Dual-Agent Photodynamic Therapy with Optical Clearing Eradicates Pigmented Melanoma in Preclinical Tumor Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Optical Clearing Agent
2.2. Cutaneous Melanoma Model
2.3. PDT Treatment
2.4. Treatment Response Evaluation
2.5. Histology Analysis
2.6. Statistical Analysis
2.7. PDT Vascular Effects
3. Results
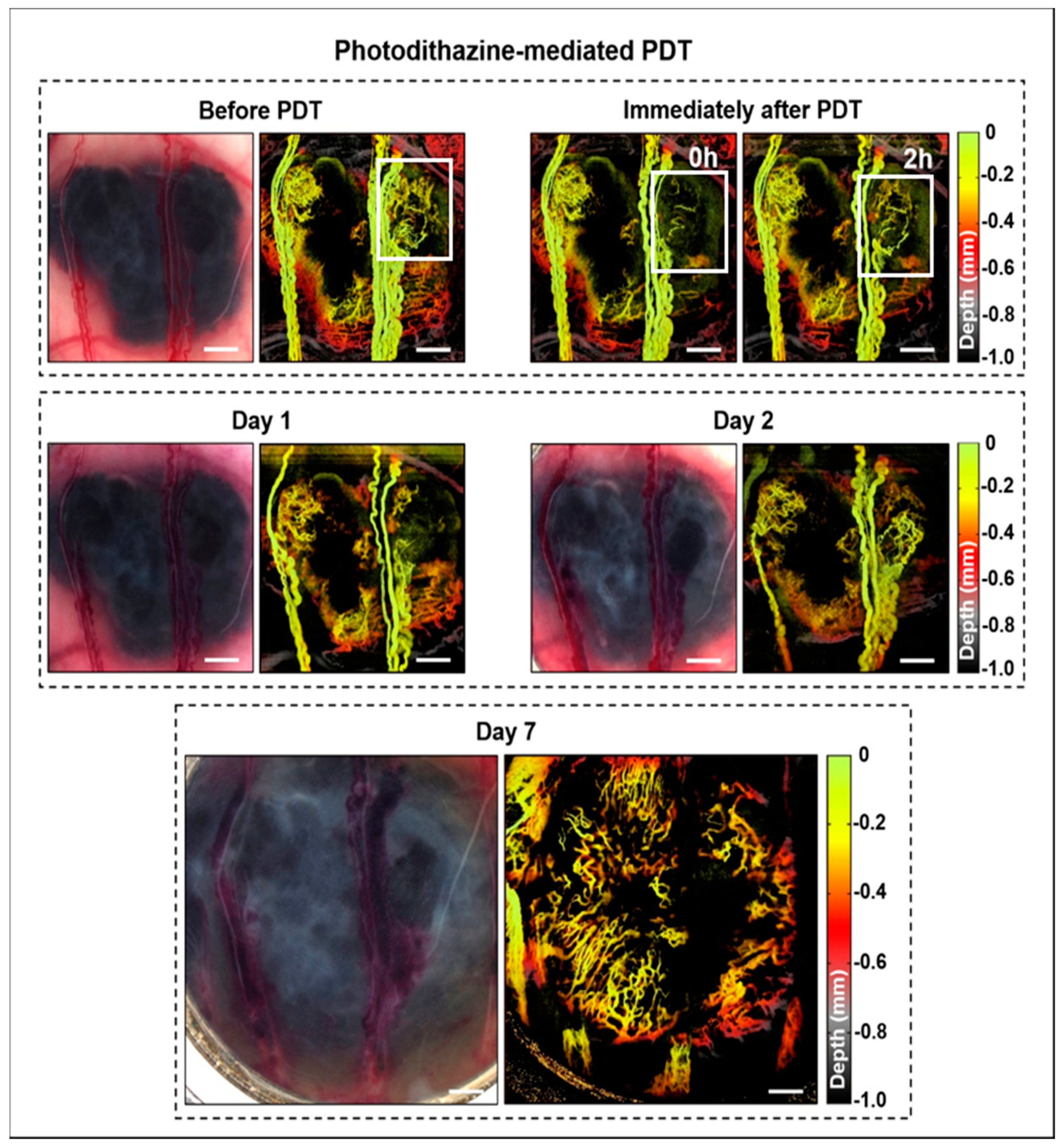
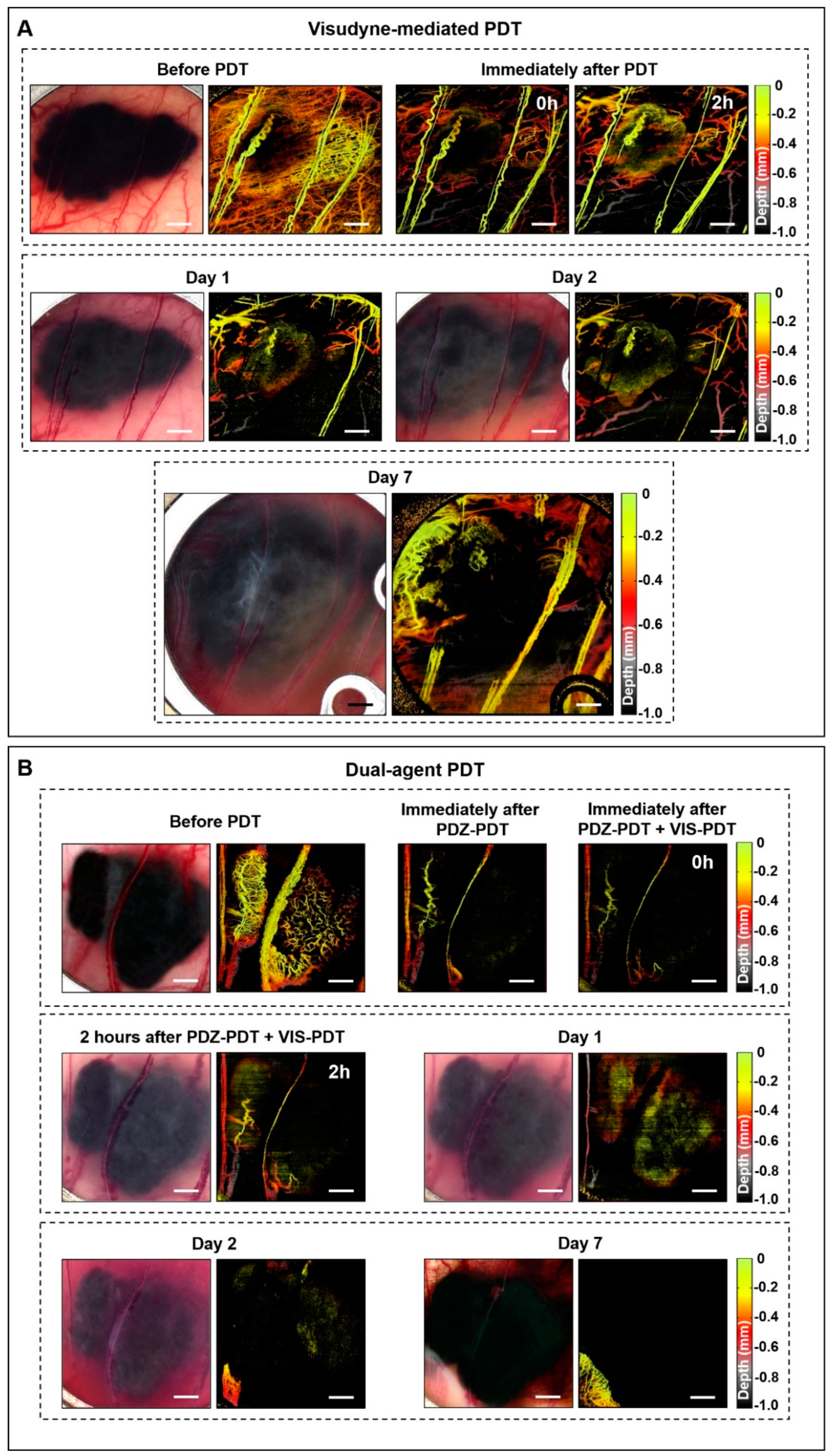
