Plasmacytoid Dendritic Cell Impairment in Metastatic Melanoma by Lactic Acidosis
Abstract
1. Introduction
2. Results
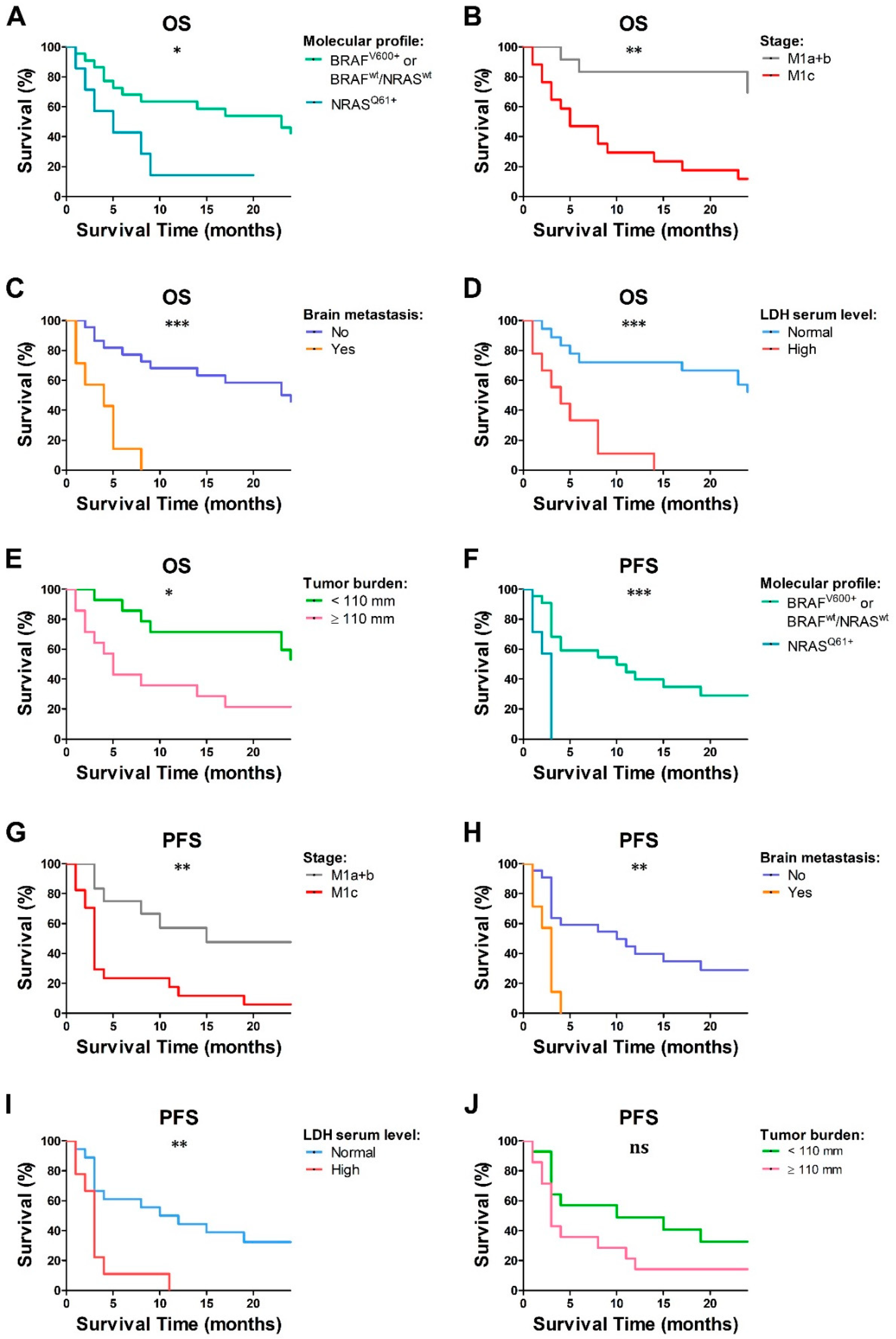
2.1. Clinical Features and Outcome of the MM Cohort
2.2. Peripheral Blood Immune Populations and pDC Function Were Impaired in Chemo-Naïve MM Patients
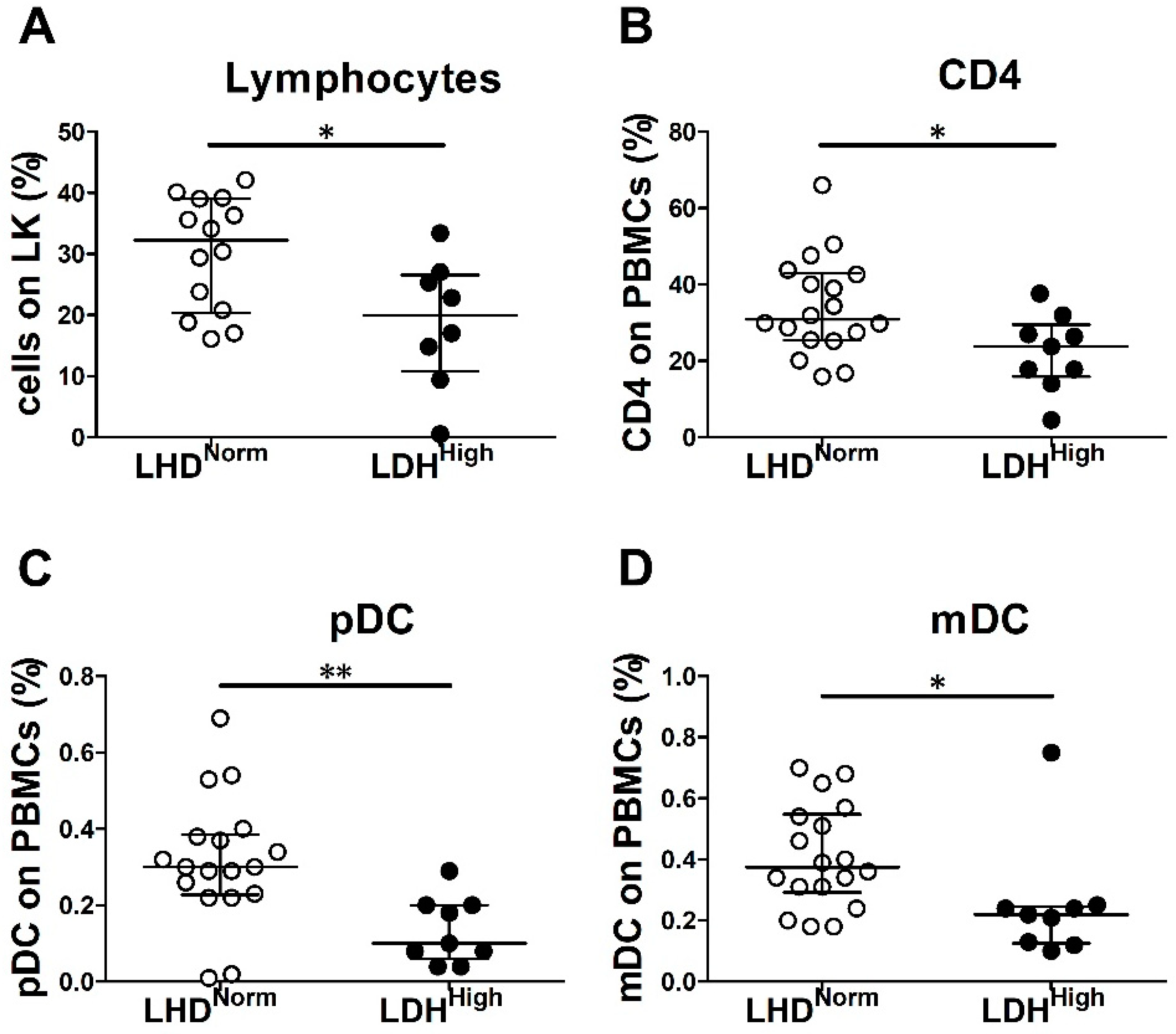
2.3. LDH Level and Tumor Burden Were Associated with Decreased Frequency of Peripheral Blood Immune Cells in Chemo-Naïve MM Patients
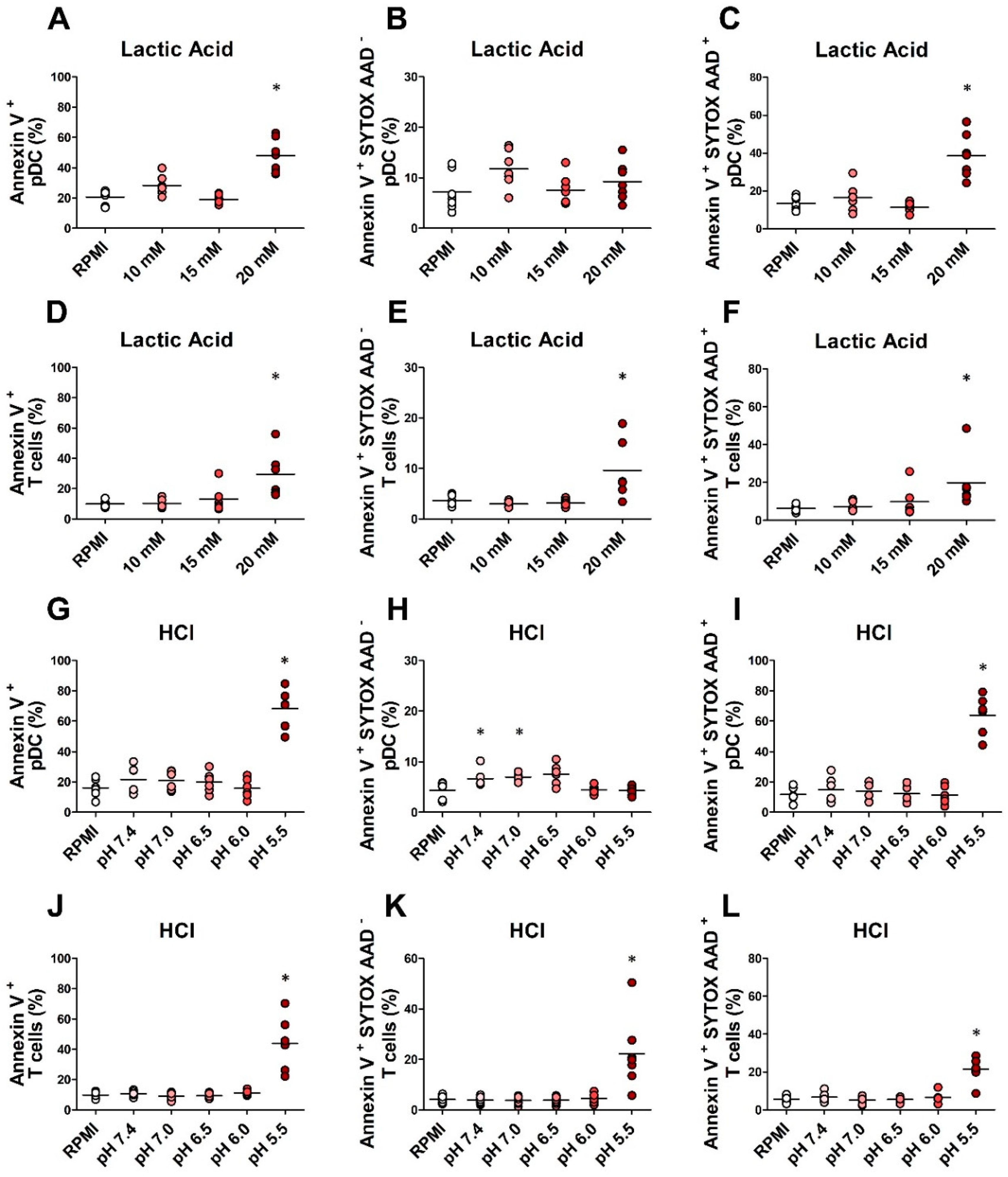
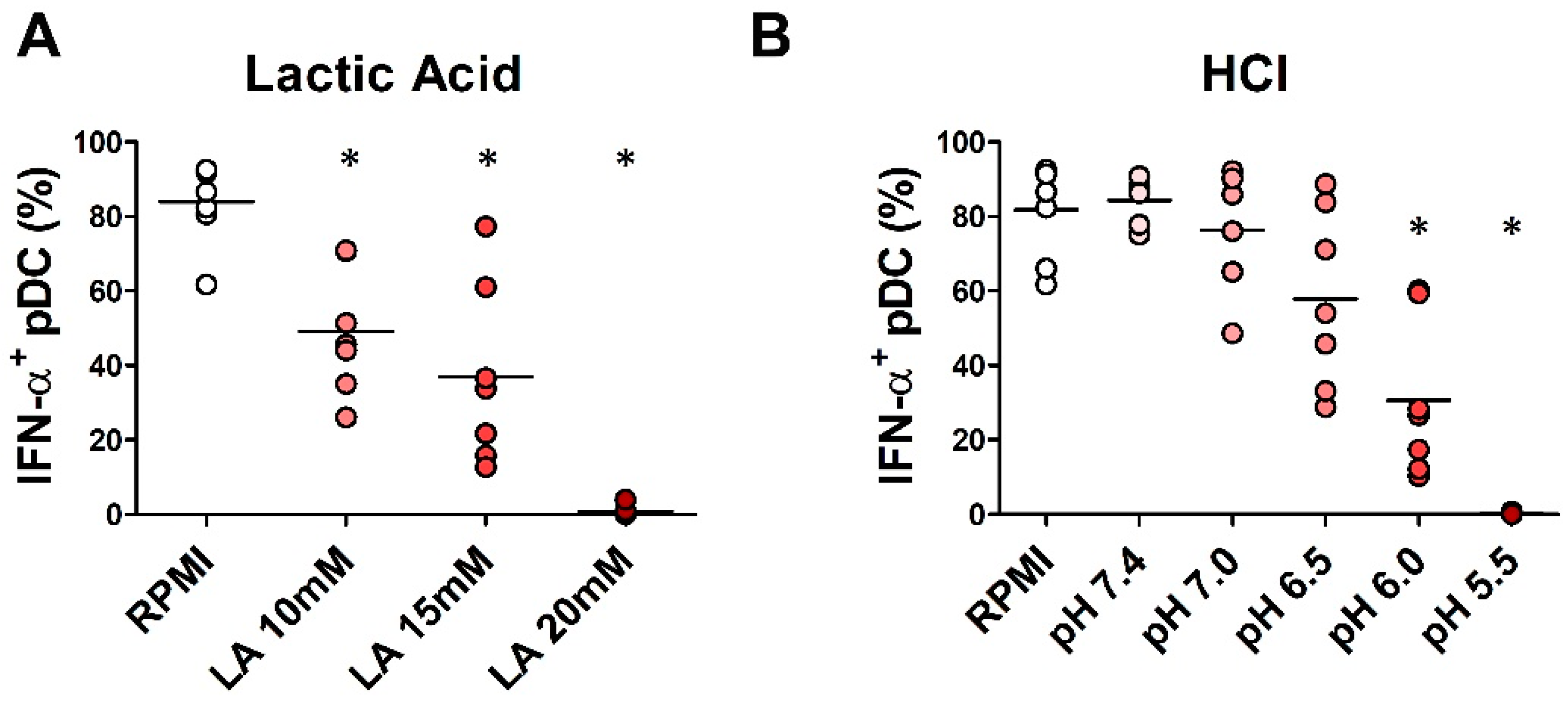
2.4. In Vitro Exposure to Lactic Acidosis Impaired the Viability and Function of Fully Differentiated pDCs
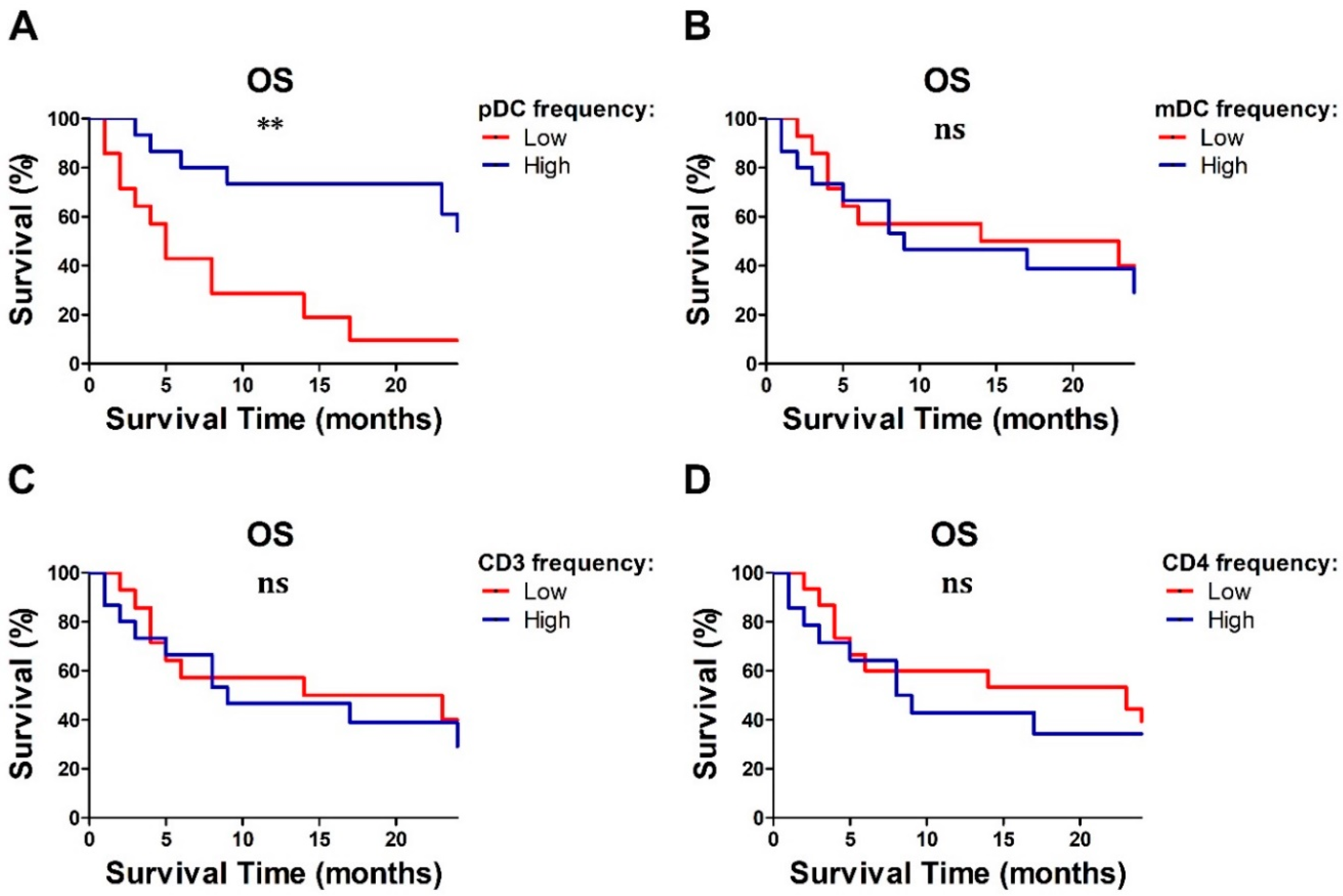
2.5. Baseline Lymphocyte and pDC Frequencies Predicted MM Outcome
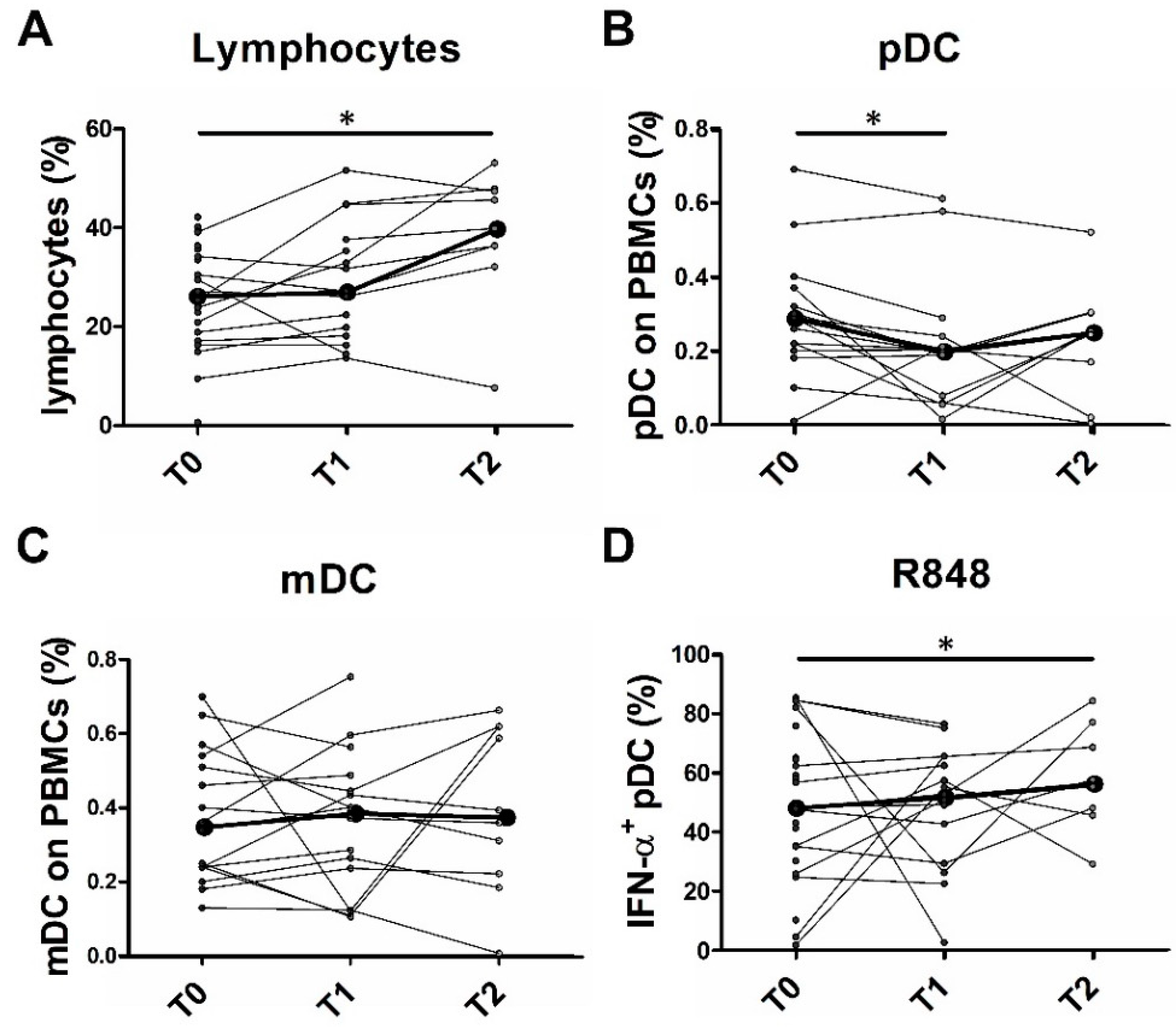
2.6. Partial pDC Recovery after BRAFi and MEKi Administration
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Human Subjects and Blood Specimen Processing
4.3. Peripheral Blood Mononuclear Cell Stimulation
4.4. Purification, Culture, and Stimulation of Peripheral Blood pDCs and T Lymphocytes
4.5. Flow-Cytometric Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Corrie, P.; Hategan, M.; Fife, K.; Parkinson, C. Management of melanoma. Br. Med. Bull. 2014, 111, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Ugurel, S.; Rohmel, J.; Ascierto, P.A.; Flaherty, K.T.; Grob, J.J.; Hauschild, A.; Larkin, J.; Long, G.V.; Lorigan, P.; McArthur, G.A.; et al. Survival of patients with advanced metastatic melanoma: The impact of novel therapies-update 2017. Eur. J. Cancer 2017, 83, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef]
- Hauschild, A.; Grob, J.J.; Demidov, L.V.; Jouary, T.; Gutzmer, R.; Millward, M.; Rutkowski, P.; Blank, C.U.; Miller, W.H., Jr.; Kaempgen, E.; et al. Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012, 380, 358–365. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Weber, J.S.; Maio, M.; Neyns, B.; Harmankaya, K.; Chin, K.; Cykowski, L.; de Pril, V.; Humphrey, R.; Lebbe, C. Four-year survival rates for patients with metastatic melanoma who received ipilimumab in phase II clinical trials. Ann. Oncol. 2013, 24, 2174–2180. [Google Scholar] [CrossRef]
- Schadendorf, D.; Hodi, F.S.; Robert, C.; Weber, J.S.; Margolin, K.; Hamid, O.; Patt, D.; Chen, T.T.; Berman, D.M.; Wolchok, J.D. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J. Clin. Oncol. 2015, 33, 1889–1894. [Google Scholar] [CrossRef]
- Topalian, S.L.; Sznol, M.; McDermott, D.F.; Kluger, H.M.; Carvajal, R.D.; Sharfman, W.H.; Brahmer, J.R.; Lawrence, D.P.; Atkins, M.B.; Powderly, J.D.; et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 2014, 32, 1020–1030. [Google Scholar] [CrossRef]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Bastholt, L.; Bataille, V.; Del Marmol, V.; Dreno, B.; Fargnoli, M.C.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 2: Treatment—Update 2019. Eur. J. Cancer 2019. [Google Scholar] [CrossRef]
- Frederick, D.T.; Piris, A.; Cogdill, A.P.; Cooper, Z.A.; Lezcano, C.; Ferrone, C.R.; Mitra, D.; Boni, A.; Newton, L.P.; Liu, C.; et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin. Cancer Res. 2013, 19, 1225–1231. [Google Scholar] [CrossRef]
- Boni, A.; Cogdill, A.P.; Dang, P.; Udayakumar, D.; Njauw, C.N.; Sloss, C.M.; Ferrone, C.R.; Flaherty, K.T.; Lawrence, D.P.; Fisher, D.E.; et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010, 70, 5213–5219. [Google Scholar] [CrossRef]
- Sumimoto, H.; Imabayashi, F.; Iwata, T.; Kawakami, Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J. Exp. Med. 2006, 203, 1651–1656. [Google Scholar] [CrossRef]
