Non-Invasive Assessment of Hypoxia and Neovascularization with MRI for Identification of Aggressive Breast Cancer
Abstract
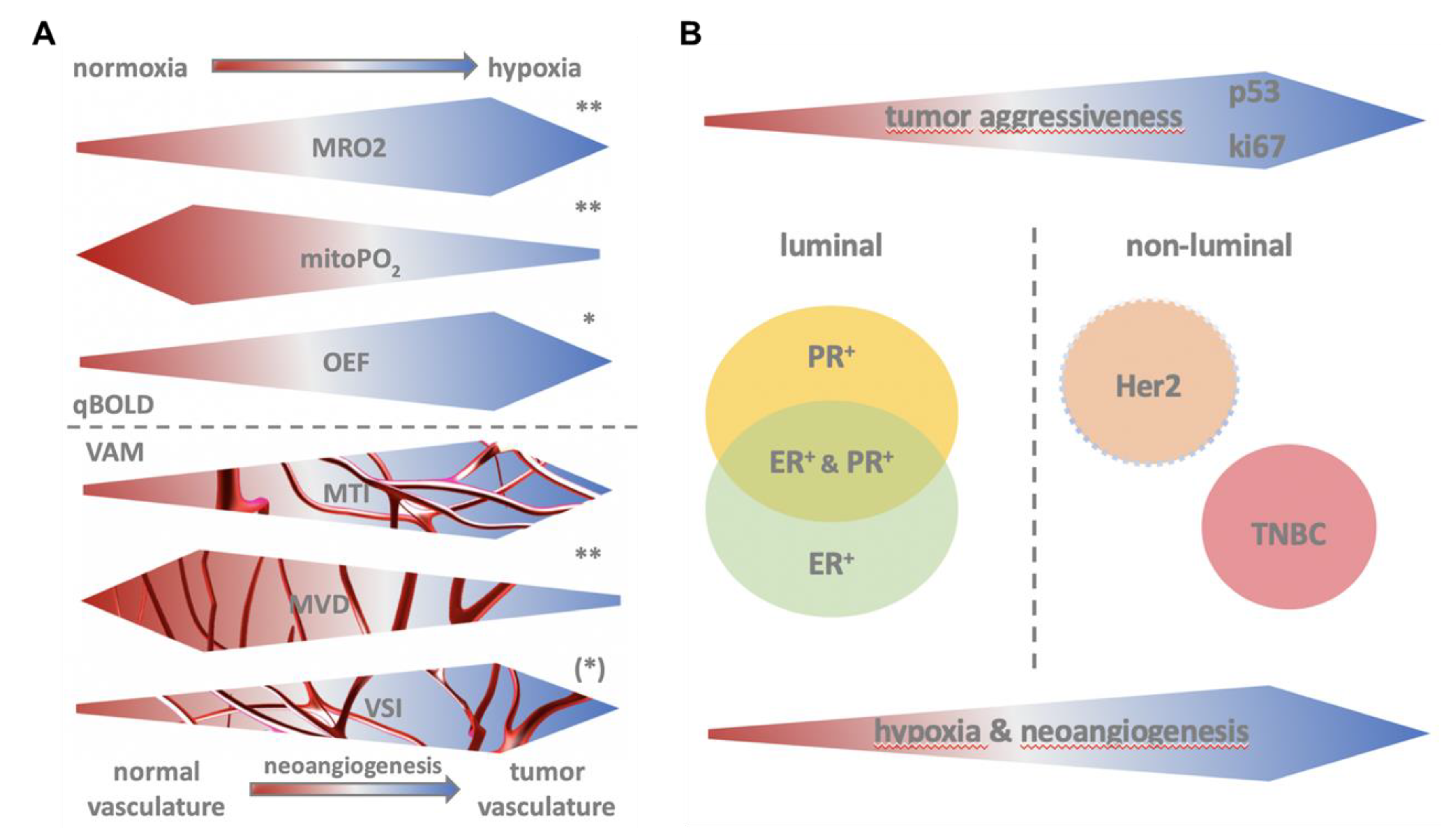
1. Introduction
2. Materials and Methods
2.1. Patients
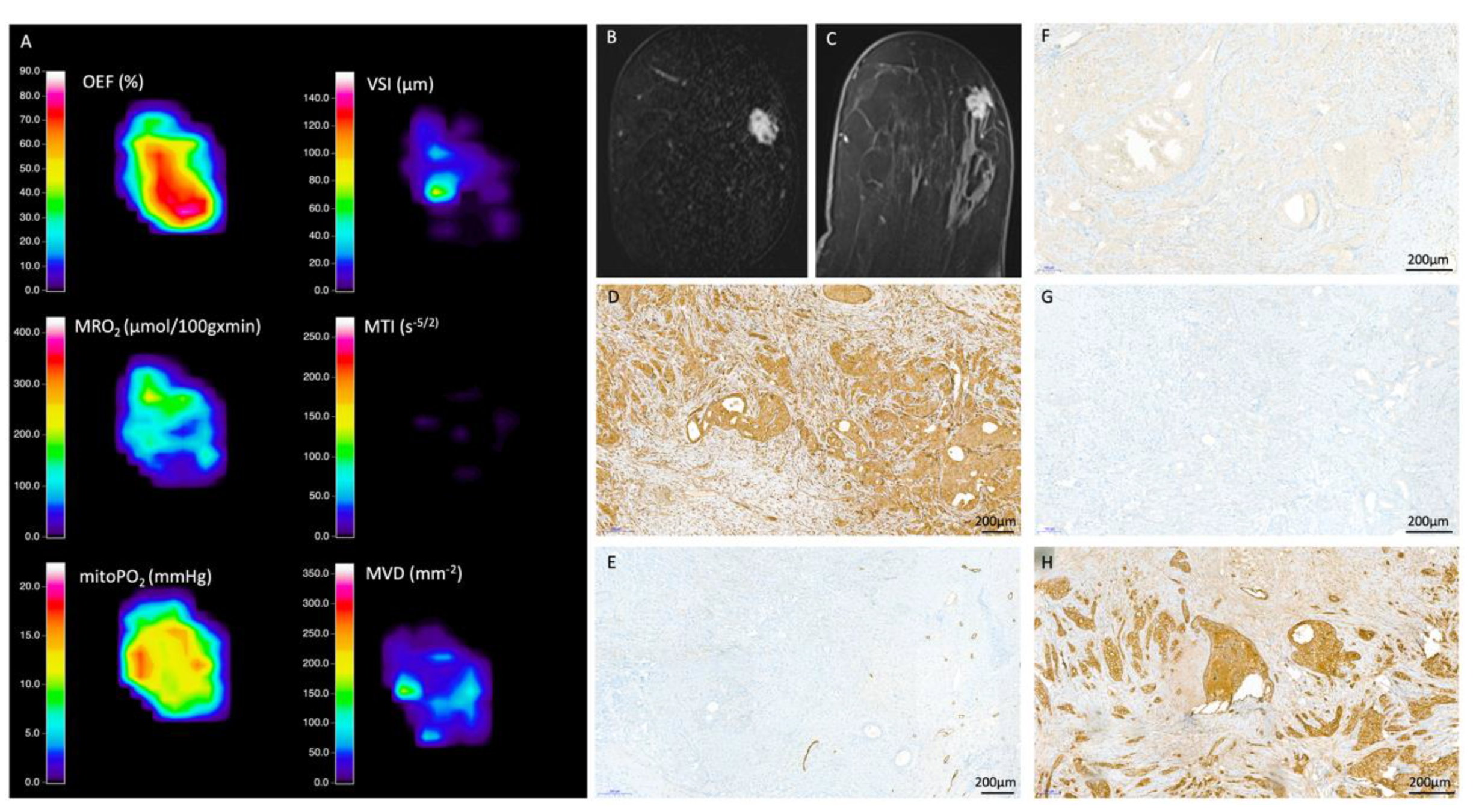
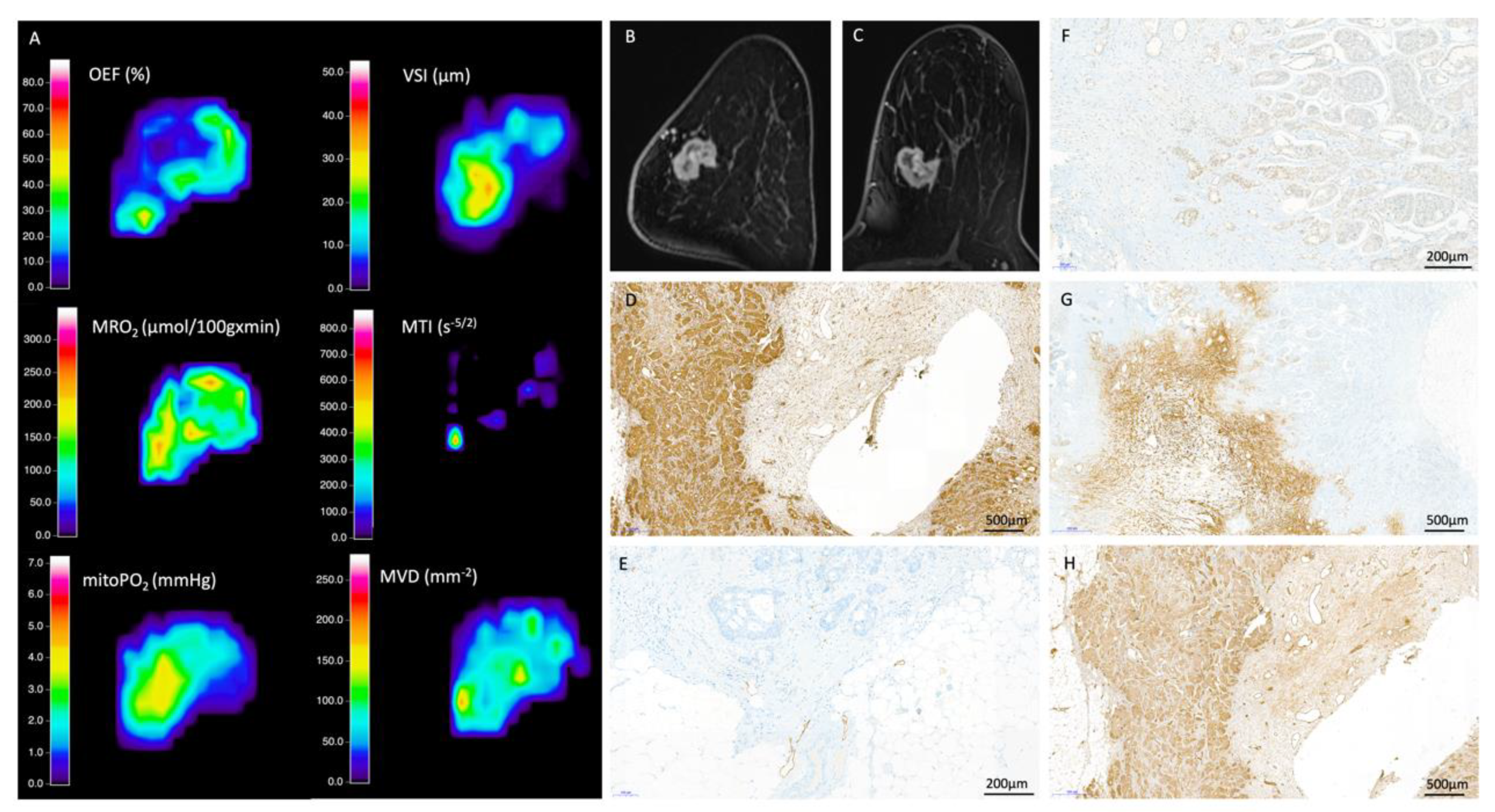
2.2. MRI Data Acquisition, Processing, and Biomarker Calculation
2.3. Quantitative Data Extraction
2.4. Histopathological Reference Standard
2.5. Statistical Analysis
3. Results
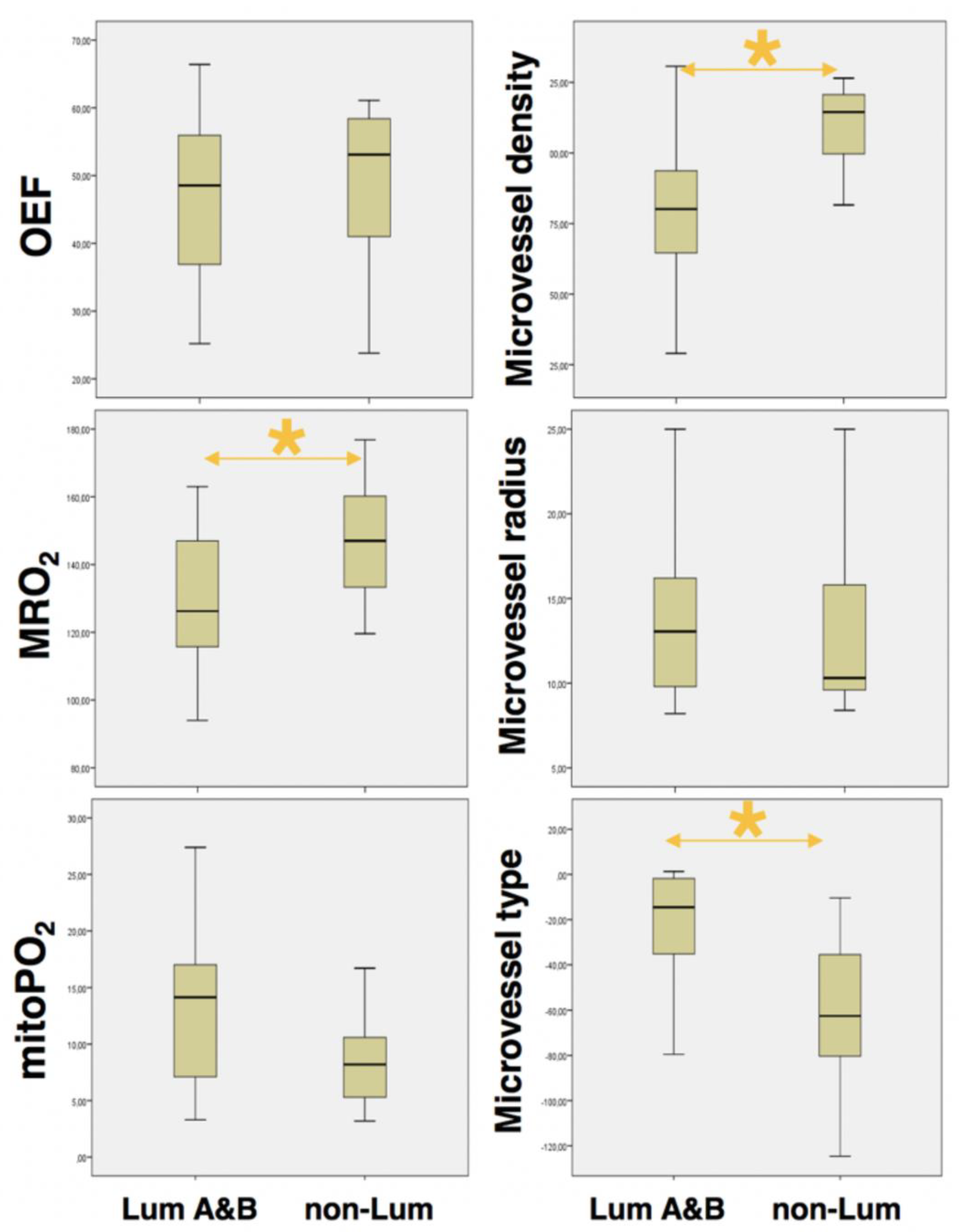
3.1. Identification of Aggressive Breast Cancer
3.2. QBOLD Mapping and Histopathological Markers
3.3. VAM Mapping and Histopathological Markers
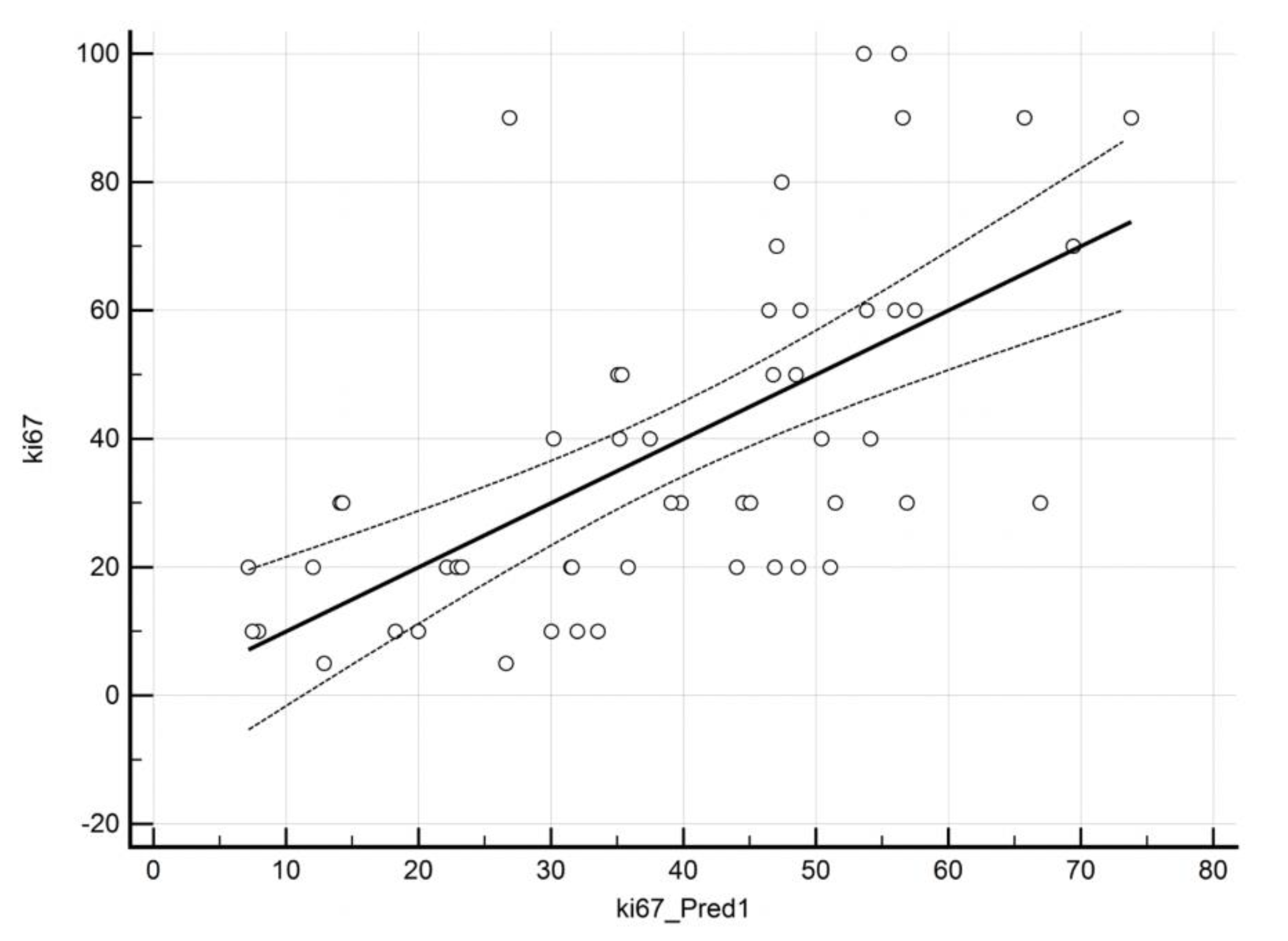
3.4. Prediction of ki67 by QBOLD and VAM Imaging
3.5. Prediction of Hormone Receptors and p53 by QBOLD and VAM Imaging
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zardavas, D.; Irrthum, A.; Swanton, C.; Piccart, M. Clinical management of breast cancer heterogeneity. Nat. Rev. Clin. Oncol. 2015, 12, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Wirapati, P.; Sotiriou, C.; Kunkel, S.; Farmer, P.; Pradervand, S.; Haibe-Kains, B.; Desmedt, C.; Ignatiadis, M.; Sengstag, T.; Schutz, F.; et al. Meta-analysis of gene expression profiles in breast cancer: Toward a unified understanding of breast cancer subtyping and prognosis signatures. Breast Cancer Res. 2008, 10, R65. [Google Scholar] [CrossRef]
