The Role of Steroid Hormone Receptors in Urothelial Tumorigenesis
Abstract
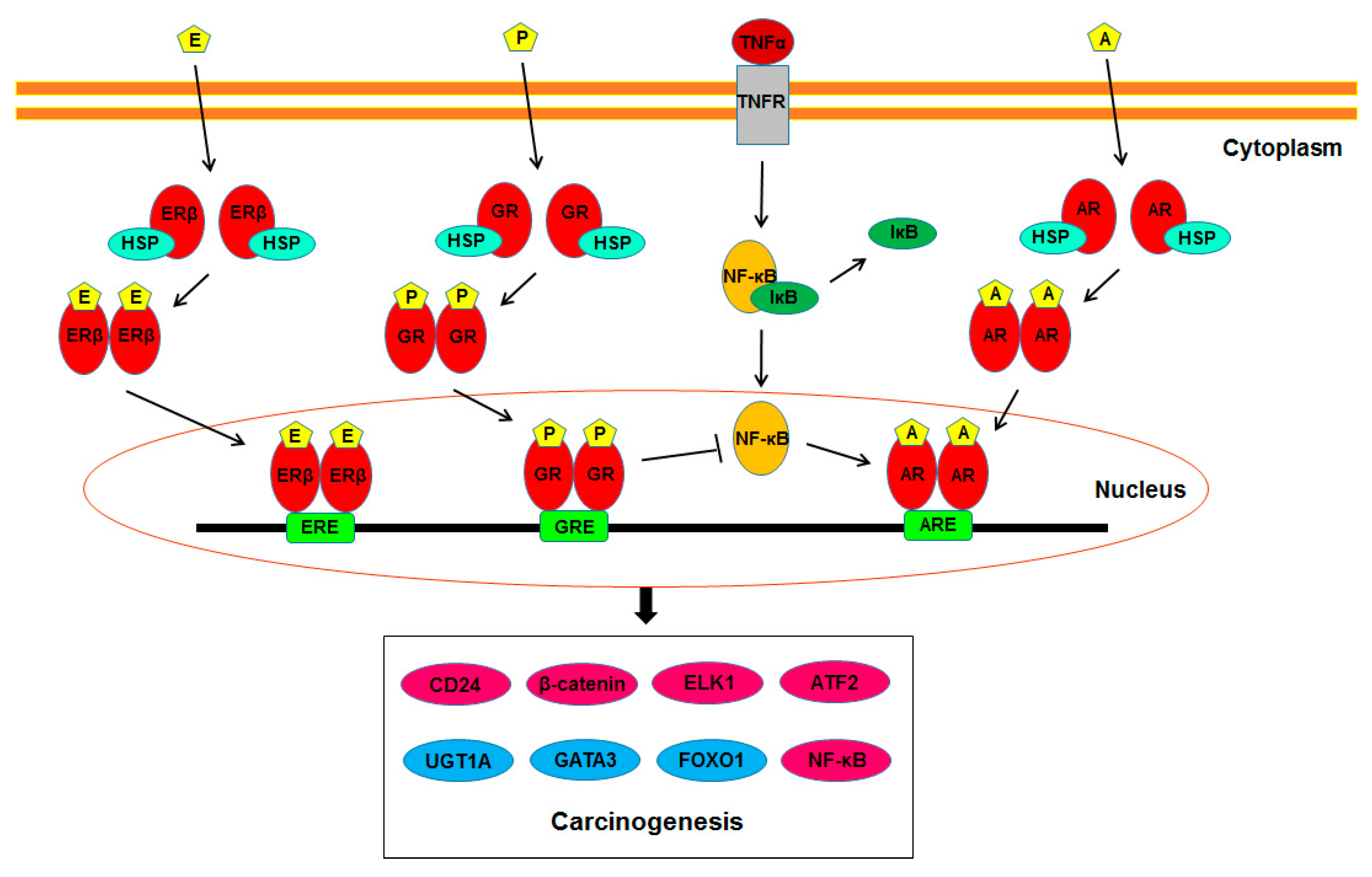
1. Introduction
2. AR
3. ERs
4. GR
5. PR
6. VDR
7. Molecules Modulated by Steroid Hormone Receptor Signaling in Urothelial Cells
7.1. UDP-Glucuronosyltransferases (UGTs)
7.2. GATA3
7.3. FOXO1
7.4. CD24
7.5. β-Catenin
7.6. ELK1
7.7. ATF2
7.8. NF-κB
8. Conclusions
Funding
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Rouprêt, M.; Babjuk, M.; Compérat, E.; Zigeuner, R.; Sylvester, R.J.; Burger, M.; Cowan, N.C.; Gontero, P.; Van Rhijn, B.W.G.; Mostafid, A.H.; et al. European Association of Urology guidelines on upper urinary tract urothelial carcinoma: 2017 update. Eur. Urol. 2018, 73, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Shiota, M.; Yokomizo, A.; Takeuchi, A.; Imada, K.; Kiyoshima, K.; Inokuchi, J.; Tatsugami, K.; Ohga, S.; Nakamura, K.; Honda, H.; et al. Secondary bladder cancer after anticancer therapy for prostate cancer: Reduced comorbidity after androgen-deprivation therapy. Oncotarget 2015, 6, 14710–14719. [Google Scholar] [CrossRef]
- Izumi, K.; Taguri, M.; Miyamoto, H.; Hara, Y.; Kishida, T.; Chiba, K.; Murai, T.; Hirai, K.; Suzuki, K.; Fujinami, K.; et al. Androgen deprivation therapy prevents bladder cancer recurrence. Oncotarget 2014, 5, 12665–12674. [Google Scholar] [CrossRef]
- Shiota, M.; Kiyoshima, K.; Yokomizo, A.; Takeuchi, A.; Kashiwagi, E.; Dejima, T.; Takahashi, R.; Inokuchi, J.; Tatsugami, K.; Eto, M. Suppressed recurrent bladder cancer after androgen suppression with androgen deprivation therapy or 5α-reductase inhibitor. J. Urol. 2017, 197, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Izumi, K.; Ito, Y.; Miyamoto, H.; Miyoshi, Y.; Ota, J.; Moriyama, M.; Murai, T.; Hayashi, H.; Inayama, Y.; Ohashi, K.; et al. Expression of androgen receptor in non-muscle-invasive bladder cancer predicts the preventive effect of androgen deprivation therapy on tumor recurrence. Oncotarget 2016, 7, 14153–14160. [Google Scholar] [CrossRef] [PubMed]
- Boorjian, S.; Ugras, S.; Mongan, N.P.; Gudas, L.J.; You, X.; Tickoo, S.K.; Scherr, D.S. Androgen receptor expression is inversely correlated with pathologic tumor stage in bladder cancer. Urology 2004, 64, 383–388. [Google Scholar] [CrossRef]
- Kauffman, E.C.; Robinson, B.D.; Downes, M.J.; Powell, L.G.; Lee, M.M.; Scherr, D.S.; Gudas, L.J.; Mongan, N.P. Role of androgen receptor and associated lysine-demethylase coregulators, LSD1 and JMJD2A, in localized and advanced human bladder cancer. Mol. Carcinog. 2011, 50, 931–944. [Google Scholar] [CrossRef]
- Tuygun, C.; Kankaya, D.; Imamoglu, A.; Sertcelik, A.; Zengin, K.; Oktay, M.; Sertcelik, N. Sex-specific hormone receptors in urothelial carcinomas of the human urinary bladder: A comparative analysis of clinicopathological features and survival outcomes according to receptor expression. Urol. Oncol. 2011, 29, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, H.; Yao, J.L.; Chaux, A.; Zheng, Y.; Hsu, I.; Izumi, K.; Chang, C.; Messing, E.M.; Netto, G.J.; Yeh, S. Expression of androgen and oestrogen receptors and its prognostic significance in urothelial neoplasm of the urinary bladder. BJU Int. 2012, 109, 1716–1726. [Google Scholar] [CrossRef] [PubMed]
