Study of the Female Sex Survival Advantage in Melanoma—A Focus on X-Linked Epigenetic Regulators and Immune Responses in Two Cohorts
Abstract
1. Introduction
2. Results
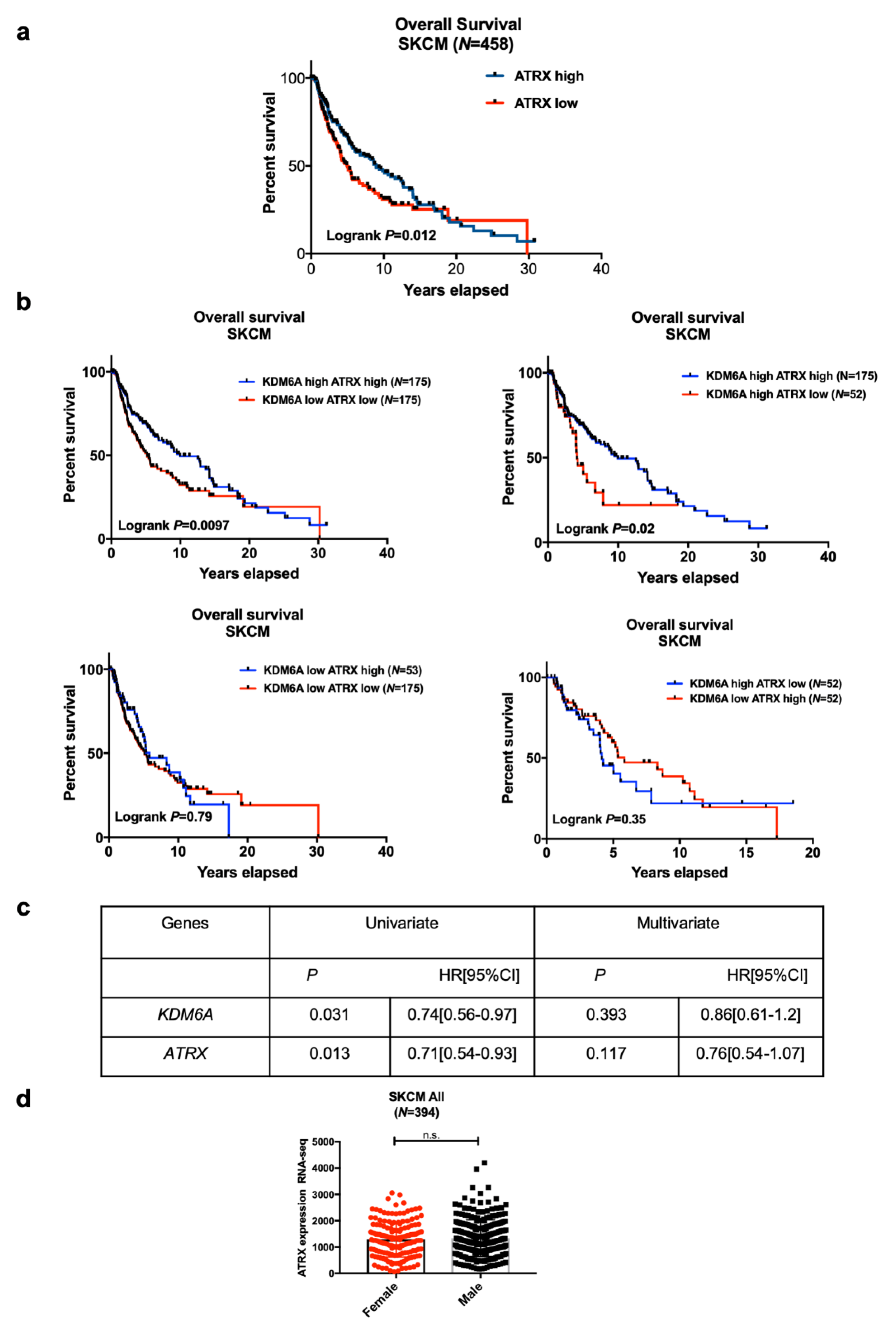
2.1. High KDM6A and ATRX Expression Are Associated with Improved Survival in Female Compared to Male Patients with Melanoma in the TCGA Data Set
LMC Analysis (Validation Cohort)
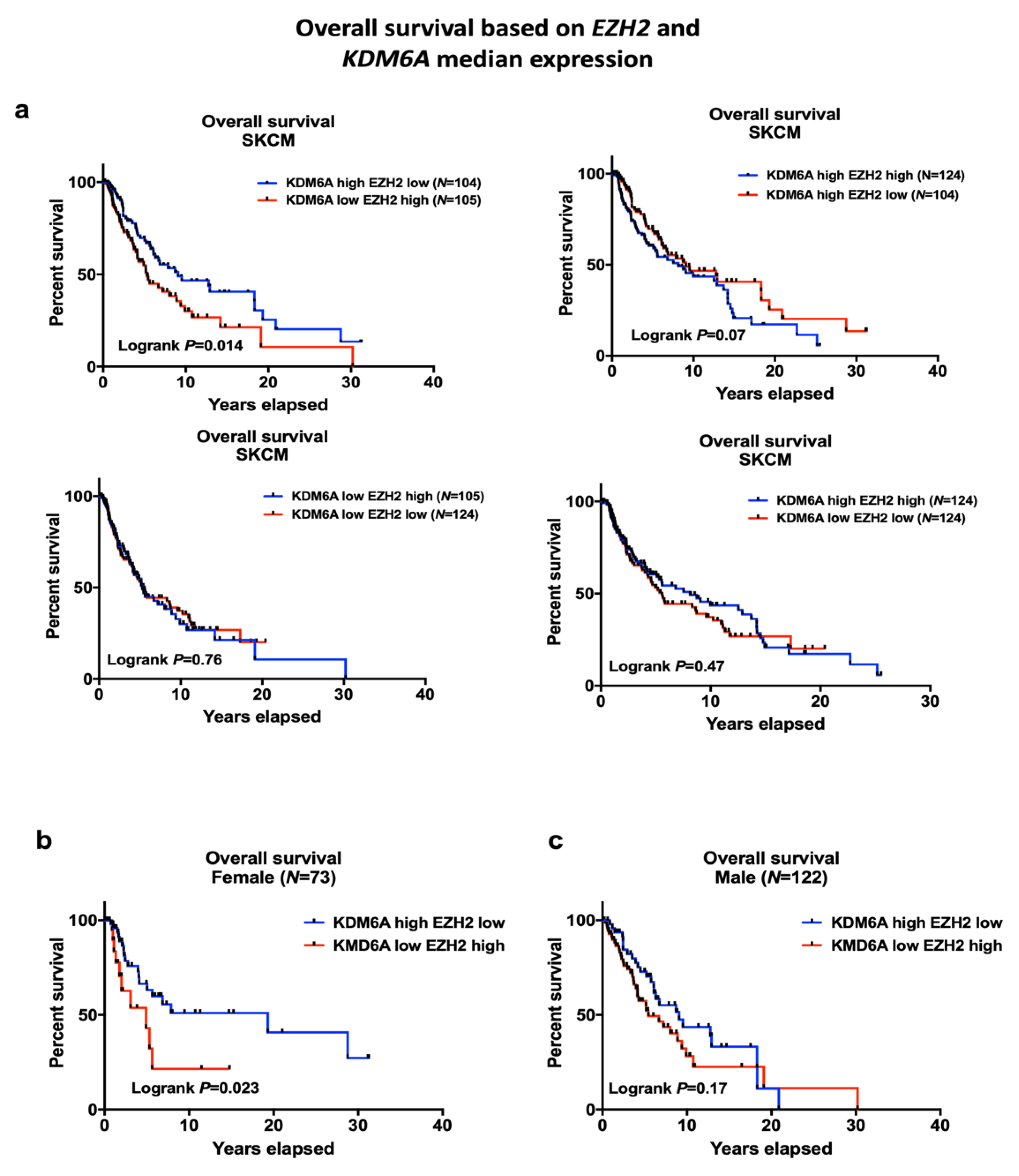
2.2. EZH2 Levels Impact Survival in High KDM6A Patients and Have Significant Associations with Sex-Biased Overall Survival (OS)
LMC Analysis (Validation Cohort)
2.3. The Tissue Distribution of KDM6A and ATRX Is Different to that of KDM6B
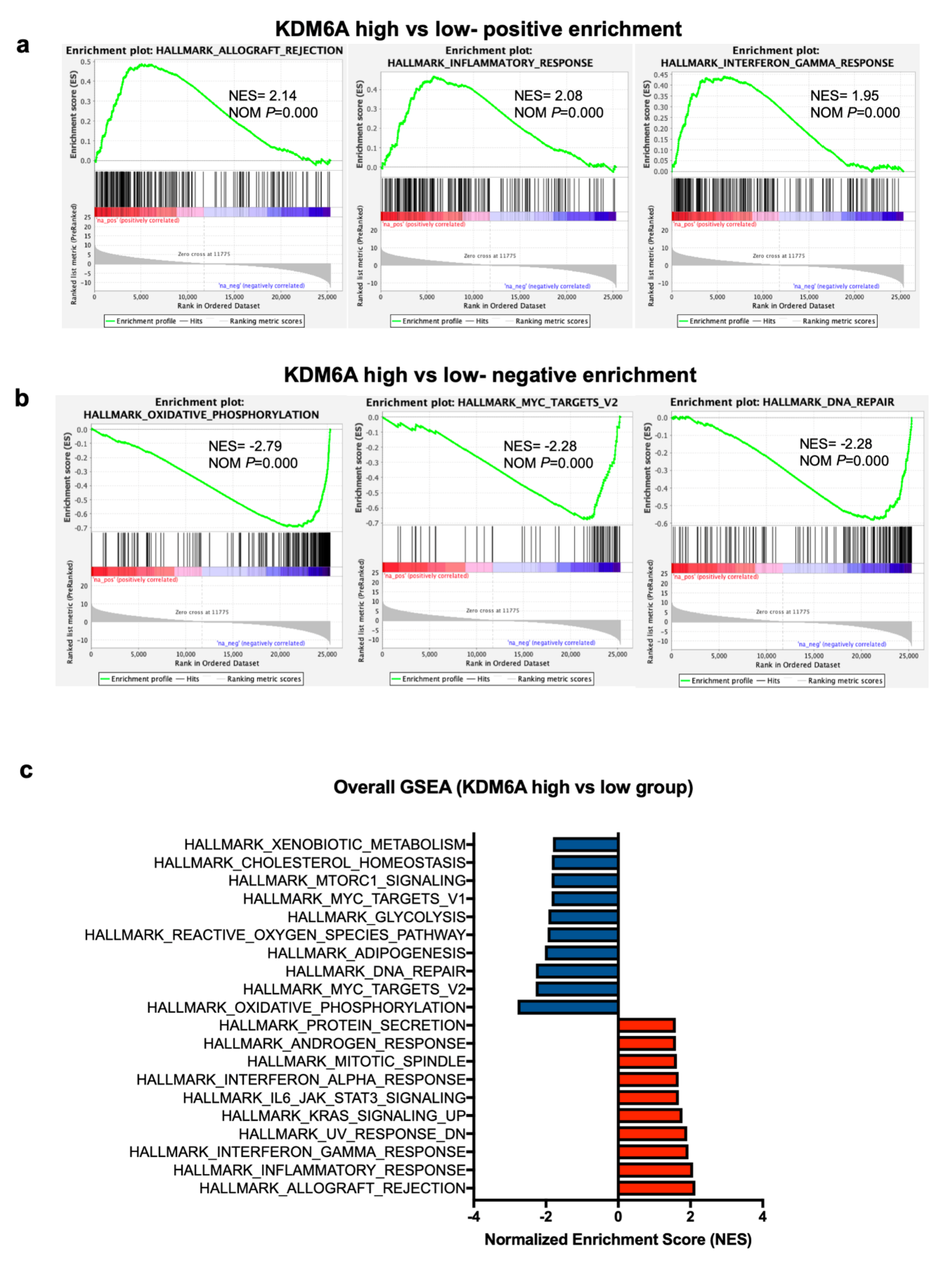
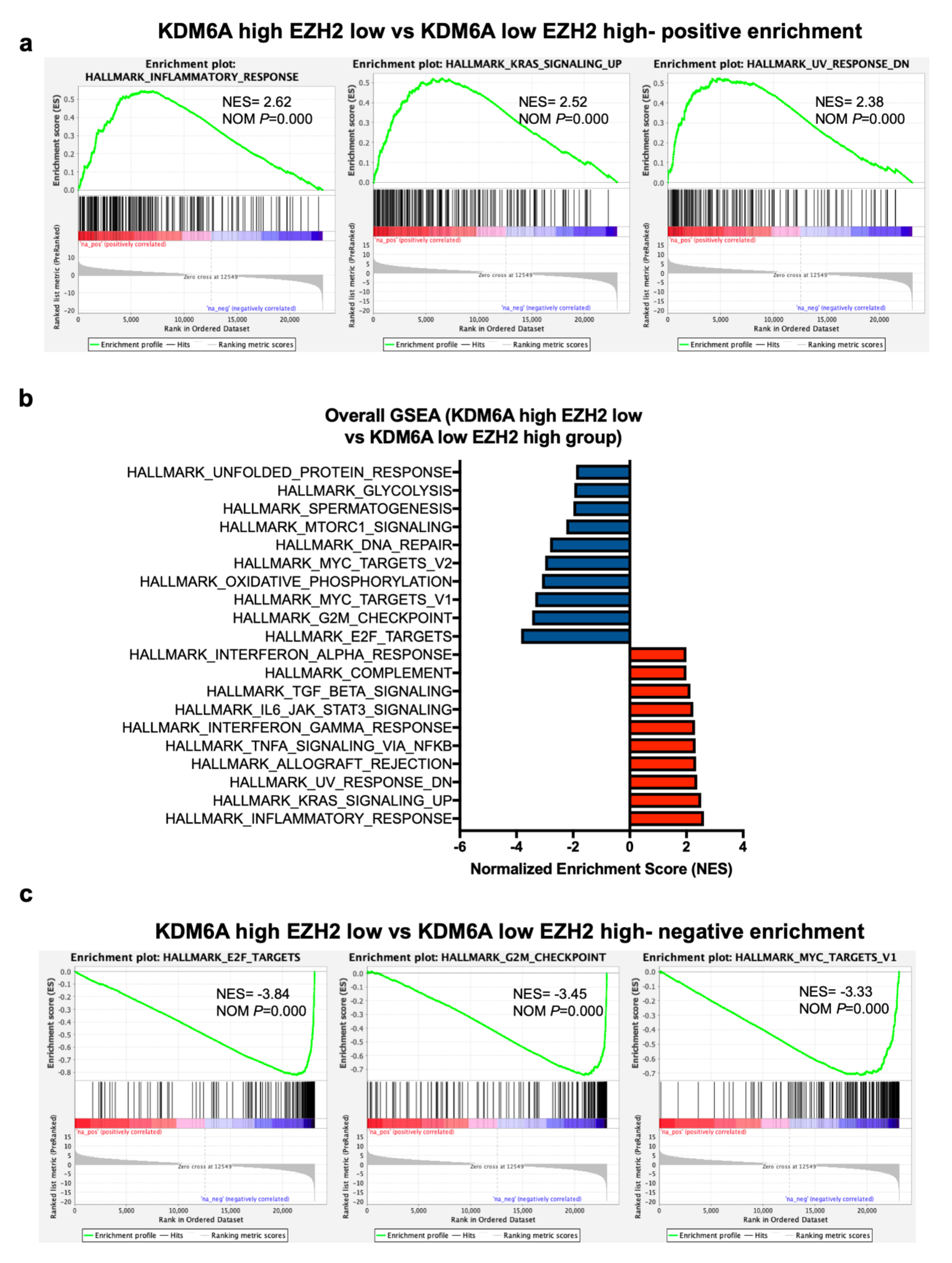
2.4. Gene Set Expression Analysis (GSEA) Has Revealed Immune Pathways to Be Positively Associated with KDM6A
2.5. GSEA Analysis Shows that ATRX Acts on Similar Gene Sets to KDM6A Except for Immune Regulatory Pathways
2.6. GSEA Analysis Identifies a Positive Enrichment of Immune Pathways in KDM6A High EZH2 Low Group Similar to KDM6A High Group
2.7. Association of KDM6A and ATRX on Immune Responses in Melanoma Patients
LMC Analysis (Validation Cohort)
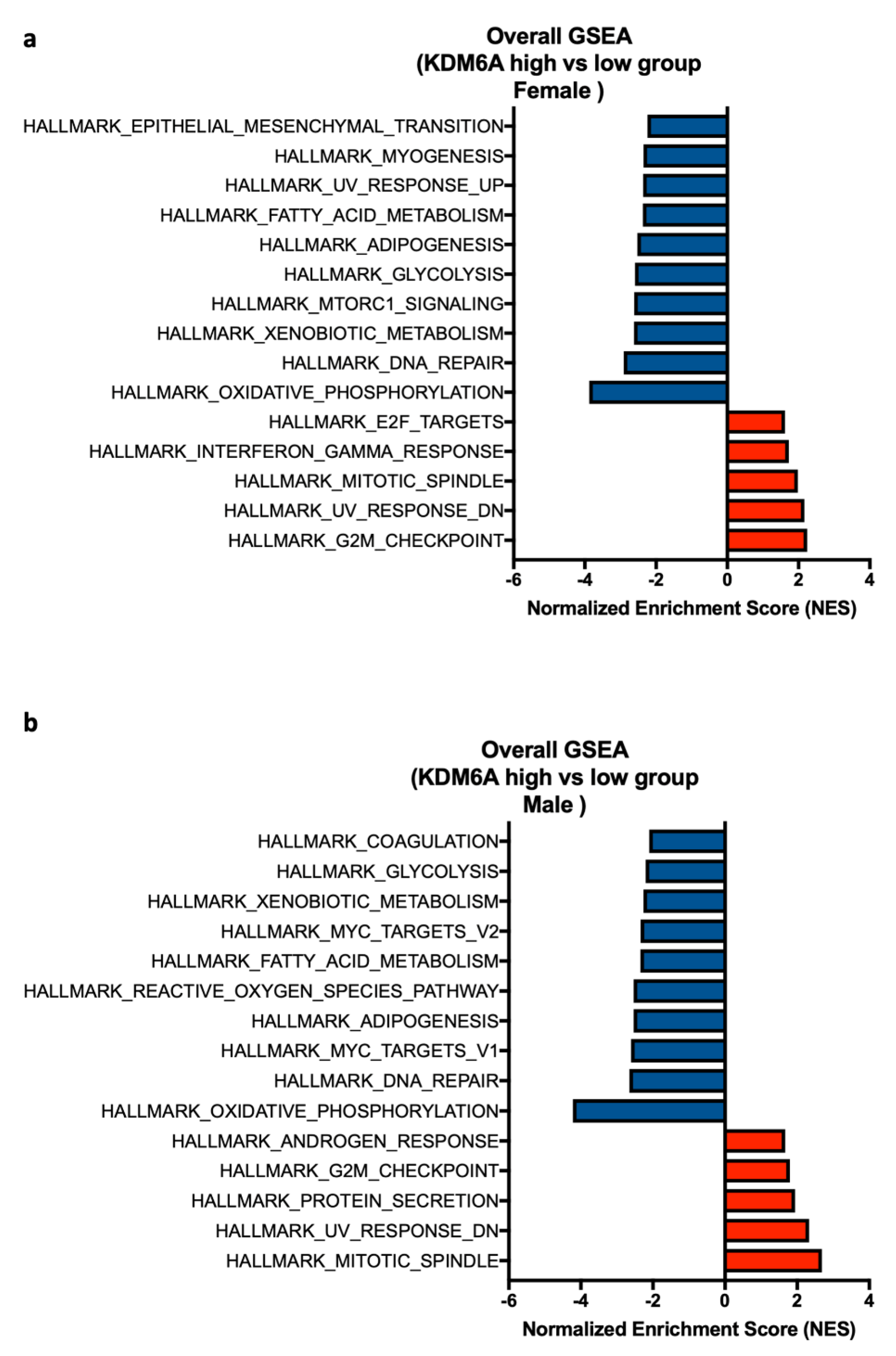
2.8. Exploration of the Basis for Increased TILs in Females Compared to Males
3. Discussion
4. Materials and Methods
4.1. Data from Skin Cutaneous Melanoma (SKCM) Cohort
4.2. RNA-seq Analysis
4.3. Gene Set Enrichment Analysis (GSEA)
4.4. Statistical Methods
4.5. Data from the Leeds Melanoma Cohort (LMC)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Joosse, A.; Collette, S.; Suciu, S.; Nijsten, T.; Patel, P.M.; Keilholz, U.; Eggermont, A.M.; Coebergh, J.W.; de Vries, E. Sex is an independent prognostic indicator for survival and relapse/progression-free survival in metastasized stage III to IV melanoma: A pooled analysis of five European organisation for research and treatment of cancer randomized controlled trials. J. Clin. Oncol. 2013, 31, 2337–2346. [Google Scholar] [CrossRef]
- Joosse, A.; Collette, S.; Suciu, S.; Nijsten, T.; Lejeune, F.; Kleeberg, U.R.; Coebergh, J.W.; Eggermont, A.M.; de Vries, E. Superior outcome of women with stage I/II cutaneous melanoma: Pooled analysis of four European Organisation for Research and Treatment of Cancer phase III trials. J. Clin. Oncol. 2012, 30, 2240–2247. [Google Scholar] [CrossRef]