- Huber, K.E.; Carey, L.A.; Wazer, D.E. Breast cancer molecular subtypes in patients with locally advanced disease: Impact on prognosis, patterns of recurrence, and response to therapy. Semin. Radiat. Oncol. 2009, 19, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Haynes, B.; Sarma, A.; Nangia-Makker, P.; Shekhar, M.P. Breast cancer complexity: Implications of intratumoral heterogeneity in clinical management. Cancer Metastasis Rev. 2017, 36, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Martelotto, L.G.; Ng, C.K.; Piscuoglio, S.; Weigelt, B.; Reis-Filho, J.S. Breast cancer intra-tumor heterogeneity. Breast Cancer Res. 2014, 16, 210. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.W.; Jimenez, C.R.; Boven, E. Breast cancer classification by proteomic technologies: Current state of knowledge. Cancer Treat Rev. 2014, 40, 129–138. [Google Scholar] [CrossRef]
- Vaupel, P. Hypoxia and aggressive tumor phenotype: Implications for therapy and prognosis. Oncologist 2008, 13 (Suppl. 3), 21–26. [Google Scholar] [CrossRef]
- Ruan, K.; Song, G.; Ouyang, G. Role of hypoxia in the hallmarks of human cancer. J. Cell Biochem. 2009, 107, 1053–1062. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hockel, M.; Schlenger, K.; Mitze, M.; Schaffer, U.; Vaupel, P. Hypoxia and Radiation Response in Human Tumors. Semin. Radiat. Oncol. 1996, 6, 3–9. [Google Scholar] [CrossRef]
