Characterization of Driver Mutations in Anaplastic Thyroid Carcinoma Identifies RAS and PIK3CA Mutations as Negative Survival Predictors
Abstract
1. Introduction
2. Results
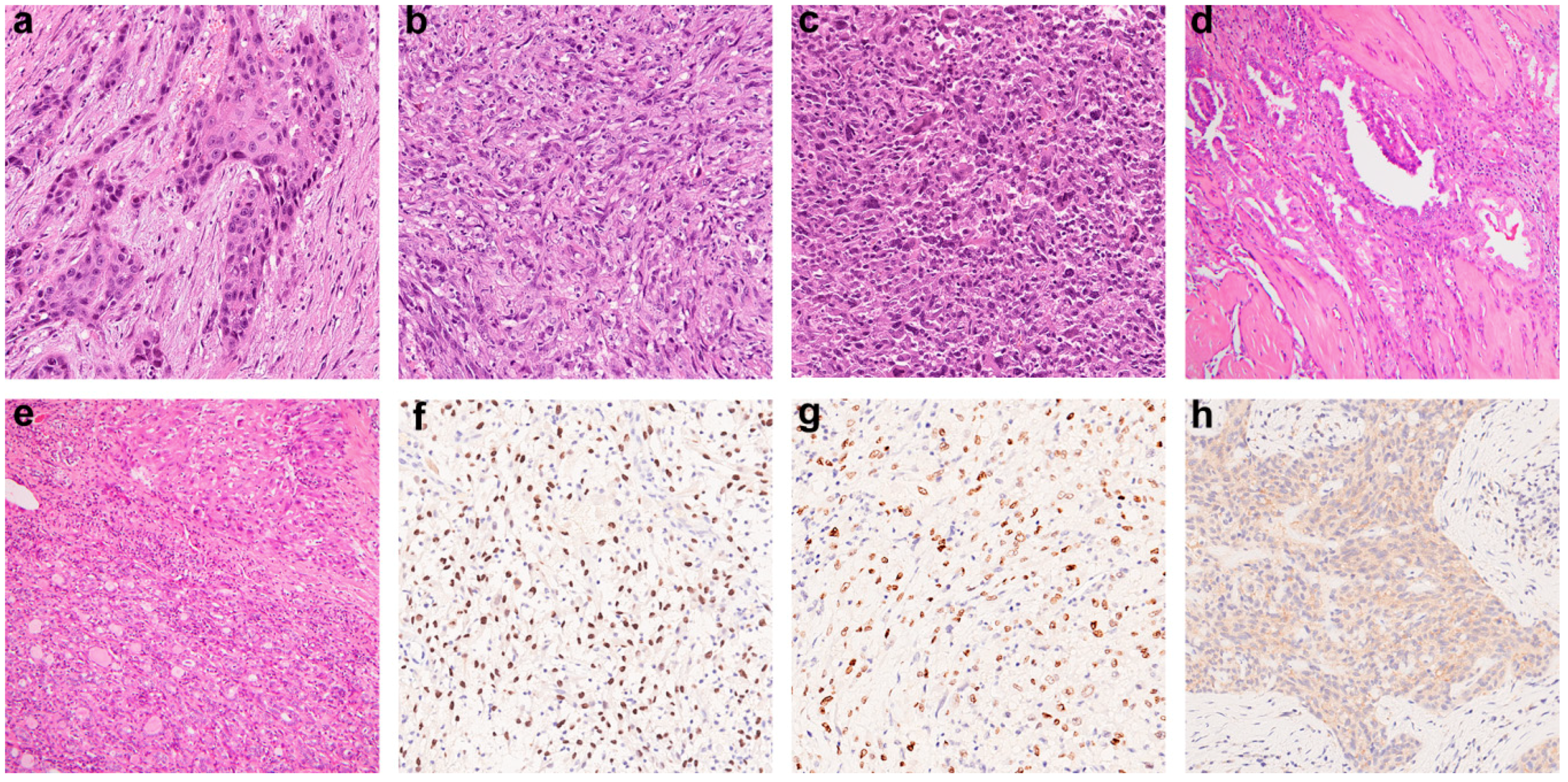
2.1. Clinicopathological Features of ATC
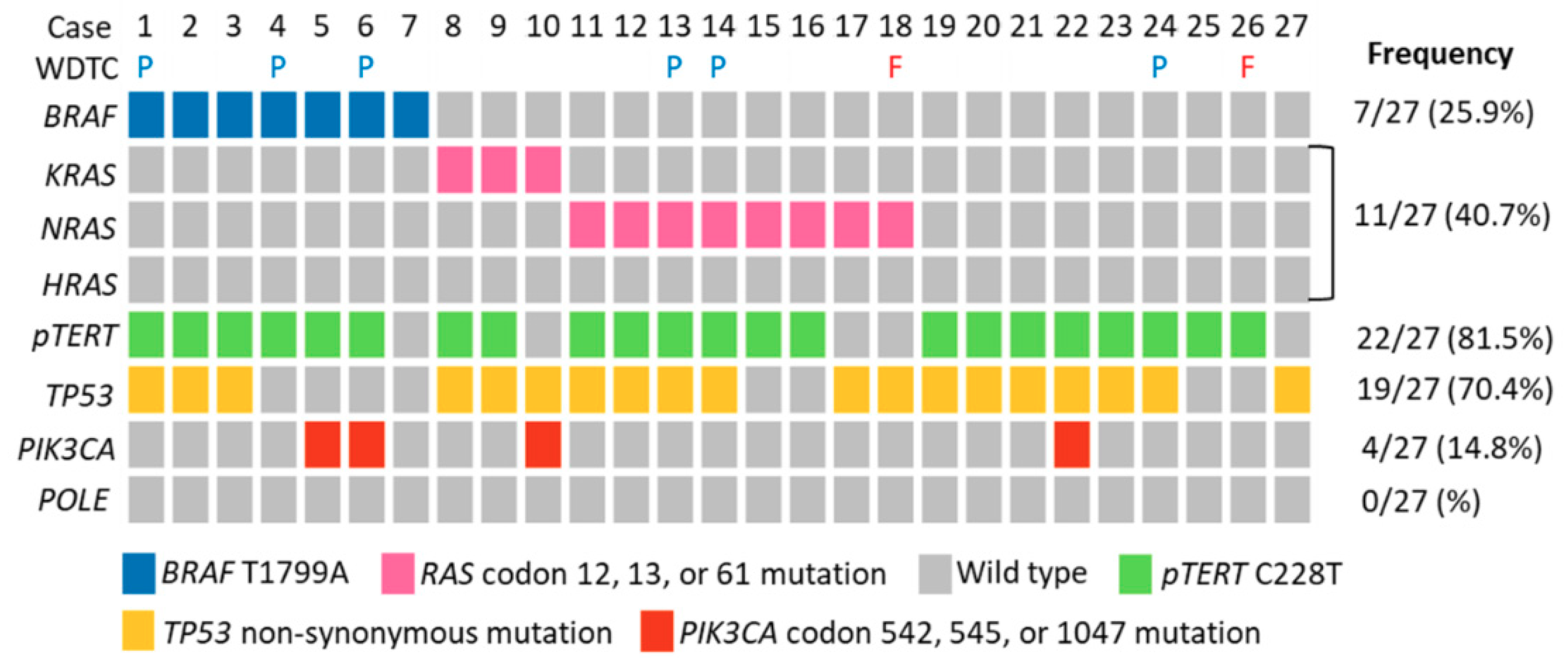
2.2. Molecular Profiles of ATC
2.3. Survival Analysis of ATC Cohort
3. Discussion
| References | Case No. | BRAFV600E | RAS | TP53 | TERT | PIK3CA | POLE | MMR Alternation b |
|---|---|---|---|---|---|---|---|---|
| Current Study | 27 | 25.9% | 40.7% | 70.4% | 81.5% | 14.8% | 0% | 0% |
| Xu 2020 [8] a | 102 | 43.1% | 22% | 63% | 75% | 18% | 4% | 8% |
| Khan 2019 [16] | 90 | 32% | 26% | 65.6% | 32% | 12.2% | na | na |
| Duan 2019 [10] | 25 | 56% | 24% | 60% | 56% | 44% | na | na |
| Yoo 2019 [9] | 27 | 40.1% | 44.4% | 48.1% | 55.6% | 11.1% | na | na |
| Ravi 2019 [33] | 11 | 18% | 18% | 55% | 36% | 18% | 9% | 9% |
| Chen 2018 [17] | 12 | 25% | 33% | 25% | na | 0% | na | na |
| Bonhomme 2017 [13] | 94 | 12.8% | 42.6% | 54.4% | 54% | 6.4% | na | na |
| Kunstman 2015 [15] | 22 | 27.3% | 27.3% | 27.3% | na | 9.1% | na | 13.6% |
4. Materials and Methods
4.1. Case Selection
4.2. Construction of Tissue Microarray and Immunohistochemistry
4.3. DNA Extraction
4.4. Targeted Next-Generation Sequencing (NGS)
4.5. Mass Spectrometry for PI3KCA Mutations
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, F.-C.; Lin, H.-T.; Lin, S.-F.; Kuo, C.-F.; Chung, T.-T.; Yu, H.-P. Nationwide cohort study on the epidemiology and survival outcomes of thyroid cancer. Oncotarget 2017, 8, 78429–78451. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Molinaro, E.; Romei, C.; Biagini, A.; Sabini, E.; Agate, L.; Mazzeo, S.; Materazzi, G.; Sellari-Franceschini, S.; Ribechini, A.; Torregrossa, L.; et al. Anaplastic thyroid carcinoma: From clinicopathology to genetics and advanced therapies. Nat. Rev. Endocrinol. 2017, 13, 644–660. [Google Scholar] [CrossRef]
- Brignardello, E.; Gallo, M.; Baldi, I.; Palestini, N.; Piovesan, A.; Grossi, E.; Ciccone, G.; Boccuzzi, G. Anaplastic thyroid carcinoma: Clinical outcome of 30 consecutive patients referred to a single institution in the past 5 years. Eur. J. Endocrinol. 2007, 156, 425–430. [Google Scholar] [CrossRef]