- Enninga, E.A.L.; Moser, J.C.; Weaver, A.L.; Markovic, S.N.; Brewer, J.D.; Leontovich, A.A.; Hieken, T.J.; Shuster, L.; Kottschade, L.A.; Olariu, A.; et al. Survival of cutaneous melanoma based on sex, age, and stage in the United States, 1992–2011. Cancer Med. 2017, 6, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shih, J.; Tran, A.; Mullane, A.; Thomas, C.; Aydin, N.; Misra, S. Gender-Based Differences and Barriers in Skin Protection Behaviors in Melanoma Survivors. J. Ski. Cancer 2016, 2016, 3874572. [Google Scholar] [CrossRef] [PubMed]
- Hersey, P.; Sillar, R.W.; Howe, C.G.; Burton, R.C.; Darbar, S.V.; Foster, H.M.; Collins, S.M.; Bradley, D.E.; Owens, D. Factors related to the presentation of patients with thick primary melanomas. Med. J. Aust. 1991, 154, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Courtenay, W.H.; Keeling, R.P. Men, gender, and health: Toward an interdisciplinary approach. J. Am. Coll. Health 2000, 48, 243–246. [Google Scholar] [CrossRef]
- Gupta, S.; Artomov, M.; Goggins, W.; Daly, M.; Tsao, H. Gender Disparity and Mutation Burden in Metastatic Melanoma. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef]
- Dika, E.; Patrizi, A.; Lambertini, M.; Manuelpillai, N.; Fiorentino, M.; Altimari, A.; Ferracin, M.; Lauriola, M.; Fabbri, E.; Campione, E.; et al. Estrogen Receptors and Melanoma: A Review. Cells 2019, 8, 1463. [Google Scholar] [CrossRef]
- Morgese, F.; Berardi, R.; Sampaolesi, C.; Torniai, M.; Marcantognini, G.; Giacchetti, A.; Serresi, S.; Onofri, A.; Bittoni, A.; Pilone, A.; et al. Gender differences and outcome of melanoma patients. J. Transl. Med. 2015, 13, P13. [Google Scholar] [CrossRef][Green Version]
- Lasithiotakis, K.; Leiter, U.; Meier, F.; Eigentler, T.; Metzler, G.; Moehrle, M.; Breuninger, H.; Garbe, C. Age and gender are significant independent predictors of survival in primary cutaneous melanoma. Cancer 2008, 112, 1795–1804. [Google Scholar] [CrossRef]
- Dunford, A.; Weinstock, D.M.; Savova, V.; Schumacher, S.E.; Cleary, J.P.; Yoda, A.; Sullivan, T.J.; Hess, J.M.; Gimelbrant, A.A.; Beroukhim, R.; et al. Tumor-suppressor genes that escape from X-inactivation contribute to cancer sex bias. Nat. Genet. 2017, 49, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Gorlov, I.; Orlow, I.; Ringelberg, C.; Hernando, E.; Ernstoff, M.S.; Cheng, C.; Her, S.; Parker, J.S.; Thompson, C.L.; Gerstenblith, M.R.; et al. Identification of gene expression levels in primary melanoma associated with clinically meaningful characteristics. Melanoma Res. 2018, 28, 380–389. [Google Scholar] [CrossRef]