- Tsutsui, S.; Kume, M.; Era, S. Prognostic value of microvessel density in invasive ductal carcinoma of the breast. Breast Cancer 2003, 10, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Al Murri, A.M.; Wilson, C.; Lannigan, A.; Doughty, J.C.; Angerson, W.J.; McArdle, C.S.; McMillan, D.C. Evaluation of the relationship between the systemic inflammatory response and cancer-specific survival in patients with primary operable breast cancer. Br. J. Cancer 2007, 96, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, P.; Barbareschi, M.; Verderio, P.; Boracchi, P.; Caffo, O.; Dalla Palma, P.; Meli, S.; Weidner, N.; Gasparini, G. Prognostic value of intratumoral microvessel density, a measure of tumor angiogenesis, in node-negative breast carcinoma--results of a multiparametric study. Breast Cancer Res. Treat 1995, 36, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Foekens, J.A.; Peters, H.A.; Grebenchtchikov, N.; Look, M.P.; Meijer-van Gelder, M.E.; Geurts-Moespot, A.; van der Kwast, T.H.; Sweep, C.G.; Klijn, J.G. High tumor levels of vascular endothelial growth factor predict poor response to systemic therapy in advanced breast cancer. Cancer Res. 2001, 61, 5407–5414. [Google Scholar] [PubMed]
