Impact of Extraskeletal Metastases on Skeletal-Related Events in Metastatic Castration-Resistant Prostate Cancer with Bone Metastases
Abstract
1. Introduction
2. Results
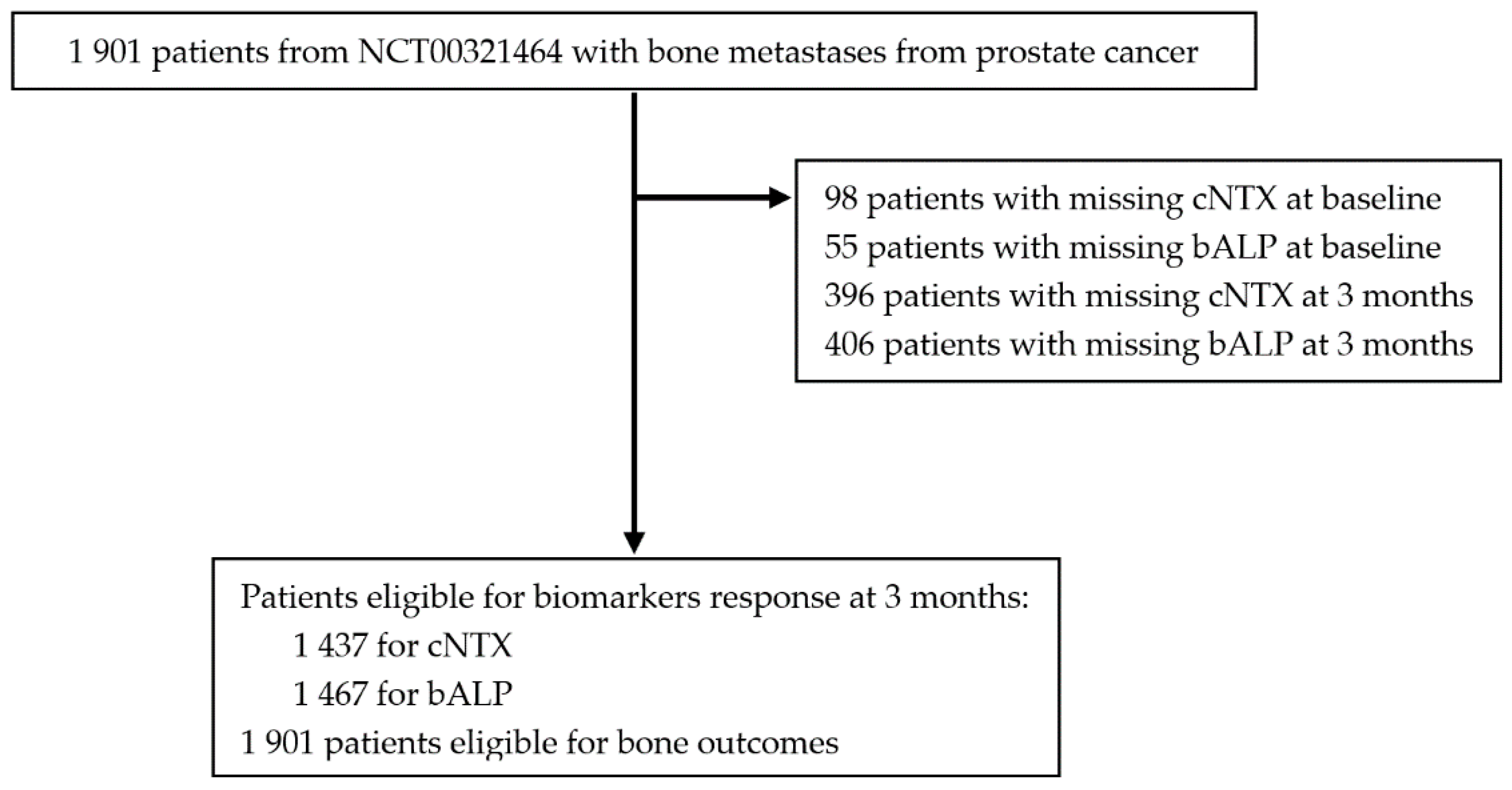
2.1. Cohort Description
2.2. Bone Disease-Specific Characteristics
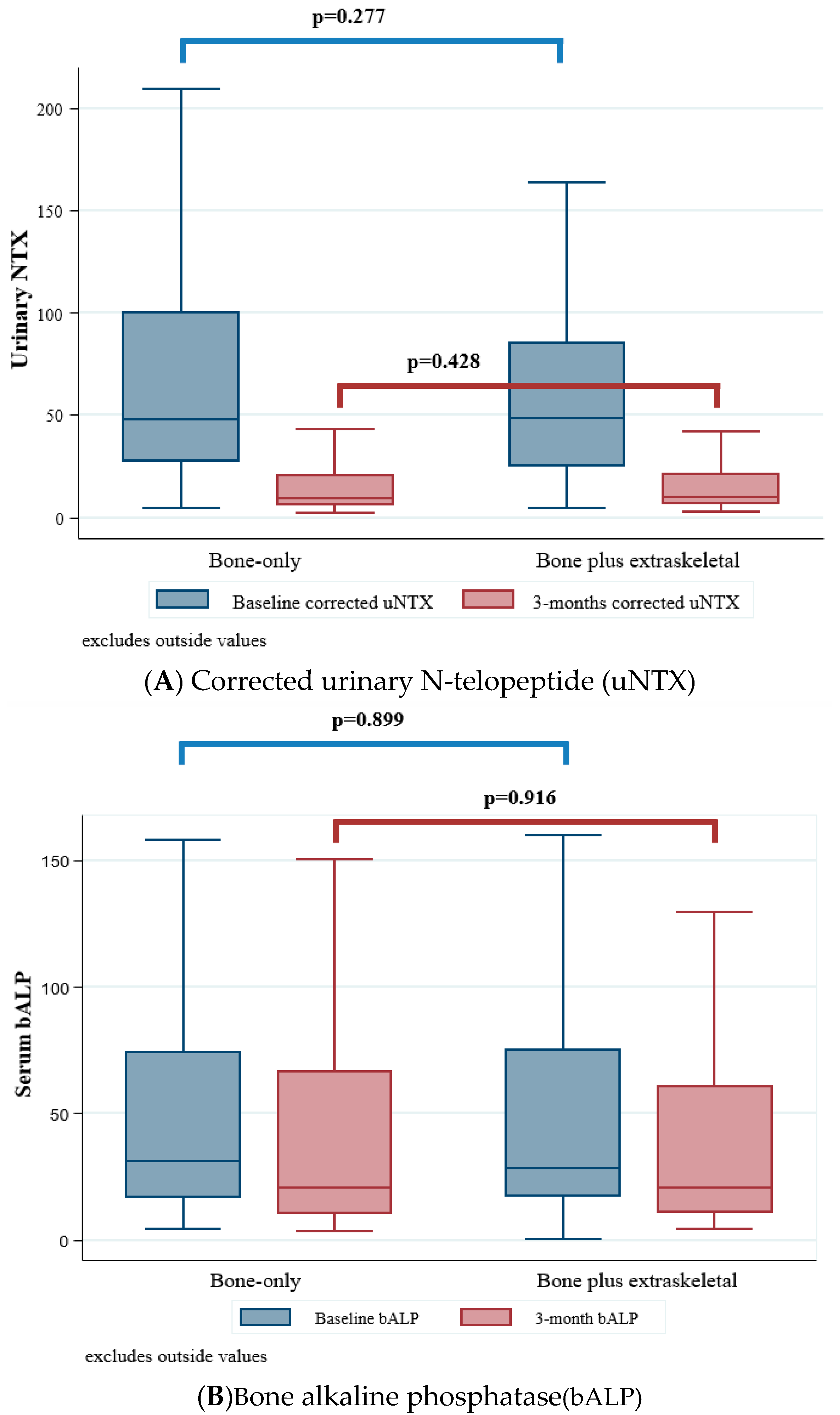
