Colorectal Cancer and Bone Tissue: Fantastic Relations and Where to Find Them
Abstract
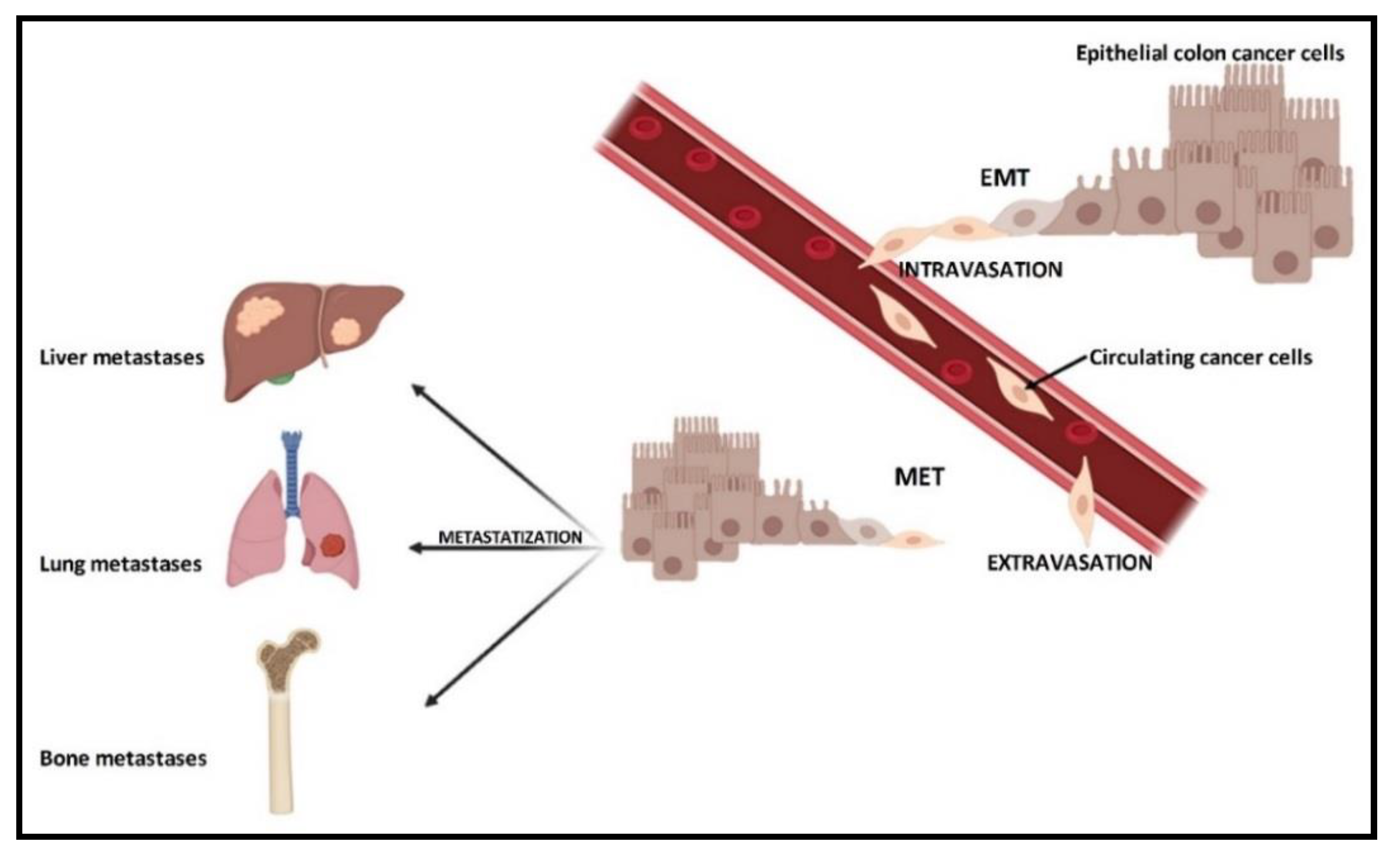
1. Introduction
2. CRC and Bone Metastases
3. CRC and Bone Marrow
4. Bone Morphogenetic Proteins (BMPs)
4.1. BMP9
4.2. BMP5
5. Osteoprotegerin
6. Osteopontin
7. Other Proteins
7.1. Bone Sialoprotein
7.2. Tartrate-Resistant Acid Phosphatase
7.3. Runt-Related Transcription Factor 2
7.4. Trasforming Growth Factor β1
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ahmadi, A.; Hashemi Nazari, S.S.; Mobasheri, M. Does ethnicity affect survival following colorectal cancer? A prospective, cohort study using Iranian cancer registry. Med. J. Islamic Repub. Iran 2014, 28, 83. [Google Scholar]
- Tillmans, L.S.; Vierkant, R.A.; Wang, A.H.; Jewel Samadder, N.; Lynch, C.F.; Anderson, K.E.; French, A.J.; Haile, R.W.; Harnack, L.J.; Potter, J.D.; et al. Associations between cigarette smoking, hormone therapy, and folate intake with incident colorectal cancer by TP53 protein expression level in a population-based cohort of older women. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2014, 23, 350–355. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hardy, R.G.; Meltzer, S.J.; Jankowski, J.A. ABC of colorectal cancer. Molecular basis for risk factors. BMJ 2000, 321, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Kinzler, K.W.; Vogelstein, B. Lessons from hereditary colorectal cancer. Cell 1996, 87, 159–170. [Google Scholar] [CrossRef]
- Brenner, B.M.; Rosenberg, D. High-throughput SNP/CGH approaches for the analysis of genomic instability in colorectal cancer. Mutat. Res. 2010, 693, 46–52. [Google Scholar] [CrossRef]
- Fidler, I.J. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef]
- Boyer, B.; Thiery, J.P. Epithelium-mesenchyme interconversion as example of epithelial plasticity. Acta Pathol. Microbiol. Immunol. Scand. 1993, 101, 257–268. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Okuda, R.; Sekine, K.; Hisamatsu, D.; Ueno, Y.; Takebe, T.; Zheng, Y.W.; Taniguchi, H. Tropism of cancer stem cells to a specific distant organ. In Vivo 2014, 28, 361–365. [Google Scholar]
- Li, F.; Tiede, B.; Massague, J.; Kang, Y. Beyond tumorigenesis: Cancer stem cells in metastasis. Cell Res. 2007, 17, 3–14. [Google Scholar] [CrossRef]
- Behbod, F.; Rosen, J.M. Will cancer stem cells provide new therapeutic targets? Carcinogenesis 2005, 26, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Itatani, Y.; Kawada, K.; Inamoto, S.; Yamamoto, T.; Ogawa, R.; Taketo, M.M.; Sakai, Y. The Role of Chemokines in Promoting Colorectal Cancer Invasion/Metastasis. Int. J. Mol. Sci. 2016, 17, 643. [Google Scholar] [CrossRef] [PubMed]
