Examination of Prognostic Factors Affecting Long-Term Survival of Patients with Stage 3/4 Gallbladder Cancer without Distant Metastasis
Abstract
1. Introduction
2. Results
2.1. Frequency and R0 Resection Rate Based on Local Factors of the Primary Tumor Site in Patients with Stage 3/4 GBC without Distant Metastasis
2.2. Long-Term Outcomes Based on the Degree of Liver and HDL Invasion
2.3. Prognostic Factors in Patients with Stage 3/4 GBC without Distant Metastasis
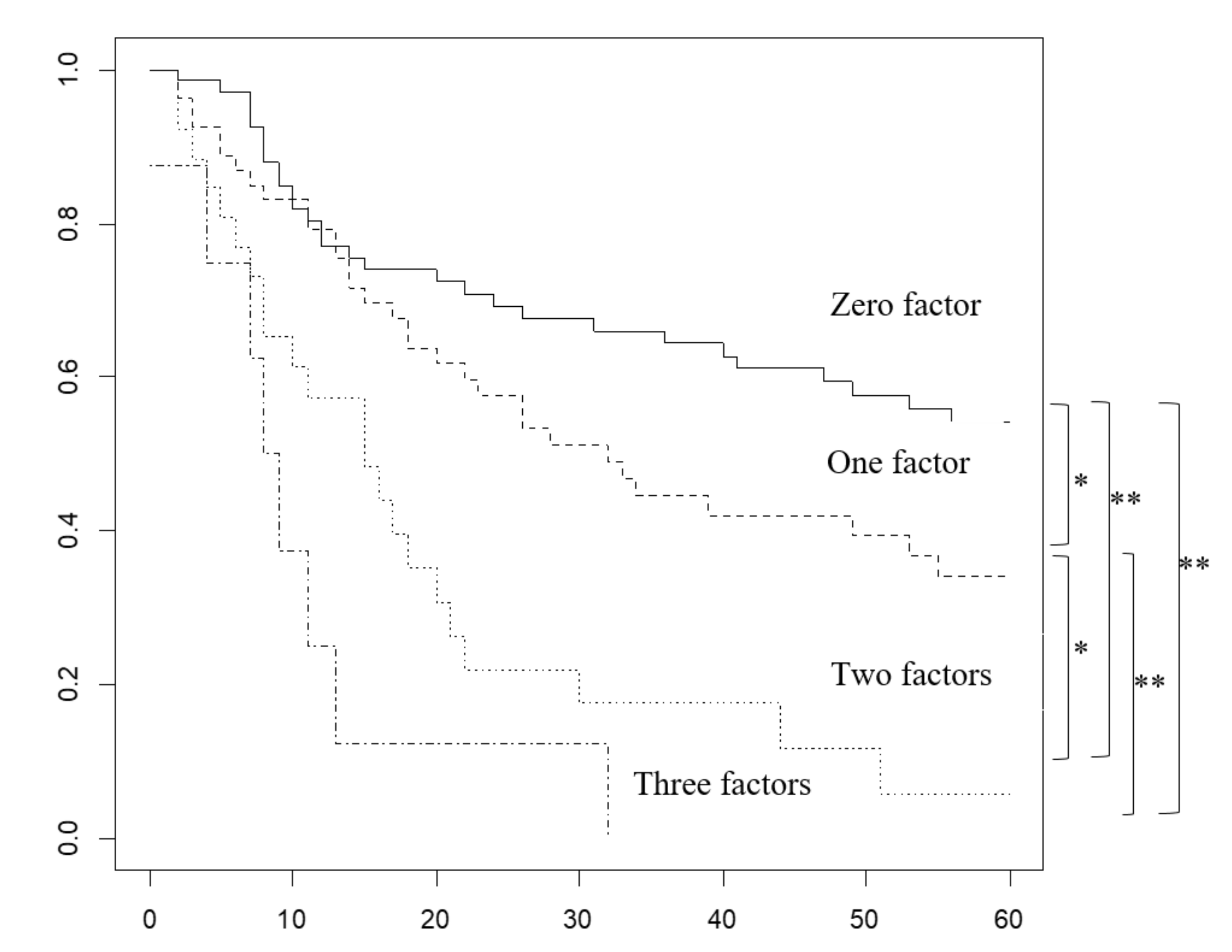
2.4. Overall Survival Rate Based on the Number of Prognostic Factors Predicted Preoperatively
2.5. Characteristics and Recurrence in Groups Based on the Number of Prognostic Factors
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Surgical Policy
4.3. Postoperative Morbidity
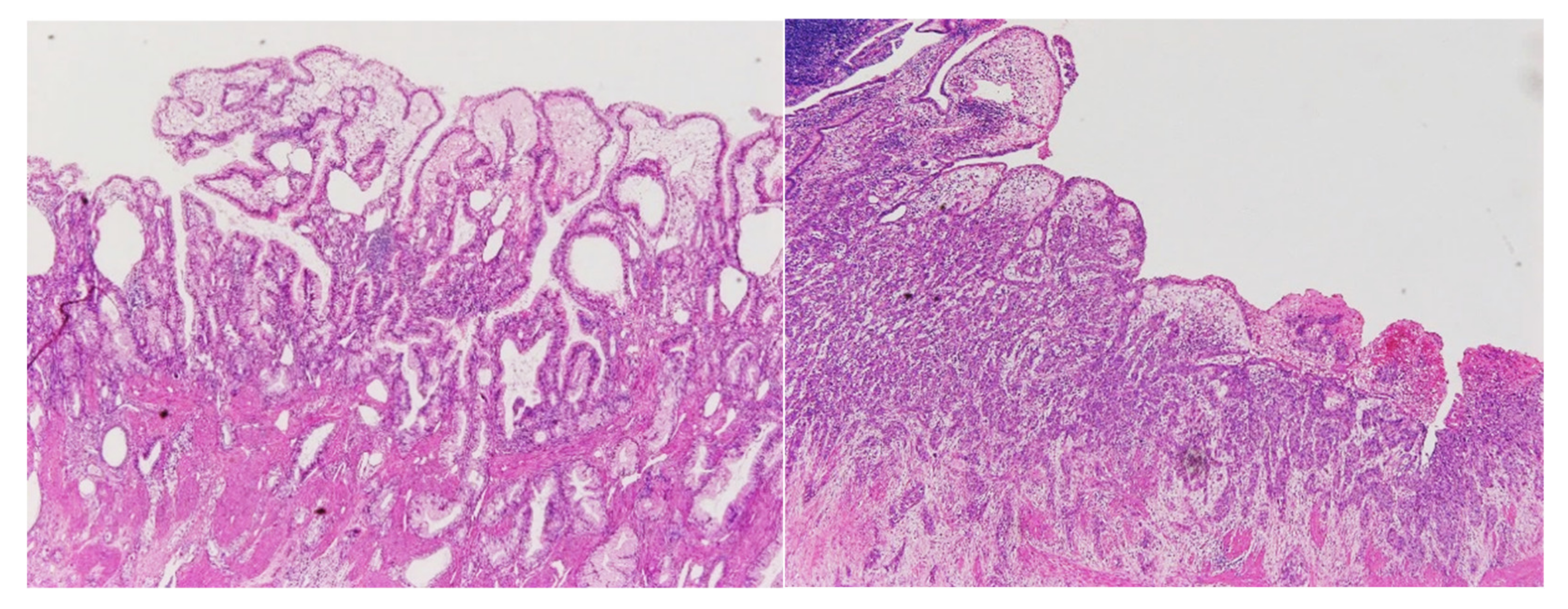
4.4. Pathological Examination
4.5. Adjuvant Chemotherapy
4.6. Follow-up
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hundal, R.; Shaffer, E.A. Gallbladder cancer: Epidemiology and outcome. Clin. Epidemiol. 2014, 6, 99–109. [Google Scholar] [CrossRef]
- Miyazaki, M.; Yoshitomi, H.; Miyakawa, S.; Uesaka, K.; Unno, M.; Endo, I.; Ota, T.; Ohtsuka, M.; Kinoshita, H.; Shimada, K.; et al. Clinical practice guidelines for the management of biliary tract cancers 2015: The 2nd English edition. J. Hepatobiliary Pancreat. Sci. 2015, 22, 249–273. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B. AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: New York, NY, USA, 2017; pp. 303–309. [Google Scholar]
- Miyazaki, M.; Ohtsuka, M.; Miyakawa, S.; Nagino, M.; Yamamoto, M.; Kokudo, N.; Sano, K.; Endo, I.; Unno, M.; Chijiiwa, K.; et al. Classification of biliary tract cancers established by the Japanese Society of Hepato-Biliary-Pancreatic Surgery: 3(rd) English edition. J. Hepatobiliary Pancreat. Sci. 2015, 22, 181–196. [Google Scholar] [CrossRef] [PubMed]
- De Savornin Lohman, E.A.J.; van der Geest, L.G.; de Bitter, T.J.J.; Nagtegaal, I.D.; van Laarhoven, C.; van den Boezem, P.; van der Post, C.S.; de Reuver, P.R. Re-resection in Incidental Gallbladder Cancer: Survival and the Incidence of Residual Disease. Ann. Surg. Oncol. 2020, 27, 1132–1142. [Google Scholar] [CrossRef]
