–586. [Google Scholar] [CrossRef]
- Chopinet, L.; Roduit, C.; Rols, M.; Dague, E. Destabilization induced by electropermeabilization analyzed by atomic force microscopy. BBA Biomembr. 2013, 1828, 2223–2229. [Google Scholar] [CrossRef]
- Pakhomova, O.N.; Khorokhorina, V.A.; Bowman, A.M.; Rodaite, R. Oxidative effects of nanosecond pulsed electric field exposure in cells and cell-free media. Arch. Biochem. Biophys. 2012, 527, 55–64. [Google Scholar] [CrossRef]
- Olga, M.; Michel, O.; Pakhomov, A.G.; Casciola, M.; Saczko, J.; Kulbacka, J. Electropermeabilization does not correlate with plasma membrane lipid oxidation. Bioelectrochemistry 2019, 132, 107433. [Google Scholar]
- Romeo, S.; Wu, Y.; Levine, Z.A.; Gundersen, M.A.; Vernier, P.T. Water influx and cell swelling after nanosecond electropermeabilization. Biochim. Biophys. Acta 2013, 1828, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- Teissie, J.; Eynard, N.; Gabriel, B.; Rols, M.P. Electropermeabilization of cell membranes. Adv. Drug Deliv. Rev. 1999, 35, 3–19. [Google Scholar] [CrossRef]
- Rols, M.-P.; Delteil, C.; Golzio, M.; Teissie, J. Control by ATP and ADP of voltage-induced mammalian-cell-membrane permeabilization, gene transfer and resulting expression. Eur. J. Biochem. 1998, 254, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, R.; Wylie, D.E.; Schuster, S.M. Transfer of Monoclonal Antibodies into Mammalian Cells by Electroporation. J. Biol. Chem. 1989, 264, 15494–15500. [Google Scholar]
- Rols, M. Electropermeabilization, a physical method for the delivery of therapeutic molecules into cells. Biochim. Biophys. Acta 2006, 1758, 423–428. [Google Scholar] [CrossRef]
- Sözer, E.B.; Pocetti, C.F.; Vernier, P.T. Transport of charged small molecules after electropermeabilization—Drift and diffusion. BMC Biophys. 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Rols, M.; Teissie, J. Electropermeabilization of mammalian cells Quantitative analysis of the phenomenon. Biophys. J. 1990, 58, 1089–1098. [Google Scholar] [CrossRef]
- Aycock, K.N.; Davalos, R.V. Irreversible Electroporation: Background, Theory, and Review of Recent Developments in Clinical Oncology. Bioelectricity. 2019, 1, 214–234. [Google Scholar] [CrossRef]
- Saulis, G. Pore disappearance in a cell after electroporation: Theoretical simulation and comparison with experiments. Biophys. J. 1997, 73, 1299–1309. [Google Scholar] [CrossRef]
- Gehl, J.; Skovsgaard, T.; Mir, L.M. Vascular reactions to in vivo electroporation: Characterization and consequences for drug and gene delivery. Biochim. Biophys. Acta Gen. Subj. 2002, 1569, 51–58. [Google Scholar] [CrossRef]
- Blazek, A.D.; Paleo, B.J.; Weisleder, N. Plasma Membrane Repair: A Central Process for Maintaining Cellular Homeostasis. Physiology 2015, 30, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Selma, C.; Zupanic, A.; Kranjc, S.; Al Sakere, B.; Miklavcic, D.; Leroy-Willig, A.; Mir, L.M. The influence of skeletal muscle anisotropy on electroporation: In vivo study and numerical modeling. Med. Biol. Eng. Comput. 2010, 48, 637–648. [Google Scholar]
- Miklavcic, D.; Pavselj, N. Electric Properties of Tissues. In Wiley Encyclopedia of Biomedical Engineering; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 1–12. ISBN 9780471740360. [Google Scholar]
- Pavlin, M.; Miklavc, D. Effective Conductivity of a Suspension of Permeabilized Cells: A Theoretical Analysis. Biophys. J. 2003, 85, 719–729. [Google Scholar] [CrossRef]
- Corovic, S.; Lackovic, I.; Sustaric, P.; Sustar, T.; Rodic, T.; Miklavcic, D. Modeling of electric field distribution in tissues during electroporation. Biomed. Eng. Online 2013, 12, 1–27. [Google Scholar] [CrossRef]
- Haemmerich, D.; Staelin, S.T.; Tsai, J.Z.; Tungjitkusolmun, S.; Mahvi, D.M.; Webster, J.G. In vivo electrical conductivity of hepatic tumours. Physiol. Meas. 2003, 24, 251–260. [Google Scholar] [CrossRef][Green Version]
- Pavselj, N.; Bregar, Z.; Vidmar, E.M.; Miklavcic, D. The Course of Tissue Permeabilization Studied on a Mathematical Model of a Subcutaneous Tumor in Small Animals. IEEE Trans. Biomed. Eng. 2005, 52, 1373–1380. [Google Scholar] [CrossRef]
- Pavlin, M.; Kanduser, M.; Rebers, M.; Pucihar, G.; Hart, F.X.; Magjarevic, R.; Miklavcic, D. Effect of Cell Electroporation on the Conductivity of a Cell Suspension. Biophys. J. 2005, 88, 4378–4390. [Google Scholar] [CrossRef]
- Davalos, R.V.; Otten, D.M.; Mir, L.M.; Rubinsky, B. Electrical Impedance Tomography for Imaging Tissue Electroporation. IEEE Trans. Biomed. Eng. 2004, 51, 761–767. [Google Scholar] [CrossRef]
- Kranjc, M.; Bajd, F.; Sersa, I.; Woo, E.J.; Miklavcic, D. Ex Vivo and In Silico Feasibility Study of Monitoring Electric Field Distribution in Tissue during Electroporation Based Treatments. PLoS ONE 2012, 7, e45737. [Google Scholar] [CrossRef][Green Version]
- Kranjc, M.; Kranjc, S.; Bajd, F.; Serša, G.; Serša, I.; Miklavčič, D. Predicting irreversible electroporation-induced tissue damage by means of magnetic resonance electrical impedance tomography. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Epstein, B.R.; Foster, K.R. Anisotropy in the dielectric properties of skeletal muscle. Med. Biol. Eng. Comput. 1983, 21, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Pavšelj, N.; Miklavčič, D. Numerical modeling in electroporation-based biomedical applications. Radiol. Oncol. 2008, 42, 159–168. [Google Scholar] [CrossRef]