- Bradley, S.D.; Chen, Z.; Melendez, B.; Talukder, A.; Khalili, J.S.; Rodriguez-Cruz, T.; Liu, S.; Whittington, M.; Deng, W.; Li, F.; et al. BRAFV600E Co-opts a Conserved MHC Class I Internalization Pathway to Diminish Antigen Presentation and CD8 + T-cell Recognition of Melanoma. Cancer Immunol. Res. 2015, 3, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Dunn, I.S.; Durda, P.J.; Butera, D.; Rose, L.B.; Haggerty, T.J.; Benson, E.M.; Kurnick, J.T. Role of the mitogen-activated protein kinase signaling pathway in the regulation of human melanocytic antigen expression. Mol. Cancer Res. 2006, 4, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Wilmott, J.S.; Long, G.V.; Howle, J.R.; Haydu, L.E.; Sharma, R.N.; Thompson, J.F.; Kefford, R.F.; Hersey, P.; Scolyer, R.A. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin. Cancer Res. 2012, 18, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Henry, T.; Baranda, S.J.; Frleta, D.; Manches, O.; Bogunovic, D.; Bhardwaj, N. Inhibition of both BRAF and MEK in BRAF(V600E) mutant melanoma restores compromised dendritic cell (DC) function while having differential direct effects on DC properties. Cancer Immunol. Immunother. 2013, 62, 811–822. [Google Scholar] [CrossRef]
- Hong, D.S.; Vence, L.; Falchook, G.; Radvanyi, L.G.; Liu, C.; Goodman, V.; Legos, J.J.; Blackman, S.; Scarmadio, A.; Kurzrock, R.; et al. BRAF(V600) inhibitor GSK2118436 targeted inhibition of mutant BRAF in cancer patients does not impair overall immune competency. Clin. Cancer Res. 2012, 18, 2326–2335. [Google Scholar] [CrossRef]
- Hauschild, A.; Larkin, J.; Ribas, A.; Dreno, B.; Flaherty, K.T.; Ascierto, P.A.; Lewis, K.D.; McKenna, E.; Zhu, Q.; Mun, Y.; et al. Modeled Prognostic Subgroups for Survival and Treatment Outcomes in BRAF V600-Mutated Metastatic Melanoma: Pooled Analysis of 4 Randomized Clinical Trials. JAMA Oncol. 2018, 4, 1382–1388. [Google Scholar] [CrossRef]
- Sabbatino, F.; Wang, Y.; Scognamiglio, G.; Favoino, E.; Feldman, S.A.; Villani, V.; Flaherty, K.T.; Nota, S.; Giannarelli, D.; Simeone, E.; et al. Antitumor Activity of BRAF Inhibitor and IFNalpha Combination in BRAF-Mutant Melanoma. J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef]
- Takeda, K.; Kaisho, T.; Akira, S. Toll-like receptors. Annu. Rev. Immunol. 2003, 21, 335–376. [Google Scholar] [CrossRef] [PubMed]
- Von Marschall, Z.; Scholz, A.; Cramer, T.; Schäfer, G.; Schirner, M.; Oberg, K.; Wiedenmann, B.; Höcker, M.; Rosewicz, S. Effects of interferon alpha on vascular endothelial growth factor gene transcription and tumor angiogenesis. J. Natl. Cancer Inst. 2003, 95, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Gutman, M.; Bucana, C.D.; Sanchez, R.; Llansa, N.; Fidler, I.J. Interferons alpha and beta down-regulate the expression of basic fibroblast growth factor in human carcinomas. Proc. Natl. Acad. Sci. USA 1995, 92, 4562–4566. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wei, H.; Sun, R.; Tian, Z. IFNalpha regulates NK cell cytotoxicity through STAT1 pathway. Cytokine 2003, 23, 190–199. [Google Scholar] [CrossRef]
- Marrack, P.; Kappler, J.; Mitchell, T. Type I interferons keep activated T cells alive. J. Exp. Med. 1999, 189, 521–530. [Google Scholar] [CrossRef]
- Curtsinger, J.M.; Valenzuela, J.O.; Agarwal, P.; Lins, D.; Mescher, M.F. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J. Immunol. 2005, 174, 4465–4469. [Google Scholar] [CrossRef]
- Blackwell, S.E.; Krieg, A.M. CpG-A-induced monocyte IFN-gamma-inducible protein-10 production is regulated by plasmacytoid dendritic cell-derived IFN-alpha. J. Immunol. 2003, 170, 4061–4068. [Google Scholar] [CrossRef]
