Abstract
The myeloproliferative neoplasms (MPNs) are acquired hematological stem cell neoplasms characterized by driver mutations in JAK2, CALR, or MPL. Additive mutations may appear in predominantly epigenetic regulator, RNA splicing and signaling pathway genes. These molecular mutations are a hallmark of diagnostic, prognostic, and therapeutic assessment in patients with MPNs. Over the past decade, next generation sequencing (NGS) has identified multiple somatic mutations in MPNs and has contributed substantially to our understanding of the disease pathogenesis highlighting the role of clonal evolution in disease progression. In addition, disease prognostication has expanded from encompassing only clinical decision making to include genomics in prognostic scoring systems. Taking into account the decreasing costs and increasing speed and availability of high throughput technologies, the integration of NGS into a diagnostic, prognostic and therapeutic pipeline is within reach. In this review, these aspects will be discussed highlighting their role regarding disease outcome and treatment modalities in patients with MPNs.
1. Introduction
The BCR-ABL negative myeloproliferative neoplasms (MPNs) essential thrombocythemia (ET), polycythemia vera (PV), and myelofibrosis (MF) including primary myelofibrosis (PMF) and secondary myelofibrosis (SMF) (post-ET MF/post-PV MF) are characterized by uncontrolled clonal proliferation of the hematopoietic stem and progenitor cells [1]. Patients with MPNs have a huge morbidity and co-morbidity burden due to a high risk of cardiovascular and cerebrovascular complications. Chronic inflammation in MPNs is associated with increased levels of circulating cytokines and reactive oxygen species leading to genetic instability favoring clonal evolution followed by premature atherosclerosis, increased risk of thrombohemorrhagic complications, leukemic transformation, and development of second cancer [2,3,4,5,6,7,8].
The somatic driver mutations JAK2V617F, CALR and MPL are included in the diagnostic criteria for MPNs and account for the majority of cases with the remaining cases termed triple negative [9,10,11,12,13,14,15,16,17,18]. JAK2V617F positive MPNs may develop in a biological continuum from the early cancer stages (ET, PV) to advanced MF over decades implying an increase in the JAK2V617F mutational load [9,19]. In addition to the three driver mutations, acquisition of additional mutations occurs frequently in patients with MPNs [20].
There is a spectrum of treatment options in MPNs primarily focusing on alleviating symptom burden, reducing thrombotic complications, and targeting the malignant clone [21,22,23,24,25,26,27,28,29,30,31,32,33]. Early treatment at the time of diagnosis, where patients have the least tumor burden, has been argued to be a prerequisite to prevent clonal evolution, subclone formation, and additional mutations [27,34,35,36,37].
In the past decade, there has been a tremendous increase in knowledge of the complex mutational landscape in MPNs, which has revolutionized research leading to an unprecedented change in diagnosis, prognosis, classification, treatment, outcome, and response evaluation [20,38]. In the present review, past and present advances in genomics are highlighted with focus on next generation sequencing (NGS) regarding disease progression, prediction of outcome, and treatment planning in patients with chronic phase MPNs.
2. Genomics in MPNs
2.1. NGS Analysis of Somatic Gene Mutations
The dominant gain of function mutation JAK2V617F is present in most patients with MPNs including approximately 98% of patients with PV and 50–60% of patients with ET or PMF [39,40]. The valine to phenylalanine substitution at position 617 of the JAK2 gene leads to constitutive activation of the JAK-STAT signaling pathway transforming hematopoietic cells to cytokine-independent growth, thereby promoting tumorigenesis, tumor progression, and inflammation [41,42]. The remaining 2% of PV patients carry somatic driver mutations in JAK2 exon 12 [43,44]. Another driver mutation, the thrombopoietin receptor gene MPL, is found in up to 5 or 10% of ET or PMF patients, respectively, with MPLW515L/K being the most common mutation [14,15,18,45,46,47]. As the JAK2V617F mutation, the MPL mutation confers constitutive, cytokine-independent activation of the JAK-STAT pathway [14].
Somatic driver mutations in CALR located in exon 9 resulting in a mutant protein with a novel C-terminal were revealed by exome sequencing in 2013 in one of the first NGS studies with mutation profiling of more than one patient with MPNs [12,13]. CALR mutations, of which a 52 bp deletion and a 5 bp insertion are the most prevalent, are found in up to 25 or 30% of patients with ET or PMF, respectively [12,13,48].
Non-driver somatic mutations implicated in the disease pathogenesis of MPNs belong to various functional classes. Mutations in the epigenetic regulator genes can be divided into DNA methylation (DNMT3A, IDH1, IDH2, TET2) and chromatin modifiers (ASXL1, BCOR, BCORL1, EZH2, KMT2A, KMT2C, KMT2D, SUZ12). In addition, mutations have been observed in genes related to RNA splicing (SF3B1, SRSF2, ZRSR2, U2AF1), signaling pathway genes (CBL, FLT3, GNAS, KIT, KRAS, NF1, NRAS, PTPN11, SH2B3), transcription factors (CEBPA, CUX1, ETV6, GATA1, GATA2, IKZF1, NFE2, NPM1, RUNX1, SETBP1), tumor suppressors (CDKN2A, NOTCH1, PHF6, RB1, TP53), DNA damage response pathway (ATM, PPM1D) or cohesin complex (STAG2) [49,50,51,52,53,54,55,56,57]. The most frequent non-driver mutations are found in the epigenetic regulator genes TET2, ASXL1, DNMT3A, and EZH2 and in the RNA splicing genes SRSF2 and U2AF1. Gene mutation frequencies obtained from NGS studies are listed in Table 1 and described for the most common mutations below.

Table 1.
The most common additional gene mutations in MPNs elaborated by NGS studies.
Mutations in TET2 occur in the entire coding sequence and result in loss of catalytic function leading to DNA hypermethylation [70,71,72]. TET2 mutations are often seen in all disease entities with 10–20% of ET, 15–30% of PV, 10–15% of PMF patients and most frequently in patients with SMF (20–40%) [58,59,60,62,63,64,66,73]. Nonsense and frameshift mutations in exon 13 are the most common mutations in ASXL1 resulting in loss of function and a truncated protein [74]. ASXL1 mutations occur in 5–10% of patients with ET or PV, 20–45% of patients with PMF and 10–25% of patients with SMF [58,59,60,62,63,64,65,67,68,75]. Although most mutations in DNMT3A are observed in exon 23, mutations occur in the entire coding region resulting in loss of catalytic activity and altered methylation patterns [71,72,76]. DNMT3A mutations are most frequent in patients with PMF (5–15%) and PV (3–15%), and less observed in ET (<10%) and SMF (<5%) [58,59,60,61,62,63,64,65,77]. EZH2 mutations are generally loss of function mutations that can be detected throughout the coding sequence and cause protein truncation [78,79]. EZH2 mutations are primarily seen in SMF (5–15%) and PMF (3–12%), and in less than 5% of patients with ET or PV [59,61,62,63,64,65,66,68]. Finally, U2AF1 mutations alter their 3′ splice acceptor preferences and SRSF2 mutations result in skewed mRNA motif recognition, both mutations leading to mis-splicing of several genes [80,81]. U2AF1 mutations are observed in 5–20% of patients with PMF or SMF and are only rarely seen in patients with ET or PV (<5%) [58,59,60,61,62,63,65,66,82,83]. Most mutations in SRSF2 are detected in PMF (10–35%) and in less than 5% of patients with ET, PV, or SMF [58,59,60,61,62,63,66,67,68,84]. The remaining additional mutations listed above occur with frequencies lower than approximately 10% in all disease entities [20,58,59,60,61,62,63,64,65,85].
2.2. Interaction of Somatic Gene Mutations Refined by NGS Analysis
A number of mutations co-occur frequently or are mutually exclusive in patients with MPNs. JAK2V617F are frequently associated with mutations in the epigenetic regulator genes, ASXL1 [63,64,67,86,87,88], DNMT3A [61,63,86], EZH2 [64,67,87,88,89], IDH2 [61,64,67,86], or TET2 [63,64,86,87,88] and to a lesser extent with mutations in CBL [86,89], CUX1 [88], IDH1 [67,86], NOTCH1 [61], NRAS [86,89], RUNX1 [88], SF3B1 [86,88,89], SETBP1 [88], SH2B3 [86], SRSF2 [67,89], TP53 [89], U2AF1 [60,89], or ZRSF2 [88,90]. Mutations in CALR are reported to co-occur with ASXL1 [67,83,88,91], DNMT3A [64,89], TET2 [75,77,89,91], or U2AF1 [83,90,91] and less with EZH2 [91], IDH2 [75,83], NRAS [91], RUNX1 [90], SF3B1 [91], SRSF2 [83,90], or TP53 [75,91]. Mutations in MPL are found associated with ASXL1 [67] or SRSF2 [60].
Mutations in epigenetic regulator genes result in deregulated gene expression, aberrant cell function and disease, and these genes are often co-mutated with each other or with mutations in other functional classes such as RNA splicing, signaling pathways or transcription factors. Focusing on non-driver somatic mutations, a number of NGS studies have shown a frequent co-existence of mutations in ASXL1 with EZH2 [20,59,61,62] or U2AF1 [13,20,60,62], and less often with DNMT3A [64], IDH2 [59], RUNX1, KIT [59], CBL [20,60], SRSF2, NRAS [20], or SETBP1 [60]. Although ASXL1 and EZH2 are related to the PRC2 complex, they are not mutually exclusive [92,93,94]. Another frequently mutated epigenetic regulator gene in MPNs, TET2 is not as frequently co-mutated as ASXL1 and is found associated with SRSF2 [13], SH2B3 [59], SUZ12, CBL, or PTPN11 [60]. Other NGS studies showed concomitant mutations in IDH1/2 with SRSF2 [13,60,89] or U2AF1 [89], U2AF1 with PTPN11 [60] or CBL [62], EZH2 with SETBP1 or CBL [62], and NRAS with SETBP1 or NF1 [62].
The two epigenetic regulator genes IDH1/2 and TET2 are almost never co-mutated but mutually exclusive and share the same functional mechanisms by altering the hypermethylation signature of hematopoietic cells. IDH enzymes normally produce α-ketoglutarate (αKG), however, cancer cells with mutated IDH1/2 produce 2-hydroxyglutarate (2-HG) which is detrimental to TET2, because TET2 uses αKG as a co-activator, resulting in TET2 having impaired functionality [95]. Importantly, mutations in IDH1/2 or TET2 are not equally distributed across disease entities in that IDH1/2 are more frequent in blast phase disease whereas TET2 occurs with equal frequency in chronic and blast phase disease [54].
Driver mutations are in most cases mutually exclusive, however, patients with two driver mutations have been observed. JAK2V617F mutations have been reported to co-exist with MPL mutations in all three disease entities [15,96,97,98] or with CALR mutations [99,100]. In a targeted NGS study of 1 patient with ET, JAK2V617F and MPLW515 were co-mutated, and interestingly, these mutations were not found by standard molecular screening [101].
Non-driver and driver mutations in signaling pathway genes are generally mutually exclusive, but may co-occur and then usually in different clones [54]. Concomitant mutations in the signaling pathway genes JAK2V617F and SH2B3 have been demonstrated in blast-phase MPN and more rarely in chronic phase disease [102]. With rare exceptions, spliceosome mutations are mutually exclusive of each other and lead to abnormal splicing, exon skipping and impaired hematopoiesis [53,103]. This is in accordance with a NGS study of 182 patients with PMF by Tefferi et al., who found that SRSF2 rarely coexists with U2AF1 [60].
3. NGS in the Diagnosis and Prognosis of MPNs
3.1. Development and Progression of Somatic Gene Mutations in Patients with MPNs
Development of disease processes in hematological neoplasms may usually begin several years before clinical manifestation [104]. Mutations in MPNs arise in the hematopoietic stem and progenitor cells (HSPCs) in the bone marrow, where a number of cells may acquire somatic mutations that pass on to the next generation of cells [105,106]. Several of these mutations have a benign effect or are deleterious and will become extinct, however, few mutations increase proliferation and fitness of the cells resulting in increased clonal expansion [107,108]. This process is named Clonal Hematopoiesis of Indeterminate Potential (CHIP) defined by the presence of somatic mutations with an allele burden of more than 2%, but without presence of a hematological abnormality or malignancy [109]. This premalignant stage may be characterized by one or more somatic mutations in genes associated with hematological neoplasms, such as predominantly TET2, DNMT3A, ASXL1, but also JAK2, SF3B1, SRSF2, TP53 or PPM1D [104,110,111,112,113] and is present in 10–20% of healthy, older individuals over age 70, but only in 1% of healthy individuals below age 50 [104,111]. Interestingly, one study of 3067 blood donors aged 17–70 and 1152 unselected individuals aged 60–98 years only observed the spliceosome mutations SF3B1 and SRSF2 in those aged over 70 years suggesting that these clones expand later in life [114]. Jaiswal et al. found a 50 fold higher risk of developing a hematological malignancy in individuals with a mutant allele burden > 10% [111], indicating the importance of variant allele frequency assessment in prognostication and prediction of disease progression.
Autoimmune diseases, smoking, chemotherapy, and chronic inflammation may impose a higher selective pressure on the HSPC pool with a more rapid outgrowth of mutant clones, thereby facilitating the development of CHIP. This inflammatory environment leads to impaired fitness, accelerated aging, and exhaustion of the HSPC pool [115,116,117,118,119]. Individuals with CHIP have a higher risk of developing a myeloid neoplasm, but are also at higher risk of developing cardiovascular diseases, type 2 diabetes, or second cancer and have a higher all-cause mortality [105,107,111,119]. Clonal expansion of the founding clone from CHIP towards a hematological neoplasm may include linear acquisition of cooperating mutations yielding subclone formation or mutations that develop in parallel to the founding clone [120] together with an increasing variant allele frequency [111]. These mutations may include JAK2, CALR or MPL [113,121]. In a NGS study of 197 patients with MPNs, Lundberg et al. presented a model of clonal evolution from CHIP to initiation of MPNs showing that most initial mutational hits occur in JAK2V617F or CALR or in the epigenetic regulator genes DNMT3A or TET2 [120]. Although NGS studies using DNA from colony formation assays or monitoring of the mutant allele burden enable assessment of the clonal hierarchy, single cell sequencing is a great tool to unveil the signature of genetically distinct subclones during clonal evolution and disease development. In the first single cell exome sequencing study in MPNs performed by Hou et al. in 2012, principal component analysis and mathematical modeling of data from 58 cells from a JAK2V617F negative patient with ET indicated monoclonal evolution of the disease [122].
3.1.1. From CHIP to More Advanced Stages of MPNs
Non-driver mutations may chronologically precede or follow the acquisition of driver mutations in the clonal evolution from CHIP to early and blast phase MPN. Lundberg et al. performed NGS on 197 patients with MPNs and showed that mutations in TET2 or DNMT3A were often present in early founding clones acquired before JAK2V617F, while mutations in ASXL1, EZH2, or IDH1/2 were often acquired after JAK2V617F [120]. Similarly, in a comprehensive NGS study of 2035 patients with MPNs, Grinfeld et al. determined the relative probability of a gene to occur first or second in a gene pair relative to JAK2V617F. DNMT3A, SH2B3, SF3B1, or CUX1 most likely occurred first, whereas genes such as ASXL1, IDH1/2, NFE2, EZH2, NRAS, TET2, TP53, or PPM1D most likely occurred second [20]. In contrast to JAK2V167F, mutations in CALR are suggested to be early events with additional mutations being secondary events [12,13].
The transformation of early phase MPN to more advanced stages and ultimately acute myeloid leukemia (AML) is usually associated with clonal expansion and acquisition of further mutations. Mutations in ASXL1, EZH2, IDH1/2, SRSF2, or TET2 may frequently occur in the MF transformation phase, and RUNX1 or TP53 in the transformation phase to AML [62,121]. A study using whole genome and capture sequencing provided evidence for clonal expansion in one patient with PMF transforming to AML. Based on variant allele frequencies, they revealed a founding clone in JAK2V617F or U2AF1 and three subclones with the MYB subclone developing parallel to the nested subclones harboring ASXL1/HCFC1 and RUNX1/IDH1, the latter expanding during transformation to AML [123]. A low or an increasing allele frequency in an early event mutation such as DNMT3A may persist for years without signs of hematological disease [104]; however, an increasing allele frequency with loss of the wild type allele in a late event mutation—TP53—usually have a deleterious effect leading to rapid clonal expansion and AML [120].
Another NGS study of 50 JAK2V617F positive patients with ET or PV demonstrated a decrease in the JAK2V617F allele burden during 3 years of follow-up in parallel with an increasing allele burden of other mutations in two patients suggesting clonal competition. However, 12 out of the 24 patients with disease progression were treated with hydroxyurea, which accordingly also might have impacted the decrease in the JAK2V617F allele burden [86]. Although increasing allele frequency of the JAK2V617F mutation has been associated with fibrotic progression or thrombosis in patients with MPNs [124,125,126], low JAK2V617F allele burden has been observed in PMF transforming to blast phase [127,128,129]. These findings indicate progression of the disease in line with other reports showing that leukemic transformation in JAK2V617F positive MPNs usually occur in JAK2V617F wild type cells [130,131]. Furthermore, low JAK2V617F allele burden might be related to a possible co-occurring mutation in MPL [97], however, a study showed that low JAK2V617F allele burden was associated with poor survival without any co-occurring MPL mutation [128] suggesting the presence of an overriding malignant JAK2V617F negative subclone [127], calling for NGS studies to be performed upfront to reveal these co-occurrences. Interestingly, in their NGS study of 2035 patients with MPNs, Grinfeld and colleagues found that clone size for most genes has no impact on outcome suggesting that the most aggressive subclone determined outcome [20].
3.1.2. Implication of Mutation Order on MPN Phenotype
The temporal order of acquisition of mutations in JAK2V617F and epigenetic regulator genes influences the phenotypic presentation of the MPN disease. In an NGS study from 2015, Ortmann and colleagues described the order of mutations in 48 JAK2V617F and TET2 mutated patients with MPNs. Among JAK2-first patients, there was an overrepresentation of homozygous PV patients at younger age having a higher risk of thrombotic events, while TET2-first patients were common among both ET and heterozygous PV patients at older age [132]. Later the same year, Nangalia et al. showed that seven of 10 JAK2-first patients presented with PV and six of 6 DNMT3A-first patients with ET, however, they found no association between DNMT3A-first and age or thrombotic risk [133]. In agreement, the NGS study of 2035 patients with MPNs by Grinfeld et al. showed that JAK2V617F was more likely an early event in patients with PV or MF and a secondary event in those with ET [20]. Stratified by mutation type, the authors found that DNMT3A, TET2, ASXL1, or EZH2 mutations occurred more often first in patients with ET compared to patients with PV or MF, however, TET2 or DNMT3A were more likely also an early event in patients with MF [20].
In Figure 1, the main pathogenetic associations discovered by NGS are depicted.
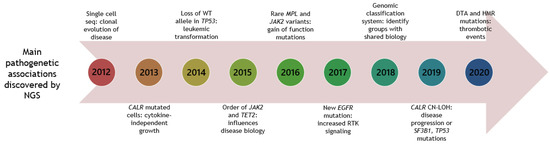
Figure 1.