- Davalos, R.V.; Mir, L.M.; Rubinsky, B. Tissue ablation with irreversible electroporation. Ann. Biomed. Eng. 2005, 33, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Knavel, E.M.; Brace, C.L. Tumor ablation: Common modalities and general practices. Tech. Vasc. Interv. Radiol. 2013, 16, 192–200. [Google Scholar] [CrossRef]
- Jiang, C.; Davalos, R.V.; Bischof, J.C. A Review of Basic to Clinical Studies of Irreversible Electroporation Therapy. IEEE Trans. Biomed. Eng. 2015, 62, 4–20. [Google Scholar] [CrossRef]
- Schoellnast, H.; Monette, S.; Ezell, P.C.; Maybody, M.; Erinjeri, J.P.; Stubblefield, M.D.; Single, G.; Solomon, S.B. The delayed effects of irreversible electroporation ablation on nerves. Eur. Radiol. 2013, 23, 375–380. [Google Scholar] [CrossRef]
- Maor, E.; Rubinsky, B. Endovascular Nonthermal Irreversible Electroporation: A Finite Element Analysis. J. Biomech. Eng. 2010, 132, 1–7. [Google Scholar] [CrossRef]
- Davalos, R.V.; Rubinsky, B.; Mir, L.M. Theoretical analysis of the thermal effects during in vivo tissue electroporation. Bioelectrochemistry 2003, 61, 99–107. [Google Scholar] [CrossRef]
- Wendler, J.; Fischbach, K.; Ricke, J.; Jürgens, J.; Fischbach, F.; Köllermann, J.; Porsch, M.; Baumunk, D.; Schostak, M.; Liehr, U.; et al. Irreversible Electroporation (IRE): Standardization of Terminology and Reporting Criteria for Analysis and Comparison. Pol. J. Radiol. 2016, 81, 54–64. [Google Scholar] [CrossRef]
- Nielsen, K.; Scheffer, H.J.; Vieveen, J.M.; Van Tilborg, A.A.J.M.; Meijer, S.; Van Kuijk, C.; Tol, M.P. Van Den Anaesthetic management during open and percutaneous irreversible electroporation. Br. J. Anaesth. 2014, 113, 985–992. [Google Scholar] [CrossRef]
- Qin, Z.; Zeng, J.; Liu, G.; Long, X.; Fang, G.; Li, Z.; Xu, K.; Niu, L. Irreversible Electroporation Ablation of an Unresectable Fibrous Sarcoma With 2 Electrodes: A Case Report. Technol. Cancer Res. Treat. 2017, 16, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Deodhar, A.; Dickfeld, T.; Single, G.W.; Raymond, H.; Sofocleous, C.T.; Maybody, M.; Solomon, S.B.; Therapies, I.; Sloan, M.; Cancer, K.; et al. Irreversible Electroporation Near the Heart: Ventricular Arrhythmias Can Be Prevented With ECG Synchronization. AJR Am. J. Roentgenol. 2011, 196, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Hyuk, L.; Choi, S.; Jai, H.; Eun, C.; Kim, S.; Keum, B.; Seok, Y.; Yoon, S.; Jeen, T.; et al. EUS-guided irreversible electroporation using endoscopic needle- electrode in porcine pancreas. Surg. Endosc. 2019, 33, 658–662. [Google Scholar]
- Maor, E.; Ivorra, A.; Mitchell, J.J.; Rubinsky, B. Vascular smooth muscle cells ablation with endovascular non thermal irreversible electroporation. J. Vasc. Interv. Radiol. 2011, 21, 1708–1715. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.T.; Zhou, V.X.; Rubinsky, B. Using non-thermal irreversible electroporation to create an in vivo niche for exogenoue cell engrafment. Biotechniques 2017, 62, 229–231. [Google Scholar] [CrossRef]
- Schmidt, C.R.; Shires, P.; Mootoo, M. Real-time ultrasound imaging of irreversible electroporation in a porcine liver model adequately characterizes the zone of cellular necrosis. Hpb. J. 2012, 14, 98–102. [Google Scholar] [CrossRef][Green Version]
- Vroomen, L.G.P.H.; Scheffer, H.J.; Melenhorst, M.C.A.M. MR and CT imaging characteristics and ablation zone volumetry of locally advanced pancreatic cancer treated with irreversible electroporation. Eur. Radiol. 2017, 27, 2521–2531. [Google Scholar] [CrossRef]
- Neal, R.E., II; Cheung, W.; Kavnoudias, H.; Thomson, K.R. Spectrum of imaging and characteristics for liver tumors treated with irreversible electroporation. J. Biomed. Sci. Eng. 2012, 5, 813–818. [Google Scholar] [CrossRef]
- Deodhar, A.; Monette, S.; Single, G.W.; Hamilton, W.C.; Thornton, R.; Maybody, M.; Coleman, J.A.; Solomon, S.B. Renal Tissue Ablation With Irreversible Electroporation: Preliminary Results in a Porcine Model. Urology 2011, 77, 754–760. [Google Scholar] [CrossRef]
- Johann Jakob, W.; Maciej, P.; Markus, P.; Andreas, J. Urinary Tract Effects After Multifocal Nonthermal Irreversible Electroporation of the Kidney: Acute and Chronic Monitoring by Magnetic Resonance Imaging, Intravenous Urography and Urinary Cytology. Cardiovasc. Interv. Radiol. 2012, 35, 921–926. [Google Scholar]
- Mara, D.X.; Zondervan, J.; De Bruin, M.; van Lienden, K.P. Feasibility and safety of irreversible electroporation (IRE) in patients with small renal masses: Results of a prospective study. Urol. Oncol. Semin. Orig. Investig. 2019, 37, 183.e1–183.e8. [Google Scholar]
- Trimmer, C.K.; Khosla, A.; Morgan, M.; Stephenson, S.L.; Ozayar, A.; Cadeddu, J.A. Minimally Invasive Percutaneous Treatment of Small Renal Tumors with Irreversible Electroporation: A Single-Center Experience. J. Vasc. Interv. Radiol. 2015, 26, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Canvasser, N.E.; Sorokin, I.; Lay, A.H.; Morgan, M.S.C.; Ozayar, A.; Trimmer, C.; Cadeddu, J.A. Irreversible electroporation of small renal masses: Suboptimal oncologic efficacy in an early series. World J. Urol. 2017, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- EAU Guidelines. In Proceedings of the EAU Annual Congress Amsterdam 2020, Amsterdam, The Netherlands, 17–26 July 2020; ISBN 978-94-92671-07-3.