- Blakely, A.M.; Wong, P.; Chu, P.; Warner, S.G.; Raoof, M.; Singh, G.; Fong, Y.; Melstrom, L.G. Intraoperative bile spillage is associated with worse survival in gallbladder adenocarcinoma. J. Surg. Oncol. 2019, 120, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Oneda, E.; Abu Hilal, M.; Zaniboni, A. Biliary Tract Cancer: Current Medical Treatment Strategies. Cancers 2020, 12, 1237. [Google Scholar] [CrossRef] [PubMed]
- Nishio, H.; Ebata, T.; Yokoyama, Y.; Igami, T.; Sugawara, G.; Nagino, M. Gallbladder cancer involving the extrahepatic bile duct is worthy of resection. Ann. Surg. 2011, 253, 953–960. [Google Scholar] [CrossRef]
- Qu, K.; Chang, H.L.; Liu, S.N.; Liu, C.; Xu, X.S.; Wang, R.T.; Zhou, L.; Tian, F.; Wei, J.C.; Tai, M.H.; et al. Prognosis and management for gallbladder cancer with hepatic invasion: Long-term results of 139 patients from a single center in China. Asian Pac. J. Cancer Prev. 2012, 13, 1015–1018. [Google Scholar] [CrossRef][Green Version]
- Sakata, J.; Shirai, Y.; Wakai, T.; Ajioka, Y.; Hatakeyama, K. Number of positive lymph nodes independently determines the prognosis after resection in patients with gallbladder carcinoma. Ann. Surg. Oncol. 2010, 17, 1831–1840. [Google Scholar] [CrossRef]
- Liu, G.J.; Li, X.H.; Chen, Y.X.; Sun, H.D.; Zhao, G.M.; Hu, S.Y. Radical lymph node dissection and assessment: Impact on gallbladder cancer prognosis. World J. Gastroenterol. 2013, 19, 5150–5158. [Google Scholar] [CrossRef]
- Amini, N.; Spolverato, G.; Kim, Y.; Gupta, R.; Margonis, G.A.; Ejaz, A.; Pawlik, T.M. Lymph node status after resection for gallbladder adenocarcinoma: Prognostic implications of different nodal staging/scoring systems. J. Surg. Oncol. 2015, 111, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Bai, D.S.; Zhou, B.H.; Chen, P.; Qian, J.J.; Jin, S.J.; Jiang, G.Q. Positive relationship between number of negative lymph nodes and duration of gallbladder cancer cause-specific survival after surgery. Cancer Manag. Res. 2018, 10, 6961–6969. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.C.; Park, S.J.; Lee, S.D.; Han, S.S.; Kim, S.H. Effects of Sarcopenia on Prognosis After Resection of Gallbladder Cancer. J. Gastrointest. Surg. 2020, 24, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Regimbeau, J.M.; Fuks, D.; Bachellier, P.; Le Treut, Y.P.; Pruvot, F.R.; Navarro, F.; Chiche, L.; Farges, O. Prognostic value of jaundice in patients with gallbladder cancer by the AFC-GBC-2009 study group. Eur. J. Surg. Oncol. 2011, 37, 505–512. [Google Scholar] [CrossRef]