- Sundermeyer, M.L.; Meropol, N.J.; Rogatko, A.; Wang, H.; Cohen, S.J. Changing patterns of bone and brain metastases in patients with colorectal cancer. Clin. Colorectal Cancer 2005, 5, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Khattak, M.A.; Martin, H.L.; Beeke, C.; Price, T.; Carruthers, S.; Kim, S.; Padbury, R.; Karapetis, C.S. Survival differences in patients with metastatic colorectal cancer and with single site metastatic disease at initial presentation: Results from South Australian clinical registry for advanced colorectal cancer. Clin. Colorectal Cancer 2012, 11, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E. Skeletal complications of malignancy. Cancer 1997, 80, 1588–1594. [Google Scholar] [CrossRef]
- Kawamura, H.; Yamaguchi, T.; Yano, Y.; Hozumi, T.; Takaki, Y.; Matsumoto, H.; Nakano, D.; Takahashi, K. Characteristics and Prognostic Factors of Bone Metastasis in Patients With Colorectal Cancer. Dis. Colon Rectum 2018, 61, 673–678. [Google Scholar] [CrossRef]
- Babu, M.C.S.; Garg, S.; Lakshmaiah, K.C.; Babu, K.G.; Kumar, R.V.; Loknatha, D.; Abraham, L.J.; Rajeev, L.K.; Lokesh, K.N.; Rudresha, A.H.; et al. Colorectal cancer presenting as bone metastasis. J. Cancer Res. Ther. 2017, 13, 80–83. [Google Scholar] [CrossRef]
- Santini, D.; Tampellini, M.; Vincenzi, B.; Ibrahim, T.; Ortega, C.; Virzi, V.; Silvestris, N.; Berardi, R.; Masini, C.; Calipari, N.; et al. Natural history of bone metastasis in colorectal cancer: Final results of a large Italian bone metastases study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 2072–2077. [Google Scholar] [CrossRef]
- Sun, C.; Deng, Y.; Zhou, H.; Hu, Z.Q. Risk factors for the development of metachronous bone metastasis in colorectal cancer patients after curative resection. Int. J. Surg. 2015, 21, 145–149. [Google Scholar] [CrossRef]
- Li, A.; Kasmann, L.; Rades, D.; Fu, C. A Scoring System to Predict the Development of Bone Metastasis After Radical Resection of Colorectal Cancer. Anticancer Res. 2017, 37, 5169–5172. [Google Scholar] [CrossRef]
- Baek, S.J.; Hur, H.; Min, B.S.; Baik, S.H.; Lee, K.Y.; Kim, N.K. The Characteristics of Bone Metastasis in Patients with Colorectal Cancer: A Long-Term Report from a Single Institution. World J. Surg. 2016, 40, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhu, Z.H.; Guo, W.J.; Zhang, N.; Cai, Y.Y.; Ying, J.S.; Xiao, M. Retrospective study of predictors of bone metastasis in colorectal cancer patients. J. Bone Oncol. 2017, 9, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Kanthan, R.; Loewy, J.; Kanthan, S.C. Skeletal metastases in colorectal carcinomas: A Saskatchewan profile. Dis. Colon Rectum 1999, 42, 1592–1597. [Google Scholar] [CrossRef] [PubMed]
- Hinganu, D.; Eva, I.; Stan, C.I.; Hinganu, M.V. Morphological aspects of the rectal neovascularization in colorectal cancer—Anatomical-surgical and imaging implications. Rom. J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2016, 57, 161–165. [Google Scholar]