- Kebebew, E.; Greenspan, F.S.; Clark, O.H.; Woeber, K.A.; McMillan, A. Anaplastic thyroid carcinoma. Treatment outcome and prognostic factors. Cancer 2005, 103, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.-K.; Lee, S.; Kim, S.-j.; Jee, H.-G.; Kim, B.-A.; Cho, H.; Song, Y.S.; Cho, S.W.; Won, J.-K.; Shin, J.-Y.; et al. Comprehensive analysis of the transcriptional and mutational landscape of follicular and papillary thyroid cancers. PLoS Genet. 2016, 12, e1006239. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, Y.E.; Nikiforova, M.N. Molecular genetics and diagnosis of thyroid cancer. Nat. Rev. Endocrinol. 2011, 7, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Akbani, R.; Aksoy, B.A.; Ally, A.; Arachchi, H.; Asa Sylvia, L.; Auman, J.T.; Balasundaram, M.; Balu, S.; Baylin, S.B.; et al. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef]
- Xu, B.; Fuchs, T.L.; Dogan, S.; Landa, I.; Katabi, N.; Fagin, J.A.; Tuttle, R.M.T.; Sherman, E.J.; Gill, A.; Ghossein, R. Dissecting anaplastic thyroid carcinoma (ATC): A comprehensive clinical, histologic, immunophenotypic, and molecular study of 360 cases. Thyroid 2020, in press. [Google Scholar] [CrossRef]
- Yoo, S.-K.; Song, Y.S.; Lee, E.K.; Hwang, J.; Kim, H.H.; Jung, G.; Kim, Y.A.; Kim, S.-j.; Cho, S.W.; Won, J.-K.; et al. Integrative analysis of genomic and transcriptomic characteristics associated with progression of aggressive thyroid cancer. Nat. Commun. 2019, 10, 2764–2775. [Google Scholar] [CrossRef]
- Duan, H.; Li, Y.; Hu, P.; Gao, J.; Ying, J.; Xu, W.; Zhao, D.; Wang, Z.; Ye, J.; Lizaso, A.; et al. Mutational profiling of poorly differentiated and anaplastic thyroid carcinoma by the use of targeted next-generation sequencing. Histopathology 2019, 75, 890–899. [Google Scholar] [CrossRef]
- Deeken-Draisey, A.; Yang, G.-Y.; Gao, J.; Alexiev, B.A. Anaplastic thyroid carcinoma: An epidemiologic, histologic, immunohistochemical, and molecular single-institution study. Hum. Pathol. 2018, 82, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Oishi, N.; Kondo, T.; Ebina, A.; Sato, Y.; Akaishi, J.; Hino, R.; Yamamoto, N.; Mochizuki, K.; Nakazawa, T.; Yokomichi, H.; et al. Molecular alterations of coexisting thyroid papillary carcinoma and anaplastic carcinoma: Identification of TERT mutation as an independent risk factor for transformation. Mod. Pathol. 