- Wildbaum, G.; Netzer, N.; Karin, N. Plasmid DNA encoding IFN-gamma-inducible protein 10 redirects antigen-specific T cell polarization and suppresses experimental autoimmune encephalomyelitis. J. Immunol. 2002, 168, 5885–5892. [Google Scholar] [CrossRef]
- Vescovi, R.; Monti, M.; Moratto, D.; Paolini, L.; Consoli, F.; Benerini, L.; Melocchi, L.; Calza, S.; Chiudinelli, M.; Rossi, G.; et al. Collapse of the Plasmacytoid Dendritic Cell Compartment in Advanced Cutaneous Melanomas by Components of the Tumor Cell Secretome. Cancer Immunol. Res. 2019, 7, 12–28. [Google Scholar] [CrossRef]
- Gottfried, E.; Kunz-Schughart, L.A.; Ebner, S.; Mueller-Klieser, W.; Hoves, S.; Andreesen, R.; Mackensen, A.; Kreutz, M. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood 2006, 107, 2013–2021. [Google Scholar] [CrossRef]
- Ding, J.; Karp, J.E.; Emadi, A. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: Interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomark. 2017, 19, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Wind, F.; Negelein, E. THE METABOLISM OF TUMORS IN THE BODY. J. Gen. Physiol. 1927, 8. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23. [Google Scholar] [CrossRef]
- Bronte, V. Tumor cells hijack macrophages via lactic acid. Immunol. Cell Biol. 2014, 92, 647–649. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.; Consoli, F.; Vescovi, R.; Bugatti, M.; Vermi, W. Human Plasmacytoid Dendritic Cells and Cutaneous Melanoma. Cells 2020, 9, 417. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Bruce, A.T.; Sheehan, K.C.; Shankaran, V.; Uppaluri, R.; Bui, J.D.; Diamond, M.S.; Koebel, C.M.; Arthur, C.; White, J.M.; et al. A critical function for type I interferons in cancer immunoediting. Nat. Immunol. 2005, 6, 722–729. [Google Scholar] [CrossRef]
- Borden, E.C. Interferons alpha and beta in cancer: Therapeutic opportunities from new insights. Nat. Rev. Drug Discov. 2019, 18, 219–234. [Google Scholar] [CrossRef]
- Le Mercier, I.; Poujol, D.; Sanlaville, A.; Sisirak, V.; Gobert, M.; Durand, I.; Dubois, B.; Treilleux, I.; Marvel, J.; Vlach, J.; et al. Tumor promotion by intratumoral plasmacytoid dendritic cells is reversed by TLR7 ligand treatment. Cancer Res. 2013, 73, 4629–4640. [Google Scholar] [CrossRef]
- Megjugorac, N.J.; Young, H.A.; Amrute, S.B.; Olshalsky, S.L.; Fitzgerald-Bocarsly, P. Virally stimulated plasmacytoid dendritic cells produce chemokines and induce migration of T and NK cells. J. Leukoc. Biol. 2004, 75, 504–514. [Google Scholar] [CrossRef]
- Zbytek, B.; Carlson, J.A.; Granese, J.; Ross, J.; Mihm, M.C., Jr.; Slominski, A. Current concepts of metastasis in melanoma. Expert Rev. Dermatol. 2008, 3, 569–585. [Google Scholar] [CrossRef]
- Karagiannis, P.; Fittall, M.; Karagiannis, S.N. Evaluating biomarkers in melanoma. Front. Oncol. 2014, 4, 383. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Grob, J.J.; Nathan, P.; Ribas, A.; Robert, C.; Schadendorf, D.; Lane, S.R.; Mak, C.; Legenne, P.; Flaherty, K.T.; et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: A pooled analysis of individual patient data from randomised trials. Lancet Oncol. 2016, 17, 1743–1754. [Google Scholar] [CrossRef]
- Caronni, N.; Simoncello, F.; Stafetta, F.; Guarnaccia, C.; Ruiz-Moreno, J.S.; Opitz, B.; Galli, T.; Proux-Gillardeaux, V.; Benvenuti, F. Downregulation of Membrane Trafficking Proteins and Lactate Conditioning Determine Loss of Dendritic Cell Function in Lung Cancer. Cancer Res. 2018, 78, 1685–1699. [Google Scholar] [CrossRef]