- Salovaara, R.; Roth, S.; Loukola, A.; Launonen, V.; Sistonen, P.; Avizienyte, E.; Kristo, P.; Jarvinen, H.; Souchelnytskyi, S.; Sarlomo-Rikala, M.; et al. Frequent loss of SMAD4/DPC4 protein in colorectal cancers. Gut 2002, 51, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Itatani, Y.; Kawada, K.; Fujishita, T.; Kakizaki, F.; Hirai, H.; Matsumoto, T.; Iwamoto, M.; Inamoto, S.; Hatano, E.; Hasegawa, S.; et al. Loss of SMAD4 from colorectal cancer cells promotes CCL15 expression to recruit CCR1+ myeloid cells and facilitate liver metastasis. Gastroenterology 2013, 145, 1064–1075. [Google Scholar] [CrossRef]
- Losi, L.; Bouzourene, H.; Benhattar, J. Loss of Smad4 expression predicts liver metastasis in human colorectal cancer. Oncol. Rep. 2007, 17, 1095–1099. [Google Scholar] [CrossRef][Green Version]
- Kiyasu, Y.; Kawada, K.; Hirai, H.; Ogawa, R.; Hanada, K.; Masui, H.; Nishikawa, G.; Yamamoto, T.; Mizuno, R.; Itatani, Y.; et al. Disruption of CCR1-mediated myeloid cell accumulation suppresses colorectal cancer progression in mice. Cancer Lett. 2020, 487, 53–62. [Google Scholar] [CrossRef]
- Assi, R.; Mukherji, D.; Haydar, A.; Saroufim, M.; Temraz, S.; Shamseddine, A. Metastatic colorectal cancer presenting with bone marrow metastasis: A case series and review of literature. J. Gastrointest. Oncol. 2016, 7, 284–297. [Google Scholar] [CrossRef]
- Anner, R.M.; Drewinko, B. Frequency and significance of bone marrow involvement by metastatic solid tumors. Cancer 1977, 39, 1337–1344. [Google Scholar] [CrossRef]
- Chuwa, H.; Kassam, N.M.; Wambura, C.; Sherman, O.A.; Surani, S. Disseminated Carcinomatosis of Bone Marrow in an African Man with Metastatic Descending Colon Carcinoma. Cureus 2020, 12, e7593. [Google Scholar] [CrossRef] [PubMed]
- Viehl, C.T.; Weixler, B.; Guller, U.; Dell-Kuster, S.; Rosenthal, R.; Ramser, M.; Banz, V.; Langer, I.; Terracciano, L.; Sauter, G.; et al. Presence of bone marrow micro-metastases in stage I-III colon cancer patients is associated with worse disease-free and overall survival. Cancer Med. 2017, 6, 918–927. [Google Scholar] [CrossRef]
- Torsvik, A.; Bjerkvig, R. Mesenchymal stem cell signaling in cancer progression. Cancer Treat. Rev. 2013, 39, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, G.; Kawada, K.; Nakagawa, J.; Toda, K.; Ogawa, R.; Inamoto, S.; Mizuno, R.; Itatani, Y.; Sakai, Y. Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression via CCR5. Cell Death Dis. 2019, 10, 264. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; Sasaki, S.; Mukaida, N.; Baba, T. Blockade of the chemokine receptor, CCR5, reduces the growth of orthotopically injected colon cancer cells via limiting cancer-associated fibroblast accumulation. Oncotarget 2016, 7, 48335–48345. [Google Scholar] [CrossRef] [PubMed]
- Halama, N.; Zoernig, I.; Berthel, A.; Kahlert, C.; Klupp, F.; Suarez-Carmona, M.; Suetterlin, T.; Brand, K.; Krauss, J.; Lasitschka, F.; et al. Tumoral Immune Cell Exploitation in Colorectal Cancer Metastases Can Be Targeted Effectively by Anti-CCR5 Therapy in Cancer Patients. Cancer Cell 2016, 29, 587–601. [Google Scholar] [CrossRef]
- Wu, M.Y.; Li, C.J.; Yiang, G.T.; Cheng, Y.L.; Tsai, A.P.; Hou, Y.T.; Ho, Y.C.; Hou, M.F.; Chu, P.Y. Molecular Regulation of Bone Metastasis Pathogenesis. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 46, 1423–1438. [Google Scholar] [CrossRef]