- Zhong, J.; Wah, T.M. Renal ablation: Current management strategies and controversies. Chin. Clin. Oncol. 2019, 8, 6–13. [Google Scholar] [CrossRef]
- Duffey, B.G.; Anderson, J.K. Current and future technology for minimally invasive ablation of renal cell carcinoma. Indian J. Urol. 2010, 26, 410–417. [Google Scholar]
- Donaldson, I.A.; Alonzi, R.; Barratt, D.; Barret, E.; Berge, V.; Bott, S.; Bottomley, D.; Eggener, S.; Ehdaie, B.; Emberton, M.; et al. Focal Therapy: Patients, Interventions, and Outcomes—A Report from a Consensus Meeting. Eur. Urol. 2015, 67, 771–777. [Google Scholar] [CrossRef]
- Blazevski, A.; Scheltema, M.J.; Amin, A.; Thompson, J.; Lawrentschuk, N.; Stricker, P.D. Irreversible electroporation (IRE): A narrative review of the development of IRE from the laboratory to a prostate cancer tratment. BJU Int. 2019, 120, 1–28. [Google Scholar] [CrossRef]
- Van Den Bos, W.; Jurhill, R.R.; De Bruin, D.M.; Postema, A.W.; Wagstaff, P.G.K.; Muller, B.G.; Varkarakis, I.M.; Skolarikos, A.; Zondervan, P.J.; Pes, M.P.L.; et al. Histopathological outcomes after irreversible electroporation in prostate cancer; Results of an ablate-and-resect study. J. Urol. 2016, 196, 552–559. [Google Scholar] [CrossRef]
- Guenther, E.; Klein, N.; Zapf, S.; Weil, S.; Schlosser, C.; Rubinsky, B.; Stehling, M.K. Prostate cancer treatment with Irreversible Electroporation (IRE): Safety, efficacy and clinical experience in 471 treatments. PLoS ONE 2019, 14, e0215093. [Google Scholar] [CrossRef]
- Blazevski, A.; Scheltema, M.J.; Yuen, B.; Masand, N. Oncological and Quality-of-life Outcomes Following Focal Irreversible Electroporation as Primary Treatment for Localised Prostate Cancer: A Biopsy-monitored Prospective Cohort. Eur. Urol. Oncol. 2019, 207, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Wang, H.; Zhao, Y. First Human Trial of High-Frequency Irreversible Electroporation Therapy for Prostate Cancer. Technol. Cancer Res. Treat. 2018, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Scheltema, M.J.; Van Den Bos, W.; Siriwardana, A.R.; Anton, M.F.; Shnier, R.; Delprado, W.; Stricker, P.D. Feasibility and safety of focal irreversible electroporation as salvage treatment for localized radio-recurrent prostate cancer. BJU Int. 2017, 120, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bos, W.; Scheltema, M.J.; Siriwardana, A.R.; Kalsbeek, A.M.F.; Thompson, J.E.; Ting, F.; Maret, B.; Haynes, A.; Shnier, R.; Delprado, W.; et al. Focal irreversible electroporation as primary treatment for localized prostate cancer. BJU Int. 2018, 121, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.; Gunther, E.; Zapf, S.; El-idrissi, R.; Atta, J.; Stehling, M. Prostate cancer infiltrating the bladder sphincter successfully treated with Electrochemotherapy: A case report. Clin. Case Rep. 2017, 5, 2127–2132. [Google Scholar] [CrossRef] [PubMed]
- Low, L.; Mander, A.; Mccann, K.; Dearnaley, D.; Tjelle, T. DNA Vaccination with Electroporation Induces Increased Antibody Responses in Patients with Prostate Cancer. Hum. Gene Ther. 2009, 20, 1269–1278. [Google Scholar] [CrossRef]
- Eriksson, F.; Tötterman, T.; Maltais, A.; Pisa, P. DNA vaccine coding for the rhesus prostate specific antigen delivered by intradermal electroporation in patients with relapsed prostate cancer. Vaccine 2013, 31, 3843–3848. [Google Scholar] [CrossRef]
- Van den Bos, W.; De Bruin, D.; Veelo, D.; Postema, A.; Muller, B.G.; Varkarakis, I.M. Quality of Life and Safety Outcomes Following Irreversible Electroporation Treatment for Prostate Cancer: Results from a Phase I-Ii Study. Cancer Sci. Ther. 2015, 7, 312–321. [Google Scholar]
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Mason, M.; Metcalfe, C.; Holding, P.; Davis, M.; Peters, T.J.; Turner, E.L.; Martin, R.M.; et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N. Engl. J. Med. 2016, 372, 1415–1424. [Google Scholar] [CrossRef]
- Trial, N.; Haglind, E.; Carlsson, S.; Stranne, J.; Wallerstedt, A.; Wildera, U.; Thorsteinsdottir, T.; Lagerkvist, M.; Damber, J.; Bjartell, A.; et al. Urinary Incontinence and Erectile Dysfunction After Robotic Versus Open Radical Prostatectomy: A Prospective, Controlled, Nonrandomised Trial. Eur. Urol. 2015, 68, 216–225. [Google Scholar]
- Van Der Wielen, G.J.; Mulhall, J.P.; Incrocci, L. Erectile dysfunction after radiotherapy for prostate cancer and radiation dose to the penile structures: A critical review. Radiother. Oncol. 2007, 84, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Pickles, T.; Berthelet, E.; Agranovich, A.; Kwan, W.; Tyldesley, S.; Mckenzie, M.; Keyes, M.; Morris, J.; Pai, H. Urinary incontinence in prostate cancer patients treated with external beam radiotherapy. Radiother. Oncol. 2005, 74, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Srimathveeravalli, G.; Silk, M.; Wimmer, T.; Monette, S.; Kimm, S.; Maybody, M.; Solomon, S.B.; Coleman, J.; Durack, J.C. Feasibility of Catheter-Directed Intraluminal Irreversible Electroporation of Porcine Ureter and Acute Outcomes in Response to Increasing Energy Delivery. J. Vasc. Interv. Radiol. 2015, 26, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Srimathveeravalli, G.; Cornelis, F.; Wimmer, H.; Monette, S.; Kimm, S.Y.; Maybody, M.; Solomon, S.B.; Coleman, J.A.; Durac, J.C. The Normal Porcine Ureter Retains Lumen Wall Integrity but not Patency Following Catheter Directed Irreversible Electroporation: Imaging and Histologic Assessment Over 28 Days. J. Vasc. Interv. Radiol. 2018, 28, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Ivey, J.W.; Latouche, E.L.; Sano, M.B.; Rossmeisl, J.H.; Davalos, R.V.; Verbridge, S.S. Targeted cellular ablation based on the morphology of malignant cells. Sci. Rep. 2015, 5, 1–17. [Google Scholar] [CrossRef]
- Ringel-scaia, V.M.; Beitel-white, N.; Lorenzo, M.F.; Brock, R.M.; Huie, K.E.; Coutermarsh-Ott, S.; Eden, K.; Mcdaniel, D.K.; Verbridge, S.S.; Rossmeisl, J.H.; et al. High-frequency irreversible electroporation is an effective tumor ablation strategy that induces immunologic cell death and promotes systemic anti-tumor immunity. EBioMedicine 2019, 44, 112–125. [Google Scholar] [CrossRef]
- Byron, C.R.; Dewitt, M.R.; Latouche, E.L.; Davalos, R.V.; Robertson, J.L. Treatment of Infiltrative Superficial Tumors in Awake Standing Horses Using Novel High-Frequency Pulsed Electrical Fields. Front. Vet. Sci. 2019, 6, 1–9. [Google Scholar] [CrossRef]