- Mashhadi, R.; Pourmand, G.; Kosari, F.; Mehrsai, A.; Salem, S.; Pourmand, M.R.; Alatab, S.; Khonsari, M.; Heydari, F.; Beladi, L.; et al. Role of steroid hormone receptors in formation and progression of bladder carcinoma: A case-control study. Urol. J. 2014, 11, 1968–1973. [Google Scholar] [PubMed]
- Kashiwagi, E.; Fujita, K.; Yamaguchi, S.; Fushimi, H.; Ide, H.; Inoue, S.; Mizushima, T.; Reis, L.O.; Sharma, R.; Netto, G.J.; et al. Expression of steroid hormone receptors and its prognostic significance in urothelial carcinoma of the upper urinary tract. Cancer Biol. Ther. 2016, 17, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Yasui, M.; Kawahara, T.; Takamoto, D.; Izumi, K.; Uemura, H.; Miyamoto, H. Distribution of androgen receptor expression in the urinary bladder. Int. J. Urol. 2019, 26, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Ide, H.; Inoue, S.; Miyamoto, H. Histopathological and prognostic significance of the expression of sex hormone receptors in bladder cancer: A meta-analysis of immunohistochemical studies. PLoS ONE 2017, 12, e0174746. [Google Scholar] [CrossRef] [PubMed]
- Sikic, D.; Breyer, J.; Hartmann, A.; Burger, M.; Erben, P.; Denzinger, D.; Eckstein, M.; Stöhr, R.; Wach, S.; Wullich, B.; et al. High androgen receptor mRNA expression is independently associated with prolonged cabcer-specific and recurrence-free survival in stage T1 bladder cancer. Trans. Oncol. 2017, 10, 340–345. [Google Scholar] [CrossRef]
- Yasui, M.; Kawahara, T.; Izumi, K.; Yao, M.; Ishiguro, Y.; Ishiguro, H.; Uemura, H.; Miyoshi, Y. Androgen receptor mRNA expression is a predictor for recurrence-free survival in non-muscle invasive bladder cancer. BMC Cancer 2019, 19, 331. [Google Scholar] [CrossRef]
- Liu, C.H.; Huang, J.D.; Huang, S.W.; Hour, T.C.; Huang, Y.K.; Hsueh, Y.M.; Chiou, H.Y.; Lee, T.C.; Jan, K.Y.; Chen, C.J.; et al. Androgen receptor gene polymorphism may affect the risk of urothelial carcinoma. J. Biomed. Sci. 2008, 15, 261–269. [Google Scholar] [CrossRef]
- Teng, X.Y.; Liu, G.Q.; Diao, X.L.; Wu, Z.Y.; Li, L.; Zhang, W.; Zhang, X.; Su, Q. CAG repeats in the androgen receptor gene are shorter in patients with pulmonary, esophageal or bladder carcinoma and longer in women with uterine leiomyoma. Oncol. Rep. 2010, 23, 811–818. [Google Scholar]
- Zhuang, Y.H.; Blauer, M.; Tammela, T.; Tuohimaa, P. Immunodetection of androgen receptor in human urinary bladder cancer. Histopathology 1997, 30, 556–562. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Imai, Y.; Noda, S.; Matsuyama, C.; Shimizu, A.; Kamai, T. Sex steroid hormone receptors in bladder cancer: Usefulness in differential diagnosis and implications in histogenesis of bladder cancer. Urol. Oncol. 2019, 37, 353.e9–353.e15. [Google Scholar] [CrossRef]
