Meta-Analysis Reveals Significant Sex Differences in Chronic Lymphocytic Leukemia Progression in the Eµ-TCL1 Transgenic Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Cohorts and Housing Facilities
2.2. Mouse Blood Analyses
2.3. Survival Determination
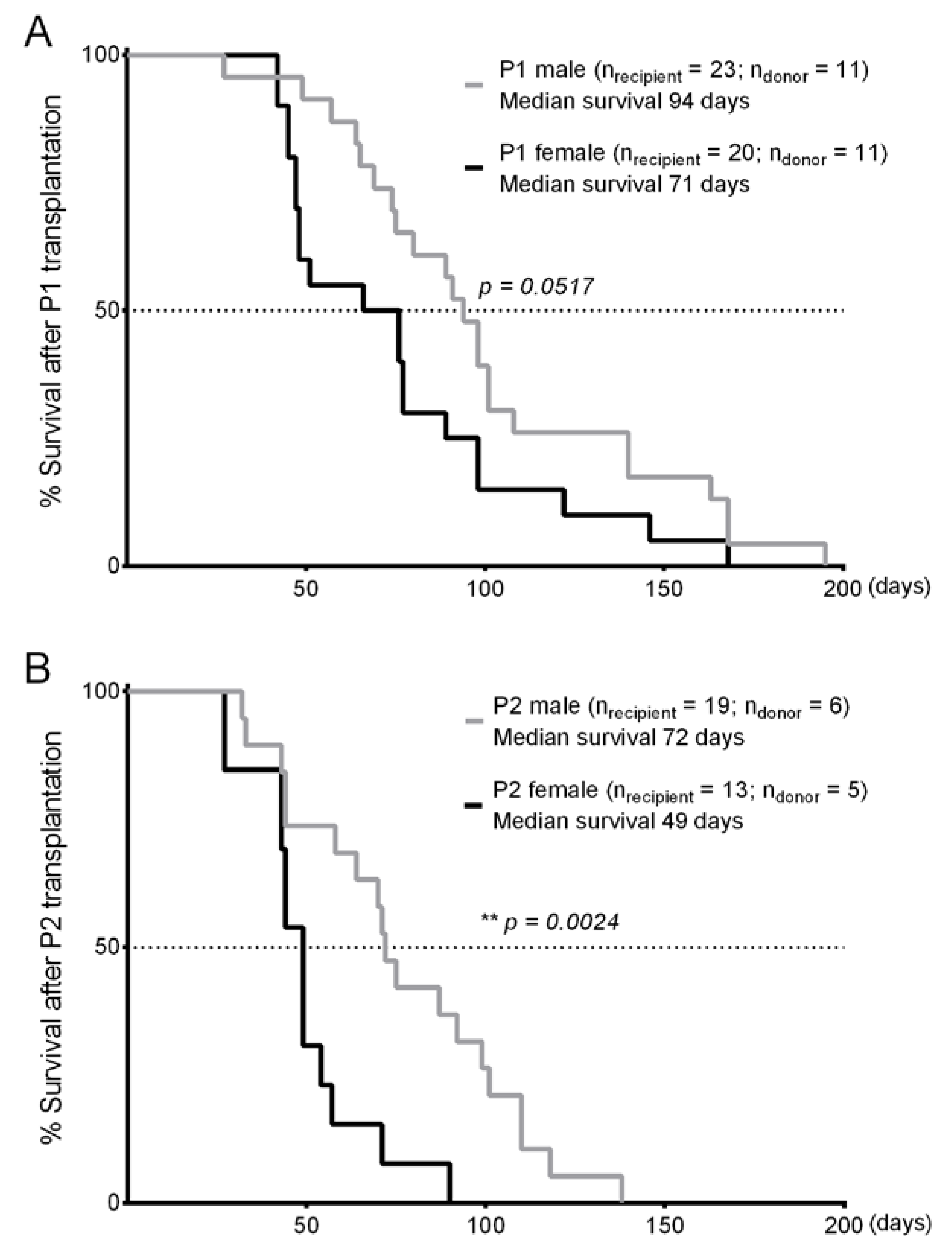
2.4. Syngeneic Adoptive Transplantation
2.5. Statistical Analysis
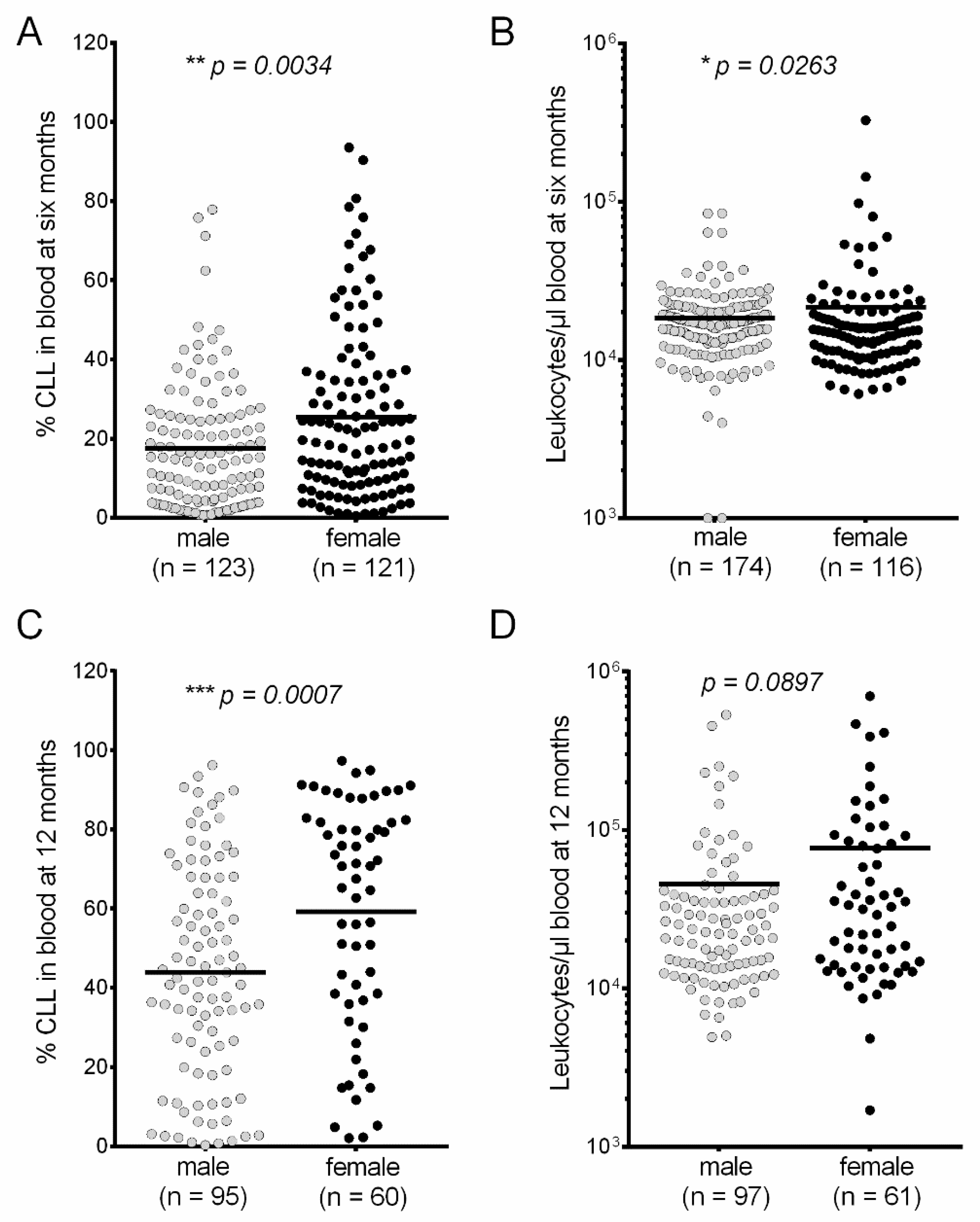
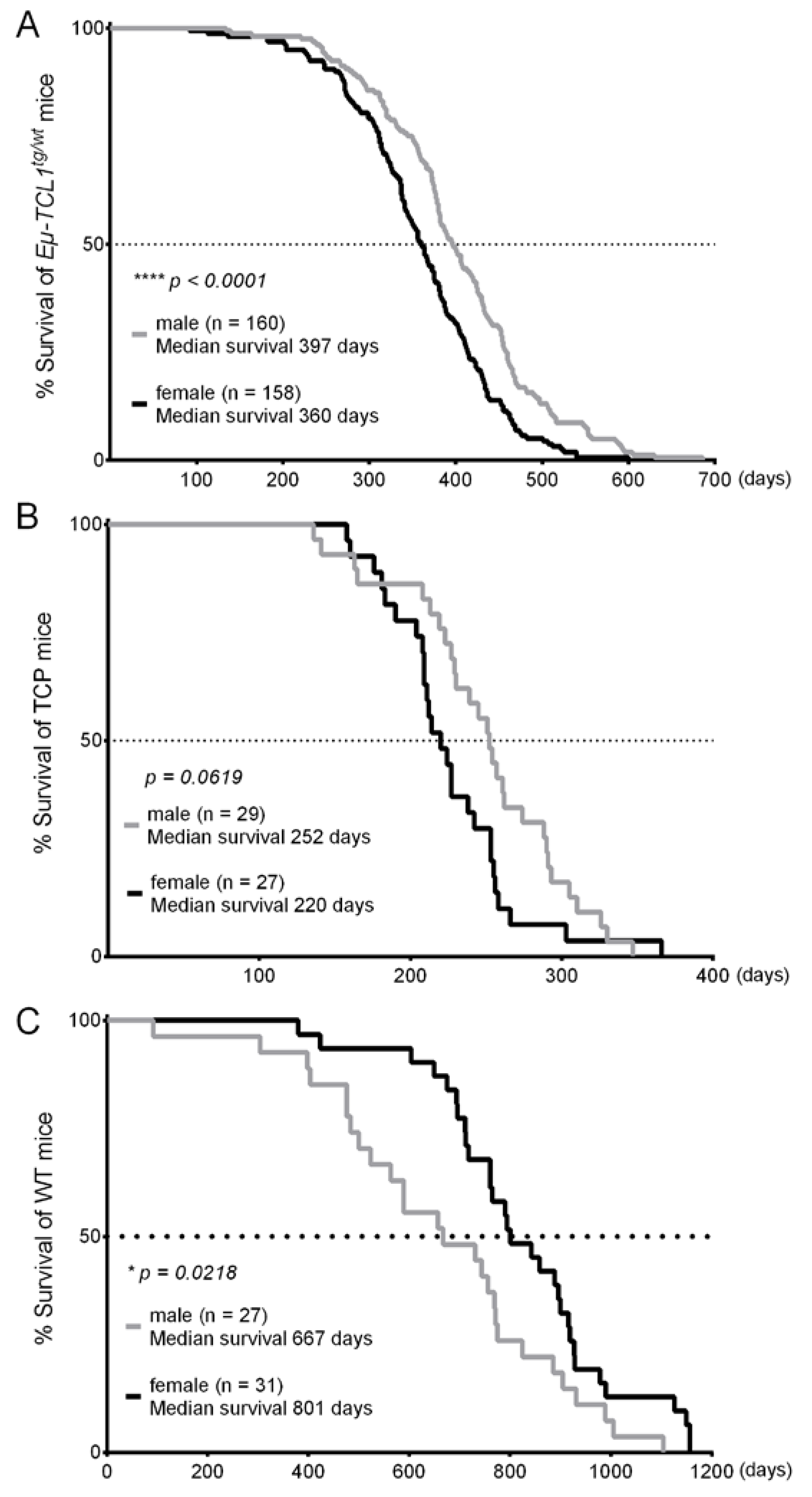
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hallek, M.; Shanafelt, T.D.; Eichhorst, B. Chronic lymphocytic leukaemia. Lancet 2018, 391, 1524–1537. [Google Scholar] [CrossRef]
- Hallek, M. Chronic lymphocytic leukemia: 2020 update on diagnosis, risk stratification and treatment. Am. J. Hematol. 2019, 94, 1266–1287. [Google Scholar] [CrossRef]
- Bichi, R.; Shinton, S.A.; Martin, E.S.; Koval, A.; Calin, G.A.; Cesari, R.; Russo, G.; Hardy, R.R.; Croce, C.M. Human chronic lymphocytic leukemia modeled in mouse by targeted tcl1 expression. Proc. Natl. Acad. Sci. USA 2002, 99, 6955–6960. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.J.; Lucas, D.M.; Muthusamy, N.; Smith, L.L.; Edwards, R.B.; De Lay, M.D.; Croce, C.M.; Grever, M.R.; Byrd, J.C. Characterization of the tcl-1 transgenic mouse as a preclinical drug development tool for human chronic lymphocytic leukemia. Blood 2006, 108, 1334–1338. [Google Scholar] [CrossRef] [PubMed]
- Pekarsky, Y.; Drusco, A.; Kumchala, P.; Croce, C.M.; Zanesi, N. The long journey of tcl1 transgenic mice: Lessons learned in the last 15 years. Gene Expr. J. Liver Res. 2015, 16, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, G.; Bertilaccio, M.T.; Ghia, P.; Klein, U. Mouse models in the study of chronic lymphocytic leukemia pathogenesis and therapy. Blood 2014, 124, 1010–1019. [Google Scholar] [CrossRef]
- Bresin, A.; D’Abundo, L.; Narducci, M.G.; Fiorenza, M.T.; Croce, C.M.; Negrini, M.; Russo, G. Tcl1 transgenic mouse model as a tool for the study of therapeutic targets and microenvironment in human B-cell chronic lymphocytic leukemia. Cell Death Dis. 2016, 7, e2071. [Google Scholar] [CrossRef]