- Arena, C.B.; Sano, M.B.; Rossmeisl, J.H., Jr.; Caldwell, J.L.; Garcia, P.A.; Rylander, M.N.; Davalos, R.V. High-frequency irreversible electroporation (H-FIRE) for non-thermal ablation without muscle contraction. Biomed. Eng. Online 2011, 10, 1–20. [Google Scholar] [CrossRef]
- Reberšek, M.; Connor, R.O.; Miklavc, D.; Dermol–Černe, J.; Polajz, T. Cancellation effect is present in high-frequency reversible and irreversible electroporation. Bioelectrochemistry 2020, 132, 1–11. [Google Scholar]
- Pandit, H.; Hong, Y.K.; Li, Y.; Rostas, J.; Pulliam, Z. Evaluating the Regulatory Immunomodulation Effect of Irreversible Electroporation (IRE) in Pancreatic Adenocarcinoma. Ann. Surg. Oncol. 2019, 26, 800–8006. [Google Scholar] [CrossRef]
- Scheffer, H.J.; Stam, A.G.M.; Geboers, B.; Vroomen, L.G.P.H.; Ruarus, A.; De Bruijn, B.; Van Den Tol, M.P.; Kazemier, G.; Meijerink, M.R.; de Gruijl, T.D. Irreversible electroporation of locally advanced pancreatic cancer transiently alleviates immune suppression and creates a window for antitumor T cell activation. Oncoimmunology 2019, 8, 1652532. [Google Scholar] [CrossRef] [PubMed]
- Perminaite, E.; Zinkevičienė, A. Antitumor Response and Immunomodulatory Effects of Sub-Microsecond Irreversible Electroporation and Its Combination with Calcium Electroporation. Cancers 2019, 11, 1–18. [Google Scholar]
- Beitel-White, N.; Martin, R.C.G.; Li, Y.; Brock, R.M.; Allen, I.C.; Davalos, R. V Real-time prediction of patient immune cell modulation during irreversible electroporation therapy. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, J.S.S.; Ray, P.; Hayashi, T.; Whisenant, T.C. Irreversible Electroporation Combined with Checkpoint Blockade and TLR7 Stimulation Induces Anti-Tumor Immunity in a Murine Pancreatic Cancer Model. Cancer Immunol. Res. 2019, 7, 1714–1726. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Lin, X. T-cell activation and immune memory enhancement induced by irreversible electroporation in pancreatic cancer. Clin. Transl. Med. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, K.; Li, W.; Qiu, X.; Ma, B.; Fan, Q.; Li, Z. Immunologic Response to Tumor Ablation with Irreversible Electroporation. PLoS ONE 2012, 7, e48749. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wen, X.; Tian, L.; Li, T.; Xu, C.; Wen, X.; Melancon, M.P.; Gupta, S.; Shen, B.; Peng, W.; et al. Irreversible electroporation reverses resistance to immune checkpoint blockade in pancreatic cancer. Nat. Commun. 2019, 10, 899. [Google Scholar] [CrossRef]
- White, S.B.; Zhang, Z.; Chen, J.; Gogineni, V.R.; Larson, A.C. Early Immunologic Response of Irreversible Electroporation versus Cryoablation in a Rodent Model of Pancreatic Cancer. J. Vasc. Interv. Radiol. 2018, 29, 1–6. [Google Scholar] [CrossRef]
- Ii, R.E.N.; Rossmeisl, J.H., Jr.; Robertson, J.L.; Arena, C.B.; Davis, E.M.; Singh, R.N.; Stallings, J.; Davalos, R.V. Improved Local and Systemic Anti-Tumor Efficacy for Irreversible Electroporation in Immunocompetent versus Immunodeficient Mice. PLoS ONE 2013, 8, e64559. [Google Scholar]
- Palucka, A.K.; Coussens, L.M. Review The Basis of Oncoimmunology. Cell 2016, 164, 1233–1247. [Google Scholar] [CrossRef]
- Mir, L.M.; Orlowski, S.; Belehradek, J., Jr.; Paoletti, C. Electrochemotherapy Potentiation of Antitumour Effect of Bleomycin by Local Electric Pulses. Eur. J. Cancer 1991, 27, 68–72. [Google Scholar] [CrossRef]
- Miklavčič, D.; Mali, B.; Kos, B.; Heller, R.; Serša, G. Electrochemotherapy: From the drawing board into medical practice. Biomed. Eng. Online 2014, 13, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, S.K.; Vissing, M.; Gehl, J. A Comprehensive Review of Calcium Electroporation—A Novel Cancer Treatment Modality. Cancers (Basel) 2020, 12, 290. [Google Scholar] [CrossRef] [PubMed]
- Mir, L.M. Therapeutic perspectives of in vivo cell electropermeabilization. Bioelectrochemistry 2000, 53, 1–10. [Google Scholar] [CrossRef]
- Calvet, C.Y.; Famin, D.; André, F.M.; Mir, L.M. Electrochemotherapy with bleomycin induces hallmarks of immunogenic cell death in murine colon cancer cells. Oncoimmunology 2014, 3, e28131. [Google Scholar] [CrossRef]
- Sersa, G.; Teissie, J.; Cemazar, M.; Signori, E.; Kamensek, U.; Marshall, G.; Miklavcic, D. Electrochemotherapy of tumors as in situ vaccination boosted by immunogene electrotransfer. Cancer Immunol. Immunother. 2015, 64, 1315–1327. [Google Scholar] [CrossRef]
- Probst, U.; Fuhrmann, I.; Beyer, L.; Wiggermann, P. Electrochemotherapy as a new modality in interventional oncology: A review. Technol. Cancer Res. Treat. 2018, 17, 153303381878532. [Google Scholar] [CrossRef]
- Bellard, E.; Markelc, B.; Pelofy, S.; Le, F.; Sersa, G.; Teissié, J.; Cemazar, M.; Golzio, M. Intravital microscopy at the single vessel level brings new insights of vascular modification mechanisms induced by electropermeabilization. J. Control. Release 2012, 163, 396–403. [Google Scholar] [CrossRef]
- Markelc, B.; Sersa, G.; Cemazar, M. Differential Mechanisms Associated with Vascular Disrupting Action of Electrochemotherapy: Intravital Microscopy on the Level of Single Normal and Tumor Blood Vessels. PLoS ONE 2013, 8, e59557. [Google Scholar] [CrossRef]
- Barve, A.; Jin, W.; Cheng, K. Prostate cancer relevant antigens and enzymes for targeted drug delivery. J. Control. Release 2014, 187, 118–132. [Google Scholar] [CrossRef]
- Vera-donoso, D.; Mar, J.; Rivero-buceta, E.; Vidaurre-agut, C.; Moreno-manzano, V.; Botella, P. PSMA-Targeted Mesoporous Silica Nanoparticles for Selective Intracellular Delivery of Docetaxel in Prostate Cancer Cells. ACS Omega 2019, 4, 1281–1291. [Google Scholar]
- Fang, Y.; Wu, J.; Li, T.; Liu, W.; Gao, L.; Luo, Y. Nanoparticle mediated chemotherapy of hormone refractory prostate cancer with a novel combi-molecule. Am. J. Transl. Res. 2015, 7, 1440–1449. [Google Scholar] [PubMed]
- Ueki, T.; Uemura, H.; Nagashima, Y.; Ohta, S.; Ishiguro, H.; Kubota, Y. Antitumour effect of electrochemotherapy with bleomycin on human prostate cancer xenograft. BJUI 2008, 102, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, R.J.; Oosterlinck, W.; Holmang, S.; Sydes, M.R.; Birtle, A.; Gudjonsson, S.; De Nunzio, C.; Okamura, K.; Kaasinen, E.; Oddens, J.R.; et al. Systematic Review and Individual Patient Data Meta-analysis of Randomized Trials Comparing a Single Immediate Instillation of Chemotherapy After Transurethral Resection with Transurethral Resection Alone in Patients with Stage pTa–pT1 Urothelial Carcino. Eur. Urol. 2016, 69, 231–244. [Google Scholar] [CrossRef]