- Chun, Y.J.; Jeung, H.C.; Park, H.S.; Park, J.S.; Rha, S.Y.; Choi, H.J.; Lee, J.H.; Jeon, T.J. Significance of Metabolic Tumor Volume and Total Lesion Glycolysis Measured Using (1)(8)F-FDG PET/CT in Locally Advanced and Metastatic Gallbladder Carcinoma. Yonsei Med. J. 2019, 60, 604–610. [Google Scholar] [CrossRef]
- Lim, H.; Seo, D.W.; Park, d.H.; Lee, S.S.; Lee, S.K.; Kim, M.H.; Hwang, S. Prognostic factors in patients with gallbladder cancer after surgical resection: Analysis of 279 operated patients. J. Clin. Gastroenterol. 2013, 47, 443–448. [Google Scholar] [CrossRef]
- Kayahara, M.; Nagakawa, T.; Nakagawara, H.; Kitagawa, H.; Ohta, T. Prognostic factors for gallbladder cancer in Japan. Ann. Surg. 2008, 248, 807–814. [Google Scholar] [CrossRef]
- Higuchi, R.; Ota, T.; Araida, T.; Kajiyama, H.; Yazawa, T.; Furukawa, T.; Yoshikawa, T.; Takasaki, K.; Yamamoto, M. Surgical approaches to advanced gallbladder cancer: A 40-year single-institution study of prognostic factors and resectability. Ann. Surg. Oncol. 2014, 21, 4308–4316. [Google Scholar] [CrossRef]
- Miura, F.; Asano, T.; Amano, H.; Toyota, N.; Wada, K.; Kato, K.; Takada, T.; Takami, H.; Ohira, G.; Matsubara, H. New prognostic factor influencing long-term survival of patients with advanced gallbladder carcinoma. Surgery 2010, 148, 271–277. [Google Scholar] [CrossRef]
- Chen, C.; Geng, Z.; Shen, H.; Song, H.; Zhao, Y.; Zhang, G.; Li, W.; Ma, L.; Wang, L. Long-Term Outcomes and Prognostic Factors in Advanced Gallbladder Cancer: Focus on the Advanced T Stage. PLoS ONE 2016, 11, e0166361. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, J.M.; Lee, J.Y.; Kim, S.H.; Han, J.K.; Choi, B.I.; Choi, J.Y. Analysis of enhancement pattern of flat gallbladder wall thickening on MDCT to differentiate gallbladder cancer from cholecystitis. AJR Am. J. Roentgenol. 2008, 191, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Kaza, R.K.; Gulati, M.; Wig, J.D.; Chawla, Y.K. Evaluation of gall bladder carcinoma with dynamic magnetic resonance imaging and magnetic resonance cholangiopancreatography. Australas Radiol. 2006, 50, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.P.; Ni, J.M.; Zhang, Z.Y.; Lu, F.Q.; Li, B.; Jin, H.H.; Dai, T. Preoperative Evaluation of Malignant Perihilar Biliary Obstruction: Negative-Contrast CT Cholangiopancreatography and CT Angiography Versus MRCP and MR Angiography. AJR Am. J. Roentgenol. 2015, 205, 780–788. [Google Scholar] [CrossRef]