- Kontos, S.; Kominea, A.; Melachrinou, M.; Balampani, E.; Sotiropoulou-Bonikou, G. Inverse expression of estrogen receptor-β and nuclear factor-κB in urinary bladder carcinogenesis. Int. J. Urol. 2010, 17, 801–809. [Google Scholar] [CrossRef]
- Ishiguro, H.; Kawahara, T.; Zheng, Y.; Netto, G.J.; Miyamoto, H. Reduced glucocorticoid receptor expression predicts bladder tumor recurrence and progression. Am. J. Clin. Pathol. 2014, 142, 157–164. [Google Scholar] [CrossRef]
- Sahin, M.O.; Canda, A.E.; Yorukoglu, K.; Mungan, M.U.; Sade, M.; Kirkali, Z. 1,25 Dihydroxyvitamin D3 receptor expression in superficial transitional cell carcinoma of the bladder: A possible prognostic factor? Eur. Urol. 2005, 47, 52–57. [Google Scholar]
- Jóźwicki, W.; Brozyna, A.A.; Siekiera, J.; Slominski, A.T. Expression of vitamin D receptor (VDR) positively correlates with survival of urothelial bladder cancer patients. Int. J. Mol. Sci. 2015, 16, 24369–24386. [Google Scholar] [CrossRef]
- Okajima, E.; Hiramatsu, T.; Iriya, K.; Ijuin, M.; Matsushima, S. Effects of sex hormones on development of urinary bladder tumours in rats induced by N-butyl-N-(4-hydroxybutyl) nitrosamine. Urol. Res. 1975, 3, 73–79. [Google Scholar] [CrossRef]
- Imada, S.; Akaza, H.; Ami, Y.; Koiso, K.; Ideyama, Y.; Takenaka, T. Promoting effects and mechanisms of action of androgen in bladder carcinogenesis in male rats. Eur. Urol. 1997, 31, 360–364. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, Q.; Huang, X.; Yang, J.; Xu, Y.; Zhang, G. The effects of early versus delayed castration targeting androgen on prolonging survival in a mouse model of bladder cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 10283–10293. [Google Scholar]
- Miyamoto, H.; Yang, Z.; Chen, Y.T.; Ishiguro, H.; Uemura, H.; Kubota, Y.; Nagashima, Y.; Chang, Y.J.; Hu, Y.C.; Tsai, M.Y.; et al. Promotion of bladder cancer development and progression by androgen receptor signals. J. Natl. Cancer Inst. 2007, 99, 558–568. [Google Scholar] [CrossRef]
- Hsu, J.W.; Hsu, I.; Xu, D.; Miyamoto, H.; Liang, L.; Wu, X.R.; Shyr, C.R.; Chang, C. Decreased tumorigenesis and mortality from bladder cancer in mice lacking urothelial androgen receptor. Am. J. Pathol. 2013, 182, 1811–1820. [Google Scholar] [CrossRef]
- Johnson, D.T.; Hooker, E.; Luong, R.; Yu, E.J.; He, Y.; Gonzalgo, M.L.; Sun, Z. Conditional expression of the androgen receptor increases susceptibility of bladder cancer in mice. PLoS ONE 2016, 11, e0148851. [Google Scholar] [CrossRef]
- Li, Y.; Ishiguro, H.; Kawahara, T.; Miyamoto, Y.; Izumi, K.; Miyamoto, H. GATA3 in the urinary bladder: Suppression of neoplastic transformation and down-regulation by androgens. Am. J. Cancer Res. 2014, 4, 461–473. [Google Scholar]
- Kawahara, T.; Inoue, S.; Kashiwagi, E.; Chen, J.; Ide, H.; Mizushima, T.; Li, Y.; Zheng, Y.; Miyamoto, H. Enzalutamide as an androgen receptor inhibitor prevents urothelial tumorigenesis. Am. J. Cancer Res. 2017, 7, 2041–2050. [Google Scholar] [CrossRef]
- Inoue, S.; Ide, H.; Mizushima, T.; Jiang, G.; Kawahara, T.; Miyamoto, H. ELK1 promotes urothelial tumorigenesis in the presence of an activated androgen receptor. Am. J. Cancer Res. 2018, 8, 2325–2336. [Google Scholar]