- Lerner, S.P.; Tangen, C.M.; Sucharew, H.; Wood, D.; Crawford, E.D. Failure to achieve a complete response to induction BCG therapy is associated with increased risk of disease worsening and death in patients with high risk non-muscle invasive bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2009, 27, 155–159. [Google Scholar] [CrossRef]
- Wirth, M.; Plattner, V.; Gabor, F. Strategies to improve drug delivery in bladder cancer therapy. Expert Opin. Drug Deliv. 2015, 6, 727–744. [Google Scholar] [CrossRef]
- Qiu, H.; Guo, H.; Li, D.; Hou, Y.; Kuang, T.; Ding, J. Intravesical Hydrogels as Drug Reservoirs. Trends Biotechnol. 2019, 38, 1–4. [Google Scholar] [CrossRef]
- Guo, H.; Li, F.; Xu, W.; Chen, J.; Hou, Y.; Wang, C.; Ding, J.; Chen, X. Mucoadhesive Cationic Polypeptide Nanogel with Enhanced Penetration for Efficient Intravesical Chemotherapy of Bladder Cancer. Adv. Sci. 2018, 5, 1–10. [Google Scholar] [CrossRef]
- Tomlinson, B.; Lin, T.; Era, M.D.; Pan, C.; Comprehensive, D. Nanotechnology in bladder cancer: Current state of development and clinical practice. Nanomedicine 2015, 10, 1189–1201. [Google Scholar] [CrossRef]
- Lugnani, F.; Mazza, G.; Cerulli, N.; Rossi, W.; Stephen, R. Iontophoresis of Drugs in the Bladder Wall: Equipment and Preliminary Studies. Artif. Organs 1993, 17, 8–17. [Google Scholar] [CrossRef]
- Colombo, R.; Lev, A.; Da Pozzo, L.F.; Freschi, M.; Gallus, G.; Rigatti, P. A new approach using local combined microwave hepertermia and chemotherapy in superficial transitional bladder carcinoma treatment. J. Urol. 1995, 153, 959–963. [Google Scholar] [CrossRef]
- Arends, T.J.H.; Nativ, O.; Maffezzini, M.; De Cobelli, O.; Canepa, G.; Verweij, F.; Moskovitz, B.; Van Der Heijden, A.G.; Witjes, J.A.; Boorjian, S. Results of a Randomised Controlled Trial Comparing Intravesical Chemohyperthermia with Mitomycin C Versus Bacillus Calmette-Guerin for Adjuvant Treatment of Patients with Intermediate- and High-risk Non—Muscle-invasive Bladder Cancer. Eur. Urol. 2016, 96, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Arends, T.J.H.; Van Der Heijden, A.G.; Urology, A.; Witjes, J.A. Combined chemohyperthermia: The 10-years monocentric experience in 160 non-muscle invasive bladder cancer patients. J. Urol. 2014, 192, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Di Stasi, S.M.; Giannantoni, A.; Giurioli, A.; Valenti, M.; Zampa, G.; Storti, L.; Attisani, F.; De Carolis, A.; Capelli, G.; Vespasiani, G.; et al. Sequential BCG and electromotive mitomycin versus BCG alone for high-risk superficial bladder cancer: A randomised controlled trial. Lancet Oncol. 2006, 7, 43–51. [Google Scholar] [CrossRef]
- Kubota, Y.; Mir, L.M.; Nakada, T.; Sasagawa, I.; Suzuki, H.; Aoyama, N. Successful treatment of metastatic skin lesions with electrochemotherapy. J. Urol. 1998, 160, 1426. [Google Scholar] [CrossRef]
- Ogihara, M.; Yamaguchi, O. Potentiation of effects of anticancer agents by local electric pulses in murine bladder cancer. Urol. Res. 2000, 28, 391–397. [Google Scholar] [CrossRef]
- Vásquez, J.L.; Gehl, J.; Hermann, G.G. Electroporation enhances mitomycin C cytotoxicity on T24 bladder cancer cell line: A potential improvement of intravesical chemotherapy in bladder cancer. Bioelectrochemistry 2012, 88, 127–133. [Google Scholar] [CrossRef]
- Kubota, L.E.Y.; Nakada, T.; Yanai, H.; Kakizaki, H.; Sasagawa, I.; Watanabe, M. Electropermeabilization in bladder cancer chemotherapy. Cancer Chemother. Pharmacol. 1996, 39, 67–70. [Google Scholar] [CrossRef]
- Hansen, E.L.; Sozer, E.B.; Romeo, S.; Frandsen, S.K.; Vernier, P.T.; Gehl, J. Dose-Dependent ATP depletion and cancer cell death following calcium electroporation, relative effect of calcium concentration and electric field strength. PLoS ONE 2015, 10, e0122973. [Google Scholar]
- Frandsen, S.K.; Gehl, J. Effect of calcium electroporation in combination with metformin in vivo and correlation between viability and intracellular ATP level after calcium electroporation in vitro. PLoS ONE 2017, 12, e0181839. [Google Scholar] [CrossRef]
- Vasquez, J.L.; Ibsen, P.; Lindberg, H.; Gehl, J. In Vitro and In Vivo Experiments on Electrochemotherapy for Bladder Cancer. J. Urol. 2015, 193, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Herr, H.W.; Dalbagni, G. Defining Bacillus Calmette-Guerin refractory superficial bladder tumors. J. Urol. 2003, 169, 1706–1708. [Google Scholar] [CrossRef] [PubMed]
- Rosazza, C.; Meglic, S.H.; Zumbusch, A.; Rols, M.-P.; Miklavcic, D. Gene Electrotransfer: A Mechanistic Perspective. Curr. Gene Ther. 2016, 16, 98–129. [Google Scholar] [CrossRef] [PubMed]
- Bodles-Brakhop, A.M.; Heller, R.; Draghia-Akli, R. Electroporation for the delivery of DNA-based vaccines and immunotherapeutics: Current clinical developments. Mol. Ther. 2009, 17, 585–592. [Google Scholar] [CrossRef]
- Titomirov, A.V.; Sukharev, S.; Kistanova, E. In vivo electroporation and stable transformation of skin cells of newborn mice by plasmid DNA. BBA Gene Struct. Expr. 1991, 1088, 131–134. [Google Scholar] [CrossRef]
- Aihara, H.; Miyazaki, J.I. Gene transfer into muscle by electroporation in vivo. Nat. Biotechnol. 1998, 16, 867–870. [Google Scholar] [CrossRef]
- Suzuki, T.; Shin, B.C.; Fujikura, K.; Matsuzaki, T.; Takata, K. Direct gene transfer into rat liver cells by in vivo electroporation. FEBS Lett. 1998, 425, 436–440. [Google Scholar] [CrossRef]
- Nishi, T.; Yoshizato, K.; Yamashiro, S.; Takeshima, H.; Sato, K.; Hamada, K.; Kitamura, I.; Yoshimura, T.; Saya, H.; Kuratsu, J.; et al. High-Efficiency in Vivo Gene Transfer Using Intraarterial Plasmid DNA Injection following in Vivo Electroporation. Cancer Res. 1996, 56, 1050–1055. [Google Scholar]
- Heller, R.; Jaroszeski, M.; Atkin, A.; Moradpour, D.; Gilbert, R.; Wands, J.; Nicolau, C. In vivo gene electroinjection and expression in rat liver. FEBS Lett. 1996, 389, 225–228. [Google Scholar] [CrossRef]
- Daud, A.I.; DeConti, R.C.; Andrews, S.; Urbas, P.; Riker, A.I.; Sondak, V.K.; Munster, P.N.; Sullivan, D.M.; Ugen, K.E.; Messina, J.L.; et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J. Clin. Oncol. 2008, 26, 5896–5903. [Google Scholar] [CrossRef]