- Comito, G.; Iscaro, A.; Bacci, M.; Morandi, A.; Ippolito, L.; Parri, M.; Montagnani, I.; Raspollini, M.R.; Serni, S.; Simeoni, L.; et al. Lactate modulates CD4(+) T-cell polarization and induces an immunosuppressive environment, which sustains prostate carcinoma progression via TLR8/miR21 axis. Oncogene 2019, 38, 3681–3695. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, D.; Bhattacharya, R.; Sinha, B.P.; Liu, C.S.C.; Ghosh, A.R.; Rahaman, O.; Bandopadhyay, P.; Sarif, J.; D’Rozario, R.; Paul, S.; et al. Lactate Induces Pro-tumor Reprogramming in Intratumoral Plasmacytoid Dendritic Cells. Front. Immunol. 2019, 10, 1878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, G.; Xu, Z.G.; Tu, H.; Hu, F.; Dai, J.; Chang, Y.; Chen, Y.; Lu, Y.; Zeng, H.; et al. Lactate Is a Natural Suppressor of RLR Signaling by Targeting MAVS. Cell 2019, 178, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.T.; Garnett, M.J.; Roe, S.M.; Lee, S.; Niculescu-Duvaz, D.; Good, V.M.; Jones, C.M.; Marshall, C.J.; Springer, C.J.; Barford, D.; et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 2004, 116, 855–867. [Google Scholar] [CrossRef]
- Johannessen, C.M.; Boehm, J.S.; Kim, S.Y.; Thomas, S.R.; Wardwell, L.; Johnson, L.A.; Emery, C.M.; Stransky, N.; Cogdill, A.P.; Barretina, J.; et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature 2010, 468, 968–972. [Google Scholar] [CrossRef]
- Wagle, N.; Emery, C.; Berger, M.F.; Davis, M.J.; Sawyer, A.; Pochanard, P.; Kehoe, S.M.; Johannessen, C.M.; Macconaill, L.E.; Hahn, W.C.; et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J. Clin. Oncol. 2011, 29, 3085–3096. [Google Scholar] [CrossRef]
- Janovec, V.; Aouar, B.; Font-Haro, A.; Hofman, T.; Trejbalova, K.; Weber, J.; Chaperot, L.; Plumas, J.; Olive, D.; Dubreuil, P.; et al. The MEK1/2-ERK Pathway Inhibits Type I IFN Production in Plasmacytoid Dendritic Cells. Front. Immunol. 2018, 9, 364. [Google Scholar] [CrossRef]
- Ott, P.A.; Bhardwaj, N. Impact of MAPK Pathway Activation in BRAF(V600) Melanoma on T Cell and Dendritic Cell Function. Front. Immunol. 2013, 4, 346. [Google Scholar] [CrossRef] [PubMed]
- Chevolet, I.; Speeckaert, R.; Schreuer, M.; Neyns, B.; Krysko, O.; Bachert, C.; Van Gele, M.; van Geel, N.; Brochez, L. Clinical significance of plasmacytoid dendritic cells and myeloid-derived suppressor cells in melanoma. J. Transl. Med. 2015, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Failli, A.; Legitimo, A.; Orsini, G.; Romanini, A.; Consolini, R. Numerical defect of circulating dendritic cell subsets and defective dendritic cell generation from monocytes of patients with advanced melanoma. Cancer Lett. 2013, 337, 184–192. [Google Scholar] [CrossRef]
- Kadowaki, N.; Antonenko, S.; Lau, J.Y.; Liu, Y.J. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J. Exp. Med. 2000, 192, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Aspord, C.; Leccia, M.T.; Charles, J.; Plumas, J. Plasmacytoid dendritic cells support melanoma progression by promoting Th2 and regulatory immunity through OX40L and ICOSL. Cancer Immunol. Res. 2013, 1, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Faget, J.; Bendriss-Vermare, N.; Gobert, M.; Durand, I.; Olive, D.; Biota, C.; Bachelot, T.; Treilleux, I.; Goddard-Leon, S.; Lavergne, E.; et al. ICOS-ligand expression on plasmacytoid dendritic cells supports breast cancer progression by promoting the accumulation of immunosuppressive CD4+ T cells. Cancer Res. 2012, 72, 6130–6141. [Google Scholar] [CrossRef]
- Hartmann, E.; Wollenberg, B.; Rothenfusser, S.; Wagner, M.; Wellisch, D.; Mack, B.; Giese, T.; Gires, O.; Endres, S.; Hartmann, G. Identification and functional analysis of tumor-infiltrating plasmacytoid dendritic cells in head and neck cancer. Cancer Res. 2003, 63, 6478–6487. [Google Scholar]
- Dunn, G.P.; Koebel, C.M.; Schreiber, R.D. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 2006, 6, 836–848. [Google Scholar] [CrossRef]