2017, 30, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Bonhomme, B.; Godbert, Y.; Perot, G.; Al Ghuzlan, A.; Bardet, S.; Belleannée, G.; Crinière, L.; Do Cao, C.; Fouilloux, G.; Guyetant, S.; et al. Molecular pathology of anaplastic thyroid carcinomas: A retrospective study of 144 cases. Thyroid 2017, 27, 682–692. [Google Scholar] [CrossRef]
- Jeon, M.J.; Chun, S.-M.; Kim, D.; Kwon, H.; Jang, E.K.; Kim, T.Y.; Kim, W.B.; Shong, Y.K.; Jang, S.J.; Song, D.E.; et al. Genomic alterations of anaplastic thyroid carcinoma detected by targeted massive parallel sequencing in a BRAF V600E mutation-prevalent area. Thyroid 2016, 26, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Kunstman, J.W.; Juhlin, C.C.; Goh, G.; Brown, T.C.; Stenman, A.; Healy, J.M.; Rubinstein, J.C.; Choi, M.; Kiss, N.; Nelson-Williams, C.; et al. Characterization of the mutational landscape of anaplastic thyroid cancer via whole-exome sequencing. Hum. Mol. Genet. 2015, 24, 2318–2329. [Google Scholar] [CrossRef]
- Khan, S.A.; Ci, B.; Xie, Y.; Gerber, D.E.; Beg, M.S.; Sherman, S.I.; Cabanillas, M.E.; Busaidy, N.L.; Burtness, B.A.; Heilmann, A.M.; et al. Unique mutation patterns in anaplastic thyroid cancer identified by comprehensive genomic profiling. Head Neck 2019, 41, 1928–1934. [Google Scholar] [CrossRef]
- Chen, H.; Luthra, R.; Routbort, M.J.; Patel, K.P.; Cabanillas, M.E.; Broaddus, R.R.; Williams, M.D. Molecular profile of advanced thyroid carcinomas by next-generation sequencing: Characterizing tumors beyond diagnosis for targeted therapy. Mol. Cancer Ther. 2018, 17, 1575–1584. [Google Scholar] [CrossRef]
- Landa, I.; Ibrahimpasic, T.; Boucai, L.; Sinha, R.; Knauf, J.A.; Shah, R.H.; Dogan, S.; Ricarte-Filho, J.C.; Krishnamoorthy, G.P.; Xu, B.; et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J. Clin. Investig. 2016, 126, 1052–1066. [Google Scholar] [CrossRef]
- Xu, B.; Ghossein, R. Genomic landscape of poorly differentiated and anaplastic thyroid carcinoma. Endocr. Pathol. 2016, 27, 205–212. [Google Scholar] [CrossRef]