2.3. Bone Marker Variation
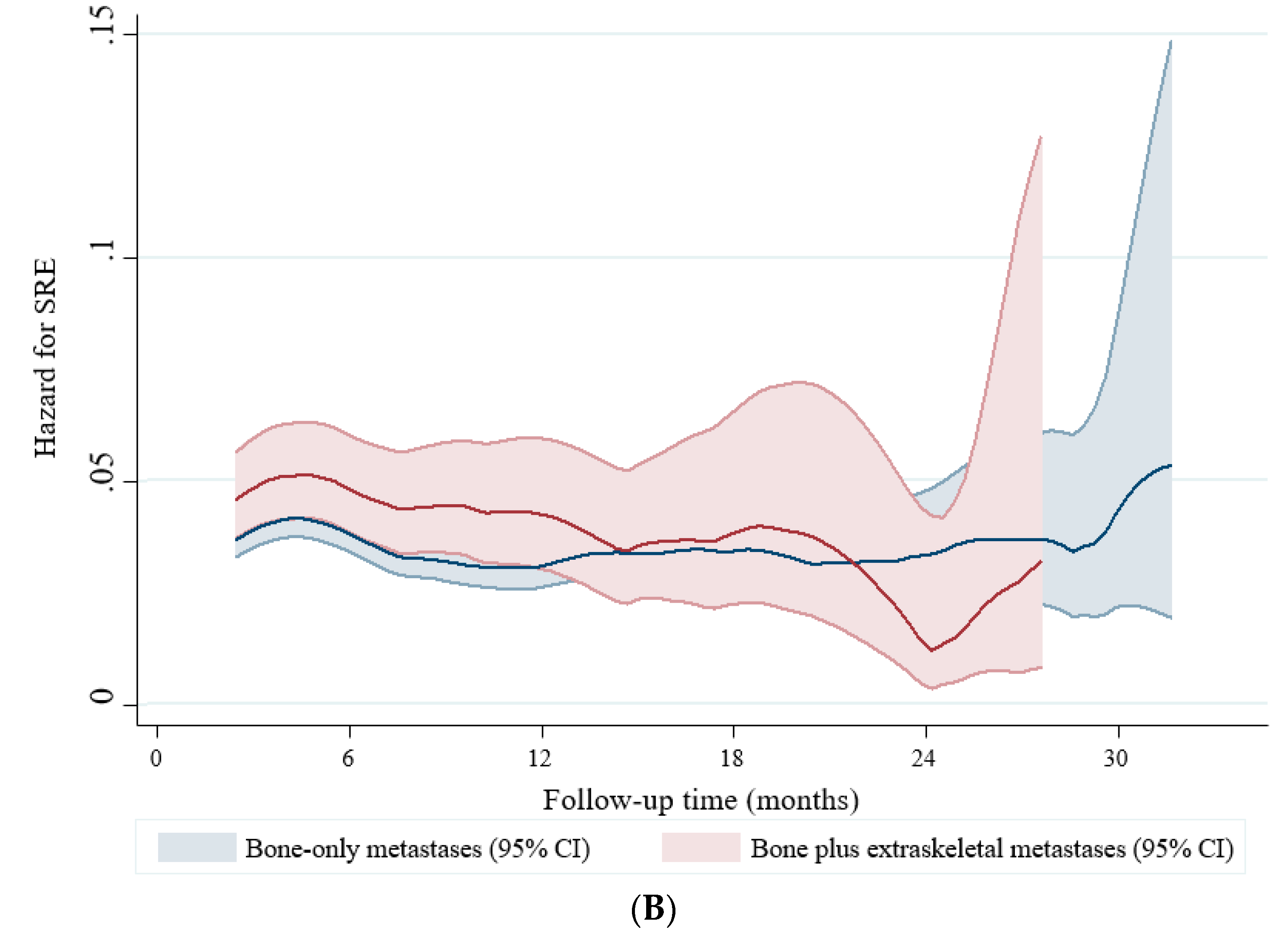
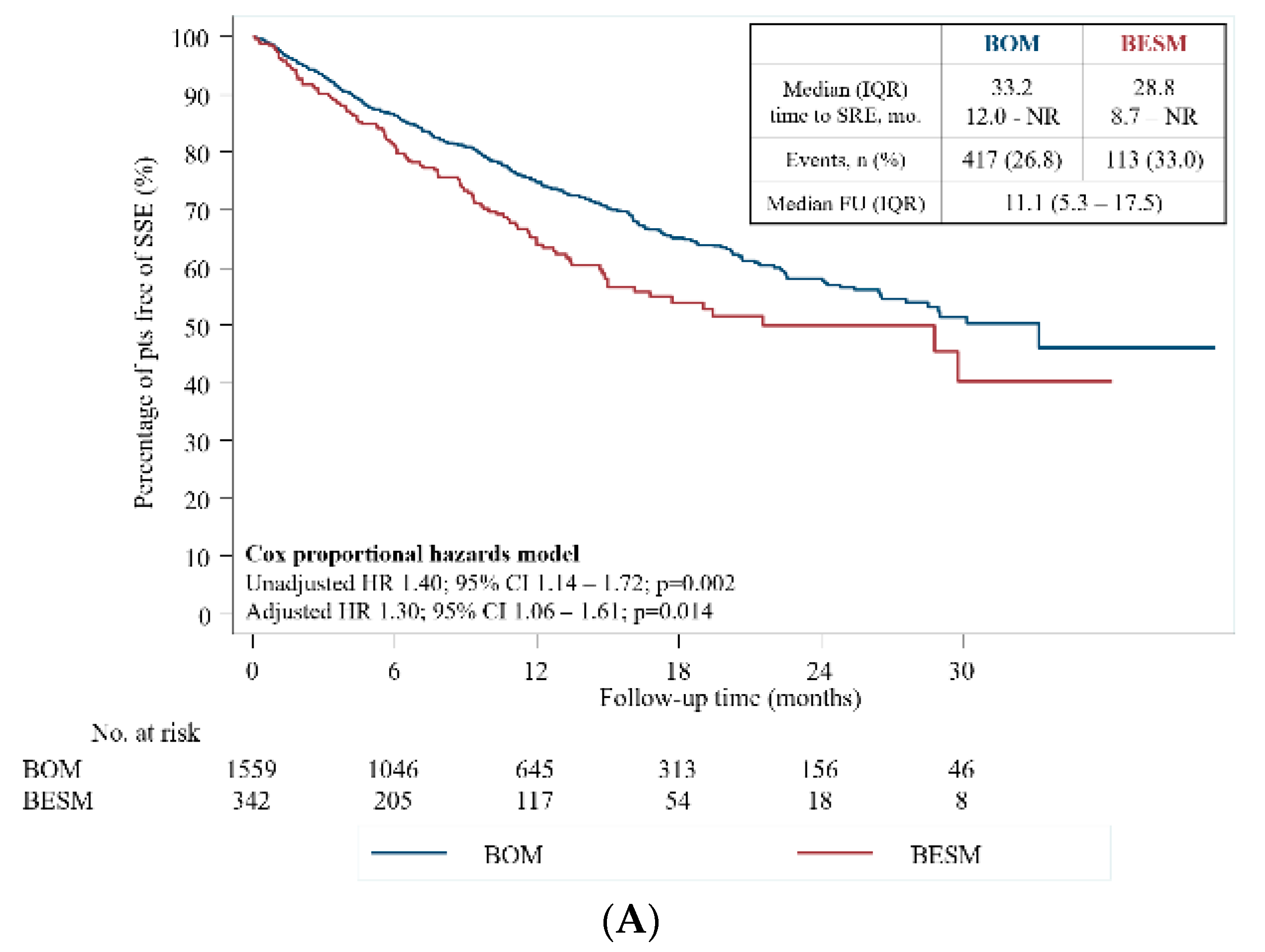
2.4. Skeletal-Related Events
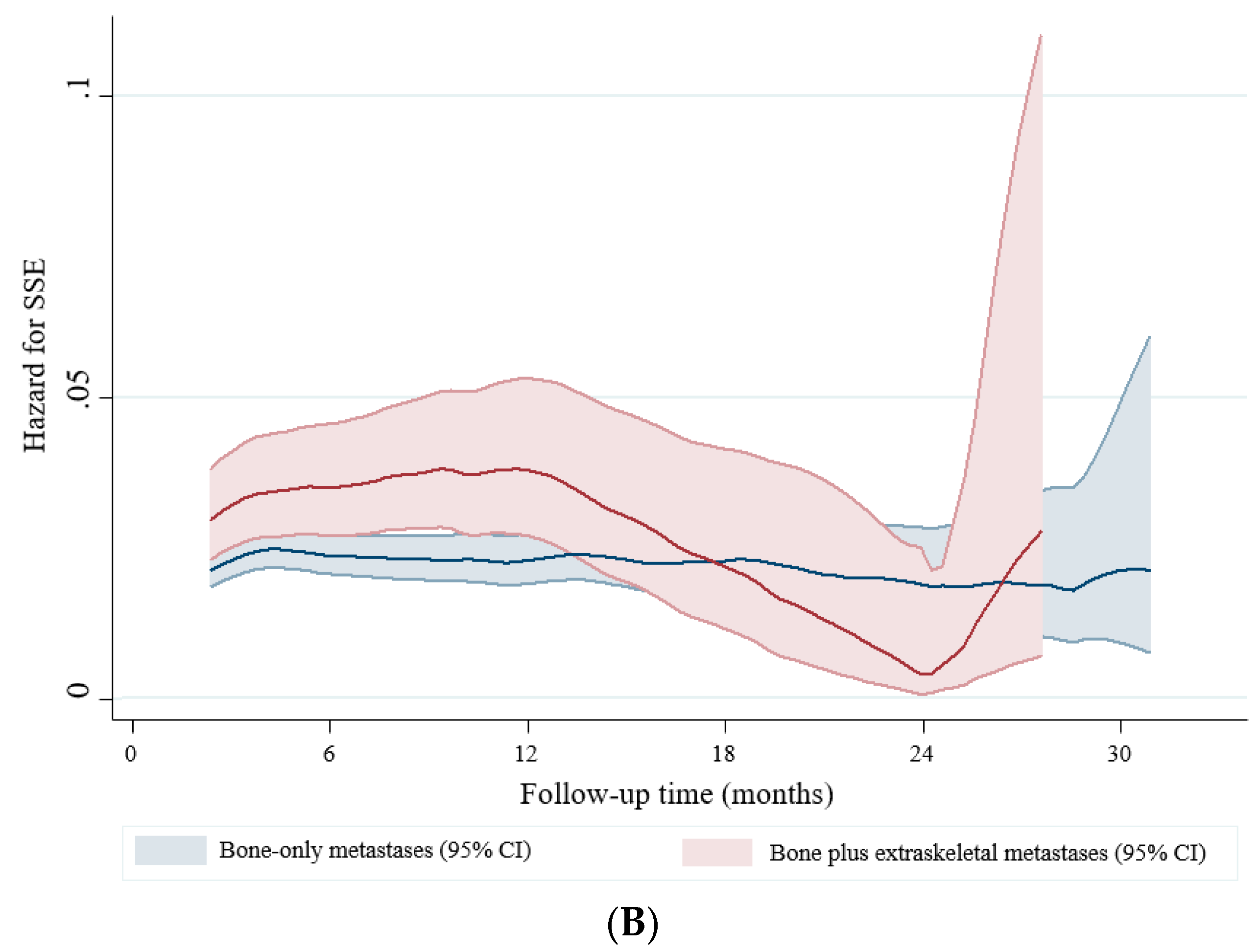
2.5. Symptomatic Skeletal Events
2.6. Overall Survival
3. Discussion
4. Materials and Methods
4.1. Design and Study Population
4.2. Study Aim and Outcomes
4.3. Bone Marker Determination
4.4. Statistical Analysis
4.5. Ethical Statement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saad, F.; Gleason, D.M.; Murray, R.; Tchekmedyian, S.; Venner, P.; Lacombe, L.; Chin, J.L.; Vinholes, J.J.; Goas, J.A.; Chen, B. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J. Natl. Cancer Inst. 2002, 94, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- European Commission ECIS—European Cancer Information System: Data Explorer. Available online: https://ecis.jrc.ec.europa.eu/index.php (accessed on 17 April 2020).