- Majer, O.; Liu, B.; Woo, B.J.; Kreuk, L.S.M.; Van Dis, E.; Barton, G.M. Release from UNC93B1 reinforces the compartmentalized activation of select TLRs. Nature 2019, 575, 371–374. [Google Scholar] [CrossRef]
- Majer, O.; Liu, B.; Kreuk, L.S.M.; Krogan, N.; Barton, G.M. UNC93B1 recruits syntenin-1 to dampen TLR7 signalling and prevent autoimmunity. Nature 2019, 575, 366–370. [Google Scholar] [CrossRef]
- See, P.; Dutertre, C.A.; Chen, J.; Günther, P.; McGovern, N.; Irac, S.E.; Gunawan, M.; Beyer, M.; Händler, K.; Duan, K.; et al. Mapping the Human DC Lineage Through the Integration of High-Dimensional Techniques. Science 2017, 356. [Google Scholar] [CrossRef] [PubMed]
- Siegal, F.P.; Kadowaki, N.; Shodell, M.; Fitzgerald-Bocarsly, P.A.; Shah, K.; Ho, S.; Antonenko, S.; Liu, Y.J. The nature of the principal type 1 interferon-producing cells in human blood. Science 1999, 284, 1835–1837. [Google Scholar] [CrossRef] [PubMed]
- Alculumbre, S.G.; Saint-Andre, V.; Di Domizio, J.; Vargas, P.; Sirven, P.; Bost, P.; Maurin, M.; Maiuri, P.; Wery, M.; Roman, M.S.; et al. Diversification of human plasmacytoid predendritic cells in response to a single stimulus. Nat. Immunol. 2018, 19, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Wimmers, F.; Subedi, N.; van Buuringen, N.; Heister, D.; Vivie, J.; Beeren-Reinieren, I.; Woestenenk, R.; Dolstra, H.; Piruska, A.; Jacobs, J.F.M.; et al. Single-cell analysis reveals that stochasticity and paracrine signaling control interferon-alpha production by plasmacytoid dendritic cells. Nat. Commun. 2018, 9, 3317. [Google Scholar] [CrossRef] [PubMed]
- Corzo, C.A.; Condamine, T.; Lu, L.; Cotter, M.J.; Youn, J.I.; Cheng, P.; Cho, H.I.; Celis, E.; Quiceno, D.G.; Padhya, T.; et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med. 2010, 207, 2439–2453. [Google Scholar] [CrossRef] [PubMed]
- Balch, C.M.; Soong, S.J.; Atkins, M.B.; Buzaid, A.C.; Cascinelli, N.; Coit, D.G.; Fleming, I.D.; Gershenwald, J.E.; Houghton, A., Jr.; Kirkwood, J.M.; et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J. Clin. 2004, 54, 131–149; quiz 134–182. [Google Scholar] [CrossRef]
- Diem, S.; Kasenda, B.; Spain, L.; Martin-Liberal, J.; Marconcini, R.; Gore, M.; Larkin, J. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br. J. Cancer 2016, 114, 256–261. [Google Scholar] [CrossRef]
- Bajwa, G.; DeBerardinis, R.J.; Shao, B.; Hall, B.; Farrar, J.D.; Gill, M.A. Cutting Edge: Critical Role of Glycolysis in Human Plasmacytoid Dendritic Cell Antiviral Responses. J. Immunol. 2016, 196, 2004–2009. [Google Scholar] [CrossRef]
- Saas, P.; Varin, A.; Perruche, S.; Ceroi, A. Recent insights into the implications of metabolism in plasmacytoid dendritic cell innate functions: Potential ways to control these functions. F1000Research 2017, 6, 456. [Google Scholar] [CrossRef]
- Fischer, G.M.; Vashisht Gopal, Y.N.; McQuade, J.L.; Peng, W.; DeBerardinis, R.J.; Davies, M.A. Metabolic strategies of melanoma cells: Mechanisms, interactions with the tumor microenvironment, and therapeutic implications. Pigment Cell Melanoma Res. 2018, 31, 11–30. [Google Scholar] [CrossRef]
- Tasdogan, A.; Faubert, B.; Ramesh, V.; Ubellacker, J.M.; Shen, B.; Solmonson, A.; Murphy, M.M.; Gu, Z.; Gu, W.; Martin, M.; et al. Metabolic heterogeneity confers differences in melanoma metastatic potential. Nature 2020, 577, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Zhang, Z.; Jv, H.; Hu, J.; Ruan, M.; Zhang, C. Culture supernatants of oral cancer cells induce impaired IFN-alpha production of pDCs partly through the down-regulation of TLR-9 expression. Arch. Oral Biol. 2018, 93, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Sisirak, V.; Vey, N.; Goutagny, N.; Renaudineau, S.; Malfroy, M.; Thys, S.; Treilleux, I.; Labidi-Galy, S.I.; Bachelot, T.; Dezutter-Dambuyant, C.; et al. Breast cancer-derived transforming growth factor-beta and tumor necrosis factor-alpha compromise interferon-alpha production by tumor-associated plasmacytoid dendritic cells. Int. J. Cancer 2013, 133, 771–778. [Google Scholar] [CrossRef]