- Karp, N.A.; Mason, J.; Beaudet, A.L.; Benjamini, Y.; Bower, L.; Braun, R.E.; Brown, S.D.M.; Chesler, E.J.; Dickinson, M.E.; Flenniken, A.M.; et al. Prevalence of sexual dimorphism in mammalian phenotypic traits. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Zheng, S.; El-Naggar, A.K.; Kim, E.S.; Kurie, J.M.; Lozano, G. A genetic mouse model for metastatic lung cancer with gender differences in survival. Oncogene 2007, 26, 6896–6904. [Google Scholar] [CrossRef]
- Naugler, W.E.; Sakurai, T.; Kim, S.; Maeda, S.; Kim, K.; Elsharkawy, A.M.; Karin, M. Gender disparity in liver cancer due to sex differences in myd88-dependent il-6 production. Science 2007, 317, 121–124. [Google Scholar] [CrossRef]
- Zhai, Y.; Haresi, A.J.; Huang, L.; Lang, D. Differences in tumor initiation and progression of melanoma in the braf(ca);tyr-creert2;pten(f/f) model between male and female mice. Pigment Cell Melanoma Res. 2020, 33, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Fedorchenko, O.; Stiefelhagen, M.; Peer-Zada, A.A.; Barthel, R.; Mayer, P.; Eckei, L.; Breuer, A.; Crispatzu, G.; Rosen, N.; Landwehr, T.; et al. Cd44 regulates the apoptotic response and promotes disease development in chronic lymphocytic leukemia. Blood 2013, 121, 4126–4136. [Google Scholar] [CrossRef] [PubMed]
- Reinart, N.; Nguyen, P.H.; Boucas, J.; Rosen, N.; Kvasnicka, H.M.; Heukamp, L.; Rudolph, C.; Ristovska, V.; Velmans, T.; Mueller, C.; et al. Delayed development of chronic lymphocytic leukemia in the absence of macrophage migration inhibitory factor. Blood 2013, 121, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.H.; Fedorchenko, O.; Rosen, N.; Koch, M.; Barthel, R.; Winarski, T.; Florin, A.; Wunderlich, F.T.; Reinart, N.; Hallek, M. Lyn kinase in the tumor microenvironment is essential for the progression of chronic lymphocytic leukemia. Cancer Cell 2016, 30, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Knittel, G.; Rehkamper, T.; Korovkina, D.; Liedgens, P.; Fritz, C.; Torgovnick, A.; Al-Baldawi, Y.; Al-Maarri, M.; Cun, Y.; Fedorchenko, O.; et al. Two mouse models reveal an actionable parp1 dependence in aggressive chronic lymphocytic leukemia. Nat. Commun. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lucas, F.; Rogers, K.A.; Harrington, B.K.; Pan, A.; Yu, L.; Breitbach, J.; Bundschuh, R.; Goettl, V.M.; Hing, Z.A.; Kanga, P.; et al. Emu-tcl1xmyc: A novel mouse model for concurrent cll and B-cell lymphoma. Clin. Cancer Res. 2019, 25, 6260–6273. [Google Scholar] [CrossRef]
- Lutzny, G.; Kocher, T.; Schmidt-Supprian, M.; Rudelius, M.; Klein-Hitpass, L.; Finch, A.J.; Durig, J.; Wagner, M.; Haferlach, C.; Kohlmann, A.; et al. Protein kinase c-beta-dependent activation of nf-kappab in stromal cells is indispensable for the survival of chronic lymphocytic leukemia B cells in vivo. Cancer Cell 2013, 23, 77–92. [Google Scholar] [CrossRef]
- Nguyen, P.H.; Niesen, E.; Hallek, M. New roles for B cell receptor associated kinases: When the B cell is not the target. Leukemia 2019, 33, 576–587. [Google Scholar] [CrossRef]
- Dong, S.; Harrington, B.K.; Hu, E.Y.; Greene, J.T.; Lehman, A.M.; Tran, M.; Wasmuth, R.L.; Long, M.; Muthusamy, N.; Brown, J.R.; et al. Pi3k p110delta inactivation antagonizes chronic lymphocytic leukemia and reverses T cell immune suppression. J. Clin. Investig. 2019, 129, 122–136. [Google Scholar] [CrossRef]
- Zaborsky, N.; Gassner, F.J.; Hopner, J.P.; Schubert, M.; Hebenstreit, D.; Stark, R.; Asslaber, D.; Steiner, M.; Geisberger, R.; Greil, R.; et al. Exome sequencing of the tcl1 mouse model for cll reveals genetic heterogeneity and dynamics during disease development. Leukemia 2019, 33, 957–968. [Google Scholar] [CrossRef]
- Cheng, F. Gender dimorphism creates divergent cancer susceptibilities. Trends Cancer 2016, 2, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.F.; Bongartz, T.; Liu, M.; Kalari, K.R.; Goss, P.E.; Shepherd, L.E.; Goetz, M.P.; Kubo, M.; Ingle, J.N.; Wang, L.; et al. Estrogen, snp-dependent chemokine expression and selective estrogen receptor modulator regulation. Mol. Endocrinol. 2016, 30, 382–398. [Google Scholar] [CrossRef] [PubMed]