- Qadeer, Z.A.; Harcharik, S.; Valle-Garcia, D.; Chen, C.; Birge, M.B.; Vardabasso, C.; Duarte, L.F.; Bernstein, E. Decreased expression of the chromatin remodeler ATRX associates with melanoma progression. J. Investig. Dermatol. 2014, 134, 1768–1772. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Cho, Y.W.; Yu, L.R.; Yu, H.; Veenstra, T.D.; Ge, K. Identification of JmjC domain-containing UTX and JMJD3 as histone H3 lysine 27 demethylases. Proc. Natl. Acad. Sci. USA 2007, 104, 18439–18444. [Google Scholar] [CrossRef] [PubMed]
- Tiffen, J.; Gallagher, S.J.; Hersey, P. EZH2: An emerging role in melanoma biology and strategies for targeted therapy. Pigment Cell Melanoma Res. 2014, 28, 21–30. [Google Scholar] [CrossRef]
- Tiffen, J.C.; Gallagher, S.J.; Tseng, H.Y.; Filipp, F.V.; Fazekas de St. Groth, B.; Hersey, P. EZH2 as a mediator of treatment resistance in melanoma. Pigment Cell Melanoma Res. 2016, 29, 500–507. [Google Scholar] [CrossRef]
- Comet, I.; Riising, E.M.; Leblanc, B.; Helin, K. Maintaining cell identity: PRC2-mediated regulation of transcription and cancer. Nat. Rev. Cancer 2016, 16, 803–810. [Google Scholar] [CrossRef]
- Walport, L.J.; Hopkinson, R.J.; Vollmar, M.; Madden, S.K.; Gileadi, C.; Oppermann, U.; Schofield, C.J.; Johansson, C. Human UTY(KDM6C) is a male-specific N-methyl lysyl demethylase. J. Biol. Chem. 2014, 289, 18302–18313. [Google Scholar] [CrossRef]
- Schulz, W.A.; Lang, A.; Koch, J.; Greife, A. The histone demethylase UTX/KDM6A in cancer: Progress and puzzles. Int. J. Cancer 2019, 145, 614–620. [Google Scholar] [CrossRef]
- Dyer, M.A.; Qadeer, Z.A.; Valle-Garcia, D.; Bernstein, E. ATRX and DAXX: Mechanisms and Mutations. Cold Spring Harb. Perspect. Med. 2017, 7. [Google Scholar] [CrossRef]
- Voon, H.P.; Wong, L.H. New players in heterochromatin silencing: Histone variant H3.3 and the ATRX/DAXX chaperone. Nucleic Acids Res. 2016, 44, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Sarma, K.; Cifuentes-Rojas, C.; Ergun, A.; Del Rosario, A.; Jeon, Y.; White, F.; Sadreyev, R.; Lee, J.T. ATRX directs binding of PRC2 to Xist RNA and Polycomb targets. Cell 2014, 159, 869–883. [Google Scholar] [CrossRef]