Timeline of NGS studies in patients with MPNs, unravelling main pathogenetic associations. Year is shown in circular boxes. Year 2020 is until June 30. CN-LOH: copy neutral-loss of heterozygosity. DTA: DNMT3A, TET2, ASXL1. HMR: high-molecular risk mutations (ASXL1, EZH2, IDH1/2, SRSF2). RTK: receptor tyrosine kinase.
With the advent of NGS technologies and its increasing use in clinical routine, the identification of CHIP associated mutations has been possible and may support clinical decision making in MPNs. In addition, NGS may be applied to follow clonal expansion, thereby identifying individuals at risk of developing a hematological malignancy or progressing to advanced stages of the disease.
3.2. NGS in the Diagnostic Decision-Making in MPNs
According to the revised 2016 WHO criteria, in triple-negative patients, testing for the most frequent additional mutations (e.g., ASXL1, DNMT3A, TET2, EZH2, IDH1/2, SRSF2) may be helpful to determine the clonal nature of the disease and complement the morphological criteria [38,65,134]. In 2018, the European Leukemia Network expert panel recommended that testing for mutations in the “high molecular risk” (HMR) mutations ASXL1, EZH2, IDH1/2 and SRSF2 should be performed in patients negative for the driver mutations in JAK2, CALR, or MPL. Although only testing for the 5 HMR mutations in triple-negative MF was recommended, testing for these mutations in triple-negative ET and for TP53, TET2, DNMT3A, and CBL in both disease entities was a matter of debate and may be performed by each institutions own preference [135]. Another advantage of using NGS for mutation detection in triple-negative MPNs is the possibility of simultaneous testing of rare variants in JAK2, CALR, or MPL otherwise not detected by conventional assays.
At the time of diagnosis of MPNs, single gene analyses of the driver mutations in JAK2, CALR, or MPL have been performed for more than a decade by conventional molecular biological tools. However, with NGS becoming increasingly implemented in the diagnostic laboratories, the application of NGS for concomitant detection of driver and non-driver mutations at the time of diagnosis for all patients suspected for MPNs may likely be routine in the years to come. Distinction between MPN subtypes is challenging and relies on clinical and morphological criteria. Besides the type of variant, studies have shown that the number [13,58,65,66] and order of mutations [20,132,133] may affect the phenotype, and thereby the specific subtype of the disease. Interestingly, classification of MPN subtypes has been performed in the NGS study by Grinfeld et al. Based on clinical variables, germline, and somatic mutations, they were able to predict if patients were diagnosed with ET or PV, or with chronic phase disease or MF [20]. Thus, upfront application of NGS can assist in confirming the diagnosis and may allow for simultaneous assessment of the molecular complexity of the disease. With increased coverage and sensitivity as well as lower costs, it seems likely that NGS will be part of the diagnostic testing algorithm in most laboratories in the near future.
3.3. Implication of Somatic Gene Mutations on Prognosis, Risk Stratification and Outcome Revealed by NGS
The genomic landscape of patients with MPNs is highly complex with mutations in both driver and non-driver genes conferring an increased risk of disease progression and transformation to AML affecting prognosis, molecular risk stratification, and outcome. For nearly a decade, several NGS studies have been performed in patients with MPNs providing a thorough investigation of the implication of gene mutations on these aspects. In the following, these studies will be reviewed in more detail with focus on non-driver mutations. In Table 2, the majority of NGS studies conducted from 2012–2020 in patients with MPNs is listed.

Table 2.
NGS studies in patients with MPNs.
The first NGS studies on more than one patient with MPNs appeared simultaneously in 2013, when Nangalia et al. and Klampfl et al. described the new CALR mutations in two separate studies [12,13]. In their series of 151 patients with MPNs, Nangalia et al. included 62 patients with ET and provided evidence for a significantly higher rate of transformation to MF, higher platelet counts, and lower hemoglobin levels in CALR mutated ET patients compared to those with JAK2V617F [13]. Similar results were reported in follow-up studies of 176 and 89 CALR mutated patients with ET [136,137], however, although no evidence of a higher transformation rate was found in these studies, Grinfeld et al. found an increased risk of transformation in their NGS study of 2035 MPN patients of whom 1321 had ET [20]. Accordingly, the higher power of the NGS studies by Grinfeld et al. and Nangalia et al. may account for the differences in risk of transformation. The study by Klampfl et al., which was mainly performed by Sanger sequencing and fragment analysis, included 311 ET patients and 203 patients with PMF showing higher platelet counts and lower leukocyte levels in CALR mutated patients in both cohorts together with lower hemoglobin in ET. In addition, CALR mutated ET patients have longer overall survival (OS) and a lower risk of thrombosis compared to JAK2V617F positive patients, while CALR mutated PMF patients have longer OS compared with both JAK2V617F and MPL positive patients [12]. These findings have been confirmed in later studies [137,138,139,140]. However, a study of 139 CALR type 1 mutated patients with MF demonstrate that additional mutations in ASXL1, EZH2, IDH1/2 or SRSF2 influenced survival and disease outcome [83].
3.3.1. High Molecular Risk Mutations
Several studies have provided evidence for the adverse impact of somatic mutations in ASXL1, EZH2, IDH1/2, and SRSF2 on shorter OS or leukemia-free survival in patients with PMF. The first simultaneous analysis of these five mutations was described in 2013 by Vannucchi et al. using Sanger sequencing in a series of 879 patients with PMF. They reported the significance of these mutations in regard to premature death and leukemic transformation and suggested them to be included in future studies [187]. In 2014, Guglielmelli et al. reported results from a NGS study of 167 patients with MF highlighting the detrimental impact of mutations in ASXL1, EZH2, IDH1/2 or SRSF2. In MF, a new molecular prognostic classification was for the first time proposed, where patients having a mutation in any of these five genes were classified as an HMR group [138]. These findings were confirmed by the same group in 2018 in a large NGS study of 805 patients ≤70 years with PMF [68]. Other NGS studies have shown conflicting results regarding HMR mutations. In a series of 165 patients with PET-MF and 194 patients with PPV-MF, Rotunno et al. only provided evidence for a detrimental effect of SRSF2 in patients with PET-MF but not in PPV-MF. Surprisingly, there was no difference in outcome regarding the remaining 4 HMR mutations. The authors suggested that the mutational events occurring in PMF are different from those implicated in the transformation to SMF [67], although, Spiegel et al. found an association between HMR mutations and shorter OS in 100 patients with MF [82]. However, as opposed to the study by Rotunno et al., the study by Spiegel et al. was performed on both PMF and SMF patients, possibly accounting for the discrepancies. Nevertheless, Tamari et al. found no association between HMR mutations and OS in their NGS study of 100 patients with PMF or SMF [176]. Finally, and not in agreement with the study by Rotunno et al., Courtier at al reported an association between mutations in SRSF2 and shorter OS in patients with PMF but not in SMF [62]. These results may support the suggestion that SMF and PMF are not two separate disease entities but should be considered as one disease entity in the biological MPN continuum, however, this is a matter of debate. Although HMR mutations have predicted poor prognosis in MF, these mutations have also been associated with poor prognosis in patients with ET and PV. In an NGS study of 50 patients with ET and PV by Luque Paz et al., patients with at least one HMR mutation at diagnosis or an increasing allele burden (relative increase of at least 20%) of at least one additional gene mutation showed disease progression after 3 years [86].
Individually, HMR mutations, in particular ASXL1 or SRSF2, have been related to inferior outcome in predominantly patients with MF but also in patients with ET or PV [94,188,189,190,191]. Several NGS studies observed shorter OS or leukemia-free survival in MF patients with mutations in ASXL1 [60,62,165], SRSF2 [60,62,88,165], or EZH2 [64,82]. Intriguingly, CALR type 1 mutations in PMF are not only related to longer OS but may also ameliorate the poor prognosis of ASXL1 and SRSF2 mutations [192,193]. In particular, PMF patients with CALR¯/ASXL1+ mutational status have an inferior survival [192]. In NGS studies of patients with ET or PV, inferior OS or higher risk of leukemic transformation have been associated with mutations in ASXL1 [63,146,164], SRSF2 [146,164], IDH1/2 [164], or EZH2 (only ET) [186]. However, in a single gene study of 107 ET patients with ASXL1 mutations, they found no impact of ASXL1 on OS [194]. In Table 3, the clinical significance of somatic mutations in MPNs refined by NGS is reported.

Table 3.
The clinical significance of the most common additional gene mutations in MPNs elaborated by NGS studies.
3.3.2. Other Groups of Adverse Mutations
Other groups of mutations have been proposed in the prognostication of patients with MPNs. In an NGS study from 2016 by Tefferi et al. of 182 patients with PMF, overall survival was reduced in ASXL1, SRSF2, CBL, and KIT mutated cases and leukemia-free survival was reduced in patients with mutations in SRSF2, RUNX1, CEBPA, and SH2B3. Accordingly, these observations led to the reporting of ASXL1, SRSF2, CBL, KIT, RUNX1, CEBPA, and SH2B3 as an adverse group of mutations associated with inferior OS and leukemia-free survival regardless of JAK2, CALR, or MPL mutation status [60]. In addition, mutations in U2AF1 were associated with anemia and thrombocytopenia, and SRSF2 with anemia [60]. Interestingly, Tefferi et al. reported shorter OS of PMF patients with the U2AF1Q157 mutation compared to U2AF1S34 mutated patients or U2AF1 unmutated patients [195] leading to the inclusion of the U2AF1Q157 mutation as an HMR mutation in their GIPSS prognostic model in patients with PMF [165]. Besides their NGS study of patients with PMF, Tefferi et al. reported the prognosis of adverse mutations in an NGS study also from 2016 of 183 patients with ET and 133 patients with PV from the Mayo clinic followed by validation in an Italian cohort of 174 ET patients and 215 PV patients. In ET, IDH2 and SH2B3 were associated with inferior OS, EZH2 and TP53 with shorter leukemia-free survival, and SF3B1 and U2AF1 with shorter myelofibrosis-free survival. In PV, ASXL1 and SRSF2 were associated with inferior OS, SRSF2 and IDH2 with shorter leukemia-free survival and SRSF2 with shorter myelofibrosis-free survival. Based on these observations, SH2B3, SF3B1, U2AF1, TP53, IDH2, and EZH2 were included as adverse mutations in ET and ASXL1, SRSF2, and IDH2 as adverse mutations in PV. Presence of at least one of these adverse mutations compared with other mutations or no mutations was associated with reduced OS, shorter leukemia-free survival and myelofibrosis-free survival in both disease entities [59]. Recently, the same group presented another NGS study also including patients from the Mayo and Italian cohorts, in total 502 ET and 404 PV patients. The authors developed a mutation enhanced prognostic system consisting of adverse mutations including SF3B1, SRSF2, TP53, and U2AF1 in ET and SRSF2 in PV affecting OS, leukemia-free survival or myelofibrosis-free survival [186]. Thus, the authors confirmed the prognostic relevance of mutations in SF3B1, TP53, and U2AF1 in ET and SRSF2 in PV [59,186]. In a very recent NGS study of 464 patients with MF, Coltro et al. provided evidence for an association of mutations in the RAS pathway genes CBL, KRAS, and NRAS with shorter OS and leukemia-free survival [57].
3.3.3. Fibrotic Progression
In 2018 and 2020, two NGS studies by Bartels et al. reported the implication of fibrotic progression in 64 patients with PV and 104 patients with prefibrotic PMF, respectively. In the study of PV patients, they observed a higher risk of fibrotic progression in patients with additive mutations such as DNMT3A, IDH2, SRSF2, or U2AF1, however mutations in TET2 were not implicated in disease progression [89]. In prefibrotic PMF, they highlighted that mutations in SRSF2, U2AF1, SF3B1, IDH1/2, or EZH2 in the pre-fibrotic stage were independent risk factors for rapid fibrotic progression. Although mutations in ASXL1 are risk factors when acquired during disease progression, mutations in ASXL1, DNMT3A, or TET2 in prefibrotic PMF were not associated with development of fibrosis [84] unless acquired after the driver mutation (ASXL1 only). Of note, the follow-up time was only three years possibly accounting for the lacking association. Interestingly, in both studies, the allele burden of driver mutations was not associated with disease progression [84,89].
3.3.4. TET2 Mutations and Order of Mutations
The prognostic impact of TET2 is debated and conflicting results exists [86,196,197]. The first NGS study reporting the implication of TET2 mutations in MPNs appeared in 2014, when Lundberg et al. provided evidence for shorter OS and increased risk of leukemic transformation in 23 TET2 mutated MPNs [120]. In the NGS study by Tefferi et al., they showed an association between TET2 mutations and thrombosis in patients with ET independently of both age and driver mutation [59]. Interestingly, Segura-Diaz performed a targeted case-control study of 55 age-matched patients with PV and provided evidence for mutations in TET2 and higher risk of cardiovascular disease and thrombotic events [185]. However, in two other NGS studies, no influence of TET2 mutations on OS in patients with MF [64] or disease progression in ET or PV was observed [86]. Recently, Kralovics et al. performed targeted NGS on 163 patients from their Proud-PV cohort receiving ropeginterferon alpha-2b [182]. They found a higher baseline JAK2V617F allele burden in TET2 mutated patients compared to TET2 wild type, although statistical significance was not reached (p < 0.09). Nevertheless, ET and PV patients with the TET2 mutation had a significantly higher baseline JAK2V617F allele burden compared with TET2 wild type cases as reported in the serial single gene sequencing study of 40 ET and 43 PV JAK2V617F positive patients performed by Quintas-Cardama et al. [198]. These results suggest a more adverse prognosis of TET2 mutated patients with ET or PV.
In the NGS study by Ortmann et al. in 2015, the order of acquisition of JAK2V617F and TET2 was comprehensively investigated in two different cohorts of patients with MPNs. The first cohort included 246 patients and the follow-up cohort 918 patients. In total, 48 patients presented with mutations in both JAK2V617F and TET2. As previously noted, although JAK2V617F first patients were predominantly PV patients of younger age, they have a higher risk of thrombotic events and present with abnormal blood counts compared with TET2 first patients [132]. Furthermore, the transcriptional consequence of the JAK2V617F mutation in TET2 first cells revealed increased proliferation of hematopoietic stem and progenitor cells. It was concluded that the order of JAK2V617F and TET2 mutations might influence the acquisition of additional mutations and the impact of JAK2V617F on the proliferation rate, thereby affecting disease pathogenesis [132]. Thus, it seems likely that the conflicting results may be attributed to the time point of TET2 acquisition.
3.3.5. TP53 and PPM1D Mutations
Mutations in TP53 are rare during the chronic phase of MPNs [120] but increases rapidly during leukemic transformation [162,199]. In accordance, Lundberg et al. found in their NGS study a particular unfavorable impact of acquisition of TP53 mutations on leukemic transformation and OS [120] also observed by Grinfeld et al. in their large NGS study [20]. Similarly, in patients with MF, TP53 mutations were reported to result in shorter OS in three NGS studies [61,62,88]. In 254 chronic phase MPNs treated with cytoreductive drugs, Kubesova et al. found no association between low burden TP53 mutations and leukemic transformation suggesting other factors such as genomic instability or hematopoietic exhaustion may lead to clonal expansion and leukemic transformation [162].
In their large series of 2035 patients with MPNs, Grinfeld et al. observed that PPM1D was the eighth most mutated gene. PPM1D is a known regulator of p53 and has been associated with the development of other cancers [200,201]. It may be speculated that MPN patients with PPM1D mutations may be more prone to development of second cancer. Importantly, several other MPN-associated mutations such as ASXL1, SH2B3, TET2, JAK2, TP53, KRAS, NRAS, and U2AF1 are found in other cancers as well [92,202,203,204,205]. Indeed, studies have shown that patients with MPNs have a higher risk of developing second cancer [206,207,208].
3.3.6. Prognostic Genomic Classification Models
In the study by Grinfeld et al. addressed above, the authors presented a prognostic genomic classification model applied on the 2035 patients and validated on an external cohort of 270 MPN patients [20]. Although prognostic predictive scoring systems such as IPSS [209], DIPSS [210], MIPSS [68], GIPSS [165] and MYSEC-PM [211] have been developed in the past, only patients with MF were included. Integrating 63 clinical, demographic, cytogenetic and genomic features, the authors identified eight different genomic subgroups enabling personalized prediction of outcome in patients with ET, PV, and MF [20]. The first group was characterized by TP53 mutations or aneuploidy and a dismal prognosis, and the second by mutations in one or more of 18 myeloid genes especially spliceosome, epigenetic, or RAS genes with increased risk of disease progression or death. Patients not belonging to one of these two groups were classified according to their driver mutation either CALR and chr20q-, MPL with higher risk of AML transformation, homozygous JAK2 or NFE2 mutations with increased risk of MF transformation, or a heterozygous JAK2 mutation mostly with favorable outcome. The seventh subgroup comprised other clonal mutations, and the eight included patients with no mutations and predominantly a benign outcome [20]. The authors demonstrate a model with good risk prediction that correlated well with outcome. Thus, these data show that including genomic data in clinical decision-making may improve prognostic models in MPNs.
3.3.7. Additional Mutations in Relation to Sex
Although male and female sex have a significant impact on cancer prognosis and treatment response, sex-related molecular signatures have rarely been investigated in cancer patients [212]. Recently, in a series of 227 patients with MPNs, Karantanos et al. found a higher number of additional mutations in men compared with women. Moreover, an increase of >0.5% per year of the JAK2V617F allele burden compared with <0.5% per year was associated with shorter OS in females, which was not observed in men. The authors concluded that male sex in patients with MPNs is an independent prognostic risk factor for poor outcome caused by an increased number of non-driver mutations, especially HMR mutations including U2AF1 [181].
3.3.8. Co-Occurring Non-Driver Somatic Mutations in Prognostication
Despite the prognostic impact of co-occurring non-driver mutations has been described in AML [213], it has only been sparsely investigated in MPNs, which may be attributed to the lower number of mutations in MPNs compared with AML. In a Sanger sequencing study, Lasho et al. reported a significant clustering of mutations in SRSF2 with IDH1/2 mutations, however, the prognostic relevance of mutations in SRSF2 was independent of IDH1/2 mutations [190]. One single NGS study showed a higher occurrence of comutated non-driver mutations in JAK2V617F positive ET or PV patients with progressive disease compared to patients without. However, the specific type of co-occurring variants regarding their prognostic implication was not reported [86]. Accordingly, larger studies investigating whether the type of co-occurring additional mutations may affect prognosis and outcome are warranted in the future.
3.3.9. Impact of the Number of Mutations on Prognosis and Outcome
In accordance with the biological continuum from chronic phase ET and PV to the more advanced and critical stages of PMF or SMF, several NGS studies have found a higher number of mutations in MF patients compared to patients with ET or PV. In their exome sequencing study, Nangalia et al. found a significantly higher median number of mutations in patients with PMF (13.0) compared to ET (6.5) and PV (6.5) [13]. Similarly, although in lower numbers owing to a smaller amount of analyzed genes, in a targeted NGS study of 40 patients with ET, 30 with PV, and 30 with PMF, patients with PMF had an overall mean number of 2.5 mutations/patient, patients with PV 1.63, and patients with ET 1.38 mutations/patient [58]. In line with the biological continuum from chronic phase MPN to the inferior stage of post-MPN AML, Alduaij et al. reported in their targeted NGS study a median number of 1 mutation/patient in ET/PV, 2 in MF, and 4 in post-MPN AML [65].