- Faurie, C.; Rebersek, M.; Golzio, M.; Kanduser, M.; Escoffre, J.-M.; Pavlin, M.; Teissie, J.; Miklavcic, D.; Rols, M.-P. 1CNRS Electro-mediated gene transfer and expression are controlled by the life-time of DNA/membrane complex formation. J. Gene Med. 2010, 12, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Henshaw, J.W.; Zaharoff, D.A.; Mossop, B.J.; Yuan, F. Short communication A single molecule detection method for understanding mechanisms of electric field-mediated interstitial transport of genes. Bioelectrochemistry 2006, 69, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Jackson, D.L.; Burcus, N.I.; Chen, Y.J.; Xiao, S.; Heller, R. Gene electrotransfer enhanced by nanosecond pulsed electric fields. Mol. Ther. Methods Clin. Dev. 2014, 1, 14043. [Google Scholar] [CrossRef] [PubMed]
- Markelc, B.; Skvarca, E.; Dolinsek, T.; Prevodnik, V.; Coer, A.; Sersa, G.; Cemazar, M. Inhibitor of endocytosis impairs gene electrotransfer to mouse muscle in vivo. Bioelectrochemistry 2015, 103, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Rosazza, C.; Deschout, H.; Buntz, A.; Braeckmans, K.; Rols, M.; Zumbusch, A. Endocytosis and Endosomal Trafficking of DNA After Gene Electrotransfer In Vitro. Mol. Ther. Acids 2016, 5, 1–11. [Google Scholar] [CrossRef]
- Sachdev, S.; Moreira, S.F.; Keehnen, Y.; Rems, L.; Kreutzer, M.T.; Boukany, P.E. DNA-membrane complex formation during electroporation is DNA size- dependent. BBA Biomembr. 2020, 1862, 183089. [Google Scholar] [CrossRef]
- Rosazza, C.; Escoffre, J.; Zumbusch, A.; Rols, M. The Actin Cytoskeleton Has an Active Role in the Electrotransfer of Plasmid DNA in Mammalian Cells. Mol. Ther. 2011, 19, 913–921. [Google Scholar] [CrossRef]
- Lechardeur, D.; Lukacs, G.L. Nucleocytoplasmic Transport of Plasmid DNA: A Perilous Journey from the Cytoplasm to the Nucleus. Hum. Gene Ther. 2006, 17, 882–889. [Google Scholar] [CrossRef]
- Roos, A.; Eriksson, F.; Timmons, J.A.; Gerhardt, J.; Nyman, U.; Wahren, B.; Pisa, P.; Bra, A. Skin Electroporation: Effects on Transgene Expression, DNA Persistence and Local Tissue Environment. PLoS ONE 2009, 4, e7226. [Google Scholar] [CrossRef]
- Zampaglione, I.; Simon, A.J.; Capone, S.; Finnefrock, A.C.; Casimiro, D.R.; Kath, G.S.; Tang, A.; Folgori, A.; La Monica, N.; Shiver, J.; et al. Genetic vaccination by gene electro-transfer in non-human primates. J. Drug Deliv. Sci. Technol. 2006, 16, 85–89. [Google Scholar] [CrossRef]
- Pasquet, L.; Chabot, S.; Bellard, E.; Markelc, B.; Rols, M.; Reynes, J.; Tiraby, G.; Couillaud, F.; Teissie, J.; Golzio, M. Safe and efficient novel approach for non-invasive gene electrotransfer to skin. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gibot, L.; Golberg, A. Electroporation in Scars/Wound Healing and Skin Response. In Handbook of Electroporation; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 1–18. [Google Scholar]
- Byrnes, C.K.; Malone, R.W.; Akhter, N.; Nass, P.H.; Wetterwald, A.; Cecchini, M.G.; Duncan, M.D.; Harmon, J.W. Electroporation enhances transfection efficiency in murine cutaneous wounds. Wound Repair. Regen. 2004, 12, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, B.; Cruz, Y.L.; Baldwin, M.; Coppola, D.; Heller, R. Increased perfusion and angiogenesis in a hindlimb ischemia model with plasmid FGF-2 delivered by noninvasive electroporation. Gene Ther. 2010, 17, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Mir, L.M.; Moller, P.H.; Franck, A.; Gehl, J. Electric Pulse-Mediated Gene Delivery to Various Animal Tissues. Adv. Genet. 2005, 54, 84–114. [Google Scholar]
- Bettan, M.; Ivanov, M.A.; Mir, L.M.; Boissière, F.; Delaere, P.; Scherman, D. Efficient DNA electrotransfer into tumors. Bioelectrochem. Bioenerg. 2000, 52, 83–90. [Google Scholar] [CrossRef]
- Sieni, E.; Dettin, M.; De Robertis, M.; Bazzolo, B.; Conconi, M.T.; Zamuner, A.; Marino, R.; Keller, F.; Campana, L.G.; Signori, E. The Efficiency of Gene Electrotransfer in Breast-Cancer Cell Lines Cultured on a Novel Collagen-Free 3D Scaffold. Cancers 2020, 12, 1043. [Google Scholar] [CrossRef]
- Znidar, K.; Bosnjak, M.; Semenova, N.; Pakhomova, O.; Heller, L.; Cemazar, M. Tumor cell death after electrotransfer of plasmid DNA is associated with cytosolic DNA sensor upregulation. Oncotarget 2018, 9, 18665–18681. [Google Scholar] [CrossRef][Green Version]
- Sedlar, A.; Dolinsek, T.; Markelc, B.; Prosen, L.; Kranjc, S.; Bosnjak, M.; Blagus, T.; Cemazar, M.; Sersa, G. Potentiation of electrochemotherapy by intramuscular IL-12 gene electrotransfer in murine sarcoma and carcinoma with different immunogenicity. Radiol. Oncol. 2012, 46, 302–311. [Google Scholar] [CrossRef]
- Heller, R.; Heller, L.C. Gene Electrotransfer Clinical Trials. Adv. Genet. 2015, 89, 235–262. [Google Scholar]
- Lucas, M.L.; Heller, L.; Coppola, D.; Heller, R. IL-12 plasmid delivery by in Vivo electroporation for the successful treatment of established subcutaneous B16.F10 melanoma. Mol. Ther. 2002, 5, 668–675. [Google Scholar] [CrossRef]
- Wang, X.; Rivière, I. Clinical manufacturing of CAR T cells: Foundation of a promising therapy. Mol. Ther. Oncolytics 2016, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zuckermann, M.; Hovestadt, V.; Knobbe-thomsen, C.B.; Zapatka, M.; Northcott, P.A.; Schramm, K.; Belic, J.; Jones, D.T.W.; Tschida, B.; Moriarity, B.; et al. Somatic CRISPR/Cas9-mediated tumour suppressor disruption enables versatile brain tumour modelling. Nat. Commun. 2015, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lambricht, L.; Lopes, A.; Kos, S.; Sersa, G.; Vandermeulen, G. Clinical potential of electroporation for gene therapy and DNA vaccine delivery. Expert Opin. Drug Deliv. 2015, 5247, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Choo, A.Y.; Shedlock, D.J.; Muthumani, K. Electroporation of cytokines for cancer gene therapy. Cancer Biol. Ther. 2009, 8, 2123–2125. [Google Scholar] [CrossRef][Green Version]
- Heller, R.; Lundberg, C.M.; Burcus, N.; Edelblute, C.; Guo, S. Gene electrotransfer of plasmids encoding cytokines as an effective immunotherapy approach for melanoma. J. Immunol. 2016, 196, 213–216. [Google Scholar]
- Shi, G.; Edelblute, C.; Arpag, S.; Lundberg, C.; Heller, R. IL-12 Gene Electrotransfer Triggers a Change in Immune Response within Mouse Tumors. Cancers 2018, 10, 498. [Google Scholar] [CrossRef]
- Verrax, J.; Defresne, F.; Lair, F.; Vandermeulen, G.; Rath, G.; Dessy, C.; Préat, V.; Feron, O. Delivery of soluble VEGF receptor 1 (sFlt1) by gene electrotransfer as a new antiangiogenic cancer therapy. Mol. Pharm. 2011, 8, 701–708. [Google Scholar] [CrossRef]
- Kiessling, R.; Pisa, P.; Miller, A.M.; Volkan, O. Immune Monitoring in a Phase 1 Trial of a PSA DNA Vaccine in Patients with Hormone-Refractory Prostate Cancer. J. Immunother. 2005, 28, 389–395. [Google Scholar]