- Thakur, R.; Laye, J.P.; Lauss, M.; Diaz, J.M.S.; O’Shea, S.J.; Poźniak, J.; Filia, A.; Harland, M.; Gascoyne, J.; Randerson-Moor, J.A.; et al. Transcriptomic Analysis Reveals Prognostic Molecular Signatures of Stage I Melanoma. Clin. Cancer Res. 2019, 25, 7424–7435. [Google Scholar] [CrossRef]
- Lonsdale, J.; Thomas, J.; Salvatore, M.; Phillips, R.; Lo, E.; Shad, S.; Hasz, R.; Walters, G.; Garcia, F.; Young, N.; et al. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Akbani, R.; Akdemi, K.C.; Aksoy, B.A.; Albert, M.; Ally, A.; Amin, S.B.; Arachchi, H.; Arora, A.; Auman, T.J.; Ayala, B.A.; et al. Genomic Classification of Cutaneous Melanoma. Cell 2015, 161, 1681–1696. [Google Scholar] [CrossRef]
- Li, B.; Severson, E.; Pignon, J.C.; Zhao, H.; Li, T.; Novak, J.; Jiang, P.; Shen, H.; Aster, J.C.; Rodig, S.; et al. Comprehensive analyses of tumor immunity: Implications for cancer immunotherapy. Genome Biol. 2016, 17, 174. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.W.; Broz, M.L.; Binnewies, M.; Headley, M.B.; Nelson, A.E.; Wolf, D.M.; Kaisho, T.; Bogunovic, D.; Bhardwaj, N.; Krummel, M.F. Critical Role for CD103(+)/CD141(+) Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell 2016, 30, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S.; Dai, D.; Horton, B.; Gajewski, T.F. Tumor-Residing Batf3 Dendritic Cells Are Required for Effector T Cell Trafficking and Adoptive T Cell Therapy. Cancer Cell 2017, 31, 711–723. [Google Scholar] [CrossRef]
- Edwards, J.; Wilmott, J.S.; Madore, J.; Gide, T.N.; Quek, C.; Tasker, A.; Ferguson, A.L.; Chen, J.; Hewavisenti, R.; Hersey, P.; et al. CD103+ tumor-resident CD8+ T cells are associated with improved survival in immunotherapy naive melanoma patients and expand significantly during anti-PD1 treatment. Clin. Cancer Res. 2018, 24, 3036–3045. [Google Scholar] [CrossRef]
- Luke, J.J.; Flaherty, K.T.; Ribas, A.; Long, G.V. Targeted agents and immunotherapies: Optimizing outcomes in melanoma. Nat. Rev. Clin. Oncol. 2017, 14, 463–482. [Google Scholar] [CrossRef]