- Gdowski, A.S.; Ranjan, A.; Vishwanatha, J.K. Current concepts in bone metastasis, contemporary therapeutic strategies and ongoing clinical trials. J. Exp. Clin. Cancer Res. 2017, 36, 1–13. [Google Scholar] [CrossRef]
- Fizazi, K.; Carducci, M.; Smith, M.; Damião, R.; Brown, J.; Karsh, L.; Milecki, P.; Shore, N.; Rader, M.; Wang, H.; et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet 2011, 377, 813–822. [Google Scholar] [CrossRef]
- Ford, J.A.; Jones, R.; Elders, A.; Mulatero, C.; Royle, P.; Sharma, P.; Stewart, F.; Todd, R.; Mowatt, G. Denosumab for treatment of bone metastases secondary to solid tumours: Systematic review and network meta-analysis. Eur. J. Cancer 2013, 49, 416–430. [Google Scholar] [CrossRef]
- von Moos, R.; Costa, L.; Gonzalez-Suarez, E.; Terpos, E.; Niepel, D.; Body, J.J. Management of bone health in solid tumours: From bisphosphonates to a monoclonal antibody. Cancer Treat. Rev. 2019, 76, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Halabi, S.; Kelly, W.K.; Ma, H.; Zhou, H.; Solomon, N.C.; Fizazi, K.; Tangen, C.M.; Rosenthal, M.; Petrylak, D.P.; Hussain, M.; et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J. Clin. Oncol. 2016, 34, 1652–1659. [Google Scholar] [CrossRef]
- Lipton, A.; Smith, M.R.; Fizazi, K.; Stopeck, A.T.; Henry, D.; Brown, J.E.; Shore, N.D.; Saad, F.; Spencer, A.; Warner, D.J. Changes in Bone Turnover Marker Levels and Clinical Outcomes in Patients with Advanced Cancer and Bone Metastases Treated with Bone Antiresorptive Agents. Clin. Cancer Res. 2016, 22, 5713–5721. [Google Scholar] [CrossRef]
- Costa, L.; Major, P.P. Effect of bisphosphonates on pain and quality of life in patients with bone metastases. Nat. Clin. Pract. Oncol. 2009, 6, 163–174. [Google Scholar] [CrossRef]
- Casimiro, S.; Ferreira, A.R.; Mansinho, A.; Alho, I.; Costa, L. Molecular mechanisms of bone metastasis: Which targets came from the bench to the bedside? Int. J. Mol. Sci. 2016, 17, 1415. [Google Scholar] [CrossRef]
- Guise, T.A.; Yin, J.J.; Mohammad, K.S. Role of endothelin-1 in osteoblastic bone metastases. Cancer 2003, 97 (Suppl. S3), 779–784. [Google Scholar] [CrossRef]
- Coleman, R.E.; Major, P.; Lipton, A.; Brown, J.E.; Lee, K.A.; Smith, M.; Saad, F.; Zheng, M.; Hei, Y.J.; Seaman, J.; et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J. Clin. Oncol. 2005, 23, 4925–4935. [Google Scholar] [CrossRef]
- Coleman, R.; Costa, L.; Saad, F.; Cook, R.; Hadji, P.; Terpos, E.; Garnero, P.; Brown, J.; Body, J.J.; Smith, M.; et al. Consensus on the utility of bone markers in the malignant bone disease setting. Crit. Rev. Oncol. Hematol. 2011, 80, 411–432. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.E.; Cook, R.J.; Major, P.; Lipton, A.; Saad, F.; Smith, M.; Lee, K.A.; Zheng, M.; Hel, Y.J.; Coleman, R.E. Bone turnover markers as predictors of skeketal complications in prostate cancer, lung cancer, and other solid tumors. J. Natl. Cancer Inst. 2005, 97, 59–69. [Google Scholar] [CrossRef]
- Ferreira, A.; Alho, I.; Casimiro, S.; Costa, L. Bone remodeling markers and bone metastases: From cancer research to clinical implications. BoneKEy Rep. 2015, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Himelstein, A.L.; Foster, J.C.; Khatcheressian, J.L.; Roberts, J.D.; Seisler, D.K.; Novotny, P.J.; Qin, R.; Go, R.S.; Grubbs, S.S.; O’Connor, T.; et al. Effect of longer-interval vs. standard dosing of zoledronic acid on skeletal events in patients with bone metastases: A randomized clinical trial. JAMA 2017, 317, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, L.; Liu, L.; Zhuang, J.; Tang, S.; Zhang, T.; Zhou, C.; Feng, F.; Liu, R.; Zhang, J.; et al. Efficacy and safety of de-escalation bone-modifying agents for cancer patients with bone metastases: A systematic review and meta-analysis. Cancer Manag. Res. 2018, 10, 3809–3823. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Bosserman, L.; Gao, G.; Skacel, T.; Markus, R. Denosumab treatment of prostate cancer with bone metastases and increased urine N-telopeptide levels after therapy with intravenous bisphosphonates: Results of a randomized phase II trial. J. Urol. 2013, 189, S51–S58. [Google Scholar] [CrossRef]