- Haroon Al Rasheed, M.R.; Xu, B. Molecular alterations in thyroid carcinoma. Surg. Pathol. Clin. 2019, 12, 921–930. [Google Scholar] [CrossRef]
- Acquaviva, G.; Visani, M.; Repaci, A.; Rhoden, K.J.; de Biase, D.; Pession, A.; Giovanni, T. Molecular pathology of thyroid tumours of follicular cells: A review of genetic alterations and their clinicopathological relevance. Histopathology 2018, 72, 6–31. [Google Scholar] [CrossRef]
- Wong, K.S.; Lorch, J.H.; Alexander, E.K.; Nehs, M.A.; Nowak, J.A.; Hornick, J.L.; Barletta, J.A. Clinicopathologic features of mismatch repair-deficient anaplastic thyroid carcinomas. Thyroid 2019, 29, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.M.; Laird, P.W.; Park, P.J. The landscape of microsatellite instability in colorectal and endometrial cancer genomes. Cell 2013, 155, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; Cho, J.Y.; Schellens, J.H.M.; Soria, J.C.; Wen, P.Y.; Zielinski, C.; Cabanillas, M.E.; Urbanowitz, G.; et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600–mutant anaplastic thyroid cancer. J. Clin. Oncol. 2018, 36, 7–13. [Google Scholar] [CrossRef]
- Wang, J.R.; Zafereo, M.E.; Dadu, R.; Ferrarotto, R.; Busaidy, N.L.; Lu, C.; Ahmed, S.; Gule-Monroe, M.K.; Williams, M.D.; Sturgis, E.M.; et al. Complete surgical resection following neoadjuvant dabrafenib plus trametinib in BRAFV600E-mutated anaplastic thyroid carcinoma. Thyroid 2019, 29, 1036–1043. [Google Scholar] [CrossRef]
- Khan, I.; Rhett, J.M.; O’Bryan, J.P. Therapeutic targeting of RAS: New hope for drugging the “undruggable”. Biochim. Biophys. Acta 2020, 1867, 118570. [Google Scholar] [CrossRef] [PubMed]
- Yakushina, V.D.; Lerner, L.V.; Lavrov, A.V. Gene fusions in thyroid cancer. Thyroid 2017, 28, 158–167. [Google Scholar] [CrossRef]
- ElMokh, O.; Ruffieux-Daidié, D.; Roelli, M.A.; Stooss, A.; Phillips, W.A.; Gertsch, J.; Dettmer, M.S.; Charles, R.-P. Combined MEK and PI3-kinase inhibition reveals synergy in targeting thyroid cancer in vitro and in vivo. Oncotarget 2017, 8, 24604–24620. [Google Scholar] [CrossRef]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef]