- Stadlbauer, A.; Zimmermann, M.; Bennani-Baiti, B.; Helbich, T.H.; Baltzer, P.; Clauser, P.; Kapetas, P.; Bago-Horvath, Z.; Pinker, K. Development of a Non-invasive Assessment of Hypoxia and Neovascularization with Magnetic Resonance Imaging in Benign and Malignant Breast Tumors: Initial Results. Mol. Imaging Biol. 2019, 21, 758–770. [Google Scholar] [CrossRef]
- D’Orsi, C.; Sickles, E.; Mendelson, E.; Morris, E. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Xu, C.; Kiselev, V.G.; Moller, H.E.; Fiebach, J.B. Dynamic hysteresis between gradient echo and spin echo attenuations in dynamic susceptibility contrast imaging. Magn. Reson. Med. 2013, 69, 981–991. [Google Scholar] [CrossRef]
- Stadlbauer, A.; Zimmermann, M.; Kitzwogerer, M.; Oberndorfer, S.; Rossler, K.; Dorfler, A.; Buchfelder, M.; Heinz, G. MR Imaging-derived Oxygen Metabolism and Neovascularization Characterization for Grading and IDH Gene Mutation Detection of Gliomas. Radiology 2017, 283, 799–809. [Google Scholar] [CrossRef]
- Elston, C.W.; Ellis, I.O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 1991, 19, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, I.; Hussainzada, A.; Berger, S.; Fietze, E.; Linke, J.; Siedentopf, F.; Schoenegg, W. The St. Gallen surrogate classification for breast cancer subtypes successfully predicts tumor presenting features, nodal