- Shen, S.S.; Smith, C.L.; Hsieh, J.T.; Yu, J.; Kim, I.Y.; Jian, W.; Sonpavde, G.; Ayala, G.E.; Younes, M.; Lerner, S.P. Expression of estrogen receptors-alpha and -beta in bladder cancer cell lines and human bladder tumor tissue. Cancer 2006, 106, 2610–2616. [Google Scholar] [CrossRef]
- Teng, J.; Wang, Z.Y.; Jarrard, D.F.; Bjorling, D.E. Roles of estrogen receptor α and β in modulating urothelial cell proliferation. Endocr. Relat. Cancer 2008, 15, 351–364. [Google Scholar] [CrossRef]
- Sanchez-Carbayo, M. Hypermethylation in bladder cancer: Biological pathways and translational applications. Tumour Biol. 2012, 33, 347–361. [Google Scholar] [CrossRef]
- Brait, M.; Begum, S.; Carvalho, A.L.; Dasgupta, S.; Vettore, A.L.; Czerniak, B.; Caballero, O.L.; Westra, W.H.; Sidransky, D.; Hoque, M.O. Aberrant promoter methylation of multiple genes during pathogenesis of bladder cancer. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2786–2794. [Google Scholar] [CrossRef]
- Reid, L.M.; Leav, I.; Kwan, P.W.; Russell, P.; Merk, F.B. Characterization of a human, sex steroid-responsive transitional cell carcinoma maintained as a tumor line (R198) in athymic nude mice. Cancer Res. 1984, 44, 4560–4573. [Google Scholar]
- Waalkes, M.P.; Liu, J.; Ward, J.M.; Powell, D.A.; Diwan, B.A. Urogenital carcinogenesis in female CD1 mice induced by in utero arsenic exposure is exacerbated by postnatal diethylstilbestrol treatment. Cancer Res. 2006, 66, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Hsu, I.; Yeh, C.R.; Slavin, S.; Miyamoto, H.; Netto, G.J.; Tsai, Y.C.; Muyan, M.; Wu, X.R.; Messing, E.M.; Guancial, E.A.; et al. Estrogen receptor alpha prevents bladder cancer via INPP4B inhibited akt pathway in vitro and in vivo. Oncotarget 2014, 5, 7917–7935. [Google Scholar] [CrossRef] [PubMed]
- Hsu, I.; Chuang, K.L.; Slavin, S.; Da, J.; Lim, W.X.; Pang, S.T.; O’Brien, J.H.; Yeh, S. Suppression of ERβ signaling via ERβ knockout or antagonist protects against bladder cancer development. Carcinogenesis 2014, 35, 651–661. [Google Scholar] [CrossRef] [PubMed]
- George, S.K.; Tovar-Sepulveda, V.; Shen, S.S.; Jian, W.; Zhang, Y.; Hilsenbeck, S.G.; Lerner, S.P.; Smith, C.L. Chemoprevention of BBN-induced bladder carcinogenesis by the selective estrogen receptor modulator tamoxifen. Transl. Oncol. 2013, 6, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, R.; Wada, H.; Ito, K.; Adcock, I.A. Effects of glucocorticoids on gene transcription. Eur. J. Pharmacol. 2004, 500, 51–62. [Google Scholar] [CrossRef]
- Duma, D.; Jewell, C.M.; Cidlowski, J.A. Multiple glucocorticoid receptor isoforms and mechanisms of post-translational modification. J. Steroid Biochem. Mol. Biol. 2006, 102, 11–21. [Google Scholar] [CrossRef]
- Oakley, R.H.; Jewell, C.M.; Yudt, M.R.; Bofetiado, D.M.; Cidlowski, J.A. The dominant negative activity of the human glucocorticoid receptor β isoform. Specificity and mechanisms of action. J. Biol. Chem. 1999, 274, 27857–27866. [Google Scholar] [CrossRef]