- De Savornin Lohman, E.A.J.; de Bitter, T.J.J.; van Laarhoven, C.; Hermans, J.J.; de Haas, R.J.; de Reuver, P.R. The diagnostic accuracy of CT and MRI for the detection of lymph node metastases in gallbladder cancer: A systematic review and meta-analysis. Eur. J. Radiol. 2019, 110, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Kurita, A.; Kodama, Y.; Nakamoto, Y.; Isoda, H.; Minamiguchi, S.; Yoshimura, K.; Kuriyama, K.; Sawai, Y.; Uza, N.; Hatano, E.; et al. Impact of EUS-FNA for preoperative para-aortic lymph node staging in patients with pancreatobiliary cancer. Gastrointest. Endosc. 2016, 84, 467–475.e1. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, J.M.; Lee, E.S.; Han, J.K.; Choi, B.I. Preoperative staging of gallbladder carcinoma using biliary MR imaging. J. Magn. Reson. Imaging 2015, 41, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, J.M.; Lee, J.Y.; Choi, J.Y.; Kim, S.H.; Han, J.K.; Choi, B.I. Accuracy of preoperative T-staging of gallbladder carcinoma using MDCT. AJR Am. J. Roentgenol. 2008, 190, 74–80. [Google Scholar] [CrossRef]
- Alyassin, Y.; Sayed, E.G.; Mehta, P.; Ruparelia, K.; Arshad, M.S.; Rasekh, M.; Shepherd, J.; Kucuk, I.; Wilson, P.B.; Singh, N.; et al. Application of mesoporous silica nanoparticles as drug delivery carriers for chemotherapeutic agents. Drug Discov. Today 2020. [Google Scholar] [CrossRef]
- Salati, M.; Filippi, R.; Vivaldi, C.; Caputo, F.; Leone, F.; Salani, F.; Cerma, K.; Aglietta, M.; Fornaro, L.; Sperti, E.; et al. The prognostic nutritional index predicts survival and response to first-line chemotherapy in advanced biliary cancer. Liver Int. 2020, 40, 704–711. [Google Scholar] [CrossRef]
- Tamma, R.; Annese, T.; Ruggieri, S.; Brunetti, O.; Longo, V.; Cascardi, E.; Mastropasqua, M.G.; Maiorano, E.; Silvestris, N.; Ribatti, D. Inflammatory cells infiltrate and angiogenesis in locally advanced and metastatic cholangiocarcinoma. Eur. J. Clin. Investig. 2019, 49, e13087. [Google Scholar] [CrossRef]
- Grootveld, M.; Percival, B.; Gibson, M.; Osman, Y.; Edgar, M.; Molinari, M.; Mather, M.L.; Casanova, F.; Wilson, P.B. Progress in low-field benchtop NMR spectroscopy in chemical and biochemical analysis. Anal. Chim. Acta 2019, 1067, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Araida, T.; Yoshikawa, T.; Azuma, T.; Ota, T.; Takasaki, K.; Hanyu, F. Indications for pancreatoduodenectomy in patients undergoing lymphadenectomy for advanced gallbladder carcinoma. J. Hepatobiliary Pancreat. Surg. 2004, 11, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Ota, T.; Araida, T.; Yamamoto, M.; Takasaki, K. Operative outcome and problems of right hepatic lobectomy with pancreatoduodenectomy for advanced carcinoma of the biliary tract. J. Hepatobiliary Pancreat. Surg. 2007, 14, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibanes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, D.J.; Kim, H.H.; Lee, H.J.; Yang, H.K. Comparison of complications after laparoscopy-assisted distal gastrectomy and open distal gastrectomy for gastric cancer using the Clavien-Dindo classification. Surg. Endosc. 2012, 26, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Lacy, A.M.; Tasende, M.M.; Delgado, S.; Fernandez-Hevia, M.; Jimenez, M.; De Lacy, B.; Castells, A.; Bravo, R.; Wexner, S.D.; Heald, R.J. Transanal Total Mesorectal Excision for Rectal Cancer: Outcomes after 140 Patients. J. Am. Coll. Surg. 2015, 221, 415–423. [Google Scholar] [CrossRef]
- Tokunaga, M.; Kondo, J.; Tanizawa, Y.; Bando, E.; Kawamura, T.; Terashima, M. Postoperative intra-abdominal complications assessed by the Clavien-Dindo classification following open and laparoscopy-assisted distal gastrectomy for early gastric cancer. J. Gastrointest. Surg. 2012, 16, 1854–1859. [Google Scholar] [CrossRef]
- Panhofer, P.; Ferenc, V.; Schutz, M.; Gleiss, A.; Dubsky, P.; Jakesz, R.; Gnant, M.; Fitzal, F. Standardization of morbidity assessment in breast cancer surgery using the Clavien Dindo Classification. Int. J. Surg. 2014, 12, 334–339. [Google Scholar] [CrossRef]
- Jiang, X.; Hiki, N.; Nunobe, S.; Fukunaga, T.; Kumagai, K.; Nohara, K.; Sano, T.; Yamaguchi, T. Postoperative outcomes and complications after laparoscopy-assisted pylorus-preserving gastrectomy for early gastric cancer. Ann. Surg. 2011, 253, 928–933. [Google Scholar] [CrossRef]
- Duraes, L.C.; Stocchi, L.; Steele, S.R.; Kalady, M.F.; Church, J.M.; Gorgun, E.; Liska, D.; Kessler, H.; Lavryk, O.A.; Delaney, C.P. The Relationship Between Clavien-Dindo Morbidity Classification and Oncologic Outcomes After Colorectal Cancer Resection. Ann. Surg. Oncol. 2018, 25, 188–196. [Google Scholar] [CrossRef]
- Chen, K.J.; Yang, F.C.; Qin, Y.S.; Jin, J.; Zheng, S.S. Assessment of clinical outcomes of advanced hilar cholangiocarcinoma. Hepatobiliary Pancreat. Dis. Int. 2018, 17, 155–162. [Google Scholar] [CrossRef] [PubMed]
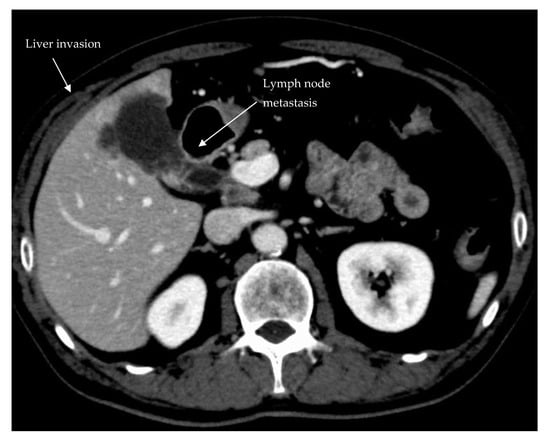
| Tumor Factors | Incidence | R0 Rate |