- Gong, Z.C.; Qian, J.; Zhang, Y.N.; Wang, W.; Kang, X.; Xu, W.; Wu, J.; Zheng, W. Colorectal cancer cells promote osteoclastogenesis and bone destruction through regulating EGF/ERK/CCL3 pathway. Biosci. Rep. 2020. [Google Scholar] [CrossRef]
- Carreira, A.C.; Alves, G.G.; Zambuzzi, W.F.; Sogayar, M.C.; Granjeiro, J.M. Bone Morphogenetic Proteins: Structure, biological function and therapeutic applications. Arch. Biochem. Biophys. 2014, 561, 64–73. [Google Scholar] [CrossRef]
- Lamplot, J.D.; Qin, J.; Nan, G.; Wang, J.; Liu, X.; Yin, L.; Tomal, J.; Li, R.; Shui, W.; Zhang, H.; et al. BMP9 signaling in stem cell differentiation and osteogenesis. Am. J. Stem Cells 2013, 2, 1–21. [Google Scholar]
- Noh, B.J.; Kim, Y.W.; Park, Y.K. A Rare Colon Cancer with Ossification: Pathogenetic Analysis of Bone Formation. Ann. Clin. Lab. Sci. 2016, 46, 428–432. [Google Scholar] [PubMed]
- Sanchez-Duffhues, G.; Hiepen, C.; Knaus, P.; Ten Dijke, P. Bone morphogenetic protein signaling in bone homeostasis. Bone 2015, 80, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Epstein, N.E. Basic science and spine literature document bone morphogenetic protein increases cancer risk. Surg. Neurol. Int. 2014, 5, S552–S560. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Liu, Y.Z.; Yin, L.J.; Chen, L.; Huang, J.; Liu, Y.; Zhang, R.X.; Zhou, L.Y.; Yang, Q.J.; Luo, J.Y.; et al. BMP9 and COX-2 form an important regulatory loop in BMP9-induced osteogenic differentiation of mesenchymal stem cells. Bone 2013, 57, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Song, T.; Wang, W.; Wang, J.; He, J.; Wu, N.; Tang, M.; He, B.; Luo, J. P38 and ERK1/2 MAPKs act in opposition to regulate BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. PLoS ONE 2012, 7, e43383. [Google Scholar] [CrossRef]
- Cuadrado, A.; Nebreda, A.R. Mechanisms and functions of p38 MAPK signalling. Biochem. J. 2010, 429, 403–417. [Google Scholar] [CrossRef]
- Luo, X.; Chen, J.; Song, W.X.; Tang, N.; Luo, J.; Deng, Z.L.; Sharff, K.A.; He, G.; Bi, Y.; He, B.C.; et al. Osteogenic BMPs promote tumor growth of human osteosarcomas that harbor differentiation defects. Lab. Investig. J. Tech. Methods Pathol. 2008, 88, 1264–1277. [Google Scholar] [CrossRef]
- Herrera, B.; van Dinther, M.; Ten Dijke, P.; Inman, G.J. Autocrine bone morphogenetic protein-9 signals through activin receptor-like kinase-2/Smad1/Smad4 to promote ovarian cancer cell proliferation. Cancer Res. 2009, 69, 9254–9262. [Google Scholar] [CrossRef]
- Duan, L.; Ye, L.; Wu, R.; Wang, H.; Li, X.; Li, H.; Yuan, S.; Zha, H.; Sun, H.; Zhang, Y.; et al. Inactivation of the phosphatidylinositol 3-kinase/Akt pathway is involved in BMP9-mediated tumor-suppressive effects in gastric cancer cells. J. Cell. Biochem. 2015, 116, 1080–1089. [Google Scholar] [CrossRef]