- Ide, H.; Inoue, S.; Miyamoto, H. The role of glucocorticoid receptor signaling in bladder cancer progression. Cancers 2018, 10, 484. [Google Scholar] [CrossRef]
- Dietrich, K.; Schned, A.; Fortuny, J.; Heaney, J.; Marsit, C.; Kelsey, K.T.; Karagas, M.R. Glucocorticoid therapy and risk of bladder cancer. Br. J. Cancer 2009, 101, 1316–1320. [Google Scholar] [CrossRef]
- Ide, H.; Inoue, S.; Mizushima, T.; Kashiwagi, E.; Zheng, Y.; Miyamoto, H. Role of glucocorticoid signaling in urothelial tumorigenesis: Inhibition by prednisone presumably through inducing glucocorticoid receptor transrepression. Mol. Carcinog. 2019, 58, 2297–2305. [Google Scholar] [CrossRef]
- Tanner, T.M.; Verrijdt, G.; Rombauts, W.; Louw, A.; Hapgood, J.P.; Claessens, F. Anti-androgenic properties of compound A, an analog of a non-steroidal plant compound. Mol. Cell. Endocrinol. 2003, 201, 155–164. [Google Scholar] [CrossRef]
- Ide, H.; Inoue, S.; Mizushima, T.; Jiang, G.; Nagata, Y.; Goto, T.; Kashiwagi, E.; Miyamoto, H. Compound A inhibits urothelial tumorigenesis via both the androgen receptor and glucocorticoid receptor signaling pathways. Am. J. Transl. Res. 2020, 12, 1779–1788. [Google Scholar] [PubMed]
- Zheng, Y.; Ishiguro, H.; Ide, H.; Inoue, S.; Kashiwagi, E.; Kawahara, T.; Jalalizadeh, M.; Reis, L.O.; Miyamoto, H. Compound A inhibits bladder cancer growth predominantly via glucocorticoid receptor transrepression. Mol. Endocrinol. 2015, 29, 1486–1497. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.M.; O’Connell, M.J.; Messing, E.M.; Reeder, J.E. Decreased bladder cancer growth in parous mice. Urology 2008, 72, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, B.J.; Amr, S.; Ezzat, S.; Saleh, D.; Gouda, I.; Loay, I.; Hifnawy, T.; Mikhail, N.N.; Abdel-Hamid, M.; Zhan, M.; et al. Estrogen exposure and bladder cancer risk in Egyptian women. Maturitas 2010, 67, 353–357. [Google Scholar] [CrossRef]
- Wang, J.P.; Leng, J.Y.; Zhang, R.K.; Zhang, L.; Zhang, B.; Jiang, W.Y.; Tong, L. Functional analysis of gene expression profiling-based prediction in bladder cancer. Oncol. Lett. 2018, 15, 8417–8842. [Google Scholar] [CrossRef]
- Liao, Y.; Huang, J.L.; Qiu, M.X.; Ma, Z.W. Impact of serum vitamin D level on risk of bladder cancer: A systemic review and meta-analysis. Tumour Biol. 2015, 36, 1567–1572. [Google Scholar] [CrossRef]
- Mittal, R.D.; Manchanda, P.K.; Bhat, S.; Bid, H.K. Association of vitamin-D receptor (Fok-I) gene polymorphism with bladder cancer in an Indian population. BJU Int. 2007, 99, 933–937. [Google Scholar] [CrossRef]
- Konety, B.R.; Lavelle, J.P.; Pirtskalaishvili, G.; Dhir, R.; Meyers, S.A.; Nguyen, T.S.; Hershberger, P.; Shurin, M.R.; Johnson, C.S.; Trump, D.L.; et al. Effects of vitamin D (calcitriol) on transitional cell carcinoma of the bladder in vitro and in vivo. J. Urol. 2001, 165, 253–258. [Google Scholar] [CrossRef]