An association between outcome and number of mutations has been demonstrated in several targeted NGS studies and in one exome sequencing study. Lundberg et al. showed in their exome sequencing study of 197 MPN patients that 2 or more mutations were associated with significantly increased risk of transformation to AML and reduced OS [120]. In agreement, two targeted NGS studies demonstrated shorter OS in patients with PMF or SMF having three or more mutations [64,82], in line with the study of 9 patients with SMF and 21 patients with PMF by Silver et al., who reported an association between adverse events and three or more mutations [157]. Likewise, in a serial single gene sequencing study of a cohort of 797 patients with PMF from Europe and Mayo clinic, Guglielmelli et al. provided evidence for a significantly shorter leukemia-free survival and OS in both cohorts in patients with two or more HMR mutations compared to one or no HMR mutations [214]. Interestingly, in their NGS study of 100 patients with SMF or PMF, Spiegel et al. observed an HMR mutation in 24% of patients with 0 to 2 mutations in contrast to 79% of patients with 3 or more mutations, the latter group having reduced OS [82].
Although Acha et al. demonstrated shorter OS in triple negative patients with ET or PMF having only one or more mutations, which might be attributed to the detrimental prognosis of triple negative patients with MPNs, Tefferi et al. also provided evidence for an association between reduced OS and one or more mutational hits in a cohort of JAK2V617F positive and negative PMF patients [60,167]. In patients with ET or PV, Tefferi et al. in their NGS study found that the number of genes is not detrimental to outcome unless specific prognostic adverse mutations are involved [59]. However, Luque Paz et al. reported shorter OS in their study of 190 ET patients with one or more mutations [170], while Andreasson et al. demonstrated shorter OS in a study of 85 PV patients with more than three mutations [63]. Accordingly, the number of mutations are of high importance in the prognostic assessment of all MPN subgroups and may be used as a risk factor in treatment planning decisions.
3.3.10. NGS and Transplantation Outcome
Patients with adverse mutations may be candidates for hematopoietic stem cell transplantation (HCT). In DIPSS intermediate risk patients, Alduaij et al. proposed early HCT in patients with an HMR profile and delayed HCT in patients with absence of an HMR profile [65]. Nevertheless, in a series of 101 patients with PMF or SMF who underwent allo-HCT, Tamari et al. reported no implication of HMR mutations or TP53 mutations on relapse free survival (RFS) or OS regardless of MIPSS score, however, mutations in DNMT3A or U2AF1 were associated with reduced RFS and U2AF1 also with shorter OS. Interestingly, variant allele frequencies of JAK2V617F, CALR, or ASXL1 had no impact on RFS or OS [176]. Recently, Stevens et al. reported no implication of ASXL1 mutations on allo-HCT outcome in an NGS study of 55 MF patients [90] in contrast to the NGS study of 169 MF patients by Kröger et al. who found a higher risk of relapse in ASXL1 mutated patients [154]. Regarding progression free survival (PFS), only IDH2 remained significant of the five HMR mutations, and no association with mutations in SRSF2, SF3B1, IDH1, TET2, DNMT3A, or EZH2 was found. No HMR mutation had an impact on OS, while CALR was associated with improved OS and PFS [154].
Although the number of mutations (≥3 vs. <3 mutations) was not associated with RFS or OS in the study by Tamari et al. [176], a threefold higher incidence of post-HCT relapse was demonstrated in patients with ≥3 mutations in the study by Stevens and colleagues, who stressed that the discrepancy could be owing to the difference in cutoff used (including vs. excluding driver mutations, respectively) [90]. Thus, conflicting results exist regarding outcome after allo-HCT calling for larger NGS studies to address this issue.
Taken together, the clinical course of patients with ET, PV, or MF relies heavily on clinical and molecular risk factors. The mutational landscape is highly complex with a vast array of mutations influencing prognosis and disease outcome. In this regard, the multigene approach of NGS is a useful tool to identify the subgroup of patients with increased genetic instability and therefore high risk of adverse outcome.
4. Use of NGS to Decipher the Mutational Landscape in MPNs in Response to Therapy
With the advancement of high throughput technologies during the past decade, there has been a tremendous progression in the understanding of MPN disease pathogenesis. As alluded to above, a prognostic predictive molecular scoring system have been developed in ET, PV, and MF with the purpose of identifying patients at risk of poor outcome. Molecular profiling at time of diagnosis may guide treatment decisions thereby tailoring therapeutic choices complying with the heterogenic presentation of the disease. Despite these advancements, there are yet no definitive cure in patients with MPNs. Below, the most widely used treatment modalities in MPNs are highlighted in parallel with improvements in therapeutic decision-making using NGS. Treatment options in MPNs are highly divergent ranging from the “wait and watch” strategy to allogenic stem cell transplantation depending on the severity stage of the disease [135]. The principal reason for treatment in MPNs is to prevent thrombohemorrhagic complications and transformation to the advanced or blast-phase stages of the disease.
4.1. Hydroxyurea
Hydroxyurea (HU) has been the choice of cytoreductive treatment for many years, however, concerns have been raised due to its mutagenic potential after long-term treatment in MPNs [215,216,217,218,219]. Conflicting results exists whether HU has any impact on the JAK2V617F mutational status, and it has been speculated if reduced JAK2V617F allele burden during treatment with HU is only a consequence of a reduction of the neutrophil cell count [220,221,222]. It is generally recognized that HU does not influence the quiescent hematopoietic stem cells, and no durable effects after treatment discontinuation have ever been observed implying that normalized cell counts will increase within days [216,223,224,225]. One targeted NGS study of HU treated MPN patients appeared in 2018, where Senin and colleagues tested a range of treatment options in ET and PV (anagrelide, busulphan, HU, P32, and interferon (IFN)). In patients treated with HU, the presence of additional mutations, especially in SRSF2 or RUNX1 at diagnosis was associated with a higher risk of developing a new mutation. Interestingly, they found no higher risk of acquiring a new mutation in patients receiving HU for more than 5 years compared with less than 5 years or no treatment. However, median follow-up was only 10 years (range 1–13) and longer follow-up time may be needed to discover any difference. In addition, they found a higher probability of cytopenia during HU in patients carrying additional mutations in DNMT3A, SRSF2, IDH1/2 or RUNX1 compared to patients without [164]. Resistance to HU has been associated with leukemic transformation and shorter OS, particularly in patients developing cytopenia [226], highlighting the value of NGS in guiding therapy. Indeed, studies in AML have provided evidence for a critical role of DNMT3A in chemotherapeutic resistance [227]. Since chemotherapeutic resistance is a major factor for drug treatment failure, it is tempting to consider how the efficacy of other treatment modalities interferes with the mutational landscape in MPNs.
4.2. Interferon Alpha
The non-leukemogenic disease modifying agent interferon-alpha2 (IFN) has been used for decades in patients with MPNs [228,229,230,231,232]. Studies have convincingly demonstrated complete hematological remission within 6 months of IFN therapy in MPN patients followed by molecular remission with a reduction of the JAK2V617F allele burden [26,28,31,33,37,155,233,234,235,236], and in some patients even a sustained deep hematological and molecular remission together with normalization of the bone marrow after discontinuation of treatment [21,22,237]. The efficacy of IFN is likely resulting from a comprehensive range of biological properties, including boosting of virtually all immune cells, selective targeting and eventually eradication of malignant cells, and an efficient activation of dormant malignant stem and progenitor cells thereby possibly breaking their resistance to therapy [235,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254].
In a series of 31 CALR mutated IFN-treated patients with ET, Verger and colleagues performed targeted sequencing and found six of 31 patients having one or more additional mutations in ASXL1, IDH1/2, TET2, or TP53. Strikingly, patients with no additional mutations had a significantly better response to IFN compared to patients presenting with ≥1 of those mutations suggesting ASXL1, IDH1/2, TET2 or TP53 may be associated with resistance to treatment [75]. A similar trend was observed in ASXL1, DNMT3A, EZH2, IDH1/2, and TET2 in the serial single gene sequencing study of 40 ET and 43 PV JAK2V617F patients by Quintas-Cardama et al., however, statistical significance was not achieved [198]. Silver et al. found in their NGS study of 30 patients with MF poor response to IFN in cases with baseline mutations in SRSF2 or ASXL1, although not statistically significant [157]. Interestingly, Ianotto and colleagues found non-driver mutations in 68% of patients who discontinued IFN compared to only 33% of patients who remained on IFN [160]. Finally, in a series of 202 and 135 MPNs studied at baseline and after 24 months of treatment with IFN or HU, Knudsen et al. reported that DNMT3A was the most frequently acquired mutation in mainly IFN treated patients not achieving clinicohematological complete response at follow-up [169]. All together, these findings strongly suggest that additional mutations may play a role in resistance to treatment with IFN.
Quintas-Cardama et al. also showed significantly higher JAK2V617F response rates to IFN in TET2 wild type patients in contrast to TET2 mutated patients [198], whereas Kralovics et al. reported no significant difference in response rates during ropeginterferon-alpha2b in their NGS study of 163 PV patients [182]. Although studies have shown that the allele burden of JAK2V617F but not TET2 decreases during IFN therapy [198,245], the allele burden of both JAK2V617F and TET2 decreased significantly during treatment with ropeginterferon-alpha2b [182]. These results suggest a heterogeneous response to IFN possibly attributed to the coexistence of different clones developing independently during therapy.
4.3. Ruxolitinib
Following the discovery of the JAK2V617F mutation in 2005, JAK1-2 inhibitor therapy was developed with ruxolitinib, a potent anti-inflammatory treatment modality, showing great benefit in reducing symptom burden and spleen size in patients with MF and PV [23,25,30,32,246,255,256,257,258] and to a lesser extent in patients with ET [259,260,261,262]. Anticipated side effects of ruxolitinib therapy such as anemia, thrombocytopenia, immunosuppression, or infections were also demonstrated in a small subset of patients [256,263,264,265]. Although ruxolitinib is not clonally selective for the malignant cells, some studies have documented a reduction of the JAK2V617F allele burden during treatment with ruxolitinib, whereas other studies showed only a modest reduction in the JAK2V617F allele burden [23,266,267,268,269,270]. In these studies, induction of remission was not experienced, however, a single exceptional case report demonstrated deep molecular remission in concert with cytogenetic remission and reversal of MF in a patient with post-PV MF [271].
In recent years, the effect of ruxolitinib on the mutational landscape in patients with MF has been elucidated using targeted NGS. In 2014, the first NGS study on the efficacy of ruxolitinib was performed by Guglielmelli et al. on 166 MF patients from the COMFORT-II trial [138]. Ruxolitinib associated spleen response and development of anemia or thrombocythemia were found unrelated to baseline HMR or LMR mutation status and to individually mutated genes [138]. Nevertheless, other studies have shown a more detrimental effect of additional mutations on the response to ruxolitinib. In their study of 95 patients with MF, Patel et al. found a lower ruxolitinib associated spleen response and shorter time to treatment discontinuation in patients with one or more mutations in ASXL1, EZH2 or IDH1/2 or with ≥3 mutations of any type [64]. Of note, SRSF2 was not included in their targeted NGS panel [64]. Likewise, Pacilli et al. found in their study of 46 MF patients that HMR status or mutations in ASXL1 at baseline resulted in loss of spleen response or shorter duration of spleen response, respectively after 3 years of ruxolitinib treatment, although the symptom response rate to ruxolitinib was not affected by baseline HMR mutations [163]. Furthermore, Spiegel et al. found a shorter time to treatment failure in MF patients with a HMR profile or with mutations in ASXL1 or EZH2, however, they found no impact of mutations in IDH1/2 or SRSF2, and no individual mutations or HMR mutations were associated with spleen response [82]. Recently, Coltro et al. performed NGS on 61 ruxolitinib treated patients with MF. After a median treatment period of 28 months, spleen response was lower in patients with mutations in the RAS/MAPK pathway genes CBL, KRAS, and NRAS [57]. Interestingly, the study by Ortmann et al. showed that the order of which JAK2 and TET2 was acquired influenced response to ruxolitinib with JAK2 first having a higher sensitivity to ruxolitinib in vitro [132].
Acquisition of mutations or clonal expansion has been reported in regard to therapeutic management. Newberry et al. provided NGS data on 62 MF patients and found an ASXL1 mutation at follow-up in 14 of 22 patients. Furthermore, after ruxolitinib discontinuation in 56 patients, they reported shorter OS and pretreatment transfusion dependence in patients with clonal expansion compared to patients without, although spleen response was not associated with clonal evolution [156]. Contradictory, among 46 ruxolitinib treated MF patients in the study by Pacilli and colleagues, all patients with acquisition of ≥1 mutation experienced loss of spleen response compared with 21% of patients without clonal evolution. Furthermore, acquisition of ≥1 non-driver mutation correlated with treatment discontinuation [163]. Whether the appearance of new clones are attributed to selective pressure by ruxolitinib or disease progression are a matter of debate and needs to be tested in larger studies. Taken together, accumulating evidence suggests that refractoriness to ruxolitinib may be, at least in part, attributed to additional mutations, in particular ASXL1 and EZH2 mutations.
In Figure 2, the main therapeutic associations discovered by NGS are depicted.
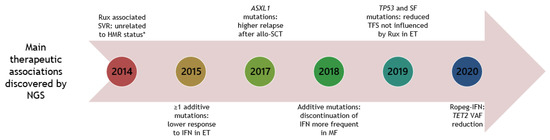
Figure 2.
Timeline of NGS studies in patients with MPNs, unravelling main therapeutic associations. Year is shown in circular boxes. Year 2020 is until June 30. Allo-SCT: allogenic-stem cell transplantation. ET: essential thrombocythemia. HMR: high-molecular risk mutations (ASXL1, EZH2, IDH1/2, SRSF2). IFN: interferon-alpha. MF: myelofibrosis. Rux: ruxolitinib. SF: splice factor. SVR: spleen volume reduction. TFS: transformation free survival. VAF: variant allele frequency. * since then other studies have not been able to demonstrate this association [64,82].
4.4. Combination Therapy with Interferon Alpha and Ruxolitinib
Unlike IFN therapy, ruxolitinib monotherapy does not modulate the quiescent hematopoietic stem cells [272,273]. Accordingly, a rational treatment approach may be combination therapy with ruxolitinib and IFN [274,275] enabling ruxolitinib to normalize a high-level of JAK-mediated pro-inflammatory cytokines [276], likely enhancing the capability of IFN to exert its effects by inhibiting clonal expansion and improving tumor immune surveillance [7,19,277]. In addition, as noted above, the induction of cell cycling of dormant hematopoietic stem cells by IFN mobilizes the malignant cells to targeted treatment with not only IFN itself but also e.g., JAK-inhibitors [253,254]. Adding a statin to combination therapy with IFN and ruxolitinib may have an even more profound effect on the malignant clone due to statins anti-inflammatory, antithrombotic, antiproliferative, and antiangiogenic properties [276,278]. It has been shown that statins selectively inhibit growth and viability of JAK2V617F MPN cells suggesting a synergistic effect with JAK-inhibitor therapy [270,279]. Indeed, the use of drug combinations has long been known to minimize therapeutic resistance [280,281]. Accordingly, studies investigating if combination therapies may counteract the adverse effects of additional mutations on treatment responses are urgently needed in the future.
4.5. Using NGS in Early Treatment Decisions
Early therapeutic intervention with IFN eventually in combination with the anti-inflammatory agent ruxolitinib at the time of diagnosis, where the tumor burden is lowest, has been proposed to be essential to impair clonal evolution, subclone formation, and development of additive mutations [2,27,34,239,282] which are likely driven by chronic inflammation [2]. In fact, early treatment with a combination of IFN and HU has been suggested for a restricted time period, since their combined effects might be highly efficacious and are foreseen to have the potential to minimize the risk of thrombosis and bleeding [283]. Applying this strategy – treatment at diagnosis as in any other cancer - the progressive disease development in the biological continuum (ET-PV-MF) with increased risk of thrombosis, resistance to therapy, and leukemic transformation may hopefully be attenuated, thereby opening the avenue for patients entering minimal residual disease with deep molecular remission and normalization of the bone marrow [7,21,22,27,34,35,235,237,274,275,276,277,284]. Thus, upfront application of NGS may drive therapeutic choices, taking into account that early treatment in patients without adverse mutations may prevent development of splenomegaly, anemia, and myelofibrosis. In particular, care should be taken in patients presenting with adverse mutations, since chemotherapy may affect clonal architecture with subclone formation and appearance of new mutations in treatment resistant clones resulting in treatment failure [285]. Hopefully, combination therapy initiated at diagnosis may ameliorate treatment resistance by targeting the malignant clone before clonal expansion occurs.
4.6. Monitoring of Disease by NGS
The pool of genetically diverse clones has a profound effect on response to therapy. During the past decade, close monitoring of the JAK2V617F or CALR allele burdens has been performed to detect those patients achieving minimal residual disease negativity rendering them eligible for treatment cessation. However, with the advent of NGS and its increasing use in clinical practice, molecular profiling of myeloid malignancy associated genes allows for sequential monitoring of the neoplastic clones during therapy, aiming for accurate assessment of disease evolution and update choice of treatment if adverse genomic changes appear.