- Wang, K.; Feng, H.; Ren, W.; Sun, X.; Luo, J.; Tang, M.; Zhou, L.; Weng, Y.; He, T.C.; Zhang, Y. BMP9 inhibits the proliferation and invasiveness of breast cancer cells MDA-MB-231. J. Cancer Res. Clin. Oncol. 2011, 137, 1687–1696. [Google Scholar] [CrossRef]
- Yuan, S.X.; Wang, D.X.; Wu, Q.X.; Ren, C.M.; Li, Y.; Chen, Q.Z.; Zeng, Y.H.; Shao, Y.; Yang, J.Q.; Bai, Y.; et al. BMP9/p38 MAPK is essential for the antiproliferative effect of resveratrol on human colon cancer. Oncol. Rep. 2016, 35, 939–947. [Google Scholar] [CrossRef]
- Li, F.S.; Huang, J.; Cui, M.Z.; Zeng, J.R.; Li, P.P.; Li, L.; Deng, Y.; Hu, Y.; He, B.C.; Shu, D.Z. BMP9 mediates the anticancer activity of evodiamine through HIF1alpha/p53 in human colon cancer cells. Oncol. Rep. 2020, 43, 415–426. [Google Scholar] [CrossRef]
- Chen, E.; Yang, F.; He, H.; Li, Q.; Zhang, W.; Xing, J.; Zhu, Z.; Jiang, J.; Wang, H.; Zhao, X.; et al. Alteration of tumor suppressor BMP5 in sporadic colorectal cancer: A genomic and transcriptomic profiling based study. Mol. Cancer 2018, 17, 176. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, M.; Belguise, K.; Yu, Z.; Wang, X.; Landesman-Bollag, E.; Seldin, D.C.; Chalbos, D.; Barille-Nion, S.; Jezequel, P.; Seldin, M.L.; et al. Epithelial-to-mesenchymal transition induced by TGF-beta1 is mediated by Blimp-1-dependent repression of BMP-5. Cancer Res. 2012, 72, 6268–6278. [Google Scholar] [CrossRef] [PubMed]
- Sarver, A.L.; French, A.J.; Borralho, P.M.; Thayanithy, V.; Oberg, A.L.; Silverstein, K.A.; Morlan, B.W.; Riska, S.M.; Boardman, L.A.; Cunningham, J.M.; et al. Human colon cancer profiles show differential microRNA expression depending on mismatch repair status and are characteristic of undifferentiated proliferative states. BMC Cancer 2009, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Li, Q.; Wang, H.; Zhang, P.; Zhao, X.; Yang, F.; Yang, J. MiR-32 promotes tumorigenesis of colorectal cancer by targeting BMP5. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 106, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Yasuda, H.; Shima, N.; Nakagawa, N.; Yamaguchi, K.; Kinosaki, M.; Mochizuki, S.; Tomoyasu, A.; Yano, K.; Goto, M.; Murakami, A.; et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA 1998, 95, 3597–3602. [Google Scholar] [CrossRef]
- Pettersen, I.; Bakkelund, W.; Smedsrod, B.; Sveinbjornsson, B. Osteoprotegerin is expressed in colon carcinoma cells. Anticancer Res. 2005, 25, 3809–3816. [Google Scholar]
- Emery, J.G.; McDonnell, P.; Burke, M.B.; Deen, K.C.; Lyn, S.; Silverman, C.; Dul, E.; Appelbaum, E.R.; Eichman, C.; DiPrinzio, R.; et al. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J. Biol. Chem. 1998, 273, 14363–14367. [Google Scholar] [CrossRef]
- Holen, I.; Shipman, C.M. Role of osteoprotegerin (OPG) in cancer. Clin. Sci. 2006, 110, 279–291. [Google Scholar] [CrossRef] [PubMed]
- De Toni, E.N.; Thieme, S.E.; Herbst, A.; Behrens, A.; Stieber, P.; Jung, A.; Blum, H.; Goke, B.; Kolligs, F.T. OPG is regulated by beta-catenin and mediates resistance to TRAIL-induced apoptosis in colon cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 4713–4718. [Google Scholar] [CrossRef] [PubMed]