|---|---|---|
| Tumor perforates the serosa or more | 61% (95) | 76% (72/95) |
| Direct invasion to the liver parenchyma, <5 mm | 17% (27) | 93% (25/27) |
| Direct invasion to the liver parenchyma, ≥5 mm, <20 mm | 17% (26) | 65% (17/26) |
| Direct invasion to the liver parenchyma, ≥20 mm | 18% (28) | 82% (23/28) |
| Invasion to the right margin, but not to the left margin of the HDL | 15% (24) | 83% (20/24) |
| Invasion to the left margin, but not to the entire HDL | 24% (37) | 63% (23/37) |
| Invasion through the HDL | 10% (16) | 56% (9/16) |
| Stomach or Duodenum invasion | 11% (17) | 65% (11/17) |
| Colon invasion | 16 (10%) | 69% (11/16) |
| Pancreas invasion | 0.6% (1) | 0% (0/1) |
| Two or more extrahepatic organs/structures | 13% (20) | 55% (11/20) |
| Main portal vein or hepatic artery | 24% (37) | 59% (22/37) |
| AJCC 8th 1–3 regional LNM | 59% (92) | 84% (77/92) |
| AJCC 8th ≥4 regional LNM | 16% (25) | 64% (16/25) |
| Factors | a Univariate | b Multivariate | |||
|---|---|---|---|---|---|
| n | 5y OS % | p Value | HR (95% CI) | p Value | |
| Period 2000–2017 (to 1985–1999) | 93/64 | 35/41 | 1.0 | 1.62 (0.90–2.92) | 0.11 |
| Age ≥67 (to <67) | 92/65 | 33/43 | 0.30 | 1.14 (0.69–1.90) | 0.60 |
| Sex (female to male) | 86/71 | 39/35 | 0.80 | 0.87 (0.53–1.43) | 0.58 |
| Incidental (to none) | 16/141 | 38/37 | 0.90 | 1.40 (0.63–3.12) | 0.41 |
| Cholecystectomy (to LBR) | 33/36 | 43/46 | 0.60 | 1.65 (0.75–3.60) | 0.13 |
| S4bS5 (to LBR) | 42/36 | 38/46 | 0.40 | 0.49 (0.21–1.15) | 0.10 |
| Hepatectomy ≥2 sections (to LBR) | 46/36 | 24/46 | 0.03 | 0.56 (0.24–1.35) | 0.20 |
| Without BDR (to with BDR) | 40/79 | 50/26 | 0.03 | 0.86 (0.35–2.09) | 0.73 |
| Pancreatoduodenectomy (to BDR) | 38/79 | 45/26 | 0.10 | 0.67 (0.34–1.34) | 0.26 |
| Blood loss ≥1400 g (to <1400 g) | 60/97 | 26/43 | 0.003 | 2.19 (1.26–3.80) | 0.005 |
| Surgery time ≥258 min (to <258 min) | 113/44 | 32/50 | 0.03 | 1.23 (0.55–2.73) | 0.61 |
| Histology papillary (to well/moderate) | 26/79 | 56/34 | 0.20 | 1.66 (0.71–3.89) | 0.24 |
| Histology poorly/others (to well/moderate) | 51/79 | 29/34 | 0.05 | 2.26 (1.33–3.84) | 0.003 |
| Liver invasion <5 mm (to no invasion) | 27/76 | 40/48 | 0.50 | 1.24 (0.56–2.72) | 0.59 |
| Liver invasion ≥5 mm (to no invasion) | 54/76 | 19/48 | <0.001 | 2.42 (1.17–5.01) | 0.017 |
| Binf1 (to no invasion) | 24/80 | 41/49 | 0.30 | 0.73 (0.31–1.70) | 0.47 |