- Libert, C.; Dejager, L.; Pinheiro, I. The X chromosome in immune functions: When a chromosome makes the difference. Nat. Rev. Immunol. 2010, 10, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S.; Gajewski, T.F. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat. Rev. Cancer 2018, 18, 139. [Google Scholar] [CrossRef] [PubMed]
- Olsen, C.M.; Thompson, J.F.; Pandeya, N.; Whiteman, D.C. Evaluation of Sex-Specific Incidence of Melanoma. JAMA Derm. 2020, 156, 1–8. [Google Scholar] [CrossRef]
- Gao, J.; Gu, J.; Pan, X.; Gan, X.; Ju, Z.; Zhang, S.; Xia, Y.; Lu, L.; Wang, X. Blockade of miR-142-3p promotes anti-apoptotic and suppressive function by inducing KDM6A-mediated H3K27me3 demethylation in induced regulatory T cells. Cell Death Dis. 2019, 10, 332. [Google Scholar] [CrossRef]
- Itoh, Y.; Golden, L.C.; Itoh, N.; Matsukawa, M.A.; Ren, E.; Tse, V.; Arnold, A.P.; Voskuhl, R.R. The X-linked histone demethylase Kdm6a in CD4+ T lymphocytes modulates autoimmunity. J. Clin. Investig. 2019, 129, 3852–3863. [Google Scholar] [CrossRef] [PubMed]
- Cribbs, A.P.; Terlecki-Zaniewicz, S.; Philpott, M.; Baardman, J.; Ahern, D.; Lindow, M.; Obad, S.; Oerum, H.; Sampey, B.; Mander, P.K.; et al. Histone H3K27me3 demethylases regulate human Th17 cell development and effector functions by impacting on metabolism. Proc. Natl. Acad. Sci. USA 2020, 117, 6056–6066. [Google Scholar] [CrossRef] [PubMed]
- Banan, B.; Xu, Z.; Gunasekaran, M.; Mohanakumar, T. Role of alloimmunity and autoimmunity in allograft rejection. Clin. Transpl. 2013, 39, 325–332. [Google Scholar]
- Cook, K.D.; Shpargel, K.B.; Starmer, J.; Whitfield-Larry, F.; Conley, B.; Allard, D.E.; Rager, J.E.; Fry, R.C.; Davenport, M.L.; Magnuson, T.; et al. T Follicular Helper Cell-Dependent Clearance of a Persistent Virus Infection Requires T Cell Expression of the Histone Demethylase UTX. Immunity 2015, 43, 703–714. [Google Scholar] [CrossRef]
- Malinczak, C.A.; Rasky, A.J.; Fonseca, W.; Schaller, M.A.; Allen, R.M.; Ptaschinski, C.; Morris, S.; Lukacs, N.W. Upregulation of H3K27 Demethylase KDM6 During Respiratory Syncytial Virus Infection Enhances Proinflammatory Responses and Immunopathology. J. Immunol. 2020, 204, 159–168. [Google Scholar] [CrossRef]
- Tiffen, J.; Gallagher, S.; Filipp, F.; Gunatilake, D.; Al Emran, A.; Cullinane, C.; Dutton-Register, K.; Aoude, L.; Hayward, N.; Chatterjee, A.; et al. EZH2 cooperates with DNA methylation to downregulate key tumour suppressors and interferon gene signatures in melanoma. J. Investig. Dermatol. 2020. [Google Scholar] [CrossRef]