- Izumi, K.; Zheng, Y.; Hsu, J.W.; Chang, C.; Miyamoto, H. Androgen receptor signals regulate UDP-glucuronosyltransferases in the urinary bladder: A potential mechanism of androgen-induced bladder carcinogenesis. Mol. Carcinog. 2013, 52, 94–102. [Google Scholar] [CrossRef]
- Izumi, K.; Li, Y.; Ishiguro, H.; Zheng, Y.; Yao, J.L.; Netto, G.J.; Miyamoto, H. Expression of UDP-glucuronosyltransferase 1A in bladder cancer: Association with prognosis and regulation by estrogen. Mol. Carcinog. 2014, 53, 314–324. [Google Scholar] [CrossRef]
- Izumi, K.; Inoue, S.; Ide, H.; Fujita, K.; Mizushima, T.; Jiang, G.; Yamaguchi, S.; Fushimi, H.; Nonomura, N.; Miyamoto, H. Uridine 5′diphospho-glucuronosyltransferase 1A expression as an independent prognosticator in urothelial carcinoma of the upper urinary tract. Int. J. Urol. 2018, 25, 429–435. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Klaene, J.J.; Li, Y.; Paonessa, J.D.; Stablewski, A.B.; Vouros, P.; Zhang, Y. The inverse relationship between bladder and liver in 4-aminobiphenyl-induced DNA damage. Oncotarget 2015, 6, 836–845. [Google Scholar] [CrossRef]
- Amin, M.B.; Trpkov, K.; Lopez-Beltran, A.; Grignon, D.; Members of the ISUP Immunohistochemistry in Diagnostic Urologic Pathology Group. Best practices recommendations in the application of immunohistochemistry in the bladder lesions: Report from the International Society of Urologic Pathology consensus conference. Am. J. Surg. Pathol. 2014, 38, e20–e34. [Google Scholar] [CrossRef]
- Miyamoto, H.; Izumi, K.; Yao, J.L.; Li, Y.; Yang, Q.; McMahon, L.A.; Gonzalez-Roibon, N.; Hicks, D.G.; Netto, G.J. GATA binding protein 3 is down-regulated in bladder cacner yet strong expression is an independent predictor of poor prognosis in invasive tumor. Hum. Pathol. 2012, 43, 2033–2040. [Google Scholar] [CrossRef]
- Inoue, S.; Mizushima, T.; Fujita, K.; Meliti, A.; Ide, H.; Yamaguchi, S.; Fushimi, H.; Netto, G.J.; Nonomura, N.; Miyamoto, H. GATA3 immunohistochemistry in urothelial carcinoma of the upper urinary tract as a urothelial marker and a prognosticator. Hum. Pathol. 2017, 64, 83–90. [Google Scholar] [CrossRef]
- Ide, H.; Mizushima, T.; Jiang, G.; Goto, T.; Nagata, Y.; Teramoto, Y.; Inoue, S.; Li, Y.; Kashiwagi, E.; Baras, A.S.; et al. FOXO1 as a tumor suppressor inactivated via AR/ERβ signals in urothelial cells. Endocr. Relat. Cancer 2020, 27, 231–244. [Google Scholar] [CrossRef]
- Ide, H.; Jiang, G.; Mizushima, T.; Fujita, K.; Inoue, S.; Yamaguchi, S.; Fushimi, H.; Nonomura, N.; Miyamoto, H. Forkhead box O1 as an indicator of prognosis is inactivated in urothelial carcinoma of the upper urinary tract. Oncol. Lett. 2019, 17, 482–487. [Google Scholar] [CrossRef]
- Ooki, A.; VandenBussche, C.J.; Kates, M.; Hahn, N.M.; Matoso, A.; McConkey, D.J.; Bivalacqua, T.J.; Hoque, M.O. CD24 regulates cancer stem cell (CSC)-like traits and a panel of CSC-related molecules serves as a non-invasive urinary biomarker for the detection of bladder cancer. Br. J. Cancer 2018, 119, 961–970. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, S.; Shen, H.; Xu, K.; Chen, J.; Li, H.; Xu, Y.; Xu, A.; Chen, B.; Kaku, H.; et al. Clinical significance of CD24 as a predictor of bladder cancer recurrence. Oncol. Lett. 2013, 6, 96–100. [Google Scholar] [CrossRef]