- Badve, S.; Collins, N.R.; Bhat-Nakshatri, P.; Turbin, D.; Leung, S.; Thorat, M.; Dunn, S.E.; Geistlinger, T.R.; Carroll, J.S.; Brown, M.; et al. Subcellular localization of activated akt in estrogen receptor- and progesterone receptor-expressing breast cancers: Potential clinical implications. Am. J. Pathol. 2010, 176, 2139–2149. [Google Scholar] [CrossRef]
- Nishimura, K.; Aizawa, S.; Nugroho, F.L.; Shiomitsu, E.; Tran, Y.T.H.; Bui, P.L.; Borisova, E.; Sakuragi, Y.; Takada, H.; Kurisaki, A.; et al. A role for klf4 in promoting the metabolic shift via tcl1 during induced pluripotent stem cell generation. Stem Cell Rep. 2017, 8, 787–801. [Google Scholar] [CrossRef] [PubMed]
- Fiorenza, M.T.; Rava, A. The tcl1 function revisited focusing on metabolic requirements of stemness. Cell Cycle 2019, 18, 3055–3063. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Raval, A.; Johnson, A.J.; Hertlein, E.; Liu, T.H.; Jin, V.X.; Sherman, M.H.; Liu, S.J.; Dawson, D.W.; Williams, K.E.; et al. Epigenetic changes during disease progression in a murine model of human chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2009, 106, 13433–13438. [Google Scholar] [CrossRef] [PubMed]
- Palamarchuk, A.; Yan, P.S.; Zanesi, N.; Wang, L.; Rodrigues, B.; Murphy, M.; Balatti, V.; Bottoni, A.; Nazaryan, N.; Alder, H.; et al. Tcl1 protein functions as an inhibitor of de novo DNA methylation in B-cell chronic lymphocytic leukemia (cll). Proc. Natl. Acad. Sci. USA 2012, 109, 2555–2560. [Google Scholar] [CrossRef]
- Hensel, J.A.; Khattar, V.; Ashton, R.; Ponnazhagan, S. Characterization of immune cell subtypes in three commonly used mouse strains reveals gender and strain-specific variations. Lab. Investig. 2019, 99, 93–106. [Google Scholar] [CrossRef]