- De Toni, E.N.; Nagel, D.; Philipp, A.B.; Herbst, A.; Thalhammer, I.; Mayerle, J.; Torok, H.P.; Brandl, L.; Kolligs, F.T. Correlation Between Baseline Osteoprotegerin Serum Levels and Prognosis of Advanced-Stage Colorectal Cancer Patients. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 45, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Birgisson, H.; Tsimogiannis, K.; Freyhult, E.; Kamali-Moghaddam, M. Plasma Protein Profiling Reveal Osteoprotegerin as a Marker of Prognostic Impact for Colorectal Cancer. Transl. Oncol. 2018, 11, 1034–1043. [Google Scholar] [CrossRef]
- Duiker, E.W.; Mom, C.H.; de Jong, S.; Willemse, P.H.; Gietema, J.A.; van der Zee, A.G.; de Vries, E.G. The clinical trail of TRAIL. Eur. J. Cancer 2006, 42, 2233–2240. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Ishikawa, T.; Iida, S.; Ishiguro, M.; Mogushi, K.; Mizushima, H.; Uetake, H.; Tanaka, H.; Sugihara, K. Clinical significance of osteoprotegerin expression in human colorectal cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 2444–2450. [Google Scholar] [CrossRef]
- Kim, H.S.; Yoon, G.; Do, S.I.; Kim, S.J.; Kim, Y.W. Down-regulation of osteoprotegerin expression as a novel biomarker for colorectal carcinoma. Oncotarget 2016, 7, 15187–15199. [Google Scholar] [CrossRef][Green Version]
- Sodek, J.; Ganss, B.; McKee, M.D. Osteopontin. Crit. Rev. Oral Biol. Med. Off. Publ. Am. Assoc. Oral Biol. 2000, 11, 279–303. [Google Scholar] [CrossRef]
- Denhardt, D.T.; Noda, M. Osteopontin expression and function: Role in bone remodeling. J. Cell. Biochem. 1998, 72, 92–102. [Google Scholar] [CrossRef]
- Likui, W.; Hong, W.; Shuwen, Z. Clinical significance of the upregulated osteopontin mRNA expression in human colorectal cancer. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2010, 14, 74–81. [Google Scholar] [CrossRef]
- Agrawal, D.; Chen, T.; Irby, R.; Quackenbush, J.; Chambers, A.F.; Szabo, M.; Cantor, A.; Coppola, D.; Yeatman, T.J. Osteopontin identified as colon cancer tumor progression marker. Comptes Rendus Biol. 2003, 326, 1041–1043. [Google Scholar] [CrossRef] [PubMed]
- Wai, P.Y.; Mi, Z.; Guo, H.; Sarraf-Yazdi, S.; Gao, C.; Wei, J.; Marroquin, C.E.; Clary, B.; Kuo, P.C. Osteopontin silencing by small interfering RNA suppresses in vitro and in vivo CT26 murine colon adenocarcinoma metastasis. Carcinogenesis 2005, 26, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Likui, W.; Hong, W.; Shuwen, Z.; Yuangang, Y.; Yan, W. The potential of osteopontin as a therapeutic target for human colorectal cancer. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2011, 15, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.L.; Lin, K.J.; Bai, A.P.; Wang, W.X.; Meng, X.K.; Su, X.L.; Hou, M.X.; Dong, P.D.; Zhang, J.J.; Wang, Z.Y.; et al. Osteopontin knockdown suppresses the growth and angiogenesis of colon cancer cells. World J. Gastroenterol. 