- Tanaka, R.; Yonemori, K.; Hirakawa, A.; Kinoshita, F.; Takahashi, N.; Hashimoto, J.; Kodaira, M.; Yamamoto, H.; Yunokawa, M.; Shimizu, C.; et al. Risk Factors for Developing Skeletal-Related Events in Breast Cancer Patients with Bone Metastases Undergoing Treatment with Bone-Modifying Agents. Oncologist 2016, 21, 508–513. [Google Scholar] [CrossRef]
- Ferreira, A.R.; Alho, I.; Shan, N.; Matias, M.; Faria, M.; Casimiro, S.; Leitzel, K.; Ali, S.; Lipton, A.; Costa, L. N-Telopeptide of Type I Collagen Long-Term Dynamics in Breast Cancer Patients with Bone Metastases: Clinical Outcomes and Influence of Extraskeletal Metastases. Oncologist 2016, 21, 1418–1426. [Google Scholar] [CrossRef]
- Chu, G.C.-Y.; Chung, L.W.K.; Gururajan, M.; Hsieh, C.-L.; Josson, S.; Nandana, S.; Sung, S.-Y.; Wang, R.; Wu, J.B.; Zhau, H.E. Regulatory signaling network in the tumor microenvironment of prostate cancer bone and visceral organ metastases and the development of novel therapeutics. Asian J. Urol. 2019, 6, 65–81. [Google Scholar] [CrossRef]
- Som, A.; Tu, S.M.; Liu, J.; Wang, X.; Qiao, W.; Logothetis, C.; Corn, P.G. Response in bone turnover markers during therapy predicts overall survival in patients with metastatic prostate cancer: Analysis of three clinical trials. Br. J. Cancer 2012, 107, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Góis, B.F.A. The Effect of Bone Collagen Fragments on Breast and Prostate Cancer Cells Biomedical Engineering. Master’s Thesis, Universidade de Lisboa, Lisboa, Portugal, March 2017. [Google Scholar]
- Shupp, A.B.; Kolb, A.D.; Mukhopadhyay, D.; Bussard, K.M. Cancer metastases to bone: Concepts, mechanisms, and interactions with bone osteoblasts. Cancers 2018, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- Gómez Rivas, J.; Carrion, D.M.; Alvarez-Maestro, M.; Cathelineau, X.; Sanchez-Salas, R.; di Lorenzo, G.; di Maio, M.; Paul, A.; Martinez-Piñeiro, L.; Sartor, O.; et al. Bone-targeted therapy in castration-resistant prostate cancer: Where do we stand? Minerva Urol. Nefrol. 2019, 71, 445–456. [Google Scholar] [CrossRef] [PubMed]
- D’Oronzo, S.; Coleman, R.; Brown, J.; Silvestris, F. Metastatic bone disease: Pathogenesis and therapeutic options: Up-date on bone metastasis management. J. Bone Oncol. 2019, 15, 100205. [Google Scholar] [CrossRef]
- Goodman, O.B.; Flaig, T.W.; Molina, A.; Mulders, P.F.A.; Fizazi, K.; Suttmann, H.; Li, J.; Kheoh, T.; de Bono, J.S.; Scher, H.I. Exploratory analysis of the visceral disease subgroup in a phase III study of abiraterone acetate in metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2014, 17, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, T.; Hongo, H.; Mizuno, R.; Oya, M. Risk stratification of castration-resistant prostate cancer patients treated with cabazitaxel. Mol. Clin. Oncol. 2018, 9, 683–688. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Overall Cohort | BOM | BESM | p-Value (BOM vs. BESM) |
|---|---|---|---|---|
| Number of patients, n (%) | 1901 (100) | 1559 (82.0) | 342 (18.0) | - |
| Demographic and clinicopathological characteristics | ||||
| Age, years | ||||
| Median P25–P75 Range | 74 65–76 35–96 | 74 65–76 35–96 | 66 64–75 45–86 | <0.001 |
| Age (years), n (%) | ||||
| ≤50 >50 to ≤65 >65 to ≤75 >75 | 14 (0.7) 615 (32.4) 726 (38.2) 546 (28.7) | 10 (0.6) 481 (30.9) 605 (38.8) 463 (29.7) | 4 (1.2) 134 (39.2) 121 (35.4) 83 (24.3) | 0.013 |
| ECOG performance status, n (%) | ||||
| ≤1 2 | 1768 (93.0) 133 (7.0) | 1448 (92.9) 111 (7.1) | 320 (93.6) 22 (6.4) | 0.652 |
| Body mass index | ||||
| Median IQR Missing, n (%) | 27.0 24.5–30.0 47 (2.5) | 26.9 24.3–29.8 39 (2.5) | 27.7 25.3–30.5 8 (2.3) | 0.003 |
| T stage at disease diagnosis, n (%) | ||||
| T1-T2 T3 T4 Missing | 725 (44.8) 693 (42.8) 200 (12.4) 283 (14.9) | 604 (45.8) 559 (42.4) 156 (11.8) 240 (15.4) | 121 (40.5) 134 (44.8) 44 (14.7) 43 (12.6) | 0.172 |
| N stage at disease diagnosis, n (%) | ||||
| N0 N1 | 1648 (86.7) 253 (13.3) | 1559 (100) 0 | 89 (26.0) 253 (74.0) | <0.001 |
| M stage at disease diagnosis, n (%) | ||||