- Lin, P.Y.; Sun, L.; Thibodeaux, S.R.; Ludwig, S.M.; Vadlamudi, R.K.; Hurez, V.J.; Bahar, R.; Kious, M.J.; Livi, C.B.; Wall, S.R.; et al. B7-h1-dependent sex-related differences in tumor immunity and immunotherapy responses. J. Immunol. 2010, 185, 2747–2753. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Wiestner, A. Targeting B cell receptor signalling in cancer: Preclinical and clinical advances. Nat. Rev. Cancer 2018, 18, 148–167. [Google Scholar] [CrossRef] [PubMed]
- Molica, S. Sex differences in incidence and outcome of chronic lymphocytic leukemia patients. Leuk. Lymphoma 2006, 47, 1477–1480. [Google Scholar] [CrossRef] [PubMed]
- Catovsky, D.; Wade, R.; Else, M. The clinical significance of patients’ sex in chronic lymphocytic leukemia. Haematologica 2014, 99, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Mauro, F.R.; Foa, R.; Giannarelli, D.; Cordone, I.; Crescenzi, S.; Pescarmona, E.; Sala, R.; Cerretti, R.; Mandelli, F. Clinical characteristics and outcome of young chronic lymphocytic leukemia patients: A single institution study of 204 cases. Blood 1999, 94, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.J.; Albesiano, E.; Zanesi, N.; Yancopoulos, S.; Sawyer, A.; Romano, E.; Petlickovski, A.; Efremov, D.G.; Croce, C.M.; Chiorazzi, N. B cell receptors in tcl1 transgenic mice resemble those of aggressive, treatment-resistant human chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2006, 103, 11713–11718. [Google Scholar] [CrossRef]
- Allain, E.P.; Venzl, K.; Caron, P.; Turcotte, V.; Simonyan, D.; Gruber, M.; Le, T.; Lévesque, E.; Guillemette, C.; Vanura, K. Sex-dependent association of circulating sex steroids and pituitary hormones with treatment-free survival in chronic lymphocytic leukemia patients. Ann. Hematol. 2018, 97, 1649–1661. [Google Scholar] [CrossRef]
- Lin, S.; Liu, Y.; Goldin, L.R.; Lyu, C.; Kong, X.; Zhang, Y.; Caporaso, N.E.; Xiang, S.; Gao, Y. Sex-related DNA methylation differences in B cell chronic lymphocytic leukemia. Biol. Sex Differ. 2019, 10, 2. [Google Scholar] [CrossRef]
- Shansky, R.M. Are hormones a "female problem" for animal research? Science 2019, 364, 825–826. [Google Scholar] [CrossRef]