- Dzionek, A.; Sohma, Y.; Nagafune, J.; Cella, M.; Colonna, M.; Facchetti, F.; Gunther, G.; Johnston, I.; Lanzavecchia, A.; Nagasaka, T.; et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J. Exp. Med. 2001, 194, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Rosen, D.B.; Ito, T.; Bover, L.; Bao, M.; Watanabe, G.; Yao, Z.; Zhang, L.; Lanier, L.L.; Liu, Y.J. Plasmacytoid dendritic cell-specific receptor ILT7-Fc epsilonRI gamma inhibits Toll-like receptor-induced interferon production. J. Exp. Med. 2006, 203, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Bover, L.; Cho, M.; Wen, X.; Hanabuchi, S.; Bao, M.; Rosen, D.B.; Wang, Y.H.; Shaw, J.L.; Du, Q.; et al. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J. Exp. Med. 2009, 206, 1603–1614. [Google Scholar] [CrossRef]
- Riboldi, E.; Daniele, R.; Parola, C.; Inforzato, A.; Arnold, P.L.; Bosisio, D.; Fremont, D.H.; Bastone, A.; Colonna, M.; Sozzani, S. Human C-type lectin domain family 4, member C (CLEC4C/BDCA-2/CD303) is a receptor for asialo-galactosyl-oligosaccharides. J. Biol. Chem. 2011, 286, 35329–35333. [Google Scholar] [CrossRef]
- Krieg, A.M. Therapeutic potential of Toll-like receptor 9 activation. Nat. Rev. Drug Discov. 2006, 5, 471–484. [Google Scholar] [CrossRef]
- Pashenkov, M.; Goess, G.; Wagner, C.; Hormann, M.; Jandl, T.; Moser, A.; Britten, C.M.; Smolle, J.; Koller, S.; Mauch, C.; et al. Phase II trial of a toll-like receptor 9-activating oligonucleotide in patients with metastatic melanoma. J. Clin. Oncol. 2006, 24, 5716–5724. [Google Scholar] [CrossRef]
- Aspord, C.; Tramcourt, L.; Leloup, C.; Molens, J.P.; Leccia, M.T.; Charles, J.; Plumas, J. Imiquimod inhibits melanoma development by promoting pDC cytotoxic functions and impeding tumor vascularization. J. Investig. Dermatol. 2014, 134, 2551–2561. [Google Scholar] [CrossRef]
- Teulings, H.E.; Tjin, E.P.M.; Willemsen, K.J.; van der Kleij, S.; Ter Meulen, S.; Kemp, E.H.; Krebbers, G.; van Noesel, C.J.M.; Franken, C.; Drijfhout, J.W.; et al. Anti-Melanoma immunity and local regression of cutaneous metastases in melanoma patients treated with monobenzone and imiquimod; a phase 2 a trial. Oncoimmunology 2018, 7, e1419113. [Google Scholar] [CrossRef] [PubMed]
- Van den Hout, M.; Koster, B.D.; Sluijter, B.J.R.; Molenkamp, B.G.; van de Ven, R.; van den Eertwegh, A.J.M.; Scheper, R.J.; van Leeuwen, P.A.M.; van den Tol, M.P.; de Gruijl, T.D. Melanoma Sequentially Suppresses Different DC Subsets in the Sentinel Lymph Node, Affecting Disease Spread and Recurrence. Cancer Immunol. Res. 2017, 5, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Sunaga, N.; Imai, H.; Shimizu, K.; Shames, D.S.; Kakegawa, S.; Girard, L.; Sato, M.; Kaira, K.; Ishizuka, T.; Gazdar, A.F.; et al. Oncogenic KRAS-induced interleukin-8 overexpression promotes cell growth and migration and contributes to aggressive phenotypes of non-small cell lung cancer. Int. J. Cancer 2012, 130, 1733–1744. [Google Scholar] [CrossRef]
- Dabrosin, N.; Sloth Juul, K.; Baehr Georgsen, J.; Andrup, S.; Schmidt, H.; Steiniche, T.; Heide Ollegaard, T.; Bonnelykke Behrndtz, L. Innate immune cell infiltration in melanoma metastases affects survival and is associated with BRAFV600E mutation status. Melanoma Res. 2019, 29, 30–37. [Google Scholar] [CrossRef] [PubMed]