- Wu, L.; Cao, J.; Cai, W.L.; Lang, S.M.; Horton, J.R.; Jansen, D.J.; Liu, Z.Z.; Chen, J.F.; Zhang, M.; Mott, B.T.; et al. KDM5 histone demethylases repress immune response via suppression of STING. PLoS Biology 2018, 16, e2006134. [Google Scholar] [CrossRef] [PubMed]
- Phung, B.; Ciesla, M.; Sanna, A.; Guzzi, N.; Beneventi, G.; Cao Thi Ngoc, P.; Lauss, M.; Cabrita, R.; Cordero, E.; Bosch, A.; et al. The X-Linked DDX3X RNA Helicase Dictates Translation Reprogramming and Metastasis in Melanoma. Cell Rep. 2019, 27, 3573–3586. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.; Ansar Ahmed, S. The Immune System Is a Natural Target for Estrogen Action: Opposing Effects of Estrogen in Two Prototypical Autoimmune Diseases. Front. Immunol. 2016, 6, 635. [Google Scholar] [CrossRef] [PubMed]
- Pierdominici, M.; Maselli, A.; Colasanti, T.; Giammarioli, A.M.; Delunardo, F.; Vacirca, D.; Sanchez, M.; Giovannetti, A.; Malorni, W.; Ortona, E. Estrogen receptor profiles in human peripheral blood lymphocytes. Immunol. Lett. 2010, 132, 79–85. [Google Scholar] [CrossRef]
- Ahnstedt, H.; McCullough, L.D. The impact of sex and age on T cell immunity and ischemic stroke outcomes. Cell. Immunol. 2019, 345, 103960. [Google Scholar] [CrossRef]
- Anaya, J. OncoLnc: Linking TCGA survival data to mRNAs, miRNAs, and lncRNAs. PeerJ Comput. Sci. 2016, 2, e67. [Google Scholar] [CrossRef]
- Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Nsengimana, J.; Laye, J.; Filia, A.; O’Shea, S.; Muralidhar, S.; Poźniak, J.; Droop, A.; Chan, M.; Walker, C.; Parkinson, L.; et al. β-Catenin-mediated immune evasion pathway frequently operates in primary cutaneous melanomas. J. Clin. Investig. 2018, 128, 2048–2063. [Google Scholar] [CrossRef] [PubMed]
- Poźniak, J.; Nsengimana, J.; Laye, J.P.; O’Shea, S.J.; Diaz, J.M.S.; Droop, A.P.; Filia, A.; Harland, M.; Davies, J.R.; Mell, T.; et al. Genetic and Environmental Determinants of Immune Response to Cutaneous Melanoma. Cancer Res. 2019, 79, 2684–2696. [Google Scholar] [CrossRef] [PubMed]


| (a) Effect of gene-gene and gene-sex interaction on OS in SKCM cohort | ||||
| Overall | KDM6A | ATRX | EZH2 | Sex |
| KDM6A | - | 0.983 | 0.033 | 0.96 |
| ATRX | - | 0.313 | 0.14 | |
| EZH2 | - | 0.02 | ||