- Klein, U.; Lia, M.; Crespo, M.; Siegel, R.; Shen, Q.; Mo, T.; Ambesi-Impiombato, A.; Califano, A.; Migliazza, A.; Bhagat, G.; et al. The dleu2/mir-15a/16-1 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell 2010, 17, 28–40. [Google Scholar] [CrossRef]
- Yin, S.; Gambe, R.G.; Sun, J.; Martinez, A.Z.; Cartun, Z.J.; Regis, F.F.D.; Wan, Y.; Fan, J.; Brooks, A.N.; Herman, S.E.M.; et al. A murine model of chronic lymphocytic leukemia based on B cell-restricted expression of sf3b1 mutation and atm deletion. Cancer Cell 2019, 35, 283–296.e5. [Google Scholar] [CrossRef]
- Clayton, J.A.; Collins, F.S. Policy: Nih to balance sex in cell and animal studies. Nature 2014, 509, 282–283. [Google Scholar] [CrossRef]
- Ozturk, S.; Roessner, P.M.; Schulze-Edinghausen, L.; Yazdanparast, H.; Kalter, V.; Lichter, P.; Hanna, B.S.; Seiffert, M. Rejection of adoptively transferred emicro-tcl1 chronic lymphocytic leukemia cells in c57bl/6 substrains or knockout mouse lines. Leukemia 2019, 33, 1514–1539. [Google Scholar] [CrossRef] [PubMed]
| No. | Ref. | Genetic Background | Additional, Unaffecting Transgene | Housing Facility | Number of Mice | Median Survival (Days) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | Male | Female | P (Log-Rank) | |||||
| 1 | [12] | B6C3 | - | EM | 41 | 17 | 24 | 378 | 339.5 | 0.0080 |
| 2 | [13] | B6C3 | - | EM | 36 | 15 | 21 | 424 | 369 | 0.0808 |
| 3 | [14] | B6C3 | - | EM | 38 | 16 | 22 | 454.5 | 376.5 | 0.0025 |
| 4 | [15] | B6 (J/N-mix) | Hemizygote CD19-Cre transgene without any loxP-flanked allele | PA | 13 | 8 | 5 | 369 | 320 | 0.9621 |
| 5 | - | B6 (J/N-mix) | LoxP-flanked mutant alleles without any Cre transgene | EM | 29 | 17 | 12 | 402 | 358 | 0.2971 |
| 6 | - | B6C3 | - | EM | 43 | 21 | 22 | 425 | 404.5 | 0.2700 |
| 7 | - | B6C3 | LoxP-flanked mutant alleles without any Cre transgene | EM | 6 | 3 | 3 | 430 | 434 | 0.6537 |
| 8 | - | B6C3 | LoxP-flanked mutant alleles without any Cre transgene | EM | 22 | 10 | 12 | 429.5 | 400.5 | 0.5522 |