- Roos, A.; Moreno, S.; Leder, C.; Pavlenko, M.; King, A.; Pisa, P. Enhancement of Cellular Immune Response to a Prostate Cancer DNA Vaccine by Intradermal Electroporation. Mol. Ther. 2006, 13, 320–327. [Google Scholar] [CrossRef]
- Ahmad, S.; Casey, G.; Sweeney, P.; Tangney, M.; Sullivan, G.C.O. Optimised electroporation mediated DNA vaccination for treatment of prostate cancer. Genet. Vaccines Ther. 2010, 8, 1–13. [Google Scholar] [CrossRef]
- Ahmad, S.; Casey, G.; Sweeney, P.; Tangney, M.; Sullivan, G.C.O. Prostate Stem Cell Antigen DNA Vaccination Breaks Tolerance to Self-antigen and Inhibits Prostate Cancer Growth. Mol. Ther. 2009, 17, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Mikata, K.; Uemura, H.; Ohuchi, H.; Ohta, S.; Nagashima, Y.; Kubota, Y. Inhibition of Growth of Human Prostate Cancer Xenograft by Transfection of p53 Gene: Gene Transfer by Electroporation. Mol. Cancer Ther. 2002, 1, 247–252. [Google Scholar] [PubMed]
- Harimoto, K.; Sugimura, K.; Lee, C.R.; Kuratsukuri, K.; Kishimoto, T. In vivo gene transfer methods in the bladder without viral vectors. Br. J. Urol. 1998, 81, 870–874. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-S.Y.; Lee, C.-F.; Hsieh, D.-S.; Chang, S.-Y. Antitumor Effects of Recombinant BCG and Interleukin-12 DNA Vaccines on Xentografted Murine Bladder Cancer. Urology 2004, 63, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Chang, S.; Hsieh, D.; Yu, D. Immunotherapy for Bladder Cancer using Recombinant Bacillus Calmette-Guerin DNA Vacciner and Interleukin-12 DNA Vaccine. J. Urol. 2004, 171, 1343–1347. [Google Scholar] [CrossRef]
- Lee, C.; Chang, S.; Hsieh, D.; Yu, D. Treatment of bladder carcinomas using recombinant BCG DNA vaccines and electroporative gene immunotherapy. Cancer Gene Ther. 2004, 11, 194–207. [Google Scholar] [CrossRef][Green Version]
- Matsubara, H.; Mizutani, Y.; Hongo, F.; Nakanishi, H.; Kimura, Y.; Ushijima, S.; Kawauchi, A.; Tamura, T.; Sakata, T.; Miki, T. Gene therapy with TRAIL against renal cell carcinoma. Mol. Cancer Ther. 2006, 5, 2165–2172. [Google Scholar] [CrossRef]
- Tamura, T.; Nishi, T.; Goto, T.; Takeshima, H.; Dev, S.B. Intratumoral Delivery of Interleukin 12 Expression Plasmids with In Vivo Electroporation Is Effective for Colon and Renal Cancer. Hum. Gene Ther. 2001, 12, 1265–1276. [Google Scholar] [CrossRef]
- Wiesinger, M.; März, J.; Kummer, M.; Schuler, G.; Dörrie, J. Clinical-Scale Production of CAR-T Cells for the Treatment of Melanoma Patients by mRNA Transfection of a CSPG4-Specific CAR under Full GMP Compliance. Cancers (Basel) 2019, 11, 1198. [Google Scholar] [CrossRef]
- Ingegnere, T.; Mariotti, F.R.; Pelosi, A.; Quintarelli, C. Human CAR NK Cells: A New Non-viral Method Allowing High Efficient Transfection and Strong Tumor Cell Killing. Front. Immunol. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Schmidt-wolf, I.G.H.; Finke, S.; Trojaneck, B.; Denkena, A.; Lefterova, P.; Schwella, N.; Heuft, H.; Prange, G.; Korte, M. Phase I clinical study applying autologous immunological effector cells transfected with the interleukin-2 gene in patients with metastatic renal cancer, colorectal cancer and lymphoma. Br. J. Cancer 1999, 81, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, A.; Arndt, C.; Bergmann, R.; Loff, S.; Cartellieri, M.; Bachmann, D.; Aliperta, R.; Hetzenecker, M.; Ludwig, F.; Albert, S.; et al. Retargeting of T lymphocytes to PSCA- or PSMA positive prostate cancer cells using the novel modular chimeric antigen receptor platform technology “UniCAR”. Oncotarget 2017, 8, 31368–31385. [Google Scholar] [CrossRef] [PubMed]
- Schepisi, G.; Cursano, M.C.; Casadei, C.; Menna, C.; Altavilla, A.; Lolli, C.; Cerchione, C.; Paganelli, G.; Santini, D.; Tonini, G.; et al. CAR-T cell therapy: A potential new strategy against prostate cancer. J. Immunother. Cancer 2019, 7, 258. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.S.; Matsushita, M.; Plotkin, J.; Riviere, I.; Sadelain, M. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI 3 kinase/AKT/Bcl-X L activation and CD8 T cell-mediated tumor eradication. Mol. Ther. 2010, 18, 413–420. [Google Scholar] [CrossRef]
- Zhang, Q.; Tian, K.; Xu, J.; Zhang, H.; Li, L.; Fu, Q.; Chai, D.; Li, H.; Zheng, J. Synergistic Effects of Cabozantinib and EGFR-Specific CAR-NK-92 Cells in Renal Cell Carcinoma. J. Immunol. Res. 2017, 2017, 6915912. [Google Scholar] [CrossRef]
- Prud’homme, G.J.; Glinka, Y.; Khan, A.S.; Draghia-akli, R. Electroporation-Enhanced Nonviral Gene Transfer for the Prevention or Treatment of Immunological, Endocrine and Neoplastic Diseases. Curr. Gene Ther. 2006, 6, 243–273. [Google Scholar] [CrossRef]
- Mahendran, R.; Mun, S.; Esuvaranath, K. Gene Therapy in Urology. In Gene Therapy Applications; InTech: London, UK, 2011. [Google Scholar]

| Type of Trial | Patients Number | Short Description | Study Outcome | Renal Function | Complications | Therapy Protocol | Ref. |
|---|---|---|---|---|---|---|---|
| Retrospective | 20 | IRE for focal treatment of cT1a renal cancer | initial treatment success rate of 90% (18/20). 17% (1/6) recurrence rate during a one-year follow-up | no significant differences in creatinine levels before and 6 weeks after IRE | 3/20 urinary retention, 2/20 perinephric hematomas, 2/20 patients suffered from pain | 3–4 electrodes, for tumors over 2.5 cm, multiple probes, 30–40 A, pulse duration 100 µs at 1 Hz, 140 pulses with electrode polarity change after 70 pulses | Trimmer et al. [65] |
| Prospective | 42 | IRE for focal treatment of cT1a renal cancer | initial treatment success rate of 93% (39/42), 83% 2-year local recurrence-free survival rate | pre and post-operative glomerular filtration rate of patients did not differ significantly | 2/42 urinary retention, 4/42 perinephric hematomas, 1/42 patient suffered from pain | 3–4 electrodes, for tumors over 2.5 cm, multiple probes 2000 V/cm, 100 µs at 1 Hz, 140 pulses with electrode polarity change after 70 pulses | Canvasser et al. [66] |
| Prospective | 10 | IRE for focal treatment of cT1a renal cancer | 10% (1/10) recurrence rate during 6 months of follow-up | no significant differences in creatinine levels in pre-IRE tests and 1 week, 3 months, 6 months, and 12 months post-IRE tests | 1/10 perinephric hematoma and pyelonephritis with fever, 1/10 blood clot in the ureter, 1/10 painful micturition, 1/10 hematuria | 6 electrodes, active tip exposure 10–25 mm, 20–40 A, 100 pulses, 90 µs | Mara et al. [64] |