| Sex | - | |||
| Primary | ||||
| KDM6A | - | 0.68 | 0.32 | 0.734 |
| ATRX | - | 0.878 | 0.971 | |
| EZH2 | - | 0.045 | ||
| Sex | - | |||
| Metastases | ||||
| KDM6A | - | 0.991 | 0.042 | 0.898 |
| ATRX | - | 0.25 | 0.115 | |
| EZH2 | - | 0.049 | ||
| Sex | - | |||
| (b) Effect of interaction on MSS in LMC (Validation cohort). | ||||
| Primary | KDM6A | ATRX | EZH2 | Sex |
| KDM6A | - | 0.05 | 0.03 | 0.41 |
| ATRX | - | 0.10 | 0.09 | |
| EZH2 | - | 0.02 | ||
| Sex | - | |||
| Genes | CD4+ T Cells | CD8+ T Cells | B Cells | DC | Neutrophil | Macrophage | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pearson | p | Pearson | p | Pearson | p | Pearson | p | Pearson | p | Pearson | p | |
| r | r | r | r | r | r | |||||||
| KDM6A Overall (N = 458) | 0.229 | <0.0001 | 0.32 | <0.0001 | 0.18 | 0.0001 | 0.16 | 0.0005 | 0.31 | <0.0001 | 0.12 | 0.008 |
| KDM6A Female (N = 162) | 0.24 | 0.0002 | 0.37 | <0.0001 | 0.31 | <0.0001 | 0.28 | 0.0002 | 0.47 | <0.0001 | 0.24 | 0.002 |
| KDM6A Male (N = 274) | 0.07 | 0.2 | 0.12 | 0.03 | 0.07 | 0.24 | 0.03 | 0.57 | 0.2 | 0.008 | 0.1 | 0.08 |
| KDM6A Metastases (N = 355) | 0.138 | 0.0096 | 0.24 | <0.0001 | 0.18 | 0.0006 | 0.152 | 0.004 | 0.3 | <0.0001 | 0.08 | 0.12 |
| KDM6A Female (N = 127) | 0.2 | 0.019 | 0.4 | <0.0001 | 0.33 | 0.0001 | 0.28 | 0.0014 | 0.45 | <0.0001 | 0.238 | 0.0074 |
| KDM6A Male (N = 218) | 0.06 | 0.32 | 0.15 | 0.022 | 0.05 | 0.43 | 0.03 | 0.56 | 0.23 | 0.0005 | 0.08 | 0.2 |
| ATRX Overall (N = 458) | 0.12 | 0.006 | 0.18 | <0.0001 | 0.12 | 0.0007 | 0.11 | 0.01 | 0.29 | <0.0001 | 0.17 | 0.0002 |
| ATRX Female (N = 162) | 0.16 | 0.03 | 0.24 | 0.0002 | 0.19 | 0.01 | 0.2 | 0.0095 | 0.38 | <0.0001 | 0.23 | 0.0002 |
| ATRX Male (N = 274) | 0.07 | 0.19 | 0.13 | 0.03 | 0.06 | 0.3 | 0.03 | 0.56 | 0.21 | 0.0004 | 0.14 | 0.01 |
| ATRX Metastases (N = 355) | 0.1669 | 0.002 | 0.0643 | 0.2665 | 0.1027 | 0.06 | 0.0586 | 0.2808 | 0.2315 | <0.0001 | 0.0852 | 0.2218 |
| ATRX Female (N = 127) | 0.2217 | 0.04 | -0.0197 | 0.9216 | 0.1657 | 0.1344 | 0.02926 | 0.7929 | 0.2268 | 0.04 | 0.1704 | 0.1235 |
| ATRX Male (N = 218) | 0.1239 | 0.07 | 0.06161 | 0.3814 | 0.0524 | 0.4521 | 0.02607 | 0.7113 | 0.2115 | 0.0024 | 0.06546 | 0.4357 |
| KMD6B Overall (N = 458) | 0.15 | 0.0007 | −0.18 | <0.0001 | −0.014 | 0.17 | -0.051 | 0.28 | −0.11 | 0.019 | 0.071 | 0.12 |