- Haraldsdottir, S.; Hampel, H.; Tomsic, J.; Frankel, W.L.; Pearlman, R.; de la Chapelle, A.; Pritchard, C.C. Colon and endometrial cancers with mismatch repair deficiency can arise from somatic, rather than germline, mutations. Gastroenterology 2014, 147, 1308–1316. [Google Scholar] [CrossRef]
- Gong, J.; Wang, C.; Lee, P.P.; Chu, P.; Fakih, M. Response to PD-1 blockade in microsatellite stable metastatic colorectal cancer harboring a POLE mutation. J. Natl. Compr. Cancer Netw. 2017, 15, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Konstantinopoulos, P.A.; Luo, W.; Liu, J.F.; Gulhan, D.C.; Krasner, C.; Ishizuka, J.J.; Gockley, A.A.; Buss, M.; Growdon, W.B.; Crowe, H.; et al. Phase II study of avelumab in patients with mismatch repair deficient and mismatch repair proficient recurrent/persistent endometrial cancer. J. Clin. Oncol. 2019, 37, 2786–2794. [Google Scholar] [CrossRef] [PubMed]
- Ravi, N.; Yang, M.; Gretarsson, S.; Jansson, C.; Mylona, N.; Sydow, S.R.; Woodward, E.L.; Ekblad, L.; Wennerberg, J.; Paulsson, K. Identification of targetable lesions in anaplastic thyroid cancer by genome profiling. Cancers 2019, 11, 402. [Google Scholar] [CrossRef] [PubMed]
- Pozdeyev, N.; Gay, L.M.; Sokol, E.S.; Hartmaier, R.; Deaver, K.E.; Davis, S.; French, J.D.; Borre, P.V.; LaBarbera, D.V.; Tan, A.-C.; et al. Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clin. Cancer Res. 2018, 24, 3059–3068. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Osamura, R.Y.; Klöppel, G.; Rosai, J. WHO Classification of Tumours of Endocrine Organs, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2017. [Google Scholar]
- Santarpia, L.; El-Naggar, A.K.; Cote, G.J.; Myers, J.N.; Sherman, S.I. Phosphatidylinositol 3-kinase/Akt and Ras/Raf-mitogen-activated protein kinase pathway mutations in anaplastic thyroid cancer. J. Clin. Endocr. Metab. 2008, 93, 278–284. [Google Scholar] [CrossRef]
- Church, D.N.; Briggs, S.E.W.; Palles, C.; Domingo, E.; Kearsey, S.J.; Grimes, J.M.; Gorman, M.; Martin, L.; Howarth, K.M.; Hodgson, S.V.; et al. DNA polymerase ε and δ exonuclease domain mutations in endometrial cancer. Hum. Mol. Genet. 2013, 22, 2820–2828. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- DePristo, M.A.; Banks, E.; Poplin, R.; Garimella, K.V.; Maguire, J.R.; Hartl, C.; Philippakis, A.A.; del Angel, G.; Rivas, M.A.; Hanna, M.; et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 2011, 43, 491–498. [Google Scholar] [CrossRef]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.S.; Thormann, A.; Flicek, P.; Cunningham, F. The ensembl variant effect predictor. Genome Biol. 2016, 17, 122. [Google Scholar] [CrossRef]