| Clinical/Molecular Features | BRAFV600+ MM (N = 16) | NRASQ61+ MM (N = 7) | BRAFwt/NRASwt (N = 6) | |||||||
| N | n | % | N | n | % | N | n | % | p | |
| Gender (males) | 16 | 12 | 75 | 7 | 5 | 71.4 | 6 | 5 | 83.3 | 1 |
| Stage | 16 | 7 | 6 | 0.5 | ||||||
| M1a | 3 | 18.7 | 1 | 14.3 | 1 | 16.7 | ||||
| M1b | 5 | 31.3 | 0 | 0 | 2 | 33.3 | ||||
| M1c | 8 | 50 | 6 | 85.7 | 3 | 50 | ||||
| Brain metastases | 16 | 3 | 18.7 | 7 | 2 | 28.6 | 6 | 2 | 33.3 | 0.6 |
| Tumor sites (≥3) | 16 | 6 | 37.5 | 7 | 3 | 42.9 | 6 | 4 | 66.7 | 0.5 |
| LDH (high *) | 16 | 4 | 25 | 6 | 3 | 50 | 5 | 2 | 40 | 0.5 |
| Therapy | 16 | 7 | 6 | |||||||
| BRAFi | 4 | 25 | ||||||||
| BRAFi + MEKi | 12 | 75 | ||||||||
| Anti-CTLA-4 | 7 | 100 | 5 | 83.3 | ||||||
| Anti-PD-1 | 1 | 16.7 | ||||||||
| N | Median (min-max) | SD | N | Median (min–max) | SD | N | Median (min–max) | SD | p | |
| Age | 16 | 58.5 (23–76) | 14.0 | 7 | 58.0 (48–76) | 10.0 | 6 | 62.0 (53–79) | 8.8 | 0.2 |
| Tumor burden (mm) | 15 | 116.5 (0–408.2) | 127.6 | 7 | 77.9 (8–260.3) | 89.0 | 6 | 136.4 (52.2–309.5) | 98.0 | 0.8 |
| Immune Cell Population | MM (N = 29) | HD (N = 25) | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Median | IQR | N | Median | IQR | p * | ||
| % pDCs on PBMCs | 29 | 0.3 | 0.2–0.3 | 24 | 0.4 | 0.4–0.6 | 0.0006 | |
| % mDCs on PBMCs | 29 | 0.3 | 0.2–0.5 | 15 | 0.5 | 0.4–0.7 | 0.03 | |
| % CD3+ on PBMCs | 29 | 58.9 | 52.6–62.3 | 15 | 58 | 50.1–66.8 | 0.9 | |
| % CD4+ on PBMCs | 29 | 28.7 | 20.1–39.0 | 15 | 34.6 | 27.4–40.8 | 0.1 | |
| % IFN-α+ pDCs | R848 | 27 | 48.5 | 30.2–65.0 | 25 | 71.5 | 64.3–74.7 | 0.004 |
| IMQ | 27 | 21.1 | 8.7–41.7 | 24 | 38.3 | 29.4–45.1 | 0.02 | |
| CpG | 26 | 9.3 | 4.3–13.9 | 22 | 11.6 | 5–15.2 | 0.4 | |
| % CXCL10+ pDCs | R848 | 24 | 78.4 | 64.8–85.3 | 19 | 80.9 | 79.1–86.8 | 0.08 |
| IMQ | 24 | 47.9 | 22.4–61.5 | 19 | 61.6 | 48.9–71.4 | 0.03 | |
| CpG | 25 | 7.1 | 3.7–17.1 | 20 | 10.2 | 2.9–14.8 | 0.7 | |
| Immune Cell Population | Normal LDH (N = 18) | High LDH * (N = 9) | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Median | IQR | N | Median | IQR | p | ||
| n° leukocytes/µL | 14 | 6805 | 5830–9270 | 9 | 9110 | 6860–12,050 | 0.08 | |
| % neutrophils on LK | 14 | 54.7 | 47.2–68.5 | 8 | 66.2 | 54.9–74.3 | 0.1 | |
| % lymphocytes on LK | 14 | 32.3 | 20.8–39 | 8 | 19.9 | 12.1–26.1 | 0.02 | |
| % monocytes on LK | 14 | 8.3 | 7.5–11 | 8 | 7.5 | 6.1–10.3 | 0.2 | |
| % eosinophils on LK | 14 | 2.1 | 1.6–2.5 | 8 | 0.8 | 0.1–3 | 0.1 | |
| % basophils on LK | 14 | 0.6 | 0.4–0.8 | 8 | 0.5 | 0.2–0.6 | 0.3 | |
| % pDCs on PBMCs | 18 | 0.3 | 0.2–0.4 | 9 | 0.1 | 0.1–0.2 | 0.003 | |
| % mDCs on PBMCs | 18 | 0.4 | 0.3–0.5 | 9 | 0.2 | 0.1–0.2 | 0.02 | |
| % CD3+ on PBMCs | 18 | 60.2 | 54.6–68.9 | 9 | 46.7 | 40.7–59.8 | 0.06 | |
| % CD4+ on PBMCs | 18 | 30.9 | 25.5–42.6 | 9 | 23.8 | 17.8–27 | 0.03 | |
| % IFN-α+ pDCs | R848 | 17 | 56.7 | 35.1–82 | 9 | 43.0 | 25.9–58 | 0.2 |
| IMQ | 17 | 27.7 | 14–56.4 | 9 | 13.6 | 5.4–27 | 0.08 | |
| CpG | 16 | 7.8 | 4.2–14.2 | 9 | 9.9 | 8.3–10.9 | 1 | |
| % CXCL10+ pDCs | R848 | 14 | 78.4 | 71.9–85.5 | 9 | 75.2 | 51.6–84.1 | 0.7 |
| IMQ | 14 | 47.3 | 23.9–57.9 | 9 | 47.5 | 19–69 | 0.4 | |
| CpG | 15 | 5.2 | 2.9–11.7 | 9 | 17.1 | 5.7–36.8 | 0.1 | |
| Immune Cell Population | N | Rho | p | |
|---|---|---|---|---|
| n° leukocytes/µL | 22 | 0.41 | 0.06 | |
| % neutrophils on LK | 21 | 0.24 | 0.30 | |
| % lymphocytes on LK | 21 | −0.48 | 0.03 | |
| % monocytes on LK | 21 | −0.08 | 0.72 | |
| % eosinophils on LK | 21 | −0.22 | 0.34 | |
| % basophils on LK | 21 | −0.44 | 0.05 | |
| % pDCs on PBMCs | 28 | −0.51 | 0.006 | |
| % mDCs on PBMCs | 28 | −0.59 | 0.001 | |
| % CD3+ on PBMCs | 28 | −0.39 | 0.04 | |