2014, 20, 10440–10448. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhang, X.; Yang, Z.H.; Sun, X.W.; Li, S.N.; Zhong, L.; Cheng, X.; Wang, Y.; Ma, Y.R. The polymorphisms of osteopontin gene and plasma osteopontin protein levels with susceptibility to colorectal carcinoma. DNA Cell Biol. 2013, 32, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Darwish, R.K.; Saad, S.; Salama, M.; El-Baradie, T.S.; Mahmoud, H.G.; Elshiwy, Y. Association of Osteopontin Gene Polymorphisms with Colorectal Cancer. Cancer Investig. 2017, 35, 71–77. [Google Scholar] [CrossRef]
- Ng, L.; Wan, T.; Chow, A.; Iyer, D.; Man, J.; Chen, G.; Yau, T.C.; Lo, O.; Foo, C.C.; Poon, J.T.; et al. Osteopontin Overexpression Induced Tumor Progression and Chemoresistance to Oxaliplatin through Induction of Stem-Like Properties in Human Colorectal Cancer. Stem Cells Int. 2015, 2015, 247892. [Google Scholar] [CrossRef]
- Burada, F.; Nicoli, E.R.; Ciurea, M.E.; Uscatu, D.C.; Ioana, M.; Gheonea, D.I. Autophagy in colorectal cancer: An important switch from physiology to pathology. World J. Gastrointest. Oncol. 2015, 7, 271–284. [Google Scholar] [CrossRef]
- Huang, R.H.; Quan, Y.J.; Chen, J.H.; Wang, T.F.; Xu, M.; Ye, M.; Yuan, H.; Zhang, C.J.; Liu, X.J.; Min, Z.J. Osteopontin Promotes Cell Migration and Invasion, and Inhibits Apoptosis and Autophagy in Colorectal Cancer by activating the p38 MAPK Signaling Pathway. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 41, 1851–1864. [Google Scholar] [CrossRef]
- Wuttke, M.; Muller, S.; Nitsche, D.P.; Paulsson, M.; Hanisch, F.G.; Maurer, P. Structural characterization of human recombinant and bone-derived bone sialoprotein. Functional implications for cell attachment and hydroxyapatite binding. J. Biol. Chem. 2001, 276, 36839–36848. [Google Scholar] [CrossRef]
- Fedarko, N.S.; Jain, A.; Karadag, A.; Van Eman, M.R.; Fisher, L.W. Elevated serum bone sialoprotein and osteopontin in colon, breast, prostate, and lung cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2001, 7, 4060–4066. [Google Scholar]
- Minkin, C. Bone acid phosphatase: Tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcif. Tissue Int. 1982, 34, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Ek-Rylander, B.; Flores, M.; Wendel, M.; Heinegard, D.; Andersson, G. Dephosphorylation of osteopontin and bone sialoprotein by osteoclastic tartrate-resistant acid phosphatase. Modulation of osteoclast adhesion in vitro. J. Biol. Chem. 1994, 269, 14853–14856. [Google Scholar]
- Nagorsen, D.; Voigt, S.; Berg, E.; Stein, H.; Thiel, E.; Loddenkemper, C. Tumor-infiltrating macrophages and dendritic cells in human colorectal cancer: Relation to local regulatory T cells, systemic T-cell response against tumor-associated antigens and survival. J. Transl. Med. 2007, 5, 62. [Google Scholar] [CrossRef]
- How, J.; Brown, J.R.; Saylor, S.; Rimm, D.L. Macrophage expression of tartrate-resistant acid phosphatase as a prognostic indicator in colon cancer. Histochem. Cell Biol. 2014, 142, 195–204. [Google Scholar] [CrossRef]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef]