involvement, recurrence patterns and disease free survival. Breast 2016, 29, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Pineda, E.; Adamo, B.; Galvan, P.; Fernandez, A.; Gaba, L.; Diez, M.; Viladot, M.; Arance, A.; Munoz, M. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast 2015, 24 (Suppl. 2), S26–S35. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thurlimann, B.; Senn, H.J.; Panel, m. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef] [PubMed]
- Manoochehri Khoshinani, H.; Afshar, S.; Najafi, R. Hypoxia: A Double-Edged Sword in Cancer Therapy. Cancer Investig. 2016, 34, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Angulo, A.M.; Morales-Vasquez, F.; Hortobagyi, G.N. Overview of resistance to systemic therapy in patients with breast cancer. Adv. Exp. Med. Biol. 2007, 608, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Denko, N.C. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat. Rev. Cancer 2008, 8, 705–713. [Google Scholar] [CrossRef]
- Pisco, A.O.; Huang, S. Non-genetic cancer cell plasticity and therapy-induced stemness in tumour relapse: ‘What does not kill me strengthens me’. Br. J. Cancer 2015, 112, 1725–1732. [Google Scholar] [CrossRef]
- Petitjean, A.; Achatz, M.I.; Borresen-Dale, A.L.; Hainaut, P.; Olivier, M. TP53 mutations in human cancers: Functional selection and impact on cancer prognosis and outcomes. Oncogene 2007, 26, 2157–2165. [Google Scholar] [CrossRef]