| % CD4+ on PBMCs | 28 | −0.45 | 0.02 | |
| % IFN-α+ pDCs | R848 | 26 | −0.36 | 0.07 |
| IMQ | 26 | −0.34 | 0.09 | |
| CpG | 25 | −0.10 | 0.62 | |
| % CXCL10+ pDCs | R848 | 23 | −0.28 | 0.20 |
| IMQ | 23 | −0.11 | 0.63 | |
| CpG | 24 | 0.11 | 0.61 | |
| Immune Cell Population | Univariate | Multivariate ° | |||||
|---|---|---|---|---|---|---|---|
| HR | p | 95% CI | HR | p | 95% CI | ||
| n° leukocytes/µL | 1.06 ** | 0.27 | 0.96–1.18 | ||||
| % neutrophils on LK | 1.01 | 0.61 | 0.96–1.07 | ||||
| % lymphocytes on LK | 0.92 | 0.01 | 0.87–0.98 | 0.95 | 0.06 | 0.90–1.00 | |
| % monocytes on LK | 1.04 | 0.73 | 0.82–1.33 | ||||
| % eosinophils on LK | 0.79 | 0.33 | 0.49–1.27 | ||||
| % basophils on LK | 0.85 * | 0.14 | 0.02–1.75 | ||||
| % pDCs on PBMCs | 0.61 * | 0.01 | 0.68–1.06 | 0.72 | 0.06 | 0.52–1.01 | |
| % mDCs on PBMCs | 0.84 * | 0.24 | 0.63–1.12 | ||||
| % CD3+ on PBMCs | 0.95 | 0.03 | 0.91–1.00 | 0.98 | 0.54 | 0.94–1.04 | |
| % CD4+ on PBMCs | 0.93 | 0.01 | 0.88–0.98 | 0.97 | 0.30 | 0.90–1.03 | |
| % IFN-α+ pDCs | R848 | 0.98 | 0.04 | 0.96–1.00 | 0.98 | 0.19 | 0.96–1.01 |
| IMQ | 0.98 | 0.07 | 0.95–1.00 | ||||
| CpG | 0.98 | 0.44 | 0.93–1.03 | ||||
| % CXCL10+ pDCs | R848 | 0.98 | 0.03 | 0.96–1.00 | 0.99 | 0.25 | 0.97–1.01 |
| IMQ | 1.00 | 0.72 | 0.97–1.02 | ||||
| CpG | 1.04 | 0.01 | 1.01–1.07 | 1.05 | 0.008 | 1.01–1.08 | |
| Immune Cell Population | Variation T1–T0 | Variation T2–T0 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | p | N | Mean | SD | p | ||
| n° leukocytes/µL | 13 | −810 | 1891 | 0.2 | 6 | −1161 | 1260 | 0.1 | |
| % neutrophils on LK | 13 | −3.2 | 10.8 | 0.3 | 6 | −10.3 | 12.7 | 0.1 | |
| % lymphocytes on LK | 13 | 3.6 | 8.9 | 0.1 | 6 | 11.0 | 12.2 | 0.05 | |
| % monocytes on LK | 13 | 1.0 | 3.6 | 0.2 | 6 | 0.1 | 1.9 | 0.8 | |
| % eosinophils on LK | 13 | 0.7 | 1.8 | 0.2 | 6 | −0.7 | 1.6 | 0.4 | |
| % basophils on LK | 13 | 0.4 | 0.5 | 0.07 | 6 | 0.0 | 0.2 | 0.6 | |
| % pDCs on PBMCs | 16 | −0.1 | 0.1 | 0.01 | 9 | −0.1 | 0.1 | 0.3 | |
| % mDCs on PBMCs | 16 | 0.0 | 0.2 | 1.0 | 9 | 0.0 | 0.2 | 1.0 | |
| % CD3+ on PBMCs | 16 | −1.4 | 10.2 | 0.6 | 9 | −4.5 | 9.7 | 0.5 | |
| % CD4+ on PBMCs | 16 | −0.1 | 6.6 | 1.0 | 9 | −0.5 | 20.6 | 0.4 | |
| % IFN-α+ pDCs | R848 | 14 | 0.3 | 37.1 | 1.0 | 7 | 19.1 | 17.7 | 0.03 |
| IMQ | 14 | −2.0 | 34.0 | 1.0 | 7 | 8.6 | 14.6 | 0.1 | |
| CpG | 12 | 3.6 | 7.6 | 0.2 | 5 | −0.1 | 9.1 | 0.9 | |
| % CXCL10+ pDCs | R848 | 12 | −9.2 | 31.9 | 0.2 | 5 | −4.1 | 9.1 | 0.4 |
| IMQ | 12 | −4.9 | 32.4 | 0.5 | 5 | 8.9 | 19.5 | 0.2 | |
| CpG | 12 | −1.6 | 15.3 | 0.8 | 5 | 1.9 | 1.5 | 0.08 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monti, M.; Vescovi, R.; Consoli, F.; Farina, D.; Moratto, D.; Berruti, A.; Specchia, C.; Vermi, W. Plasmacytoid Dendritic Cell Impairment in Metastatic Melanoma by Lactic Acidosis. Cancers 2020, 12, 2085. https://doi.org/10.3390/cancers12082085
Monti M, Vescovi R, Consoli F, Farina D, Moratto D, Berruti A, Specchia C, Vermi W. Plasmacytoid Dendritic Cell Impairment in Metastatic Melanoma by Lactic Acidosis. Cancers. 2020; 12(8):2085. https://doi.org/10.3390/cancers12082085
Chicago/Turabian StyleMonti, Matilde, Raffaella Vescovi, Francesca Consoli, Davide Farina, Daniele Moratto, Alfredo Berruti, Claudia Specchia, and William Vermi. 2020. "Plasmacytoid Dendritic Cell Impairment in Metastatic Melanoma by Lactic Acidosis" Cancers 12, no. 8: 2085. https://doi.org/10.3390/cancers12082085
APA StyleMonti, M., Vescovi, R., Consoli, F., Farina, D., Moratto, D., Berruti, A., Specchia, C., & Vermi, W. (2020). Plasmacytoid Dendritic Cell Impairment in Metastatic Melanoma by Lactic Acidosis. Cancers, 12(8), 2085. https://doi.org/10.3390/cancers12082085