- Overdevest, J.B.; Knubel, K.H.; Duex, J.E.; Thomas, S.; Nitz, M.D.; Harding, M.A.; Smith, S.C.; Frierson, H.F.; Conaway, M.; Theodorescu, D. CD24 expression is important in male urothelial tumorigenesis and metastasis in mice and is androgen regulated. Proc. Natl. Acad. Sci. USA 2012, 109, E3588–E3596. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Dancik, G.M.; Goodspeed, A.; Costello, J.C.; Owens, C.; Duex, J.E.; Theodorescu, D. GON4L drives cancer growth through a YY1-androgen receptor-CD24 axis. Cancer Res. 2016, 76, 5175–5185. [Google Scholar] [CrossRef] [PubMed]
- Shiina, H.; Igawa, M.; Shigeno, K.; Terashima, M.; Deguchi, M.; Yamanaka, M.; Ribeiro-Filho, L.; Kane, C.J.; Dahiya, R. β-Catenin mutations correlate with over expression of c-myc and cyclin D1 genes in bladder cancer. J. Urol. 2002, 168, 2220–2226. [Google Scholar] [CrossRef]
- Lin, C.; Yin, Y.; Stemler, K.; Humphrey, P.; Kibel, A.S.; Mysorekar, I.U.; Ma, L. Constitutive β-catenin activation induces male-specific tumorigenesis in the bladder urothelium. Cancer Res. 2013, 73, 5914–5925. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, Y.; Izumi, K.; Ishiguro, H.; Ye, B.; Li, F.; Miyamoto, H. Androgen activates beta-catenin signaling in bladder cancer cells. Endocr. Relat. Cancer 2013, 20, 293–304. [Google Scholar] [CrossRef]
- Kawahara, T.; Shareef, H.K.; Aljarah, A.K.; Ide, H.; Li, Y.; Kashiwagi, E.; Netto, G.J.; Zheng, Y.; Miyamoto, H. ELK1 is up-regulated by androgen in bladder cancer cells and promotes tumor progression. Oncotarget 2015, 6, 29860–29876. [Google Scholar] [CrossRef]
- Inoue, S.; Ide, H.; Fujita, K.; Mizushima, T.; Jiang, G.; Kawahara, T.; Yamaguchi, S.; Fushimi, H.; Nonomura, N.; Miyamoto, H. Expression of phospho-ELK1 and its prognostic significance in urothelial carcinoma of the upper urinary tract. Int. J. Mol. Sci. 2018, 19, 777. [Google Scholar] [CrossRef]
- Kawahara, T.; Ide, H.; Kashiwagi, E.; Patterson, J.D.; Inoue, S.; Shareef, H.K.; Aljarah, A.K.; Zheng, Y.; Barasm, A.S.; Miyamoto, H. Silodosin inhibits the growth of bladder cancer cells and enhances the cytotoxic activity of cisplatin via ELK1 inactivation. Am. J. Cancer Res. 2015, 5, 2959–2968. [Google Scholar]
- Inoue, S.; Mizushima, T.; Ide, H.; Jiang, G.; Goto, T.; Nagata, Y.; Netto, G.J.; Miyamoto, H. ATF2 promotes urothelial cancer outgrowth via cooperation with androgen receptor signaling. Endocr. Connect. 2018, 7, 1397–1408. [Google Scholar] [CrossRef]
- Inoue, S.; Ide, H.; Mizushima, T.; Jiang, G.; Netto, G.J.; Gotoh, M.; Miyamoto, H. Nuclear factor-κB promotes urothelial tumorigenesis and cancer progression via cooperation with androgen receptor signaling. Mol. Cancer Ther. 2018, 17, 1303–1314. [Google Scholar] [CrossRef]