| Binf2/3 (to no invasion) | 53/80 | 17/49 | <0.001 | 2.10 (0.99–4.46) | 0.053 |
| ≥2 organs or structure invasions (to <1) | 20/137 | 21/40 | 0.02 | 1.05 (0.51–2.20) | 0.89 |
| MPV or HA invasions (to no invasions) | 35/119 | 18/43 | <0.001 | 1.75 (0.87–3.51) | 0.11 |
| AJCC8th 1–3 regional LNM (to N0) | 92/40 | 39/47 | 0.50 | 1.34 (0.74–2.39) | 0.33 |
| AJCC8th ≥4 regional LNM (to N0) | 25/40 | 11/47 | 0.001 | 2.25 (1.07–4.74) | 0.034 |
| Residual cancer R1.2 (to R0) | 32/125 | 14/42 | 0.004 | 1.09 (0.55–2.16) | 0.81 |
| Clavien–Dindo classification ≥3 (to ≤2) | 65/92 | 29/43 | 0.02 | 1.64 (0.99–2.71) | 0.054 |
| Without adjuvant chemotherapy (to with) | 117/40 | 36/41 | 0.20 | 1.93 (1.03–3.62) | 0.041 |
| Factors | 0 Factors | 1 Factor | 2 or 3 Factors | p Value Φ |
|---|---|---|---|---|
| (n = 68) | (n = 54) | (n = 35) | ||
| Period 2000-17 | 38 (56%) | 34 (63%) | 21 (60%) | 0.73 |
| Age (y, median) | 71 | 69 | 66 | 0.20 ε |
| Female | 39 (57%) | 25 (46%) | 22 (63%) | 0.26 |
| Incidental | 10 (15%) | 4 (7.4%) | 2 (5.7%) | 0.32 |
| Liver invasion ≥5 mm | 0 #, * | 24 (44%) #, ∀ | 30 (86%) *,∀ | <0.001 |
| Invasion of the left margin, or entire area of the HDL | 0 #, * | 24 (44%) #, ∀ | 29 (83%) *, ∀ | <0.001 |
| ≥4 regional lymph node metastasis | 0 #, * | 6 (11%) #, ∀ | 19 (54%) *, ∀ | <0.001 |
| Hepatectomy (LB/GB/S45/≥2 sections) | 27/25/15/1 #, * | 6/6/17/15 # | 3/2/10/20 * | <0.001 |
| BDR (with/without/PD) | 22/29/17 #, * | 34/10/10 # | 23/1/11 * | <0.001 |
| Vascular resection | 1 (1.5%) #, * | 6 (11%) #, ∀ | 17 (49%) *, ∀ | <0.001 |
| Blood loss (g) | 765 #, * | 1025 # | 1498 * | <0.001 #, ε |
| Surgery time (min) | 261 #, * | 370 # | 400 * | <0.001 ε |
| Histology (tub1 or 2/pap/poor or others) | 30/22/16 #, * | 28/3/23 # | 21/1/12 * | <0.001 |
| ≥2 organs or structure invasions | 1 (1.5%) #, * | 6 (11%) #, ∀ | 13 (37%) *, ∀ | <0.001 |
| MPV or HA invasions | 2 (2.9%) #, * | 12 (22%) #, ∀ | 23 (66%) *, ∀ | <0.001 |
| R0 | 60 (88%) # | 48 (89%) * | 18 (51%) #, * | <0.001 |
| Clavien–Dindo classification ≥3 | 20 (29%) # | 25 (46%) * | 20 (57%) #, * | 0.018 |
| Adjuvant chemotherapy | 17 (25%) | 14 (26%) | 9 (26%) | 1.0 |
| Type of Recurrence | 0 Factors | 1 Factor | 2 or 3 Factors | p Value Φ |
|---|---|---|---|---|
| (n = 68) | (n = 54) | (n = 35) | ||
| Any type of recurrence | 32 (47%) #, * | 38 (70%) # | 26 (74%) * | 0.007 |
| Liver | 11 (16%) # | 17/52 (33%) # | 6/33 (18%) | 0.09 |
| Lymph node | 9 (16%) | 13/52 (25%) | 5/33 (15%) | 0.23 |