- Brosh, R.; Rotter, V. When mutants gain new powers: News from the mutant p53 field. Nat. Rev. Cancer 2009, 9, 701–713. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]

| Acronym | Novel MRI Parameters | Unit |
|---|---|---|
| qBOLD | quantitative blood-oxygen-level-dependent imaging | |
| OEF | oxygen extraction fraction | % |
| MRO2 | metabolic rate of oxygen | µmol/100 g × min |
| mitoPO2 | mitochondrial oxygen tension | mmHg |
| VAM | vascular architecture mapping | |
| VSI | vessel size index | µm |
| MVD | microvessel density | mm−2 |
| MTI | microvessel type indicator | s−5/2 |
| Characteristic | n (%) |
|---|---|
| Entire cohort | 53 (100%) |
| Mean patient age (SD) | 57.6 (±13.1y) |
| Mean tumor volume | 6535 mm3 |
| Tumor grade | |
| G1 | 6 (11%) |
| G2 | 27 (51%) |
| G3 | 20 (38%) |
| Luminal A/B | 40 (75.5%) |
| Mean patient age (SD) | 53.7 (±14.8y) |
| Mean tumor volume | 3144 mm3 |
| Luminal A | 9 (17%) |
| Luminal B | 31 (59%) |
| Non-luminal | 13 (24.5%) |
| Mean patient age (SD) | 60 (±11.8y) |
| Mean tumor volume | 16,968 mm3 |
| HER2-positive | 4 (7.5%) |
| TN/basal-like | 9 (17%) |
| MRI Biomarkers (unit) | Tumor Type | Value | Std. Error | p-Value |
|---|---|---|---|---|
| OEF | Non-luminal | 48.81 | ±12.6 | 0.568 |