5. Conclusions
MPNs are heterogeneous diseases not fully understood with a complex multitude of several factors such as hematological characteristics, mutational diversity, bone marrow microenvironment, stem cell biology, and clonal evolution contributing to disease pathology. Numerous phenotypes occur ranging from genuine ET to blast phase MF with quite different prognosis and outcome. In the past decade, the explosion of knowledge in genomics obtained by high-throughput sequencing has provided an abundance of useful information in patients with MPNs. Several mutational processes implicated in disease evolution, prognostication, and treatment decisions have been uncovered enabling development of molecular classification schemes and prognostic stratification models. With NGS becoming more and more implemented in clinical practice, integration of clinical data with genomic profiling data at diagnosis and during follow-up may support clinical decision-making allowing personal prediction of outcome and tailored treatment modalities for patient management.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
References
- Spivak, J.L. Myeloproliferative Neoplasms. N. Engl. J. Med. 2017, 376, 2168–2181. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, H.C. Perspectives on chronic inflammation in essential thrombocythemia, polycythemia vera, and myelofibrosis: Is chronic inflammation a trigger and driver of clonal evolution and development of accelerated atherosclerosis and second cancer? Blood 2012, 119, 3219–3225. [Google Scholar] [CrossRef] [PubMed]
- Lussana, F.; Rambaldi, A. Inflammation and myeloproliferative neoplasms. J. Autoimmun. 2017, 85, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Hermouet, S.; Bigot-Corbel, E.; Gardie, B. Pathogenesis of Myeloproliferative Neoplasms: Role and Mechanisms of Chronic Inflammation. Mediators Inflamm. 2015, 2015, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.; Farkas, D.K.; Christiansen, C.F.; Hasselbalch, H.C.; Sørensen, H.T. Chronic myeloproliferative neoplasms and subsequent cancer risk: A Danish population-based cohort study. Blood 2011, 118, 6515–6520. [Google Scholar] [CrossRef]
- Hasselbalch, H.C. Perspectives on the increased risk of second cancer in patients with essential thrombocythemia, polycythemia vera and myelofibrosis. Eur. J. Haematol. 2015, 94, 96–98. [Google Scholar] [CrossRef]
- Hasselbalch, H.C. The role of cytokines in the initiation and progression of myelofibrosis. Cytokine Growth Factor Rev. 2013, 24, 133–145. [Google Scholar] [CrossRef]
- Øbro, N.F.; Grinfeld, J.; Belmonte, M.; Irvine, M.; Shepherd, M.; Rao, T.N.; Karow, A.; Riedel, L.M.; Harris, O.; Baxter, E.J.; et al. Longitudinal Cytokine Profiling Identifies GRO-α and EGF as Potential Biomarkers of Disease Progression in Essential Thrombocythemia. HemaSphere 2020, 4. [Google Scholar] [CrossRef]
- Campbell, P.J.; Green, A.R. The myeloproliferative disorders. N. Engl. J. Med. 2006, 355, 2452–2466. [Google Scholar] [CrossRef]
- Baxter, E.J.; Scott, L.M.; Campbell, P.J.; East, C.; Fourouclas, N.; Swanton, S.; Vassiliou, G.S.; Bench, A.J.; Boyd, E.M.; Curtin, N.; et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet (Lond. Engl.) 2005, 365, 1054–1061. [Google Scholar] [CrossRef]
- Levine, R.L.; Belisle, C.; Wadleigh, M.; Zahrieh, D.; Lee, S.; Chagnon, P.; Gilliland, D.G.; Busque, L. X-inactivation-based clonality analysis and quantitative JAK2V617F assessment reveal a strong association between clonality and JAK2V617F in PV but not ET/MMM, and identifies a subset of JAK2V617F-negative ET and MMM patients with clonal hematopoiesis. Blood 2006, 107, 4139–4141. [Google Scholar] [CrossRef] [PubMed]
- Klampfl, T.; Gisslinger, H.; Harutyunyan, A.S.; Nivarthi, H.; Rumi, E.; Milosevic, J.D.; Them, N.C.C.; Berg, T.; Gisslinger, B.; Pietra, D.; et al. Somatic Mutations of Calreticulin in Myeloproliferative Neoplasms. N. Engl. J. Med. 2013, 369, 2379–2390. [Google Scholar] [CrossRef] [PubMed]
- Nangalia, J.; Massie, C.E.; Baxter, E.J.; Nice, F.L.; Gundem, G.; Wedge, D.C.; Avezov, E.; Li, J.; Kollmann, K.; Kent, D.G.; et al. Somatic CALR Mutations in Myeloproliferative Neoplasms with Nonmutated JAK2. N. Engl. J. Med. 2013, 369, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Pikman, Y.; Lee, B.H.; Mercher, T.; McDowell, E.; Ebert, B.L.; Gozo, M.; Cuker, A.; Wernig, G.; Moore, S.; Galinsky, I.; et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006, 3, e270. [Google Scholar] [CrossRef]
- Pardanani, A.D.; Levine, R.L.; Lasho, T.; Pikman, Y.; Mesa, R.A.; Wadleigh, M.; Steensma, D.P.; Elliott, M.A.; Wolanskyj, A.P.; Hogan, W.J.; et al. MPL515 mutations in myeloproliferative and other myeloid disorders: A study of 1182 patients. Blood 2006, 108, 3472–3476. [Google Scholar] [CrossRef]
- Vannucchi, A.M.; Antonioli, E.; Guglielmelli, P.; Pancrazzi, A.; Guerini, V.; Barosi, G.; Ruggeri, M.; Specchia, G.; Lo-Coco, F.; Delaini, F.; et al. Characteristics and clinical correlates of MPL 515W>L/K mutation in essential thrombocythemia. Blood 2008, 112, 844–847. [Google Scholar] [CrossRef]
- Chachoua, I.; Pecquet, C.; El-Khoury, M.; Nivarthi, H.; Albu, R.-I.; Marty, C.; Gryshkova, V.; Defour, J.-P.; Vertenoeil, G.; Ngo, A.; et al. Thrombopoietin receptor activation by myeloproliferative neoplasm associated calreticulin mutants. Blood 2016, 127, 1325–1335. [Google Scholar] [CrossRef]
- Beer, P.A.; Campbell, P.J.; Scott, L.M.; Bench, A.J.; Erber, W.N.; Bareford, D.; Wilkins, B.S.; Reilly, J.T.; Hasselbalch, H.C.; Bowman, R.; et al. MPL mutations in myeloproliferative disorders: Analysis of the PT-1 cohort. Blood 2008, 112, 141–149. [Google Scholar] [CrossRef]
- Hasselbalch, H.C. Chronic inflammation as a promotor of mutagenesis in essential thrombocythemia, polycythemia vera and myelofibrosis. A human inflammation model for cancer development? Leuk. Res. 2013, 37, 214–220. [Google Scholar] [CrossRef]
- Grinfeld, J.; Nangalia, J.; Baxter, E.J.; Wedge, D.C.; Angelopoulos, N.; Cantrill, R.; Godfrey, A.L.; Papaemmanuil, E.; Gundem, G.; MacLean, C.; et al. Classification and Personalized Prognosis in Myeloproliferative Neoplasms. N. Engl. J. Med. 2018, 379, 1416–1430. [Google Scholar] [CrossRef]
- Larsen, T.S.; Møller, M.B.; de Stricker, K.; Nørgaard, P.; Samuelsson, J.; Marcher, C.; Andersen, M.T.; Bjerrum, O.W.; Hasselbalch, H.C. Minimal residual disease and normalization of the bone marrow after long-term treatment with alpha-interferon2b in polycythemia vera. A report on molecular response patterns in seven patients in sustained complete hematological remission. Hematology 2009, 14, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.S.; Bjerrum, O.W.; Pallisgaard, N.; Andersen, M.T.; Møller, M.B.; Hasselbalch, H.C. Sustained major molecular response on interferon alpha-2b in two patients with polycythemia vera. Ann. Hematol. 2008, 87, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.N.; Vannucchi, A.M.; Kiladjian, J.-J.; Al-Ali, H.K.; Gisslinger, H.; Knoops, L.; Cervantes, F.; Jones, M.M.; Sun, K.; McQuitty, M.; et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia 2016, 30, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Verstovsek, S.; Mesa, R.A.; Gotlib, J.; Levy, R.S.; Gupta, V.; DiPersio, J.F.; Catalano, J.V.; Deininger, M.; Miller, C.; Silver, R.T.; et al. A Double-Blind, Placebo-Controlled Trial of Ruxolitinib for Myelofibrosis. N. Engl. J. Med. 2012, 366, 799–807. [Google Scholar] [CrossRef]
- Verstovsek, S.; Kantarjian, H.; Mesa, R.A.; Pardanani, A.D.; Cortes-Franco, J.; Thomas, D.A.; Estrov, Z.; Fridman, J.S.; Bradley, E.C.; Erickson-Viitanen, S.; et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N. Engl. J. Med. 2010, 363, 1117–1127. [Google Scholar] [CrossRef]
- Kiladjian, J.-J.; Cassinat, B.; Chevret, S.; Turlure, P.; Cambier, N.; Roussel, M.; Bellucci, S.; Grandchamp, B.; Chomienne, C.; Fenaux, P. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood 2008, 112, 3065–3072. [Google Scholar] [CrossRef]
- Silver, R.T.; Kiladjian, J.-J.; Hasselbalch, H.C. Interferon and the treatment of polycythemia vera, essential thrombocythemia and myelofibrosis. Expert Rev. Hematol. 2013, 6, 49–58. [Google Scholar] [CrossRef]
- Kiladjian, J.; Cassinat, B.; Turlure, P.; Cambier, N.; Roussel, M.; Bellucci, S.; Menot, M.; Massonnet, G.; Dutel, J.; Ghomari, K.; et al. High molecular response rate of polycythemia vera patients treated with pegylated interferon alpha–2a. Blood 2006, 108, 2037–2041. [Google Scholar] [CrossRef]
- Bose, P.; Verstovsek, S. Updates in the management of polycythemia vera and essential thrombocythemia. Ther. Adv. Hematol. 2019, 10, 2040620719870052. [Google Scholar] [CrossRef]
- Kiladjian, J.-J.; Zachee, P.; Hino, M.; Pane, F.; Masszi, T.; Harrison, C.N.; Mesa, R.; Miller, C.B.; Passamonti, F.; Durrant, S.; et al. Long-term efficacy and safety of ruxolitinib versus best available therapy in polycythaemia vera (RESPONSE): 5-year follow up of a phase 3 study. Lancet. Haematol. 2020, 7, e226–e237. [Google Scholar] [CrossRef]
- Gisslinger, H.; Klade, C.; Georgiev, P.; Krochmalczyk, D.; Gercheva-Kyuchukova, L.; Egyed, M.; Rossiev, V.; Dulicek, P.; Illes, A.; Pylypenko, H.; et al. Ropeginterferon alfa-2b versus standard therapy for polycythaemia vera (PROUD-PV and CONTINUATION-PV): A randomised, non-inferiority, phase 3 trial and its extension study. Lancet. Haematol. 2020, 7, e196–e208. [Google Scholar] [CrossRef]
- Verstovsek, S.; Mesa, R.A.; Gotlib, J.; Gupta, V.; DiPersio, J.F.; Catalano, J.V.; Deininger, M.W.N.; Miller, C.B.; Silver, R.T.; Talpaz, M.; et al. Long-term treatment with ruxolitinib for patients with myelofibrosis: 5-year update from the randomized, double-blind, placebo-controlled, phase 3 COMFORT-I trial. J. Hematol. Oncol. 2017, 10, 55. [Google Scholar] [CrossRef]
- Knudsen, T.A.; Hansen, D.L.; Ocias, L.F.; Bjerrum, O.W.; Brabrand, M.; El Fassi, D.; Frederiksen, M.; Kjær, L.; Kristensen, T.K.; Kruse, T.A.; et al. Long-term efficacy and safety of recombinant interferon alpha-2 vs. hydroxyures in polycythemia vera: Preliminary results from the three-year analysis of the DALIAH trial - a randomized controlled phase III clinical trial. Blood 2018, 132. [Google Scholar] [CrossRef]
- Hasselbalch, H.C. A new era for IFN-α in the treatment of Philadelphia-negative chronic myeloproliferative neoplasms. Expert Rev. Hematol. 2011, 4, 637–655. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, H.C.; Holmstrom, M.O. Perspectives on interferon-alpha in the treatment of polycythemia vera and related myeloproliferative neoplasms: Minimal residual disease and cure? Semin. Immunopathol. 2019, 41, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, H.C.; Silver, R.T. Interferon in polycythemia vera and related neoplasms. Can it become the treatment of choice without a randomized trial? Expert Rev. Hem 2015, 8, 1–7. [Google Scholar] [CrossRef]
- Pedersen, R.K.; Andersen, M.; Knudsen, T.A.; Sajid, Z.; Gudmand-Hoeyer, J.; Dam, M.J.B.; Skov, V.; Kjaer, L.; Ellervik, C.; Larsen, T.S.; et al. Data-driven analysis of JAK2V617F kinetics during interferon-alpha2 treatment of patients with polycythemia vera and related neoplasms. Cancer Med. 2020, 9, 2039–2051. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- James, C.; Ugo, V.; Le Couedic, J.-P.; Staerk, J.; Delhommeau, F.; Lacout, C.; Garcon, L.; Raslova, H.; Berger, R.; Bennaceur-Griscelli, A.; et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005, 434, 1144–1148. [Google Scholar] [CrossRef]
- Kralovics, R.; Passamonti, F.; Buser, A.S.; Teo, S.-S.; Tiedt, R.; Passweg, J.R.; Tichelli, A.; Cazzola, M.; Skoda, R.C. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med. 2005, 352, 1779–1790. [Google Scholar] [CrossRef]
- Miklossy, G.; Hilliard, T.S.; Turkson, J. Therapeutic modulators of STAT signalling for human diseases. Nat. Rev. Drug Discov. 2013, 12, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Wadleigh, M.; Cools, J.; Ebert, B.L.; Wernig, G.; Huntly, B.J.P.; Boggon, T.J.; Wlodarska, I.; Clark, J.J.; Moore, S.; et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005, 7, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.M.; Tong, W.; Levine, R.L.; Scott, M.A.; Beer, P.A.; Stratton, M.R.; Futreal, P.A.; Erber, W.N.; McMullin, M.F.; Harrison, C.N.; et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N. Engl. J. Med. 2007, 356, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Pietra, D.; Li, S.; Brisci, A.; Passamonti, F.; Rumi, E.; Theocharides, A.; Ferrari, M.; Gisslinger, H.; Kralovics, R.; Cremonesi, L.; et al. Somatic mutations of JAK2 exon 12 in patients with JAK2 (V617F)-negative myeloproliferative disorders. Blood 2008, 111, 1686–1689. [Google Scholar] [CrossRef]
- Pardanani, A.; Guglielmelli, P.; Lasho, T.L.; Pancrazzi, A.; Finke, C.M.; Vannucchi, A.M.; Tefferi, A. Primary myelofibrosis with or without mutant MPL: Comparison of survival and clinical features involving 603 patients. Leukemia 2011, 25, 1834–1839. [Google Scholar] [CrossRef][Green Version]
- Schnittger, S.; Bacher, U.; Eder, C.; Dicker, F.; Alpermann, T.; Grossmann, V.; Kohlmann, A.; Kern, W.; Haferlach, C.; Haferlach, T. Molecular analyses of 15,542 patients with suspected BCR-ABL1-negative myeloproliferative disorders allow to develop a stepwise diagnostic workflow. Haematologica 2012, 97, 1582–1585. [Google Scholar] [CrossRef]
- Bridgford, J.L.; Lee, S.M.; Lee, C.M.M.; Guglielmelli, P.; Rumi, E.; Pietra, D.; Wilcox, S.; Chhabra, Y.; Rubin, A.F.; Cazzola, M.; et al. Novel drivers and modifiers of MPL-dependent oncogenic transformation identified by deep mutational scanning. Blood 2020, 135, 287–292. [Google Scholar] [CrossRef]
- Pietra, D.; Rumi, E.; Ferretti, V.V.; Di Buduo, C.A.; Milanesi, C.; Cavalloni, C.; Sant’Antonio, E.; Abbonante, V.; Moccia, F.; Casetti, I.C.; et al. Differential clinical effects of different mutation subtypes in CALR-mutant myeloproliferative neoplasms. Leukemia 2016, 30, 431–438. [Google Scholar] [CrossRef]
- Grinfeld, J.; Nangalia, J.; Green, A.R. Molecular determinants of pathogenesis and clinical phenotype in myeloproliferative neoplasms. Haematologica 2017, 102, 7–17. [Google Scholar] [CrossRef]
- Patel, U.; Luthra, R.; Medeiros, L.J.; Patel, K.P. Diagnostic, Prognostic, and Predictive Utility of Recurrent Somatic Mutations in Myeloid Neoplasms. Clin. Lymphoma, Myeloma Leuk. 2017, 17, S62–S74. [Google Scholar] [CrossRef]
- Oz Puyan, F.; Alkan, S. The Progress of Next Generation Sequencing in the Assessment of Myeloid Malignancies. Balkan Med. J. 2019, 36, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Skoda, R.C.; Duek, A.; Grisouard, J. Pathogenesis of myeloproliferative neoplasms. Exp. Hematol. 2015, 43, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Kuo, F.C.; Dong, F. Next-generation sequencing-based panel testing for myeloid neoplasms. Curr. Hematol. Malig. Rep. 2015, 10, 104–111. [Google Scholar] [CrossRef]
- Murati, A.; Brecqueville, M.; Devillier, R.; Mozziconacci, M.-J.; Gelsi-Boyer, V.; Birnbaum, D. Myeloid malignancies: Mutations, models and management. BMC Cancer 2012, 12, 304. [Google Scholar] [CrossRef]
- Matynia, A.P.; Szankasi, P.; Shen, W.; Kelley, T.W. Molecular genetic biomarkers in myeloid malignancies. Arch. Pathol. Lab. Med. 2015, 139, 594–601. [Google Scholar] [CrossRef]
- Hsu, J.I.; Dayaram, T.; Tovy, A.; De Braekeleer, E.; Jeong, M.; Wang, F.; Zhang, J.; Heffernan, T.P.; Gera, S.; Kovacs, J.J.; et al. PPM1D Mutations Drive Clonal Hematopoiesis in Response to Cytotoxic Chemotherapy. Cell Stem Cell 2018, 23, 700–713. [Google Scholar] [CrossRef]
- Coltro, G.; Rotunno, G.; Mannelli, L.; Mannarelli, C.; Fiaccabrino, S.; Romagnoli, S.; Sant’Antonio, E.; Guglielmelli, P.; Vannucchi, A.M. RAS/MAPK Pathway Mutations are Associated with Adverse Survival Outcomes and may Predict Resistance to JAK Inhibitors in Myelofibrosis. EHA Abstr. 2020. [Google Scholar]