| Dissemination | 10 (15%) * | 10/52 (19%) ∀ | 13/33 (39%) *, ∀ | 0.02 |
| Local | 9 (16%) | 12/52 (23%) | 8/33 (24%) | 0.61 |
| Others | 1 | 4 (44%) | 3 (83%) | 0.11 |
| Diagnosed Factors | Sensitivity | Specificity | Accuracy | |||
|---|---|---|---|---|---|---|
| MRI | CT | MRI | CT | MRI | CT | |
| ≤T1a vs≥T1b [27,28] | 82.7–89.3 | NA | 100 | NA | 93.0–95.4 | NA |
| ≤T1vs≥T2 [27,28] | 88.1–94.0 | 79.3 | 79.0–84.2 | 98.8 | 87.2–90.7 | 94.7 |
| ≤T2vs≥T3 [27,28] | 83.3–90.0 | 92.7 | 80.4–89.4 | 86 | 80.2–88.4 | 89.3 |
| ≤T3vs≥T4 [27,28] | 100 | 100 | 59.5–100 | 100 | 96.5–100 | 100 |
| Liver invasion [23,24] | 87.5 | NA | 86 | NA | NA | NA |
| Biliary invasion [23,24] | 80 | NA | 100 | NA | 81–85.7 | 90.5 |
| Lymph node Mets [23,24,25,27] | 25–71 | 81.8–93.0 | 42–100 | 70–100 | NA | NA |
| Hepatic artery invasion [25] | 50–66.7 | 83.3 | 53.3–73.3 | 93.3–100 | NA | NA |
| Portal vein invasion [24] | 80 | 80–90 | 72.7–81.8 | 90.9 | NA | NA |
| Duodenal invasion [23] | 50 | NA | 100 | NA | NA | NA |
| Liver or peritoneal Mets | 93.8 | 80–100 | 93.8–100 | 80 | NA | NA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higuchi, R.; Yazawa, T.; Uemura, S.; Matsunaga, Y.; Ota, T.; Araida, T.; Furukawa, T.; Yamamoto, M. Examination of Prognostic Factors Affecting Long-Term Survival of Patients with Stage 3/4 Gallbladder Cancer without Distant Metastasis. Cancers 2020, 12, 2073. https://doi.org/10.3390/cancers12082073
Higuchi R, Yazawa T, Uemura S, Matsunaga Y, Ota T, Araida T, Furukawa T, Yamamoto M. Examination of Prognostic Factors Affecting Long-Term Survival of Patients with Stage 3/4 Gallbladder Cancer without Distant Metastasis. Cancers. 2020; 12(8):2073. https://doi.org/10.3390/cancers12082073
Chicago/Turabian StyleHiguchi, Ryota, Takehisa Yazawa, Shuichirou Uemura, Yutaro Matsunaga, Takehiro Ota, Tatsuo Araida, Toru Furukawa, and Masakazu Yamamoto. 2020. "Examination of Prognostic Factors Affecting Long-Term Survival of Patients with Stage 3/4 Gallbladder Cancer without Distant Metastasis" Cancers 12, no. 8: 2073. https://doi.org/10.3390/cancers12082073
APA StyleHiguchi, R., Yazawa, T., Uemura, S., Matsunaga, Y., Ota, T., Araida, T., Furukawa, T., & Yamamoto, M. (2020). Examination of Prognostic Factors Affecting Long-Term Survival of Patients with Stage 3/4 Gallbladder Cancer without Distant Metastasis. Cancers, 12(8), 2073. https://doi.org/10.3390/cancers12082073