| 9 | - | B6C3 | - | EM | 47 | 28 | 19 | 357 | 345 | 0.2092 |
| 10 | - | B6 (J) | - | CE | 21 | 10 | 11 | 443 | 389 | 0.0466 |
| 11 | - | B6 (J/N-mix) | - | PA | 22 | 15 | 7 | 360 | 308 | 0.0079 |
| No. | Ref. | Genetic Background | Housing Facility | Number of Mice | Median Survival (Days) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | Male | Female | P (Log-Rank) | ||||
| 1 | [15] | B6 (J/N-mix) | PA | 21 | 13 | 8 | 252 | 205.98 | 0.0223 |
| 2 | - | B6 (J) | CE | 35 | 16 | 19 | 256.5 | 227 | 0.2633 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koch, M.; Reinartz, S.; Saggau, J.; Knittel, G.; Rosen, N.; Fedorchenko, O.; Thelen, L.; Barthel, R.; Reinart, N.; Seeger-Nukpezah, T.; et al. Meta-Analysis Reveals Significant Sex Differences in Chronic Lymphocytic Leukemia Progression in the Eµ-TCL1 Transgenic Mouse Model. Cancers 2020, 12, 1980. https://doi.org/10.3390/cancers12071980
Koch M, Reinartz S, Saggau J, Knittel G, Rosen N, Fedorchenko O, Thelen L, Barthel R, Reinart N, Seeger-Nukpezah T, et al. Meta-Analysis Reveals Significant Sex Differences in Chronic Lymphocytic Leukemia Progression in the Eµ-TCL1 Transgenic Mouse Model. Cancers. 2020; 12(7):1980. https://doi.org/10.3390/cancers12071980
Chicago/Turabian StyleKoch, Maximilian, Sebastian Reinartz, Julia Saggau, Gero Knittel, Natascha Rosen, Oleg Fedorchenko, Lisa Thelen, Romy Barthel, Nina Reinart, Tamina Seeger-Nukpezah, and et al. 2020. "Meta-Analysis Reveals Significant Sex Differences in Chronic Lymphocytic Leukemia Progression in the Eµ-TCL1 Transgenic Mouse Model" Cancers 12, no. 7: 1980. https://doi.org/10.3390/cancers12071980
APA StyleKoch, M., Reinartz, S., Saggau, J., Knittel, G., Rosen, N., Fedorchenko, O., Thelen, L., Barthel, R., Reinart, N., Seeger-Nukpezah, T., Reinhardt, H. C., Hallek, M., & Nguyen, P.-H. (2020). Meta-Analysis Reveals Significant Sex Differences in Chronic Lymphocytic Leukemia Progression in the Eµ-TCL1 Transgenic Mouse Model. Cancers, 12(7), 1980. https://doi.org/10.3390/cancers12071980