- Delic, S.; Rose, D.; Kern, W.; Nadarajah, N.; Haferlach, C.; Haferlach, T.; Meggendorfer, M. Application of an NGS-based 28-gene panel in myeloproliferative neoplasms reveals distinct mutation patterns in essential thrombocythaemia, primary myelofibrosis and polycythaemia vera. Br. J. Haematol. 2016, 175, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Lasho, T.L.; Guglielmelli, P.; Finke, C.M.; Rotunno, G.; Elala, Y.; Pacilli, A.; Hanson, C.A.; Pancrazzi, A.; Ketterling, R.P.; et al. Targeted deep sequencing in polycythemia vera and essential thrombocythemia. Blood Adv. 2016, 1, 21–30. [Google Scholar] [CrossRef]
- Tefferi, A.; Lasho, T.L.; Finke, C.M.; Elala, Y.; Hanson, C.A.; Ketterling, R.P.; Gangat, N.; Pardanani, A. Targeted deep sequencing in primary myelofibrosis. Blood Adv. 2016, 1, 105–111. [Google Scholar] [CrossRef]
- Wanquet, A.; Courtier, F.; Guille, A.; Carbuccia, N.; Garnier, S.; Adelaide, J.; Gelsi-Boyer, V.; Mozziconacci, M.J.; Rey, J.; Vey, N.; et al. Mutation patterns in essential thrombocythemia, polycythemia vera and secondary myelofibrosis. Leuk. Lymphoma 2019, 60, 1289–1293. [Google Scholar] [CrossRef]
- Courtier, F.; Garnier, S.; Carbuccia, N.; Guille, A.; Adelaide, J.; Chaffanet, M.; Hirsch, P.; Paz, D.L.; Slama, B.; Vey, N.; et al. Targeted molecular characterization shows differences between primary and secondary myelofibrosis. Genes. Chromosomes Cancer 2020, 59, 30–39. [Google Scholar] [CrossRef]
- Andreasson, B.; Pettersson, H.; Wasslavik, C.; Johansson, P.; Palmqvist, L.; Asp, J. ASXL1 mutations, previous vascular complications and age at diagnosis predict survival in 85 WHO-defined polycythaemia vera patients. Br. J. Haematol. 2020, 189, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.P.; Newberry, K.J.; Luthra, R.; Jabbour, E.; Pierce, S.; Cortes, J.; Singh, R.; Mehrotra, M.; Routbort, M.J.; Luthra, M.; et al. Correlation of mutation profile and response in patients with myelofibrosis treated with ruxolitinib. Blood 2015, 126, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Alduaij, W.; McNamara, C.J.; Schuh, A.; Arruda, A.; Sukhai, M.; Kanwar, N.; Thomas, M.; Spiegel, J.; Kennedy, J.A.; Stockley, T.; et al. Clinical Utility of Next-generation Sequencing in the Management of Myeloproliferative Neoplasms: A Single-Center Experience. HemaSphere 2018, 4, e44. [Google Scholar] [CrossRef]
- Agarwal, R.; Blombery, P.; McBean, M.; Jones, K.; Fellowes, A.; Doig, K.; Forsyth, C.; Westerman, D.A. Clinicopathological differences exist between CALR- and JAK2-mutated myeloproliferative neoplasms despite a similar molecular landscape: Data from targeted next-generation sequencing in the diagnostic laboratory. Ann. Hematol. 2017, 96, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Rotunno, G.; Pacilli, A.; Artusi, V.; Rumi, E.; Maffioli, M.; Delaini, F.; Brogi, G.; Fanelli, T.; Pancrazzi, A.; Pietra, D.; et al. Epidemiology and clinical relevance of mutations in postpolycythemia vera and postessential thrombocythemia myelofibrosis: A study on 359 patients of the AGIMM group. Am. J. Hematol. 2016, 91, 681–686. [Google Scholar] [CrossRef]
- Guglielmelli, P.; Lasho, T.L.; Rotunno, G.; Mudireddy, M.; Mannarelli, C.; Nicolosi, M.; Pacilli, A.; Pardanani, A.; Rumi, E.; Rosti, V.; et al. MIPSS70: Mutation-Enhanced International Prognostic Score System for Transplantation-Age Patients With Primary Myelofibrosis. J. Clin. Oncol. 2018, 36, 310–318. [Google Scholar] [CrossRef]
- Boiocchi, L.; Hasserjian, R.P.; Pozdnyakova, O.; Wong, W.J.; Lennerz, J.K.; Le, L.P.; Dias-Santagata, D.; Iafrate, A.J.; Hobbs, G.S.; Nardi, V. Clinicopathological and molecular features of SF3B1-mutated myeloproliferative neoplasms. Hum. Pathol. 2019, 86, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pronier, E.; Delhommeau, F. Role of TET2 mutations in myeloproliferative neoplasms. Curr. Hematol. Malig. Rep. 2012, 7, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Marneth, A.E.; Mullally, A. The Molecular Genetics of Myeloproliferative Neoplasms. Cold Spring Harb. Perspect. Med. 2020, 10, a034876. [Google Scholar] [CrossRef] [PubMed]
- Langabeer, S.E.; Andrikovics, H.; Asp, J.; Bellosillo, B.; Carillo, S.; Haslam, K.; Kjaer, L.; Lippert, E.; Mansier, O.; Oppliger Leibundgut, E.; et al. Molecular diagnostics of myeloproliferative neoplasms. Eur. J. Haematol. 2015, 95, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Angona, A.; Fernández-Rodríguez, C.; Alvarez-Larrán, A.; Camacho, L.; Longarón, R.; Torres, E.; Pairet, S.; Besses, C.; Bellosillo, B. Molecular characterisation of triple negative essential thrombocythaemia patients by platelet analysis and targeted sequencing. Blood Cancer J. 2016, 6, e463. [Google Scholar] [CrossRef]
- Inoue, D.; Matsumoto, M.; Nagase, R.; Saika, M.; Fujino, T.; Nakayama, K.I.; Kitamura, T. Truncation mutants of ASXL1 observed in myeloid malignancies are expressed at detectable protein levels. Exp. Hematol. 2016, 44, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Verger, E.; Cassinat, B.; Chauveau, A.; Dosquet, C.; Giraudier, S.; Schlageter, M.H.; Ianotto, J.C.; Yassin, M.A.; Al-Dewik, N.; Carillo, S.; et al. Clinical and molecular response to interferon-α therapy in essential thrombocythemia patients with CALR mutations. Blood 2015, 126, 2585–2591. [Google Scholar] [CrossRef] [PubMed]
- Holz-Schietinger, C.; Matje, D.M.; Reich, N.O. Mutations in DNA methyltransferase (DNMT3A) observed in acute myeloid leukemia patients disrupt processive methylation. J. Biol. Chem. 2012, 287, 30941–30951. [Google Scholar] [CrossRef]
- Cottin, L.; Riou, J.; Orvain, C.; Ianotto, J.C.; Boyer, F.; Renard, M.; Truchan-Graczyk, M.; Murati, A.; Jouanneau-Courville, R.; Allangba, O.; et al. Sequential mutational evaluation of CALR -mutated myeloproliferative neoplasms with thrombocytosis reveals an association between CALR allele burden evolution and disease progression. Br. J. Haematol. 2020, 188, 935–944. [Google Scholar] [CrossRef]
- Khan, S.N.; Jankowska, A.M.; Mahfouz, R.; Dunbar, A.J.; Sugimoto, Y.; Hosono, N.; Hu, Z.; Cheriyath, V.; Vatolin, S.; Przychodzen, B.; et al. Multiple mechanisms deregulate EZH2 and histone H3 lysine 27 epigenetic changes in myeloid malignancies. Leukemia 2013, 27, 1301–1309. [Google Scholar] [CrossRef]
- Ernst, T.; Chase, A.J.; Score, J.; Hidalgo-Curtis, C.E.; Bryant, C.; Jones, A.V.; Waghorn, K.; Zoi, K.; Ross, F.M.; Reiter, A.; et al. Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders. Nat. Genet. 2010, 42, 722–726. [Google Scholar] [CrossRef]
- Shirai, C.L.; Ley, J.N.; White, B.S.; Kim, S.; Tibbitts, J.; Shao, J.; Ndonwi, M.; Wadugu, B.; Duncavage, E.J.; Okeyo-Owuor, T.; et al. Mutant U2AF1 Expression Alters Hematopoiesis and Pre-mRNA Splicing In Vivo. Cancer Cell 2015, 27, 631–643. [Google Scholar] [CrossRef]
- Kim, E.; Ilagan, J.O.; Liang, Y.; Daubner, G.M.; Lee, S.C.-W.; Ramakrishnan, A.; Li, Y.; Chung, Y.R.; Micol, J.-B.; Murphy, M.E.; et al. SRSF2 Mutations Contribute to Myelodysplasia by Mutant-Specific Effects on Exon Recognition. Cancer Cell 2015, 27, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, J.Y.; McNamara, C.; Kennedy, J.A.; Panzarella, T.; Arruda, A.; Stockley, T.; Sukhai, M.; Thomas, M.; Bartoszko, J.; Ho, J.; et al. Impact of genomic alterations on outcomes in myelofibrosis patients undergoing JAK1/2 inhibitor therapy. Blood Adv. 2017, 1, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Szuber, N.; Lasho, T.L.; Finke, C.; Hanson, C.A.; Ketterling, R.P.; Pardanani, A.; Gangat, N.; Tefferi, A. Determinants of long-term outcome in type 1 calreticulin-mutated myelofibrosis. Leukemia 2019, 33, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Bartels, S.; Faisal, M.; Büsche, G.; Schlue, J.; Hasemeier, B.; Schipper, E.; Vogtmann, J.; Westphal, L.; Lehmann, U.; Kreipe, H. Mutations associated with age-related clonal hematopoiesis in PMF patients with rapid progression to myelofibrosis. Leukemia 2020, 34, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Hussaini, M.; Zhang, H.; Shao, H.; Qin, D.; Zhang, X.; Ma, Z.; Hussnain Naqvi, S.M.; Zhang, L.; Moscinski, L.C. Comparison of the Mutational Profiles of Primary Myelofibrosis, Polycythemia Vera, and Essential Thrombocytosis. Am. J. Clin. Pathol. 2017, 147, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Luque Paz, D.; Chauveau, A.; Boyer, F.; Buors, C.; Samaison, L.; Cottin, L.; Seegers, V.; Ferec, C.; Le Marechal, C.; Gueguen, P.; et al. Sequential analysis of 18 genes in polycythemia vera and essential thrombocythemia reveals an association between mutational status and clinical outcome. Genes. Chromosomes Cancer 2017, 56, 354–362. [Google Scholar] [CrossRef]
- Byun, J.M.; Song, S.; Koh, Y.; Yoon, S.-S.; Kim, D. The Temporal Sequence and the Differences in Somatic Mutation Acquisition Determines Clinical Behaviors of JAK2-Positive Myeloproliferative Neoplasms. Anticancer Res. 2019, 39, 6273–6282. [Google Scholar] [CrossRef]
- Gill, H.; Ip, H.W.; Yim, R.; Tang, W.F.; Pang, H.H.; Lee, P.; Leung, G.M.K.; Li, J.; Tang, K.; So, J.C.C.; et al. Next-generation sequencing with a 54-gene panel identified unique mutational profile and prognostic markers in Chinese patients with myelofibrosis. Ann. Hematol. 2019, 98, 869–879. [Google Scholar] [CrossRef]
- Bartels, S.; Faisal, M.; Busche, G.; Schlue, J.; Kreipe, H.; Lehmann, U. Fibrotic progression in Polycythemia vera is associated with early concomitant driver-mutations besides JAK2. Leukemia 2018, 32, 556–558. [Google Scholar] [CrossRef]
- Stevens, E.A.; Jenkins, I.C.; Beppu, L.W.; Zhang, Q.; Salit, R.; Loeb, K.R.; Deeg, H.J.; Radich, J.P. Targeted Sequencing Improves DIPSS-Plus Prognostic Scoring in Myelofibrosis Patients Undergoing Allogeneic Transplant. Biol. Blood Marrow Transplant. 2020, 26, 1371–1374. [Google Scholar] [CrossRef]
- Stengel, A.; Jeromin, S.; Haferlach, T.; Meggendorfer, M.; Kern, W.; Haferlach, C. Detection and characterization of homozygosity of mutated CALR by copy neutral loss of heterozygosity in myeloproliferative neoplasms among cases with high CALR mutation loads or with progressive disease. Haematologica 2019, 104, e187–e190. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M. Functional and cancer genomics of ASXL family members. Br. J. Cancer 2013, 109, 299–306. [Google Scholar] [CrossRef]
- Guglielmelli, P.; Biamonte, F.; Score, J.; Hidalgo-Curtis, C.; Cervantes, F.; Maffioli, M.; Fanelli, T.; Ernst, T.; Winkelman, N.; Jones, A.V.; et al. EZH2 mutational status predicts poor survival in myelofibrosis. Blood 2011, 118, 5227–5234. [Google Scholar] [CrossRef] [PubMed]
- Gelsi-Boyer, V.; Brecqueville, M.; Devillier, R.; Murati, A.; Mozziconacci, M.-J.; Birnbaum, D. Mutations in ASXL1 are associated with poor prognosis across the spectrum of malignant myeloid diseases. J. Hematol. Oncol. 2012, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, M.E.; Abdel-Wahab, O.; Lu, C.; Ward, P.S.; Patel, J.; Shih, A.; Li, Y.; Bhagwat, N.; Vasanthakumar, A.; Fernandez, H.F.; et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell 2010, 18, 553–567. [Google Scholar] [CrossRef]
- Lasho, T.L.; Pardanani, A.; McClure, R.F.; Mesa, R.A.; Levine, R.L.; Gary Gilliland, D.; Tefferi, A. Concurrent MPL515 and JAK2V617F mutations in myelofibrosis: Chronology of clonal emergence and changes in mutant allele burden over time. Br. J. Haematol. 2006, 135, 683–687. [Google Scholar] [CrossRef]
- Nussenzveig, R.H.; Pham, H.T.; Perkins, S.L.; Prchal, J.T.; Agarwal, A.M.; Salama, M.E. Increased frequency of co-existing JAK2 exon-12 or MPL exon-10 mutations in patients with low JAK2(V617F) allelic burden. Leuk. Lymphoma 2016, 57, 1429–1435. [Google Scholar] [CrossRef]
- Pardanani, A.; Lasho, T.L.; Finke, C.M.; Tefferi, A. Infrequent occurrence of MPL exon 10 mutations in polycythemia vera and post-polycythemia vera myelofibrosis. Am. J. Hematol. 2011, 86, 701–702. [Google Scholar] [CrossRef]
- Usseglio, F.; Beaufils, N.; Calleja, A.; Raynaud, S.; Gabert, J. Detection of CALR and MPL Mutations in Low Allelic Burden JAK2 V617F Essential Thrombocythemia. J. Mol. Diagn. 2017, 19, 92–98. [Google Scholar] [CrossRef]
- Holmström, M.O.; Novotny, G.W.; Petersen, J.; Aaboe-Jørgensen, M.; Hasselbalch, H.C.; Andersen, M.H.; Nielsen, S.L.; El Fassi, D.; Schöllkopf, C. Progression of JAK2-mutant polycythemia vera to CALR-mutant myelofibrosis severely impacts on disease phenotype and response to therapy. Leuk. Lymphoma 2019, 60, 3296–3299. [Google Scholar] [CrossRef]
- Beucher, A.; Dib, M.; Orvain, C.; Bouvier, A.; Jouanneau-Courville, R.; Dobo, I.; Cottin, L.; Guardiola, P.; Rousselet, M.-C.; Blanchet, O.; et al. Next generation sequencing redefines a triple negative essential thrombocythaemia as double-positive with rare mutations on JAK2 V617 and MPL W515 hotspots. Br. J. Haematol. 2019, 186, 785–788. [Google Scholar] [CrossRef]
- Pardanani, A.; Lasho, T.; Finke, C.; Oh, S.T.; Gotlib, J.; Tefferi, A. LNK mutation studies in blast-phase myeloproliferative neoplasms, and in chronic-phase disease with TET2, IDH, JAK2 or MPL mutations. Leukemia 2010, 24, 1713–1718. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Sanada, M.; Shiraishi, Y.; Nowak, D.; Nagata, Y.; Yamamoto, R.; Sato, Y.; Sato-Otsubo, A.; Kon, A.; Nagasaki, M.; et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature 2011, 478, 64–69. [Google Scholar] [CrossRef]
- Genovese, G.; Kähler, A.K.; Handsaker, R.E.; Lindberg, J.; Rose, S.A.; Bakhoum, S.F.; Chambert, K.; Mick, E.; Neale, B.M.; Fromer, M.; et al. Clonal Hematopoiesis and Blood-Cancer Risk Inferred from Blood DNA Sequence. N. Engl. J. Med. 2014, 371, 2477–2487. [Google Scholar] [CrossRef] [PubMed]
- Bowman, R.L.; Busque, L.; Levine, R.L. Clonal Hematopoiesis and Evolution to Hematopoietic Malignancies. Cell Stem Cell 2018, 22, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Nowell, P.C. The clonal evolution of tumor cell populations. Science 1976, 194, 23–28. [Google Scholar] [CrossRef]
- Jan, M.; Ebert, B.L.; Jaiswal, S. Clonal hematopoiesis. Semin. Hematol. 2017, 54, 43–50. [Google Scholar] [CrossRef]
- Welch, J.S.; Ley, T.J.; Link, D.C.; Miller, C.A.; Larson, D.E.; Koboldt, D.C.; Wartman, L.D.; Lamprecht, T.L.; Liu, F.; Xia, J.; et al. The origin and evolution of mutations in acute myeloid leukemia. Cell 2012, 150, 264–278. [Google Scholar] [CrossRef]
- Steensma, D.P.; Bejar, R.; Jaiswal, S.; Lindsley, R.C.; Sekeres, M.A.; Hasserjian, R.P.; Ebert, B.L. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 2015, 126, 9–16. [Google Scholar] [CrossRef]
- Busque, L.; Patel, J.P.; Figueroa, M.E.; Vasanthakumar, A.; Provost, S.; Hamilou, Z.; Mollica, L.; Li, J.; Viale, A.; Heguy, A.; et al. Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nat. Genet. 2012, 44, 1179–1181. [Google Scholar] [CrossRef]
- Jaiswal, S.; Fontanillas, P.; Flannick, J.; Manning, A.; Grauman, P.V.; Mar, B.G.; Lindsley, R.C.; Mermel, C.H.; Burtt, N.; Chavez, A.; et al. Age-Related Clonal Hematopoiesis Associated with Adverse Outcomes. N. Engl. J. Med. 2014, 371, 2488–2498. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Lu, C.; Wang, J.; McLellan, M.D.; Johnson, K.J.; Wendl, M.C.; McMichael, J.F.; Schmidt, H.K.; Yellapantula, V.; Miller, C.A.; et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat. Med. 2014, 20, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Delhommeau, F.; Dupont, S.; Della Valle, V.; James, C.; Trannoy, S.; Masse, A.; Kosmider, O.; Le Couedic, J.-P.; Robert, F.; Alberdi, A.; et al. Mutation in TET2 in myeloid cancers. N. Engl. J. Med. 2009, 360, 2289–2301. [Google Scholar] [CrossRef]
- McKerrell, T.; Park, N.; Moreno, T.; Grove, C.S.; Ponstingl, H.; Stephens, J.; Crawley, C.; Craig, J.; Scott, M.A.; Hodkinson, C.; et al. Leukemia-associated somatic mutations drive distinct patterns of age-related clonal hemopoiesis. Cell Rep. 2015, 10, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Craver, B.M.; El Alaoui, K.; Scherber, R.M.; Fleischman, A.G. The critical role of inflammation in the pathogenesis and progression of myeloid malignancies. Cancers (Basel). 2018, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Ferrone, C.K.; Blydt-Hansen, M.; Rauh, M.J. Age-Associated TET2 Mutations: Common Drivers of Myeloid Dysfunction, Cancer and Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef]