| EZH2 Overall (N = 458) | −0.06 | 0.19 | 0.183 | 0.0013 | 0.084 | 0.07 | 0.069 | 0.14 | 0.18 | <0.0001 | −0.01 | 0.71 |
| Gene | CD141 | BATF3 | CD103 | CD25 | CD69 | CCR5 | CXCR3 | IFNG | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (DC) | (DC) | (DC) | (Treg) | Trem | CD8 | CD4 | ||||||||||
| Spearman | p | Spearman | p | Spearman | p | Spearman | p | Spearman | p | Spearman | p | Spearman | p | Spearman | p | |
| r | r | r | r | r | r | r | r | |||||||||
| KDM6A Overall (N = 458) | −0.03 | 0.51 | −0.08 | 0.07 | 0.01 | 0.73 | 0.3 | <0.0001 | 0.32 | <0.0001 | 0.18 | 0.0001 | 0.01 | 0.73 | 0.15 | 0.0008 |
| KDM6A Female (N = 162) | −0.03 | 0.66 | −0.02 | 0.74 | 0.05 | 0.47 | 0.41 | <0.0001 | 0.46 | <0.0001 | 0.33 | <0.0001 | 0.17 | 0.02 | 0.28 | 0.0003 |
| KDM6A Male (N = 274) | −0.06 | 0.26 | −0.14 | 0.018 | −0.04 | 0.43 | 0.16 | 0.0007 | 0.21 | 0.0006 | 0.04 | 0.49 | −0.13 | 0.03 | 0.05 | 0.41 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emran, A.A.; Nsengimana, J.; Punnia-Moorthy, G.; Schmitz, U.; Gallagher, S.J.; Newton-Bishop, J.; Tiffen, J.C.; Hersey, P. Study of the Female Sex Survival Advantage in Melanoma—A Focus on X-Linked Epigenetic Regulators and Immune Responses in Two Cohorts. Cancers 2020, 12, 2082. https://doi.org/10.3390/cancers12082082
Emran AA, Nsengimana J, Punnia-Moorthy G, Schmitz U, Gallagher SJ, Newton-Bishop J, Tiffen JC, Hersey P. Study of the Female Sex Survival Advantage in Melanoma—A Focus on X-Linked Epigenetic Regulators and Immune Responses in Two Cohorts. Cancers. 2020; 12(8):2082. https://doi.org/10.3390/cancers12082082
Chicago/Turabian StyleEmran, Abdullah Al, Jérémie Nsengimana, Gaya Punnia-Moorthy, Ulf Schmitz, Stuart J. Gallagher, Julia Newton-Bishop, Jessamy C. Tiffen, and Peter Hersey. 2020. "Study of the Female Sex Survival Advantage in Melanoma—A Focus on X-Linked Epigenetic Regulators and Immune Responses in Two Cohorts" Cancers 12, no. 8: 2082. https://doi.org/10.3390/cancers12082082
APA StyleEmran, A. A., Nsengimana, J., Punnia-Moorthy, G., Schmitz, U., Gallagher, S. J., Newton-Bishop, J., Tiffen, J. C., & Hersey, P. (2020). Study of the Female Sex Survival Advantage in Melanoma—A Focus on X-Linked Epigenetic Regulators and Immune Responses in Two Cohorts. Cancers, 12(8), 2082. https://doi.org/10.3390/cancers12082082