- García-Rostán, G.; Costa, A.M.; Pereira-Castro, I.; Salvatore, G.; Hernandez, R.; Hermsem, M.J.A.; Herrero, A.; Fusco, A.; Cameselle-Teijeiro, J.; Santoro, M. Mutation of the PIK3CA gene in anaplastic thyroid cancer. Cancer Res. 2005, 65, 10199–10207. [Google Scholar] [CrossRef]

| Age, n (%) | ||
| ≤55 | 4 (10.3) | |
| >55 | 35 (89.7) | |
| Gender, n (%) | ||
| Female | 20 (51.3) | |
| Male | 19 (48.7) | |
| Stage, n (%) a | ||
| IVA | 5 (13.9) | |
| IVB | 11 (30.6) | |
| IVC | 20 (55.6) | |
| Surgery, n (%) | ||
| Subtotal thyroidectomy | 9 (23.1) | |
| Total thyroidectomy | 24 (61.5) | |
| Biopsy only | 6 (15.4) | |
| Other Therapies b, n (%) | ||
| Chemotherapy | 7 (24.1) | |
| Radioiodine | 6 (21.4) | |
| External radiation | 7 (25) | |
| Targeted therapy | 5 (15.1) | |
| Tumor size c (cm) | 7.14 | |
| Histologic Pattern, n (%) | ||
| Epithelial | + | 28 (71.8%) |
| - | 11 (28.2%) | |
| Giant cell | + | 5 (12.8%) |
| - | 34 (87.2%) | |
| Sarcomatoid | + | 17 (43.6%) |
| - | 22 (56.4%) | |
| Coexisting WDTC, n (%) | ||
| PTC | + | 10 (25.6) |
| - | 29 (74.4) | |
| FTC | + | 3 (7.7) |
| - | 36 (92.3) | |
| Case Number | n (%) | ||||
|---|---|---|---|---|---|
| PAX8 | TTF-1 | BRAF VE1 | Retained MMR Proteins | ||
| All | 39 | 22 (56.4) | 2 (5.1) | 10 (25.6) | 39 (100) |
| Coexisting WDTC | |||||
| None | 26 | 15 (5.8) | 2 (7.7) | 4 (15.4) | 26 (100) |
| PTC | 10 | 7 (70) | 0 | 6 (60) | 10 (100) |
| FTC | 3 | 0 | 0 | 0 | 3 (100) |
| BRAF | RAS | Non-BRAF/RAS | p Value | ||
|---|---|---|---|---|---|
| Case Number | 7 | 11 | 9 | ||
| Age, n (%) | |||||
| ≤55 | 1 (14.3) | 1 (9.1) | 2 (22.2) | 0.803 | |
| >55 | 6 (85.7) | 10 (90.9) | 7 (77.8) | ||
| Gender, n (%) | |||||
| Female | 2 (28.6) | 10 (90.9) | 4 (44.4) | 0.012 * | |
| Male | 5 (71.4) | 1 (9.1) | 5 (55.6) | ||
| Stage, n (%) a | |||||
| IVA | 1 (14.3) | 0 (0) | 2 (25) | 0.445 | |
| IVB | 3 (42.9) | 4 (44.4) | 1 (12.5) | ||
| IVC | 3 (42.9) | 5 (55.6) | 5 (50) | ||