- Abegunde, S.O.; Buckstein, R.; Wells, R.A.; Rauh, M.J. An inflammatory environment containing TNFalpha favors Tet2-mutant clonal hematopoiesis. Exp. Hematol. 2018, 59, 60–65. [Google Scholar] [CrossRef]
- Fleischman, A.G.; Aichberger, K.J.; Luty, S.B.; Bumm, T.G.; Petersen, C.L.; Doratotaj, S.; Vasudevan, K.B.; LaTocha, D.H.; Yang, F.; Press, R.D.; et al. TNFalpha facilitates clonal expansion of JAK2V617F positive cells in myeloproliferative neoplasms. Blood 2011, 118, 6392–6398. [Google Scholar] [CrossRef]
- Coombs, C.C.; Zehir, A.; Devlin, S.M.; Kishtagari, A.; Syed, A.; Jonsson, P.; Hyman, D.M.; Solit, D.B.; Robson, M.E.; Baselga, J.; et al. Therapy-Related Clonal Hematopoiesis in Patients with Non-hematologic Cancers Is Common and Associated with Adverse Clinical Outcomes. Cell Stem Cell 2017, 21, 374–382. [Google Scholar] [CrossRef]
- Lundberg, P.; Karow, A.; Nienhold, R.; Looser, R.; Hao-Shen, H.; Nissen, I.; Girsberger, S.; Lehmann, T.; Passweg, J.; Stern, M.; et al. Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood 2014, 123, 2220–2228. [Google Scholar] [CrossRef]
- Zoi, K.; Cross, N.C.P. Genomics of Myeloproliferative Neoplasms. J. Clin. Oncol. 2017, 35, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Song, L.; Zhu, P.; Zhang, B.; Tao, Y.; Xu, X.; Li, F.; Wu, K.; Liang, J.; Shao, D.; et al. Single-cell exome sequencing and monoclonal evolution of a JAK2-negative myeloproliferative neoplasm. Cell 2012, 148, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Engle, E.; Fisher, D.; Miller, C.; McLellan, M.; Fulton, R.; Moore, D.; Wilson, R.; Ley, T.; Oh, S. Clonal Evolution Revealed by Whole Genome Sequencing in a Case of Primary Myelofibrosis Transformed to Secondary Acute Myeloid Leukemia. Leukemia 2015, 29, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Silver, R.T.; Vandris, K.; Wang, Y.L.; Adriano, F.; Jones, A.V.; Christos, P.J.; Cross, N.C.P. JAK2(V617F) allele burden in polycythemia vera correlates with grade of myelofibrosis, but is not substantially affected by therapy. Leuk. Res. 2011, 35, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, A.M.; Antonioli, E.; Guglielmelli, P.; Rambaldi, A.; Barosi, G.; Marchioli, R.; Marfisi, R.M.; Finazzi, G.; Guerini, V.; Fabris, F.; et al. Clinical profile of homozygous JAK2V617F mutation in patients with polycythemia vera or essential thrombocythemia. Blood 2007, 110, 840–846. [Google Scholar] [CrossRef]
- Larsen, T.S.; Pallisgaard, N.; M??ller, M.B.; Hasselbalch, H.C.; Møller, M.B.; Hasselbalch, H.C. The JAK2 V617F allele burden in essential thrombocythemia, polycythemia vera and primary myelofibrosis - Impact on disease phenotype. Eur. J. Haematol. 2007, 79, 508–515. [Google Scholar] [CrossRef]
- Tefferi, A.; Lasho, T.L.; Huang, J.; Finke, C.; Mesa, R.A.; Li, C.Y.; Wu, W.; Hanson, C.A.; Pardanani, A. Low JAK2V617F allele burden in primary myelofibrosis, compared to either a higher allele burden or unmutated status, is associated with inferior overall and leukemia-free survival. Leukemia 2008, 22, 756–761. [Google Scholar] [CrossRef]
- Guglielmelli, P.; Barosi, G.; Specchia, G.; Rambaldi, A.; Lo Coco, F.; Antonioli, E.; Pieri, L.; Pancrazzi, A.; Ponziani, V.; Delaini, F.; et al. Identification of patients with poorer survival in primary myelofibrosis based on the burden of JAK2V617F mutated allele. Blood 2009, 114, 1477–1483. [Google Scholar] [CrossRef]
- Bose, P.; Verstovsek, S. Prognosis of Primary Myelofibrosis in the Genomic Era. Clin. Lymphoma. Myeloma Leuk. 2016, 16 Suppl., S105–S113. [Google Scholar] [CrossRef]
- Theocharides, A.; Boissinot, M.; Girodon, F.; Garand, R.; Teo, S.-S.; Lippert, E.; Talmant, P.; Tichelli, A.; Hermouet, S.; Skoda, R.C. Leukemic blasts in transformed JAK2-V617F-positive myeloproliferative disorders are frequently negative for the JAK2-V617F mutation. Blood 2007, 110, 375–379. [Google Scholar] [CrossRef]
- Beer, P.A.; Delhommeau, F.; LeCouédic, J.P.; Dawson, M.A.; Chen, E.; Bareford, D.; Kušec, R.; McMullin, M.F.; Harrison, C.N.; Vannucchi, A.M.; et al. Two routes to leukemic transformation after a JAK2 mutation-positive myeloproliferative neoplasm. Blood 2010, 115, 2891–2900. [Google Scholar] [CrossRef] [PubMed]
- Ortmann, C.A.; Kent, D.G.; Nangalia, J.; Silber, Y.; Wedge, D.C.; Grinfeld, J.; Baxter, E.J.; Massie, C.E.; Papaemmanuil, E.; Menon, S.; et al. Effect of Mutation Order on Myeloproliferative Neoplasms. N. Engl. J. Med. 2015, 372, 601–612. [Google Scholar] [CrossRef]
- Nangalia, J.; Nice, F.L.; Wedge, D.C.; Godfrey, A.L.; Grinfeld, J.; Thakker, C.; Massie, C.E.; Baxter, J.; Sewell, D.; Silber, Y.; et al. DNMT3A mutations occur early or late in patients with myeloproliferative neoplasms and mutation order influences phenotype. Haematologica 2015, 100, e438–e442. [Google Scholar] [CrossRef]
- Rumi, E.; Cazzola, M. Diagnosis, risk stratification, and response evaluation in classical myeloproliferative neoplasms. Blood 2017, 129, 680–692. [Google Scholar] [CrossRef]
- Barbui, T.; Tefferi, A.; Vannucchi, A.M.; Passamonti, F.; Silver, R.T.; Hoffman, R.; Verstovsek, S.; Mesa, R.; Kiladjian, J.-J.; Hehlmann, R.; et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: Revised management recommendations from European LeukemiaNet. Leukemia 2018, 32, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Rumi, E.; Pietra, D.; Ferretti, V.; Klampfl, T.; Harutyunyan, A.S.; Milosevic, J.D.; Them, N.C.C.; Berg, T.; Elena, C.; Casetti, I.C.; et al. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood 2014, 123, 1544–1551. [Google Scholar] [CrossRef]
- Rotunno, G.; Mannarelli, C.; Guglielmelli, P.; Pacilli, A.; Pancrazzi, A.; Pieri, L.; Fanelli, T.; Bosi, A.; Vannucchi, A.M. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood 2014, 123, 1552–1555. [Google Scholar] [CrossRef]
- Guglielmelli, P.; Biamonte, F.; Rotunno, G.; Artusi, V.; Artuso, L.; Bernardis, I.; Tenedini, E.; Pieri, L.; Paoli, C.; Mannarelli, C.; et al. Impact of mutational status on outcomes in myelofibrosis patients treated with ruxolitinib in the COMFORT-II study. Blood 2014, 123, 2157–2160. [Google Scholar] [CrossRef]
- Rumi, E.; Pietra, D.; Pascutto, C.; Guglielmelli, P.; Martinez-Trillos, A.; Casetti, I.; Colomer, D.; Pieri, L.; Pratcorona, M.; Rotunno, G.; et al. Clinical effect of driver mutations of JAK2, CALR, or MPL in primary myelofibrosis. Blood 2014, 124, 1062–1069. [Google Scholar] [CrossRef]
- Tefferi, A.; Lasho, T.L.; Finke, C.M.; Knudson, R.A.; Ketterling, R.; Hanson, C.H.; Maffioli, M.; Caramazza, D.; Passamonti, F.; Pardanani, A. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: Clinical, cytogenetic and molecular comparisons. Leukemia 2014, 28, 1472–1477. [Google Scholar] [CrossRef]
- Merker, J.D.; Roskin, K.M.; Ng, D.; Pan, C.; Fisk, D.G.; King, J.J.; Hoh, R.; Stadler, M.; Okumoto, L.M.; Abidi, P.; et al. Comprehensive whole-genome sequencing of an early-stage primary myelofibrosis patient defines low mutational burden and non-recurrent candidate genes. Haematologica 2013, 98, 1689–1696. [Google Scholar] [CrossRef][Green Version]
- Tenedini, E.; Bernardis, I.; Artusi, V.; Artuso, L.; Roncaglia, E.; Guglielmelli, P.; Pieri, L.; Bogani, C.; Biamonte, F.; Rotunno, G.; et al. Targeted cancer exome sequencing reveals recurrent mutations in myeloproliferative neoplasms. Leukemia 2014, 28, 1052–1059. [Google Scholar] [CrossRef]
- Wang, L.; Swierczek, S.I.; Drummond, J.; Hickman, K.; Kim, S.J.; Walker, K.; Doddapaneni, H.; Muzny, D.M.; Gibbs, R.A.; Wheeler, D.A.; et al. Whole-exome sequencing of polycythemia vera revealed novel driver genes and somatic mutation shared by T cells and granulocytes. Leukemia 2014, 28, 935–938. [Google Scholar] [CrossRef]
- Angona, A.; Alvarez-Larrán, A.; Bellosillo, B.; Martínez-Avilés, L.; Camacho, L.; Fernández-Rodríguez, C.; Pairet, S.; Longarón, R.; Ancochea, Á.; Senín, A.; et al. Hematopoietic clonal dominance, stem cell mutations, and evolutionary pattern of JAK2V617F allele burden in polycythemia vera. Eur. J. Haematol. 2015, 94, 251–257. [Google Scholar] [CrossRef]
- Kirschner, M.M.J.; Schemionek, M.; Schubert, C.; Chatain, N.; Sontag, S.; Isfort, S.; Ortiz-Bruchle, N.; Schmitt, K.; Kruger, L.; Zerres, K.; et al. Dissecting Genomic Aberrations in Myeloproliferative Neoplasms by Multiplex-PCR and Next Generation Sequencing. PLoS ONE 2015, 10, e0123476. [Google Scholar] [CrossRef]
- Asp, J.; Andreasson, B.; Hansson, U.; Wasslavik, C.; Abelsson, J.; Johansson, P.; Palmqvist, L. Mutation status of essential thrombocythemia and primary myelofibrosis defines clinical outcome. Haematologica 2016, 101, e129–e132. [Google Scholar] [CrossRef]
- Cabagnols, X.; Favale, F.; Pasquier, F.; Messaoudi, K.; Defour, J.P.; Ianotto, J.C.; Marzac, C.; Le Couedic, J.P.; Droin, N.; Chachoua, I.; et al. Presence of atypical thrombopoietin receptor (MPL) mutations in triple-negative essential thrombocythemia patients. Blood 2016, 127, 333–342. [Google Scholar] [CrossRef]
- Jeromin, S.; Kohlmann, A.; Meggendorfer, M.; Schindela, S.; Perglerová, K.; Nadarajah, N.; Kern, W.; Haferlach, C.; Haferlach, T.; Schnittger, S. Next-generation deep-sequencing detects multiple clones of CALR mutations in patients with BCR-ABL1 negative MPN. Leukemia 2016, 30, 973–976. [Google Scholar] [CrossRef]
- Magor, G.W.; Tallack, M.R.; Klose, N.M.; Taylor, D.; Korbie, D.; Mollee, P.; Trau, M.; Perkins, A.C. Rapid Molecular Profiling of Myeloproliferative Neoplasms Using Targeted Exon Resequencing of 86 Genes Involved in JAK-STAT Signaling and Epigenetic Regulation. J. Mol. Diagnostics 2016, 18, 707–718. [Google Scholar] [CrossRef]
- Milosevic Feenstra, J.D.; Nivarthi, H.; Gisslinger, H.; Leroy, E.; Rumi, E.; Chachoua, I.; Bagienski, K.; Kubesova, B.; Pietra, D.; Gisslinger, B.; et al. Whole-exome sequencing identifies novel MPL and JAK2 mutations in triple-negative myeloproliferative neoplasms. Blood 2016, 127, 325–332. [Google Scholar] [CrossRef]
- Casolari, D.A.; Nguyen, T.; Butcher, C.M.; Iarossi, D.G.; Hahn, C.N.; Bray, S.C.; Neufing, P.; Parker, W.T.; Feng, J.; Maung, K.Z.Y.; et al. A novel, somatic, transforming mutation in the extracellular domain of Epidermal Growth Factor Receptor identified in myeloproliferative neoplasm. Sci. Rep. 2017, 7, 2467. [Google Scholar] [CrossRef]
- Chang, Y.C.; Lin, H.C.; Chiang, Y.H.; Chen, C.G.S.; Huang, L.; Wang, W.T.; Cheng, C.C.; Lin, J.; Chang, Y.F.; Chang, M.C.; et al. Targeted next-generation sequencing identified novel mutations in triple-negative myeloproliferative neoplasms. Med. Oncol. 2017, 34, 1–6. [Google Scholar] [CrossRef]
- Courtier, F.; Carbuccia, N.; Garnier, S.; Guille, A.; Adelaide, J.; Cervera, N.; Gelsi-Boyer, V.; Mozziconacci, M.-J.; Rey, J.; Vey, N.; et al. Genomic analysis of myeloproliferative neoplasms in chronic and acute phases. Haematologica 2017, 102, e11–e14. [Google Scholar] [CrossRef]
- Kröger, N.; Panagiota, V.; Badbaran, A.; Zabelina, T.; Triviai, I.; Araujo Cruz, M.M.; Shahswar, R.; Ayuk, F.; Gehlhaar, M.; Wolschke, C.; et al. Impact of Molecular Genetics on Outcome in Myelofibrosis Patients after Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2017, 23, 1095–1101. [Google Scholar] [CrossRef]
- Masarova, L.; Patel, K.P.; Newberry, K.J.; Cortes, J.; Borthakur, G.; Konopleva, M.; Estrov, Z.; Kantarjian, H.; Verstovsek, S. Pegylated interferon alfa-2a in patients with essential thrombocythaemia or polycythaemia vera: A post-hoc, median 83 month follow-up of an open-label, phase 2 trial. Lancet. Haematol. 2017, 4, e165–e175. [Google Scholar] [CrossRef]
- Newberry, K.J.; Patel, K.; Masarova, L.; Luthra, R.; Manshouri, T.; Jabbour, E.; Bose, P.; Daver, N.; Cortes, J.; Kantarjian, H.; et al. Clonal evolution and outcomes in myelofibrosis after ruxolitinib discontinuation. Blood 2017, 130, 1125–1131. [Google Scholar] [CrossRef]
- Silver, R.T.; Barel, A.C.; Lascu, E.; Ritchie, E.K.; Roboz, G.J.; Christos, P.J.; Orazi, A.; Hassane, D.C.; Tam, W.; Cross, N.C.P. The effect of initial molecular profile on response to recombinant interferon-alpha (rIFNalpha) treatment in early myelofibrosis. Cancer 2017, 123, 2680–2687. [Google Scholar] [CrossRef]
- Zaidi, U.; Shahid, S.; Fatima, N.; Ahmed, S.; Sufaida, G.; Nadeem, M.; Shamsi, T. Genomic profile of a patient with triple negative essential thrombocythemia, unresponsive to therapy: A case report and literature review. J. Adv. Res. 2017, 8, 375–378. [Google Scholar] [CrossRef]
- Ayres-Silva, J.P.; Bonamino, M.H.; Gouveia, M.E.; Monte-Mor, B.C.R.; Coutinho, D.F.; Daumas, A.H.; Solza, C.; Braggio, E.; Zalcberg, I.R. Genetic Alterations in Essential Thrombocythemia Progression to Acute Myeloid Leukemia: A Case Series and Review of the Literature. Front. Oncol. 2018, 8, 32. [Google Scholar] [CrossRef]
- Ianotto, J.-C.; Chauveau, A.; Boyer-Perrard, F.; Gyan, E.; Laribi, K.; Cony-Makhoul, P.; Demory, J.-L.; de Renzis, B.; Dosquet, C.; Rey, J.; et al. Benefits and pitfalls of pegylated interferon-α2a therapy in patients with myeloproliferative neoplasm-associated myelofibrosis: A French Intergroup of Myeloproliferative neoplasms (FIM) study. Haematologica 2018, 103, 438–446. [Google Scholar] [CrossRef]
- Ju, M.; Fu, R.; Li, H.; Liu, X.; Xue, F.; Chen, Y.; Liu, W.; Huang, Y.; Zhang, L.L.L.L.; Yang, R.; et al. Mutation profiling by targeted sequencing of “triple-negative” essential thrombocythaemia patients - supplemental. Br. J. Haematol. 2018, 181, 857–860. [Google Scholar] [CrossRef]
- Kubesova, B.; Pavlova, S.; Malcikova, J.; Kabathova, J.; Radova, L.; Tom, N.; Tichy, B.; Plevova, K.; Kantorova, B.; Fiedorova, K.; et al. Low-burden TP53 mutations in chronic phase of myeloproliferative neoplasms: Association with age, hydroxyurea administration, disease type and JAK2 mutational status. Leukemia 2018, 32, 450–461. [Google Scholar] [CrossRef]
- Pacilli, A.; Rotunno, G.; Mannarelli, C.; Fanelli, T.; Pancrazzi, A.; Contini, E.; Mannelli, F.; Gesullo, F.; Bartalucci, N.; Fattori, G.C.; et al. Mutation landscape in patients with myelofibrosis receiving ruxolitinib or hydroxyurea. Blood Cancer J. 2018, 8, 122. [Google Scholar] [CrossRef]
- Senín, A.; Fernández-Rodríguez, C.; Bellosillo, B.; Camacho, L.; Longarón, R.; Angona, A.; Besses, C.; Álvarez-Larrán, A. Non-driver mutations in patients with JAK2V617F-mutated polycythemia vera or essential thrombocythemia with long-term molecular follow-up. Ann. Hematol. 2018, 97, 443–451. [Google Scholar] [CrossRef]
- Tefferi, A.; Guglielmelli, P.; Nicolosi, M.; Mannelli, F.; Mudireddy, M.; Bartalucci, N.; Finke, C.M.; Lasho, T.L.; Hanson, C.A.; Ketterling, R.P.; et al. GIPSS: Genetically inspired prognostic scoring system for primary myelofibrosis. Leukemia 2018, 32, 1631–1642. [Google Scholar] [CrossRef]
- Tefferi, A.; Barraco, D.; Lasho, T.L.; Shah, S.; Begna, K.H.; Al-Kali, A.; Hogan, W.J.; Litzow, M.R.; Hanson, C.A.; Ketterling, R.P.; et al. Momelotinib therapy for myelofibrosis: A 7-year follow-up. Blood Cancer J. 2018, 8, 29. [Google Scholar] [CrossRef]
- Acha, P.; Xandri, M.; Fuster-Tormo, F.; Palomo, L.; Xicoy, B.; Cabezon, M.; Marce, S.; Granada, I.; Vela, D.; Sagues, M.; et al. Diagnostic and prognostic contribution of targeted NGS in patients with triple-negative myeloproliferative neoplasms. Am. J. Hematol. 2019, 94, E264–E267. [Google Scholar] [CrossRef]
- Gagelmann, N.; Ditschkowski, M.; Bogdanov, R.; Bredin, S.; Robin, M.; Cassinat, B.; Shahswar, R.; Thol, F.; Heuser, M.; Socié, G.; et al. Comprehensive clinical-molecular transplant scoring system for myelofibrosis undergoing stem cell transplantation. Blood 2019, 133, 2233–2242. [Google Scholar] [CrossRef]
- Knudsen, T.A.; Skov, V.; Werner, L.; Duke, W.; Gibson, C.J.; Nag, A.; Thorner, A.R.; Wollison, B.; Hansen, D.L.; Stauffer Larsen, T.; et al. Genomic Profiling of a Phase III Clinical Trial of Interferon Versus Hydroxyurea in MPN Patients Reveals Mutation-Specific and Treatment-Specific Patterns of Response. Blood (ASH Annu. Meet. Abstr. 2019, 134, 4202. [Google Scholar] [CrossRef]