| M0 M1 Missing | 832 (55.7) 662 (44.3) 407 (21.4) | 685 (55.9) 541 (44.1) 333 (21.4) | 147 (54.9) 121 (45.1) 74 (21.6) | 0.760 |
| Gleason score, n (%) | ||||
| ≤6 7 ≥8 | 355 (18.7) 744 (39.1) 802 (42.2) | 297 (19.0) 614 (39.4) 648 (41.6) | 58 (17.0) 130 (38.0) 154 (45.0) | 0.452 |
| PSA at disease diagnosis, ng/mL | ||||
| Median IQR | 60 19–213 | 57 18–207 | 78 23–247 | 0.021 |
| PSA at disease diagnosis, n (%) | ||||
| <10 ng/mL ≥10 ng/mL | 290 (15.3) 1611 (84.7) | 251 (16.1) 1308 (83.9) | 39 (11.4) 303 (88.6) | 0.029 |
| Time from cancer diagnosis to bone metastases, months | ||||
| Median IQR | 24.5 1.8–68.6 | 23.9 2.0–69.3 | 27.2 1.2–66.4 | 0.784 |
| Type of bone metastases, n (%) | ||||
| Lytic Blastic Mixed Unknown/Not seen | 71 (4.8) 1138 (76.5) 278 (18.7) 414 (21.8) | 57 (4.6) 961 (77.6) 221 (17.8) 320 (20.5) | 14 (5.6) 177 (71.4) 57 (23.0) 94 (27.5) | 0.109 |
| Number of bone metastases, n (%) | ||||
| ≤2 >2 to ≤4 >4 | 1255 (66.0) 329 (17.3) 317 (16.7) | 1021 (65.5) 271 (17.4) 267 (17.1) | 234 (68.4) 58 (17.0) 50 (14.6) | 0.484 |
| Type of visceral metastases, n (%) 1 | ||||
| Liver Lung Other sites | - | - | 36 (10.5) 58 (17.0) 294 (86.0) | - |
| TPrevious SRE, n (%) | ||||
| Yes No | 463 (24.4) 1438 (75.6) | 388 (24.9) 1171 (75.1) | 75 (21.9) 267 (78.1) | 0.248 |
| Treatment characteristics | ||||
| BTA type, n (%) | ||||
| Zoledronic acid Denosumab No BTA | 943 (49.6) 945 (49.7) 13 (0.7) | 764 (49.0) 783 (50.2) 12 (0.8) | 181 (52.9) 160 (46.8) 1 (0.3) | 0.291 |
| Previous bisphosphonate treatment, n (%) | ||||
| Yes No | 57 (3.0) 1844 (97.0) | 48 (3.1) 1511 (96.9) | 9 (2.6) 333 (97.4) | 0.660 |
| Castration type, n (%) | ||||
| Castration type, n (%) Chemical Surgical Both | 1256 (66.1) 92 (4.8) 553 (29.1) | 995 (63.8) 85 (5.5) 479 (30.7) | 261 (76.3) 7 (2.1) 74 (21.6) | <0.001 |
| Currently receiving CT, n (%) | ||||
| Yes No | 264 (13.9) 1637 (86.1) | 173 (11.1) 1386 (88.9) | 91 (26.6) 251 (73.4) | <0.001 |
| Previous CT, n (%) | ||||
| Yes No | 446 (23.5) 1455 (76.5) | 310 (19.9) 1249 (80.1) | 136 (39.8) 206 (60.2) | <0.001 |
| Previous RT, n (%) | ||||
| Yes No | 604 (31.8) 1297 (68.2) | 470 (30.2) 1089 (69.8) | 134 (39.2) 208 (60.8) | 0.001 |
| Previous prostatectomy, n (%) | ||||
| Yes No | 313 (16.5) 1588 (83.5) | 247 (15.8) 1312 (84.2) | 66 (19.3) 276 (80.7) | 0.119 |
| Previous antineoplastic surgery (any), n (%) | ||||
| Yes No | 832 (43.8) 1069 (56.2) | 655 (42.0) 904 (58.0) | 177 (51.8) 165 (48.3) | 0.001 |
| Characteristic | BOM | BESM | p-Value |
|---|---|---|---|
| Development of any on-study SRE, n (%) | |||
| Yes No | 583 (37.4) 976 (62.6) | 144 (42.1) 198 (57.9) | 0.105 |
| Development of symptomatic on-study SREs, n (%) | |||
| Yes No | 417 (26.8) 1142 (73.3) | 113 (33.0) 229 (67.0) | 0.019 |
| Number of SREs | |||
| Median (IQR) Min.–max. | 0 0–1 | 0 0–1 | 0.099 |
| Number of SREs, n (%) | |||
| 0 1 2 ≥3 | 976 (62.6) 383 (24.6) 127 (8.1) 73 (4.7) | 198 (57.9) 91 (26.6) 38 (11.1) 15 (4.4) | 0.224 |
| Pts with radiotherapy to bone as first SRE, n (%) 1 | |||
| Yes No | 309 (53.0) 274 (47.0) | 78 (54.2) 66 (45.8) | 0.802 |
| Pts with pathological fracture as first SRE, n (%) 1 | |||
| Yes No | 233 (40.0) 350 (60.0) | 55 (38.2) 89 (61.8) | 0.697 |
| Characteristic | Risk for SRE Development | ||
|---|---|---|---|
| HR | 95% CI | p-Value | |
| Univariate analysis | |||
| Metastatic compartment | |||
| Bone-only Bone + extraskeletal metastases | Reference 1.25 | Reference 1.04–1.51 | 0.015 |
| Age (years) | |||
| ≤50 >50 to ≤65 >65 to ≤75 >75 | Reference 0.47 0.38 0.43 | Reference 0.26–0.85 0.21–0.70 0.23–0.79 | 0.014 0.002 0.006 |
| ECOG performance status | |||
| ≤1 2 | Reference 1.36 | Reference 1.01–1.82 | 0.043 |
| Gleason score | |||
| ≤6 7 ≥8 | Reference 0.97 1.19 | Reference 0.79–1.20 0.98–1.46 | 0.795 0.977 |
| PSA at disease diagnosis, ng/mL | |||
| <10 ≥10 | Reference 1.23 | Reference 1.02–1.50 | 0.035 |
| Type of bone metastases | |||
| Lytic Blastic Mixed | Reference 0.92 0.93 | Reference 0.63–1.33 0.62–1.41 | 0.648 0.738 |
| Number of bone metastases | |||
| ≤2 >2 | Reference 1.12 | Reference 0.96–1.30 | 0.162 |
| Previous SREs | |||
| No Yes | Reference 1.45 | Reference 1.23–1.70 | <0.001 |