| Surgery, n (%) | |||||
| Subtotal Tx | 1 (14.3) | 2 (18.2) | 4 (44.4) | 0.488 | |
| Total Tx | 6 (85.7) | 8 (72.7) | 4 (44.4) | ||
| Biopsy only | 0 | 1 (9.1) | 1 (11.1) | ||
| Other Therapies b, n (%) | |||||
| Chemotherapy | 1 (16.7) | 3 (33.3) | 2 (33.3) | 0.851 | |
| Radioiodine | 1 (16.7) | 2 (22.2) | 0 (0) | 0.763 | |
| External radiation | 1 (16.7) | 4 (44.4) | 1 (20) | 0.568 | |
| Targeted therapy | 2 (33.3) | 1 (10) | 0 (0) | 0.314 | |
| Tumor size, cm | 5.1 | 7.8 | 7.5 | 0.253 | |
| Histologic Pattern, n (%) | |||||
| Epithelial | + | 6 (85.7) | 8 (72.7) | 6 (66.7) | 0.860 |
| - | 1(14.3) | 3 (27.3) | 3 (33.3) | ||
| Giant cell | + | 1 (14.3) | 1 (9.1) | 1 (11.1) | 1 |
| - | 6 (85.7) | 10 (90.9) | 8 (88.9) | ||
| Sarcomatoid | + | 1 (14.3) | 5 (45.5) | 7 (77.8) | 0.045 * |
| - | 6 (85.7) | 6 (54.5) | 2 (22.2) | ||
| Coexisting WDTC, n (%) | |||||
| PTC | + | 3 (42.9) | 2 (18.2) | 1 (11.1) | 0.369 |
| - | 4 (57.1) | 9 (81.8) | 8 (88.9) | ||
| FTC | + | 0 (0) | 1 (9.1) | 1 (11.1) | 1.000 |
| - | 7 (100) | 10 (90.9) | 8 (88.9) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, W.-A.; Liu, C.-Y.; Lin, S.-Y.; Chen, C.-C.; Hang, J.-F. Characterization of Driver Mutations in Anaplastic Thyroid Carcinoma Identifies RAS and PIK3CA Mutations as Negative Survival Predictors. Cancers 2020, 12, 1973. https://doi.org/10.3390/cancers12071973
Lai W-A, Liu C-Y, Lin S-Y, Chen C-C, Hang J-F. Characterization of Driver Mutations in Anaplastic Thyroid Carcinoma Identifies RAS and PIK3CA Mutations as Negative Survival Predictors. Cancers. 2020; 12(7):1973. https://doi.org/10.3390/cancers12071973
Chicago/Turabian StyleLai, Wei-An, Chih-Yi Liu, Shih-Yao Lin, Chien-Chin Chen, and Jen-Fan Hang. 2020. "Characterization of Driver Mutations in Anaplastic Thyroid Carcinoma Identifies RAS and PIK3CA Mutations as Negative Survival Predictors" Cancers 12, no. 7: 1973. https://doi.org/10.3390/cancers12071973
APA StyleLai, W.-A., Liu, C.-Y., Lin, S.-Y., Chen, C.-C., & Hang, J.-F. (2020). Characterization of Driver Mutations in Anaplastic Thyroid Carcinoma Identifies RAS and PIK3CA Mutations as Negative Survival Predictors. Cancers, 12(7), 1973. https://doi.org/10.3390/cancers12071973