- Luque Paz, D.L.; Mansier, O.; Riou, J.; Conejero, C.; Roy, L.; Belkhodja, C.; Ugo, V.; Giraudier, S. Positive impact of molecular analysis on prognostic scores in essential thrombocythemia: A single center prospective cohort experience. Haematologica 2019, 104, e134–e137. [Google Scholar]
- Mannina, D.; Gagelmann, N.; Badbaran, A.; Ditschkowski, M.; Bogdanov, R.; Robin, M.; Cassinat, B.; Heuser, M.; Shahswar, R.; Thol, F.; et al. Allogeneic stem cell transplantation in patients with myelofibrosis harboring the MPL mutation. Eur. J. Haematol. 2019, 103, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Nam, A.S.; Kim, K.-T.; Chaligne, R.; Izzo, F.; Ang, C.; Taylor, J.; Myers, R.M.; Abu-Zeinah, G.; Brand, R.; Omans, N.D.; et al. Somatic mutations and cell identity linked by Genotyping of Transcriptomes. Nature 2019, 571, 355–360. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.M.; Hamblin, A.; Yap, C.; Fox, S.; Boucher, R.; Panchal, A.; Alimam, S.; Dreau, H.; Howard, K.; Ware, P.; et al. The poor outcome in high molecular risk, hydroxycarbamide-resistant/intolerant ET is not ameliorated by ruxolitinib. Blood 2019, 134, 2107–2111. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Meira, A.; Buck, G.; Clark, S.-A.; Povinelli, B.J.; Alcolea, V.; Louka, E.; McGowan, S.; Hamblin, A.; Sousos, N.; Barkas, N.; et al. Unravelling Intratumoral Heterogeneity through High-Sensitivity Single-Cell Mutational Analysis and Parallel RNA Sequencing. Mol. Cell 2019, 73, 1292–1305. [Google Scholar] [CrossRef] [PubMed]
- Schischlik, F.; Jäger, R.; Rosebrock, F.; Hug, E.; Schuster, M.; Holly, R.; Fuchs, E.; Milosevic Feenstra, J.D.; Bogner, E.; Gisslinger, B.; et al. Mutational landscape of the transcriptome offers putative targets for immunotherapy of myeloproliferative neoplasms. Blood 2019, 134, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Tamari, R.; Rapaport, F.; Zhang, N.; McNamara, C.; Kuykendall, A.; Sallman, D.A.; Komrokji, R.; Arruda, A.; Najfeld, V.; Sandy, L.; et al. Impact of High-Molecular-Risk Mutations on Transplantation Outcomes in Patients with Myelofibrosis. Biol. Blood Marrow Transplant. 2019, 25, 1142–1151. [Google Scholar] [CrossRef]
- Yacoub, A.; Mascarenhas, J.; Kosiorek, H.; Prchal, J.T.; Berenzon, D.; Baer, M.R.; Ritchie, E.; Silver, R.T.; Kessler, C.; Winton, E.; et al. Pegylated interferon alfa-2a for polycythemia vera or essential thrombocythemia resistant or intolerant to hydroxyurea. Blood 2019, 134, 1498–1509. [Google Scholar] [CrossRef]
- Cassinat, B.; Verger, E.; Maslah, N.; Gauthier, N.; Vainchenker, W.; Giraudier, S.; Kiladjian, J.-J. CCND2 mutations are infrequent events in BCR-ABL1 negative myeloproliferative neoplasm patients. Haematologica 2020. [Google Scholar] [CrossRef]
- Gill, H.; Leung, G.M.K.; Yim, R.; Lee, P.; Pang, H.H.; Ip, H.-W.; Leung, R.Y.Y.; Li, J.; Panagiotou, G.; Ma, E.S.K.; et al. Myeloproliferative neoplasms treated with hydroxyurea, pegylated interferon alpha-2A or ruxolitinib: Clinicohematologic responses, quality-of-life changes and safety in the real-world setting. Hematology 2020, 25, 247–257. [Google Scholar] [CrossRef]
- Guglielmelli, P.; Carobbio, A.; Rumi, E.; De Stefano, V.; Mannelli, L.; Mannelli, F.; Rotunno, G.; Coltro, G.; Betti, S.; Cavalloni, C.; et al. Validation of the IPSET score for thrombosis in patients with prefibrotic myelofibrosis. Blood Cancer J. 2020, 10, 21. [Google Scholar] [CrossRef]
- Karantanos, T.; Chaturvedi, S.; Braunstein, E.M.; Spivak, J.; Resar, L.; Karanika, S.; Williams, D.M.; Rogers, O.; Gocke, C.D.; Moliterno, A.R. Sex determines the presentation and outcomes in MPN and is related to sex-specific differences in the mutational burden. Blood Adv. 2020, 4, 2567–2576. [Google Scholar] [CrossRef] [PubMed]
- Kralovics, R.; Klade, C.; Georgiev, P.; Krochmalczyk, D.; Gercheva-Kyuchukova, L.; Egyed, M.; Rossiev, V.; Dulicek, P.; Illes, A.; Pylypenko, H.; et al. Ropeginterferon Alpha-2b is Efficacious and Reduces Variant TET2 Allele Burden in Patients with Polycythaemia Vera and TET2 Mutation: Genetic Analysis of Phase III PROUD-PV/CONTINUATION-PV Studies. EHA Abstr. 2020. [Google Scholar]
- Mylonas, E.; Yoshida, K.; Frick, M.; Hoyer, K.; Christen, F.; Kaeda, J.; Obenaus, M.; Noerenberg, D.; Hennch, C.; Chan, W.; et al. Single-cell analysis based dissection of clonality in myelofibrosis. Nat. Commun. 2020, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Nonino, A.; Campregher, P.V.; de Souza Santos, F.P.; Mazzeu, J.F.; Pereira, R.W. Genomic characterization and prognostication applied to a Brazilian cohort of patients with myelofibrosis. Int. J. Hematol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Segura-Diaz, A.; Stuckey, R.; Florido, Y.; Gonzalez-Martin, J.M.; Lopez-Rodriguez, J.F.; Sanchez-Sosa, S.; Gonzalez-Perez, E.; Saez Perdomo, M.N.; Perera, M.D.M.; de la Iglesia, S.; et al. Thrombotic Risk Detection in Patients with Polycythemia Vera: The Predictive Role of DNMT3A/TET2/ASXL1 Mutations. Cancers (Basel) 2020, 12, 934. [Google Scholar] [CrossRef]
- Tefferi, A.; Guglielmelli, P.; Lasho, T.L.; Coltro, G.; Finke, C.M.; Loscocco, G.G.; Sordi, B.; Szuber, N.; Rotunno, G.; Pacilli, A.; et al. Mutation-enhanced international prognostic systems for essential thrombocythaemia and polycythaemia vera. Br. J. Haematol. 2020, 189, 291–302. [Google Scholar] [CrossRef]
- Vannucchi, A.M.; Lasho, T.L.; Guglielmelli, P.; Biamonte, F.; Pardanani, A.; Pereira, A.; Finke, C.; Score, J.; Gangat, N.; Mannarelli, C.; et al. Mutations and prognosis in primary myelofibrosis. Leukemia 2013, 27, 1861–1869. [Google Scholar] [CrossRef]
- Tefferi, A.; Lasho, T.L.; Hanson, C.A.; Ketterling, R.P.; Gangat, N.; Pardanani, A. Screening for ASXL1 and SRSF2 mutations is imperative for treatment decision-making in otherwise low or intermediate-1 risk patients with myelofibrosis. Br. J. Haematol. 2018, 183, 678–681. [Google Scholar] [CrossRef]
- Tefferi, A.; Lasho, T.L.; Finke, C.; Gangat, N.; Hanson, C.A.; Ketterling, R.P.; Pardanani, A. Prognostic significance of ASXL1 mutation types and allele burden in myelofibrosis. Leukemia 2018, 32, 837–839. [Google Scholar] [CrossRef]
- Lasho, T.L.; Jimma, T.; Finke, C.M.; Patnaik, M.; Hanson, C.A.; Ketterling, R.P.; Pardanani, A.; Tefferi, A. SRSF2 mutations in primary myelofibrosis: Significant clustering with IDH mutations and independent association with inferior overall and leukemia-free survival. Blood 2012, 120, 4168–4171. [Google Scholar] [CrossRef]
- Vainchenker, W.; Kralovics, R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood 2017, 129, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Guglielmelli, P.; Lasho, T.L.; Rotunno, G.; Finke, C.; Mannarelli, C.; Belachew, A.A.; Pancrazzi, A.; Wassie, E.A.; Ketterling, R.P.; et al. CALR and ASXL1 mutations-based molecular prognostication in primary myelofibrosis: An international study of 570 patients. Leukemia 2014, 28, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Nicolosi, M.; Mudireddy, M.; Szuber, N.; Finke, C.M.; Lasho, T.L.; Hanson, C.A.; Ketterling, R.P.; Pardanani, A.; Gangat, N.; et al. Driver mutations and prognosis in primary myelofibrosis: Mayo-Careggi MPN alliance study of 1,095 patients. Am. J. Hematol. 2018, 93, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Yonal-Hindilerden, I.; Daglar-Aday, A.; Akadam-Teker, B.; Yilmaz, C.; Nalcaci, M.; Yavuz, A.S.; Sargin, D. Prognostic significance of ASXL1, JAK2V617F mutations and JAK2V617F allele burden in Philadelphia-negative myeloproliferative neoplasms. J. Blood Med. 2015, 6, 157–175. [Google Scholar] [CrossRef]
- Tefferi, A.; Finke, C.M.; Lasho, T.L.; Hanson, C.A.; Ketterling, R.P.; Gangat, N.; Pardanani, A. U2AF1 mutation types in primary myelofibrosis: Phenotypic and prognostic distinctions. Leukemia 2018, 32, 2274–2278. [Google Scholar] [CrossRef]
- Tefferi, A.; Pardanani, A.; Lim, K.H.; Abdel-Wahab, O.; Lasho, T.L.; Patel, J.; Gangat, N.; Finke, C.M.; Schwager, S.; Mullally, A.; et al. TET2 mutations and their clinical correlates in polycythemia vera, essential thrombocythemia and myelofibrosis. Leukemia 2009, 23, 905–911. [Google Scholar] [CrossRef]
- Abdel-Wahab, O.; Manshouri, T.; Patel, J.; Harris, K.; Yao, J.; Hedvat, C.; Heguy, A.; Bueso-Ramos, C.; Kantarjian, H.; Levine, R.L.; et al. Genetic analysis of transforming events that convert chronic myeloproliferative neoplasms to leukemias. Cancer Res. 2010, 70, 447–452. [Google Scholar] [CrossRef]
- Quintas-Cardama, A.; Abdel-Wahab, O.; Manshouri, T.; Kilpivaara, O.; Cortes, J.; Roupie, A.-L.A.L.; Zhang, S.J.; Harris, D.; Estrov, Z.; Kantarjian, H.; et al. Molecular analysis of patients with polycythemia vera or essential thrombocythemia receiving pegylated interferon a -2a. Blood 2013, 122, 893–901. [Google Scholar] [CrossRef]
- Harutyunyan, A.; Klampfl, T.; Cazzola, M.; Kralovics, R. p53 lesions in leukemic transformation. N. Engl. J. Med. 2011, 364, 488–490. [Google Scholar] [CrossRef]
- Ruark, E.; Snape, K.; Humburg, P.; Loveday, C.; Bajrami, I.; Brough, R.; Rodrigues, D.N.; Renwick, A.; Seal, S.; Ramsay, E.; et al. Mosaic PPM1D mutations are associated with predisposition to breast and ovarian cancer. Nature 2013, 493, 406–410. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.H.; Wan, H.; Yang, R.; Wang, Z.; Feng, J.; Yang, S.; Jones, S.; Wang, S.; Zhou, W.; et al. Exome sequencing identifies somatic gain-of-function PPM1D mutations in brainstem gliomas. Nat. Genet. 2014, 46, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Bian, E.-B.; Zong, G.; Xie, Y.-S.; Meng, X.-M.; Huang, C.; Li, J.; Zhao, B. TET family proteins: New players in gliomas. J. Neurooncol. 2014, 116, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Sclafani, F.; Wilson, S.H.; Cunningham, D.; Gonzalez De Castro, D.; Kalaitzaki, E.; Begum, R.; Wotherspoon, A.; Capdevila, J.; Glimelius, B.; Rosello, S.; et al. Analysis of KRAS, NRAS, BRAF, PIK3CA and TP53 mutations in a large prospective series of locally advanced rectal cancer patients. Int. J. cancer 2020, 146, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, M.S.; Lee, L.J.; Jeon, Y.-J.; Flynn, R.A.; Stehr, H.; Hui, A.B.; Ishisoko, N.; Kildebeck, E.; Newman, A.M.; Bratman, S.V.; et al. Functional significance of U2AF1 S34F mutations in lung adenocarcinomas. Nat. Commun. 2019, 10, 5712. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, C.S.; Amici, M.; Bortolotto, Z.A.; Doherty, A.; Csaba, Z.; Fafouri, A.; Dournaud, P.; Gressens, P.; Collingridge, G.L.; Peineau, S. The role of JAK-STAT signaling within the CNS. JAK-STAT 2013, 2, e22925. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.; Farkas, D.K.; Christiansen, C.F.; Larsen, T.S.; Hasselbalch, H.C.; Stentoft, J.; Sørensen, H.T. Survival of patients with chronic myeloproliferative neoplasms and new primary cancers: A population-based cohort study. Lancet. Haematol. 2015, 2, e289–e296. [Google Scholar] [CrossRef][Green Version]
- Landtblom, A.R.; Bower, H.; Andersson, T.M.L.; Dickman, P.W.; Samuelsson, J.; Björkholm, M.; Kristinsson, S.Y.; Hultcrantz, M. Second malignancies in patients with myeloproliferative neoplasms: A population-based cohort study of 9379 patients. Leukemia 2018, 32, 2203–2210. [Google Scholar] [CrossRef]
- Pettersson, H.; Knutsen, H.; Holmberg, E.; Andréasson, B. Increased incidence of another cancer in myeloproliferative neoplasms patients at the time of diagnosis. Eur. J. Haematol. 2015, 94, 152–156. [Google Scholar] [CrossRef]
- Cervantes, F.; Dupriez, B.; Pereira, A.; Passamonti, F.; Reilly, J.T.; Morra, E.; Vannucchi, A.M.; Mesa, R.A.; Demory, J.-L.; Barosi, G.; et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood 2009, 113, 2895–2901. [Google Scholar] [CrossRef]
- Passamonti, F.; Cervantes, F.; Vannucchi, A.M.; Morra, E.; Rumi, E.; Pereira, A.; Guglielmelli, P.; Pungolino, E.; Caramella, M.; Maffioli, M.; et al. A dynamic prognostic model to predict survival in primary myelofibrosis: A study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood 2010, 115, 1703–1708. [Google Scholar] [CrossRef]
- Passamonti, F.; Giorgino, T.; Mora, B.; Guglielmelli, P.; Rumi, E.; Maffioli, M.; Rambaldi, A.; Caramella, M.; Komrokji, R.; Gotlib, J.; et al. A clinical-molecular prognostic model to predict survival in patients with post polycythemia vera and post essential thrombocythemia myelofibrosis. Leukemia 2017, 31, 2726–2731. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, L.; Chen, H.; Wang, Y.; Xu, Y.; Mao, H.; Li, J.; Mills, G.B.; Shu, Y.; Li, L.; et al. Comprehensive Characterization of Molecular Differences in Cancer between Male and Female Patients. Cancer Cell 2016, 29, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Guglielmelli, P.; Lasho, T.L.; Rotunno, G.; Score, J.; Mannarelli, C.; Pancrazzi, A.; Biamonte, F.; Pardanani, A.; Zoi, K.; Reiter, A.; et al. The number of prognostically detrimental mutations and prognosis in primary myelofibrosis: An international study of 797 patients. Leukemia 2014, 28, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, I.; Hasselbalch, H.C. Acute leukemia and myelodysplasia in patients with a Philadelphia chromosome negative chronic myeloproliferative disorder treated with hydroxyurea alone or with hydroxyurea after busulphan. Am. J. Hematol. 2003, 74, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Spivak, J.L.; Hasselbalch, H. Hydroxycarbamide: A user ’ s guide for chronic myeloproliferative disorders. Expert Rev. Anticancer Ther. 2011, 11, 403–414. [Google Scholar] [CrossRef]
- Kiladjian, J.J.; Chevret, S.; Dosquet, C.; Chomienne, C.; Rain, J.D. Treatment of polycythemia vera with hydroxyurea and pipobroman: Final results of a randomized trial initiated in 1980. J. Clin. Oncol. 2011, 29, 3907–3913. [Google Scholar] [CrossRef]
- Kissova, J.; Ovesna, P.; Penka, M.; Bulikova, A.; Kiss, I. Second Malignancies in Philadelphia-negative Myeloproliferative Neoplasms − Single-center Experience. Anticancer Res. 2014, 34, 2489–2496. [Google Scholar]
- Esteve, E.; Georgescu, V.; Heitzmann, P.; Martin, L. [Multiple skin and mouth squamous cell carcinomas related to long-term treatment with hydroxyurea]. Ann. Dermatol. Venereol. 2001, 128, 919–921. [Google Scholar]
- Spanoudakis, E.; Bazdiara, I.; Kotsianidis, I.; Margaritis, D.; Goutzouvelidis, A.; Christoforidou, A.; Tsatalas, C.; Bourikas, G. Hydroxyurea (HU) is effective in reducing JAK2V617F mutated clone size in the peripheral blood of essential thrombocythemia (ET) and polycythemia vera (PV) patients. Ann. Hematol. 2009, 88, 629–632. [Google Scholar] [CrossRef]
- Larsen, T.S.; Pallisgaard, N.; de Stricker, K.; Møller, M.B.; Hasselbalch, H.C. Limited efficacy of hydroxyurea in lowering of the JAK2 V617F allele burden. Hematology 2009, 14, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, E.; Carobbio, A.; Pieri, L.; Pancrazzi, A.; Guglielmelli, P.; Delaini, F.; Ponziani, V.; Bartalucci, N.; Tozzi, L.; Bosi, A.; et al. Hydroxyurea does not appreciably reduce JAK2 v617F allele burden in patients with polycythemia vera or essential thrombocythemia. Haematologica 2010, 95, 1435–1438. [Google Scholar] [CrossRef]
- Campbell, P.J.; Scott, L.M.; Buck, G.; Wheatley, K.; East, C.L.; Marsden, J.T.; Duffy, A.; Boyd, E.M.; Bench, A.J.; Scott, M.A.; et al. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: A prospective study. Lancet (Lond. Engl.) 2005, 366, 1945–1953. [Google Scholar] [CrossRef]
- Harrison, C.N.; Campbell, P.J.; Buck, G.; Wheatley, K.; East, C.L.; Bareford, D.; Wilkins, B.S.; van der Walt, J.D.; Reilly, J.T.; Grigg, A.P.; et al. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N. Engl. J. Med. 2005, 353, 33–45. [Google Scholar] [CrossRef]