| (%) | Luminal A/B | 46.55 | ±12.19 | |
| MRO2 * | Non-luminal | 146.93 | ±19.12 | 0.007 |
| (µmol/100 g × min) | Luminal A/B | 129.45 | ±19.7 | |
| mitoPO2 * | Non-luminal | 8.62 | ±4.06 | 0.006 |
| (mmHg) | Luminal A/B | 13.3 | ±7.13 | |
| VSI | Non-luminal | 13.81 | ±6.04 | 0.966 |
| (µm) | Luminal A/B | 13.88 | ±4.73 | |
| MVD * | Non-luminal | 110.56 | ±14.57 | <0.001 |
| (mm−2) | Luminal A/B | 81 | ±24.32 | |
| MTI * | Non-luminal | −62.13 | ±39.27 | <0.001 |
| (s−5/2) | Luminal A/B | −22.43 | ±25.6 |
| Heading | Coefficient | Std. Error | p-Value | VIF | Adjusted R2 |
|---|---|---|---|---|---|
| ki67 | 0.361 | ||||
| (constant) | 74.9 | ||||
| OEF | −0.072 | 0.039 | 0.07 | 2.55 | |
| mitoPO2 | −0.292 | 0.067 | 0.0001 | 2.35 | |
| MVD | 0.038 | 0.012 | 0.003 | 1.14 | |
| ER positivity | 0.469 | ||||
| (constant) | 332.1 | ||||
| MRO2 | −0.056 | 0.019 | 0.004 | 1.03 | |
| OEF | −0.133 | 0.035 | <0.001 | 1.25 | |
| MVD | −0.109 | 0.018 | <0.0001 | 1.46 | |
| VSI | −0.235 | 0.088 | 0.011 | 1.31 | |
| PR positivity | 0.254 | ||||
| (constant) | 218.9 | ||||
| MRO2 | −0.048 | 0.023 | 0.038 | 1.03 | |
| OEF | −0.109 | 0.041 | <0.01 | 1.13 | |
| MVD | −0.068 | 0.019 | <0.001 | 1.14 | |
| P53 positivity | 0.298 | ||||
| (constant) | −10.6 | ||||