| Castration type | |||
| Chemical Surgical Both | Reference 0.49 0.78 | Reference 0.33–0.75 0.66–0.93 | 0.001 0.004 |
| Currently receiving chemotherapy | |||
| No Yes | Reference 1.40 | Reference 1.15–1.70 | 0.001 |
| Previous chemotherapy | |||
| No Yes | Reference 1.29 | Reference 1.09–1.52 | 0.003 |
| Previous radiotherapy | |||
| No Yes | Reference 1.26 | Reference 1.09–1.47 | 0.002 |
| Previous anti-neoplastic surgery (any) | |||
| No Yes | Reference 0.96 | Reference 0.83–1.11 | 0.600 |
| Multivariate model 1; n = 1901 | |||
| Covariates: Age, ECOG PS, Gleason score, PSA at diagnosis, number of bone lesions, previous SREs, castration type, and previous surgery and radiotherapy. | |||
| Metastatic compartment | |||
| Bone-only Bone + extraskeletal metastases | Reference 1.21 | Reference 1.01–1.46 | 0.043 |
| Andersen-Gill model for multiple failure-time data | |||
| Unadjusted HR 1.28; 95% CI 1.04–1.58; p = 0.018; Adjusted HR 1.23; 95% CI 1.00–1.52; p = 0.048 | |||
| Characteristic | Risk for SSE Development | ||
|---|---|---|---|
| HR | 95% CI | p-Value | |
| Univariate analysis | |||
| Metastatic compartment | |||
| Bone-only Bone + extra-bone metastases | Reference 1.40 | Reference 1.14–1.72 | 0.002 |
| Age (years) | |||
| ≤50 >50 to ≤65 >65 to ≤75 >75 | Reference 0.53 0.38 0.34 | Reference 0.27–1.03 0.19–0.73 0.17–0.68 | 0.063 0.004 0.002 |
| ECOG performance status | |||
| ≤1 2 | Reference 1.39 | Reference 0.99–1.95 | 0.055 |
| Gleason score | |||
| ≤6 7 ≥8 | Reference 0.92 1.27 | Reference 0.72–1.19 1.01–1.62 | 1.188 1.619 |
| PSA at disease diagnosis, ng/mL | |||
| <10 ≥10 | Reference 1.29 | Reference 1.02–1.63 | 0.031 |
| Type of bone metastases | |||
| Lytic Blastic Mixed | Reference 0.99 0.90 | Reference 0.63–1.55 0.55–1.48 | 0.952 0.672 |
| Number of bone metastases | |||
| ≤2 >2 | Reference 1.07 | Reference 0.89–1.28 | 0.454 |
| Previous SREs | |||
| No Yes | Reference 1.63 | Reference 1.35–1.96 | <0.001 |
| Castration type | |||
| Chemical Surgical Both | Reference 0.37 0.67 | Reference 0.21–0.64 0.54–0.82 | <0.001 <0.001 |
| Currently receiving chemotherapy | |||
| No Yes | Reference 1.47 | Reference 1.17–1.84 | 0.001 |
| Previous chemotherapy | |||
| No Yes | Reference 1.32 | Reference 1.09–1.60 | 0.004 |
| Previous radiotherapy | |||
| No Yes | Reference 1.48 | Reference 1.24–1.76 | <0.001 |
| Previous antineoplastic surgery (any) | |||
| No Yes | Reference 0.93 | Reference 0.79–1.11 | 0.439 |
| Multivariate model; n = 1901 | |||
| Covariates: Age, ECOG PS, Gleason score, PSA at diagnosis, number of bone lesions, previous SREs, castration type, and previous surgery and radiotherapy | |||
| Metastatic compartment | |||
| Bone-only Bone + extraskeletal metastases | Reference 1.30 | Reference 1.06–1.61 | 0.014 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobo-Martins, S.; Ferreira, A.R.; Mansinho, A.; Casimiro, S.; Leitzel, K.; Ali, S.; Lipton, A.; Costa, L. Impact of Extraskeletal Metastases on Skeletal-Related Events in Metastatic Castration-Resistant Prostate Cancer with Bone Metastases. Cancers 2020, 12, 2034. https://doi.org/10.3390/cancers12082034
Lobo-Martins S, Ferreira AR, Mansinho A, Casimiro S, Leitzel K, Ali S, Lipton A, Costa L. Impact of Extraskeletal Metastases on Skeletal-Related Events in Metastatic Castration-Resistant Prostate Cancer with Bone Metastases. Cancers. 2020; 12(8):2034. https://doi.org/10.3390/cancers12082034
Chicago/Turabian StyleLobo-Martins, Soraia, Arlindo R. Ferreira, André Mansinho, Sandra Casimiro, Kim Leitzel, Suhail Ali, Allan Lipton, and Luís Costa. 2020. "Impact of Extraskeletal Metastases on Skeletal-Related Events in Metastatic Castration-Resistant Prostate Cancer with Bone Metastases" Cancers 12, no. 8: 2034. https://doi.org/10.3390/cancers12082034
APA StyleLobo-Martins, S., Ferreira, A. R., Mansinho, A., Casimiro, S., Leitzel, K., Ali, S., Lipton, A., & Costa, L. (2020). Impact of Extraskeletal Metastases on Skeletal-Related Events in Metastatic Castration-Resistant Prostate Cancer with Bone Metastases. Cancers, 12(8), 2034. https://doi.org/10.3390/cancers12082034