| Therapy | Type of Trial | Patients Number | Short Description | Urinary Continence | Erectile Function | Study Outcome | Therapy Protocol | Ref. |
|---|---|---|---|---|---|---|---|---|
| IRE | Retrospective | 429 | IRE for focally (123), sub-whole-gland (154), whole-gland (134) or for recurrent prostate cancer (63) after previous radical prostatectomy or radiation therapy, | IPSS-Score (urinary symptoms)- 72.8% of patients with no change or improvement; 27.2% with a decrease during the follow-up period (about 12 months) | IIEF-5 score–14/124 (11.3%) patients developed erectile dysfunction after IRE in 4/124 (3%) patients dysfunction persisted longer than one year | 47/429 (10.9%) of patients with prostate cancer recurrence in 72 months follow-up (27/429 in-field and 20/429 out-of-field recurrence) | 5 ± 1 electrodes; 1518.13 ± 204.05 V/cm | Guenther et al. [73] |
| IRE | Prospective | 123 | IRE for prostate gland ablation as a primary procedure; largest reported cohort of patients treated with IRE. | 80/81 (98.8%) patients remained pad free and 70/75 (93.3%) remained leak-free at 12 months after treatment. | 40/53 (76%) patients with normal erectile function, 9/53 (17%) with decreased, but enough for sexual activity, and 4/53 (7%) with total erectile dysfunction 12 months after treatment | 23/102 (22.5%) of patients with prostate cancer recurrence 12 months after treatment (10/102 (9.8%) in-field recurrence. 13/102 (12.7%) out-of-field recurrence) | 90 pulses; 1500 V/cm; 70 µs or 90 µs; 5 mm distance from vital structures; safety margin of 5 mm to 10 mm from the targeted area; | Blazevski et al. [74] |
| IRE (H-FIRE) | Prospective | 40 | H-FIRE for prostate gland ablation, as a primary procedure 8/40 patients underwent prostatectomy four weeks after treatment | 40/40 (100%) patients remained pad free | 14/14 (100%) patients with normal erectile function | high-frequency bipolar pulses can be safely applied for IRE of prostate cancer; oncological outcome was not evaluated | HF bipolar pulses; 250 bursts; 50 pulses in one burst; 1 burst/second; 10-s inter-burst delay; 2–6 needle electrodes; interelectrode distance <20 mm | Dong et al. [75] |
| IRE | Prospective | 18 | IRE for localized radio-recurrent prostate cancer, | 8/11 (72.2%) of patients remained pad-free at six months; 10/11 (90.9%) pad-free at 12 months | 2/6 (33.3%) patients with normal erectile function 6 months after treatment and 2/4 (50%) patients with normal erectile function 12 months after treatment (One patient recovered at 12 months from erectile dysfunction); a significant decrease in EPIC sexual score between baseline and six months | 2/10 (20%) of patients with prostate cancer recurrence 12 months after treatment (1/10 (10%) in-field recurrence and 1/10 (10%) out-of-field recurrence) | 90 pulses; 1500 V/cm; pulse; 70 µs or 90 µs; safety margin of 5 mm to 10 mm from the targeted area; active tip length 10-20 mm; interelectrode distance 7-19 mm | Scheltema et al. [76] |
| IRE | Prospective | 63 | IRE for prostate gland ablation, as a primary procedure | 44/45 (98%) of patients remained pad-free at 6 months 45/45 (100%) pad-free at 12 months. | 8/26 (31%) patients with erectile dysfunction at 6 months after IRE and 3/13 (23%) with erectile dysfunction 12 months after IRE.; the significant difference in EPIC sexual score between baseline and 6 months after IRE | 11/48 (22.9%) of patients with prostate cancer recurrence 12 months after treatment (7/48 (14.6%) in-field recurrence and 4/48 (8.3%) out-of-field recurrence) | 90 pulses; 1500 V/cm; 70 µs or 90 µs; safety margins of 5 mm or 10 mm from the targeted area; 5 mm distance from vital structures | Van Den Bos et al. [77] |
| ECT | Case report | 1 | ECT for recurrence of prostate cancer by a 67-year-old patient | IPSS—no significant impairment after six months (remaining mild incontinence) | IIEF-5 score restored to the pretreatment level six months after ECT (remaining mild erectile dysfunction) | MRI six months after the treatment, showed no evidence of tumor persistence | 8 min before electroporation administration of 29 mg of bleomycin i.v.; eight pulses; 100 µs; 4 Hz frequency; 1642 ± 812 V | Klein et al. [78] |
| GET | Prospective, two-arm | 30 | Patients with, biochemically recurrent prostate cancer (rising PSA but no radiological evidence of disease) received an intramuscular injection of DNA encoding PSMA+DOM C or injection followed by EP | Not evaluated | Not evaluated | Vaccination alone and with EP was well tolerated; vaccination alone showed 1.7 increase of anti-DOM IgG; vaccination with EP showed a 24.5-fold increase of anti-DOM IgG compering to baseline; responses persisted up to 18 months of follow-up | Vaccinations were administered at 0, 4, and 8 weeks, with booster doses at 24 and 48 weeks; EP was performed by two needles, which after injection served as electrodes; five pulses; 20 µs; 8.3 Hz frequency; 8 mm intra-needle distance | Low et al. [79] |
| GET | Prospective | 15 | Patients with biochemically recurrent prostate cancer without macroscopic disease received an intradermal injection of DNA encoding PSA followed by EP | Not evaluated | Not evaluated | Intradermal vaccination with skin EP is feasible; only minor discomfort occurred; vaccination with EP enhanced pre-existing or boosted PSA-specific immunity | Vaccination was administrated every four weeks for five months; EP was applied immediately after each injection with two parallel rows of needles (6 needles/row); 10 pulses: 2 (1125 V/cm; 0.05 µs) and 8 (275 V/cm; 10 µs) | Eriksson et al. [80] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiełbik, A.; Szlasa, W.; Saczko, J.; Kulbacka, J. Electroporation-Based Treatments in Urology. Cancers 2020, 12, 2208. https://doi.org/10.3390/cancers12082208
Kiełbik A, Szlasa W, Saczko J, Kulbacka J. Electroporation-Based Treatments in Urology. Cancers. 2020; 12(8):2208. https://doi.org/10.3390/cancers12082208
Chicago/Turabian StyleKiełbik, Aleksander, Wojciech Szlasa, Jolanta Saczko, and Julita Kulbacka. 2020. "Electroporation-Based Treatments in Urology" Cancers 12, no. 8: 2208. https://doi.org/10.3390/cancers12082208
APA StyleKiełbik, A., Szlasa, W., Saczko, J., & Kulbacka, J. (2020). Electroporation-Based Treatments in Urology. Cancers, 12(8), 2208. https://doi.org/10.3390/cancers12082208