- Sase, T.; Suzuki, T.; Miura, K.; Shiiba, K.; Sato, I.; Nakamura, Y.; Takagi, K.; Onodera, Y.; Miki, Y.; Watanabe, M.; et al. Runt-related transcription factor 2 in human colon carcinoma: A potent prognostic factor associated with estrogen receptor. Int. J. Cancer 2012, 131, 2284–2293. [Google Scholar] [CrossRef] [PubMed]
- Edvardsson, K.; Strom, A.; Jonsson, P.; Gustafsson, J.A.; Williams, C. Estrogen receptor beta induces antiinflammatory and antitumorigenic networks in colon cancer cells. Mol. Endocrinol. 2011, 25, 969–979. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, X.; Lei, W.; Pang, L.; Wan, C.; Shi, Z.; Zhao, L.; Nagy, T.R.; Peng, X.; Hu, J.; et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat. Med. 2009, 15, 757–765. [Google Scholar] [CrossRef]
- Zhao, Y.; Xia, S.; Cao, C.; Du, X. Effect of TGF-beta1 on Apoptosis of Colon Cancer Cells Via the ERK Signaling Pathway. J. BUON Off. J. Balk. Union Oncol. 2019, 24, 449–455. [Google Scholar]
| Molecular Factor | Mechanism of Action in Bone Tissue | Mechanism of Action in CRC | References |
|---|---|---|---|
| BMP9 | Stimulation of the production of bone tissue | Antitumoral, pro-apoptotic | [26,27,28,29,30,31,32,33,34,35,36] |
| BMP5 | Stimulation of the production of bone tissue | Antitumoral, pro-apoptotic | [37,38,39,40] |
| OPG | Protection of bone tissue from the erosive action of osteoclasts | Oncogene/oncosuppressor | [41,42,43,44,45,46,47,48,49,50,51] |
| OPN | Involvement in bone remodeling/bone turnover | Promotion of tumorigenesis | [52,53,54,55,56,57,58,59,60,61,62,63] |
| BSP | Involvement in both bone formation and bone erosion | Protumoral biomarker | [64,65] |
| TRAP | Osteoclast maturation, bone erosion | Antitumoral biomarker | [66,67,68,69] |
| RUNX2 | Involvement in osteoblastogenesis | Protumoral, anti-apoptotic | [70,71,72] |
| TGFβ1 | Regulation of the proliferation and differentiation of osteoprogenitor cells | Antitumoral | [73,74] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gigante, I.; Tutino, V.; De Nunzio, V.; Notarnicola, M. Colorectal Cancer and Bone Tissue: Fantastic Relations and Where to Find Them. Cancers 2020, 12, 2029. https://doi.org/10.3390/cancers12082029
Gigante I, Tutino V, De Nunzio V, Notarnicola M. Colorectal Cancer and Bone Tissue: Fantastic Relations and Where to Find Them. Cancers. 2020; 12(8):2029. https://doi.org/10.3390/cancers12082029
Chicago/Turabian StyleGigante, Isabella, Valeria Tutino, Valentina De Nunzio, and Maria Notarnicola. 2020. "Colorectal Cancer and Bone Tissue: Fantastic Relations and Where to Find Them" Cancers 12, no. 8: 2029. https://doi.org/10.3390/cancers12082029
APA StyleGigante, I., Tutino, V., De Nunzio, V., & Notarnicola, M. (2020). Colorectal Cancer and Bone Tissue: Fantastic Relations and Where to Find Them. Cancers, 12(8), 2029. https://doi.org/10.3390/cancers12082029