| MVD | 0.038 | 0.015 | 0.014 | 1.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bennani-Baiti, B.; Pinker, K.; Zimmermann, M.; Helbich, T.H.; Baltzer, P.A.; Clauser, P.; Kapetas, P.; Bago-Horvath, Z.; Stadlbauer, A. Non-Invasive Assessment of Hypoxia and Neovascularization with MRI for Identification of Aggressive Breast Cancer. Cancers 2020, 12, 2024. https://doi.org/10.3390/cancers12082024
Bennani-Baiti B, Pinker K, Zimmermann M, Helbich TH, Baltzer PA, Clauser P, Kapetas P, Bago-Horvath Z, Stadlbauer A. Non-Invasive Assessment of Hypoxia and Neovascularization with MRI for Identification of Aggressive Breast Cancer. Cancers. 2020; 12(8):2024. https://doi.org/10.3390/cancers12082024
Chicago/Turabian StyleBennani-Baiti, Barbara, Katja Pinker, Max Zimmermann, Thomas H. Helbich, Pascal A. Baltzer, Paola Clauser, Panagiotis Kapetas, Zsuzsanna Bago-Horvath, and Andreas Stadlbauer. 2020. "Non-Invasive Assessment of Hypoxia and Neovascularization with MRI for Identification of Aggressive Breast Cancer" Cancers 12, no. 8: 2024. https://doi.org/10.3390/cancers12082024
APA StyleBennani-Baiti, B., Pinker, K., Zimmermann, M., Helbich, T. H., Baltzer, P. A., Clauser, P., Kapetas, P., Bago-Horvath, Z., & Stadlbauer, A. (2020). Non-Invasive Assessment of Hypoxia and Neovascularization with MRI for Identification of Aggressive Breast Cancer. Cancers, 12(8), 2024. https://doi.org/10.3390/cancers12082024