- Nelius, T.; Filleur, S.; Yemelyanov, A.; Budunova, I.; Shroff, E.; Mirochnik, Y.; Aurora, A.; Veliceasa, D.; Xiao, W.; Wang, Z.; et al. Androgen receptor targets NFκB and TSP1 to suppress prostate tumor growth in vivo. Int. J. Cancer 2007, 121, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Altuwaijri, S.; Deng, F.; Chen, L.; Lal, P.; Bhanot, U.K.; Korets, R.; Wenske, S.; Lilja, H.G.; Chang, C.; et al. NF-κB regulates androgen receptor expression and prostate cancer growth. Am. J. Pathol. 2009, 175, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Cheng, H.; Lin, D.; Liu, L.; Yang, O.; Jia, L.; Fazli, L.; Gleave, M.E.; Wang, Y.; Rennie, P.; et al. The expression of glucocorticoid receptor is negatively regulated by active androgen receptor signaling in prostate tumors. Int. J. Cancer 2015, 136, E27–E38. [Google Scholar] [CrossRef] [PubMed]
| Author, Year [Reference] | Receptor | Tumor Site | Positive/Total Cases | ||
|---|---|---|---|---|---|
| Non-Tumor | Tumor | p Value | |||
| Boorjian, 2004 [8] | AR | Bladder | 32/37 (86%) | 26/49 (53%) | 0.001 * |
| Kauffman, 2011 [9] | AR | Bladder | 50/59 (84%) | 30/59 (51%) | <0.001 |
| Tuygun, 2011 [10] | AR | Bladder | 0/58 (0%) (Male) | 71/139 (51%) | <0.001 * |
| Miyamoto, 2012 [11] | AR | Bladder | 113/141 (80%) | 79/188 (42%) | <0.001 |
| Mashhadi, 2014 [12] | AR | Bladder | 0/132 (0%) | 26/120 (22%) | <0.001 |
| Kashiwagi, 2015 [13] | AR | UUT | 46/80 (58%) | 20/99 (20%) | <0.001 |
| Miyamoto, 2012 [11] | ERα | Bladder | 70/141 (50%) | 51/188 (27%) | <0.001 |
| Mashhadi, 2014 [12] | ERα | Bladder | 2/132 (2%) | 3/120 (3%) | 0.67 |
| Kashiwagi, 2015 [13] | ERα | UUT | 32/80 (40%) | 18/99 (18%) | 0.001 |
| Imai, 2019 [22] | ERα | Bladder | 33/92 (36%) | 48/125 (38%) | 0.777 * |
| Kontos, 2010 [23] | ERβ | Bladder | 27/29 (93%) | 84/111 (76%) | 0.041 * |
| Miyamoto, 2012 [11] | ERβ | Bladder | 125/141 (89%) | 93/188 (49%) | <0.001 |
| Kashiwagi, 2015 [13] | ERβ | UUT | 68/80 (85%) | 62/99 (63%) | 0.001 |
| Ishiguro,2014 [24] | GR | Bladder | 90/94 (96%) | 129/149 (87%) | 0.026 |
| Kashiwagi, 2015 [13] | GR | UUT | 67/80 (84%) | 62/99 (63%) | 0.001 |
| Mashhadi, 2014 [12] | PR | Bladder | 3/132 (2%) | 5/120 (4%) | 0.48 |
| Kashiwagi, 2015 [13] | PR | UUT | 10/80 (13%) | 16/99 (16%) | 0.487 |
| Imai, 2019 [22] | PR | Bladder | 1/92 (1%) | 4/125 (3%) | 0.398 * |
| Sahin, 2005 [25] | VDR | Bladder | 70/105 (67%) | 90/105 (86%) | 0.02 |
| Jóźwicki, 2015 [26] | VDR | Bladder | 12/12 (100%) | 62/71 (87%) | 0.345 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ide, H.; Miyamoto, H. The Role of Steroid Hormone Receptors in Urothelial Tumorigenesis. Cancers 2020, 12, 2155. https://doi.org/10.3390/cancers12082155
Ide H, Miyamoto H. The Role of Steroid Hormone Receptors in Urothelial Tumorigenesis. Cancers. 2020; 12(8):2155. https://doi.org/10.3390/cancers12082155
Chicago/Turabian StyleIde, Hiroki, and Hiroshi Miyamoto. 2020. "The Role of Steroid Hormone Receptors in Urothelial Tumorigenesis" Cancers 12, no. 8: 2155. https://doi.org/10.3390/cancers12082155
APA StyleIde, H., & Miyamoto, H. (2020). The Role of Steroid Hormone Receptors in Urothelial Tumorigenesis. Cancers, 12(8), 2155. https://doi.org/10.3390/cancers12082155