- King, K.Y.; Matatall, K.A.; Shen, C.-C.; Goodell, M.A.; Swierczek, S.I.; Prchal, J.T. Comparative long-term effects of interferon alpha and hydroxyurea on human hematopoietic progenitor cells. Exp. Hematol. 2015, 43, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Larrán, A.; Pereira, A.; Cervantes, F.; Arellano-Rodrigo, E.; Hernández-Boluda, J.C.; Ferrer-Marín, F.; Angona, A.; Gómez, M.; Muiña, B.; Guillén, H.; et al. Assessment and prognostic value of the European leukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood 2012, 119, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Guryanova, O.A.; Shank, K.; Spitzer, B.; Luciani, L.; Koche, R.P.; Garrett-Bakelman, F.E.; Ganzel, C.; Durham, B.H.; Mohanty, A.; Hoermann, G.; et al. DNMT3A mutations promote anthracycline resistance in acute myeloid leukemia via impaired nucleosome remodeling. Nat. Med. 2016, 22, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Velu, T.; Delwiche, F.; Gangji, D.; Monsieur, R.; Flament, J.; Stryckmans, P.; Wybran, J.; Bellens, R. Therapeutic effect of human recombinant interferon-alpha-2C in essential thrombocythaemia. Oncology 1985, 42 Suppl. 1, 10–14. [Google Scholar] [CrossRef]
- Ludwig, H.; Linkesch, W.; Gisslinger, H.; Fritz, E.; Sinzinger, H.; Radaszkiewicz, T.; Chott, A.; Flener, R.; Micksche, M. Interferon-alfa corrects thrombocytosis in patients with myeloproliferative disorders. Cancer Immunol. Immunother. 1987, 25, 266–273. [Google Scholar] [CrossRef]
- Hasselbalch, H. Interferon in myelofibrosis. Lancet (Lond. Engl.) 1988, 1, 355. [Google Scholar] [CrossRef]
- Silver, R.T. Recombinant interferon-alpha for treatment of polycythaemia vera. Lancet (Lond. Engl.) 1988, 2, 403. [Google Scholar] [CrossRef]
- Foa, P.; Massaro, P.; Caldiera, S.; LaTargia, M.L.; Iurlo, A.; Clerici, C.; Fornier, M.; Bertoni, F.; Maiolo, A.T. Long-term therapeutic efficacy and toxicity of recombinant interferon-alpha 2a in polycythaemia vera. Eur. J. Haematol. 1998, 60, 273–277. [Google Scholar] [CrossRef]
- Quintás-Cardama, A.; Kantarjian, H.; Manshouri, T.; Luthra, R.; Estrov, Z.; Pierce, S.; Richie, M.A.; Borthakur, G.; Konopleva, M.; Cortes, J.; et al. Pegylated interferon alfa-2a yields high rates of hematologic and molecular response in patients with advanced essential thrombocythemia and polycythemia vera. J. Clin. Oncol. 2009, 27, 5418–5424. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, J.; Hasselbalch, H.; Bruserud, O.; Temerinac, S.; Brandberg, Y.; Merup, M.; Linder, O.; Bjorkholm, M.; Pahl, H.L.; Birgegard, G. A phase II trial of pegylated interferon alpha-2b therapy for polycythemia vera and essential thrombocythemia: Feasibility, clinical and biologic effects, and impact on quality of life. Cancer 2006, 106, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Stauffer Larsen, T.; Iversen, K.F.; Hansen, E.; Mathiasen, A.B.; Marcher, C.; Frederiksen, M.; Larsen, H.; Helleberg, I.; Riley, C.H.; Bjerrum, O.W.; et al. Long term molecular responses in a cohort of Danish patients with essential thrombocythemia, polycythemia vera and myelofibrosis treated with recombinant interferon alpha. Leuk. Res. 2013, 37, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Mondello, P.; Di Mirto, C.; Cuzzocrea, S.; Arrigo, C.; Mian, M.; Pitini, V. Interferon Alpha Has a Strong Anti-tumor Effect in Philadelphia-negative Myeloproliferative Neoplasms. Clin. Lymphoma. Myeloma Leuk. 2019, 19, e489–e495. [Google Scholar] [CrossRef]
- Utke Rank, C.; Weis Bjerrum, O.; Larsen, T.S.; Kjær, L.; de Stricker, K.; Riley, C.H.; Hasselbalch, H.C. Minimal residual disease after long-term interferon-alpha2 treatment: A report on hematological, molecular and histomorphological response patterns in 10 patients with essential thrombocythemia and polycythemia vera. Leuk. Lymphoma 2016, 57, 348–354. [Google Scholar] [CrossRef]
- Hasan, S.; Lacout, C.; Marty, C.; Cuingnet, M.; Solary, E.; Vainchenker, W.; Villeval, J.-L. JAK2V617F expression in mice amplifies early hematopoietic cells and gives them a competitive advantage that is hampered by IFNalpha. Blood 2013, 122, 1464–1477. [Google Scholar] [CrossRef]
- Kiladjian, J.J.; Giraudier, S.; Cassinat, B. Interferon-alpha for the therapy of myeloproliferative neoplasms: Targeting the malignant clone. Leukemia 2016, 30, 776–781. [Google Scholar] [CrossRef]
- Riley, C.H.; Jensen, M.K.; Brimnes, M.K.; Hasselbalch, H.C.; Bjerrum, O.W.; Straten, P.T.; Svane, I.M.; Hansen, M.; Brimnes, M.K.; Hasselbalch, H.C.; et al. Increase in circulating CD4+CD25+Foxp3+ T cells in patients with Philadelphia-negative chronic myeloproliferative neoplasms during treatment with IFN-α. Eur. J. Haematol. 2011, 118, 2170–2173. [Google Scholar] [CrossRef][Green Version]
- Riley, C.H.; Hansen, M.; Brimnes, M.K.; Hasselbalch, H.C.; Bjerrum, O.W.; Straten, P.T.; Svane, I.M.; Jensen, M.K. Expansion of circulating CD56bright natural killer cells in patients with JAK2-positive chronic myeloproliferative neoplasms during treatment with interferon-α. Eur. J. Haematol. 2015, 94, 227–234. [Google Scholar] [CrossRef]
- Riley, C.H.; Brimnes, M.K.; Hansen, M.; Jensen, M.K.; Hasselbalch, H.C.; Kjaer, L.; Straten, P.T.; Svane, I.M. Interferon-α induces marked alterations in circulating regulatory T cells, NK cell subsets, and dendritic cells in patients with JAK2V617F-positive essential thrombocythemia and polycythemia vera. Eur. J. Haematol. 2016, 97, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Kovacsovics-Bankowski, M.; Kelley, T.W.; Efimova, O.; Kim, S.J.; Wilson, A.; Swierczek, S.; Prchal, J. Changes in peripheral blood lymphocytes in polycythemia vera and essential thrombocythemia patients treated with pegylated-interferon alpha and correlation with JAK2(V617F) allelic burden. Exp. Hematol. Oncol. 2015, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhang, W.; Li, Y.; Berenzon, D.; Wang, X.; Wang, J.; Mascarenhas, J.; Xu, M.; Hoffman, R. Interferon-alpha targets JAK2V617F-positive hematopoietic progenitor cells and acts through the p38 MAPK pathway. Exp. Hematol. 2010, 38, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Kiladjian, J.J.; Massé, A.; Cassinat, B.; Mokrani, H.; Teyssandier, I.; Le Couédic, J.P.; Cambier, N.; Almire, C.; Pronier, E.; Casadevall, N.; et al. Clonal analysis of erythroid progenitors suggests that pegylated interferon α-2a treatment targets JAK2 V617F clones without affecting TET2 mutant cells. Leukemia 2010, 24, 1519–1523. [Google Scholar] [CrossRef]
- Harrison, C.; Kiladjian, J.-J.; Al-Ali, H.K.; Gisslinger, H.; Waltzman, R.; Stalbovskaya, V.; McQuitty, M.; Hunter, D.S.; Levy, R.; Knoops, L.; et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N. Engl. J. Med. 2012, 366, 787–798. [Google Scholar] [CrossRef]
- Mullally, A.; Bruedigam, C.; Poveromo, L.; Heidel, F.H.; Purdon, A.; Vu, T.; Austin, R.; Heckl, D.; Breyfogle, L.J.; Kuhn, C.P.; et al. Depletion of Jak2V617F myeloproliferative neoplasm-propagating stem cells by interferon-α in a murine model of polycythemia vera. Blood 2013, 121, 3692–3702. [Google Scholar] [CrossRef]
- Hasan, S.; Cassinat, B.; Droin, N.; Le Couedic, J.P.; Favale, F.; Monte-Mor, B.; Lacout, C.; Fontenay, M.; Dosquet, C.; Chomienne, C.; et al. Use of the 46/1 haplotype to model JAK2(V617F) clonal architecture in PV patients: Clonal evolution and impact of IFNalpha treatment. Leukemia 2014, 28, 460–463. [Google Scholar] [CrossRef]
- Them, N.C.C.; Bagienski, K.; Berg, T.; Gisslinger, B.; Schalling, M.; Chen, D.; Buxhofer-Ausch, V.; Thaler, J.; Schloegl, E.; Gastl, G.A.; et al. Molecular responses and chromosomal aberrations in patients with polycythemia vera treated with peg-proline-interferon alpha-2b. Am. J. Hematol. 2015, 90, 288–294. [Google Scholar] [CrossRef]
- Passegué, E.; Ernst, P.A. IFN-alpha wakes up sleeping hematopoietic stem cells. Nat. Med. 2009, 15, 612–613. [Google Scholar] [CrossRef]
- Hasselbalch, H.C.; Kiladjian, J.J.; Silver, R.T. Interferon alfa in the treatment of philadelphia-negative chronic myeloproliferative neoplasms. J. Clin. Oncol. 2011, 29, 2011–2012. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Massaro, P.; Foa, P.; Pomati, M.; LaTargia, M.L.; Iurlo, A.; Clerici, C.; Caldiera, S.; Fornier, M.; Maiolo, A.T. Polycythemia vera treated with recombinant interferon-alpha 2a: Evidence of a selective effect on the malignant clone. Am. J. Hematol. 1997, 56, 126–128. [Google Scholar] [CrossRef]
- Essers, M.A.G.; Offner, S.; Blanco-Bose, W.E.; Waibler, Z.; Kalinke, U.; Duchosal, M.A.; Trumpp, A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature 2009, 458, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Trumpp, A.; Essers, M.; Wilson, A. Awakening dormant haematopoietic stem cells. Nat. Rev. Immunol. 2010, 10, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Verstovsek, S.; Mesa, R.A.; Gotlib, J.; Levy, R.S.; Gupta, V.; DiPersio, J.F.; Catalano, J.V.; Deininger, M.W.N.; Miller, C.B.; Silver, R.T.; et al. Efficacy, safety and survival with ruxolitinib in patients with myelofibrosis: Results of a median 2-year follow-up of COMFORT-I. Haematologica 2013, 98, 1865–1871. [Google Scholar] [CrossRef]
- Cervantes, F.; Vannucchi, A.M.; Kiladjian, J.-J.; Al-Ali, H.K.; Sirulnik, A.; Stalbovskaya, V.; McQuitty, M.; Hunter, D.S.; Levy, R.S.; Passamonti, F.; et al. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood 2013, 122, 4047–4053. [Google Scholar] [CrossRef]
- Al-Ali, H.K.; Griesshammer, M.; le Coutre, P.; Waller, C.F.; Liberati, A.M.; Schafhausen, P.; Tavares, R.; Giraldo, P.; Foltz, L.; Raanani, P.; et al. Safety and efficacy of ruxolitinib in an open-label, multicenter, single-arm phase 3b expanded-access study in patients with myelofibrosis: A snapshot of 1144 patients in the JUMP trial. Haematologica 2016, 101, 1065–1073. [Google Scholar] [CrossRef]
- Verstovsek, S.; Passamonti, F.; Rambaldi, A.; Barosi, G. A Phase 2 Study of Ruxolitinib, an Oral JAK1 and JAK2 Inhibitor, in Patients With Advanced Polycythemia Vera Who Are Refractory or Intolerant to Hydroxyurea. Cancer 2014, 120, 513–520. [Google Scholar] [CrossRef]
- Harrison, C.N.; Mead, A.J.; Panchal, A.; Fox, S.; Yap, C.; Gbandi, E.; Houlton, A.; Alimam, S.; Ewing, J.; Wood, M.; et al. Ruxolitinib vs best available therapy for ET intolerant or resistant to hydroxycarbamide. Blood 2017, 130, 1889–1897. [Google Scholar] [CrossRef]
- Verstovsek, S.; Passamonti, F.; Rambaldi, A.; Barosi, G.; Rumi, E.; Gattoni, E.; Pieri, L.; Zhen, H.; Granier, M.; Assad, A.; et al. Ruxolitinib for essential thrombocythemia refractory to or intolerant of hydroxyurea: Long-term phase 2 study results. Blood 2017, 130, 1768–1771. [Google Scholar] [CrossRef]
- Gunawan, A.; Harrington, P.; Garcia-Curto, N.; McLornan, D.; Radia, D.; Harrison, C. Ruxolitinib for the Treatment of Essential Thrombocythemia. HemaSphere 2018, 2, e56. [Google Scholar] [CrossRef] [PubMed]
- Bose, P.; Verstovsek, S. Ruxolitinib for essential thrombocythemia? Oncoscience 2017, 4, 148–149. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C. JAK inhibitors and myelofibrosis, Einstein and ruxolitinib. Haematologica 2015, 100, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Elli, E.M.; Baratè, C.; Mendicino, F.; Palandri, F.; Palumbo, G.A. Mechanisms Underlying the Anti-inflammatory and Immunosuppressive Activity of Ruxolitinib. Front. Oncol. 2019, 9, 1186. [Google Scholar] [CrossRef] [PubMed]
- Polverelli, N.; Palumbo, G.A.; Binotto, G.; Abruzzese, E.; Benevolo, G.; Bergamaschi, M.; Tieghi, A.; Bonifacio, M.; Breccia, M.; Catani, L.; et al. Epidemiology, outcome, and risk factors for infectious complications in myelofibrosis patients receiving ruxolitinib: A multicenter study on 446 patients. Hematol. Oncol. 2018. [Google Scholar] [CrossRef]
- Deininger, M.; Radich, J.; Burn, T.C.; Huber, R.; Paranagama, D.; Verstovsek, S. The effect of long-term ruxolitinib treatment on JAK2p.V617F allele burden in patients with myelofibrosis. Blood 2015, 126, 1551–1555. [Google Scholar] [CrossRef]
- Verstovsek, S.; Vannucchi, A.M.; Griesshammer, M.; Masszi, T.; Durrant, S.; Passamonti, F.; Harrison, C.N.; Pane, F.; Zachee, P.; Kirito, K.; et al. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial. Haematologica 2016, 101, 821–829. [Google Scholar] [CrossRef]
- Pardanani, A.; Vannucchi, A.M.; Passamonti, F.; Cervantes, F.; Barbui, T.; Tefferi, A. JAK inhibitor therapy for myelofibrosis: Critical assessment of value and limitations. Leukemia 2011, 25, 218–225. [Google Scholar] [CrossRef]
- Tefferi, A. JAK inhibitors for myeloproliferative neoplasms: Clarifying facts from myths. Blood 2012, 119, 2721–2730. [Google Scholar] [CrossRef]
- Griner, L.N.; Mcgraw, K.L.; Johnson, J.O.; List, A.F.; Reuther, G.W. JAK2-V617F-mediated signalling is dependent on lipid rafts and statins inhibit JAK2-V617F-dependent cell growth. Br. J. Haematol. 2013, 160, 177–187. [Google Scholar] [CrossRef]
- Pieri, L.; Pancrazzi, A.; Pacilli, A.; Rabuzzi, C.; Rotunno, G.; Fanelli, T.; Guglielmelli, P.; Fjerza, R.; Paoli, C.; Verstovsek, S.; et al. JAK2V617F complete molecular remission in polycythemia vera/essential thrombocythemia patients treated with ruxolitinib. Blood 2015, 125, 3352–3353. [Google Scholar] [CrossRef] [PubMed]
- Lane, S.W.; Mullally, A. Jak2V617F myeloproliferative neoplasm stem cells and interferon-alpha. Oncotarget 2013, 4, 500–501. [Google Scholar] [CrossRef][Green Version]
- Mullally, A.; Lane, S.W.; Ball, B.; Megerdichian, C.; Okabe, R.; Al-Shahrour, F.; Paktinat, M.; Haydu, J.E.; Housman, E.; Lord, A.M.; et al. Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer Cell 2010, 17, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, S.U.; Kjaer, L.; Bjørn, M.E.; Knudsen, T.A.; Sørensen, A.L.; Andersen, C.B.L.; Bjerrum, O.W.; Brochmann, N.; El Fassi, D.; Kruse, T.A.; et al. Safety and efficacy of combination therapy of interferon-α2 and ruxolitinib in polycythemia vera and myelofibrosis. Cancer Med. 2018, 7, 3571–3581. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, A.L.; Mikkelsen, S.U.; Knudsen, T.A.; Bjorn, M.E.; Andersen, C.L.; Bjerrum, O.W.; Brochmann, N.; Patel, D.A.; Gjerdrum, L.M.R.; El Fassi, D.; et al. Ruxolitinib and interferon-alpha2 combination therapy for patients with polycythemia vera or myelofibrosis: A phase II study. Haematologica 2019. [Google Scholar] [CrossRef]
- Hasselbalch, H.C. Perspectives on the impact of JAK-inhibitor therapy upon inflammation-mediated comorbidities in myelofibrosis and related neoplasms. Expert Rev. Hematol. 2014, 7, 203–216. [Google Scholar] [CrossRef]
- Bjorn, M.E.; Hasselbalch, H.C. Minimal residual disease or cure in MPNs? Rationales and perspectives on combination therapy with interferon-alpha2 and ruxolitinib. Expert Rev. Hematol. 2017, 10, 393–404. [Google Scholar] [CrossRef]
- Hasselbalch, H.C.; Bjørn, M.E. MPNs as Inflammatory Diseases: The Evidence, Consequences, and Perspectives. Mediat. Inflamm. 2015, 2015, 102476. [Google Scholar] [CrossRef]
- Griner, L.N.; McGraw, K.L.; Johnson, J.O.; List, A.F.; Reuther, G.W. A mechanistic rationale for the use of statins to enhance JAK inhibitor therapy in MPNs. Blood 2011, 118, 53. [Google Scholar] [CrossRef]
- Kaelin, W.G.J. The concept of synthetic lethality in the context of anticancer therapy. Nat. Rev. Cancer 2005, 5, 689–698. [Google Scholar] [CrossRef]
- Nijman, S.M.B. Synthetic lethality: General principles, utility and detection using genetic screens in human cells. FEBS Lett. 2011, 585, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, H.C. Myelofibrosis with myeloid metaplasia: The advanced phase of an untreated disseminated hematological cancer. Time to change our therapeutic attitude with early upfront treatment? Leuk. Res. 2009, 33, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Skov, V.; Dam, M.J.B.; Pedersen, R.K.; Andersen, M.; Knudsen, T.A.; Sajid, Z.; Kjær, L.; Ellervik, C.; Hasselbalch, H.C.; Ottesen, J.T. Superiority of IFN Versus HU Using a Novel Biomarker-Based Tool for Assessment of Disease Burden in MPNs. Blood (ASH Annu. Meet. Abstr.) 2019, 134, 2972. [Google Scholar] [CrossRef]
- Bjørn, M.; de Stricker, K.; Kjær, L.; Ellemann, K.; Hasselbalch, H. Combination therapy with interferon and JAK1-2 inhibitor is feasible: Proof of concept with rapid reduction in JAK2V617F-allele burden in polycythemia vera. Leuk. Res. reports 2014, 3, 73–75. [Google Scholar]
- Goldie, J.H.; Coldman, A.J. The genetic origin of drug resistance in neoplasms: Implications for systemic therapy. Cancer Res. 1984, 44, 3643–3653. [Google Scholar]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).