3.1. Window Chamber Model Responses
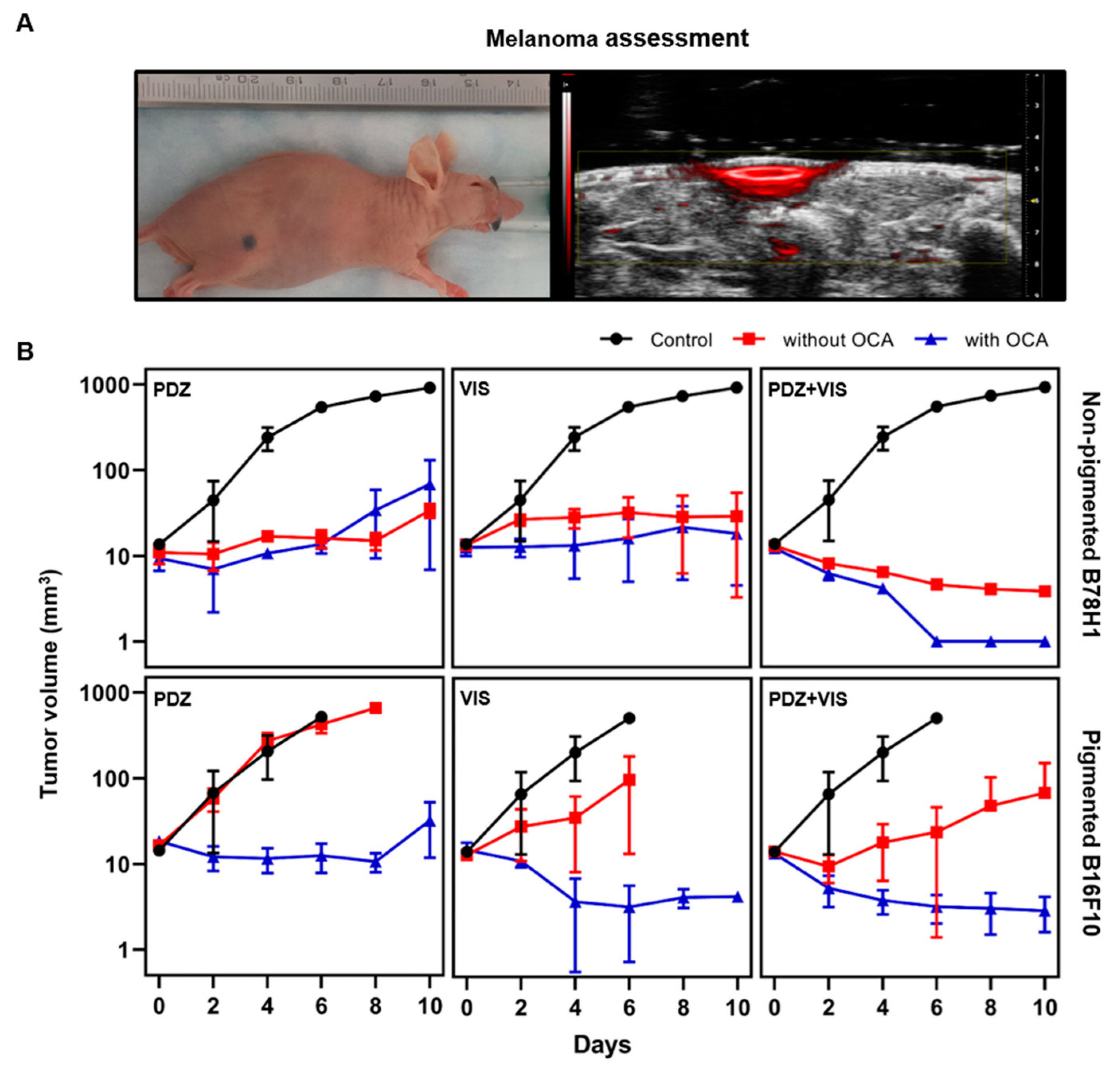
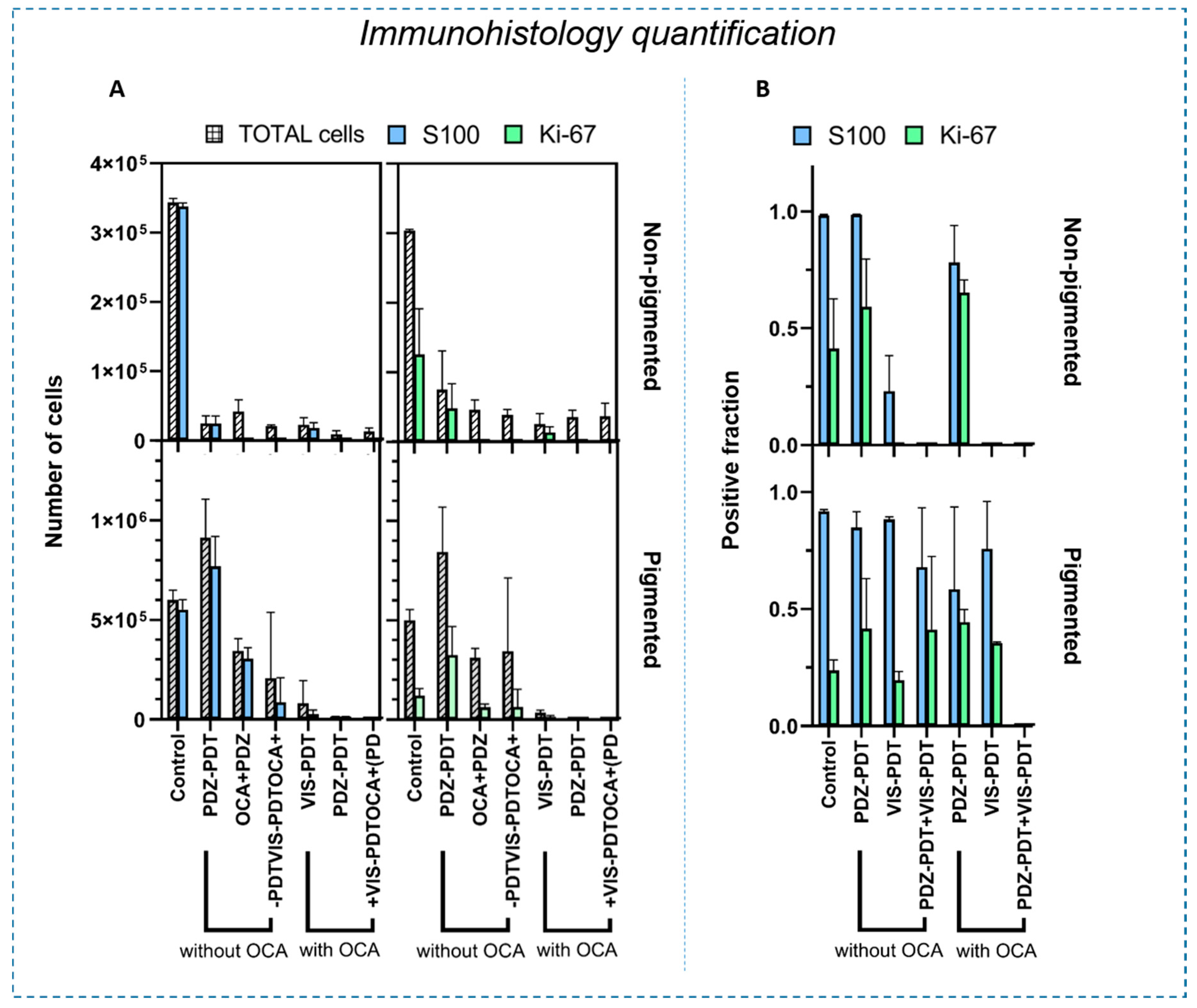
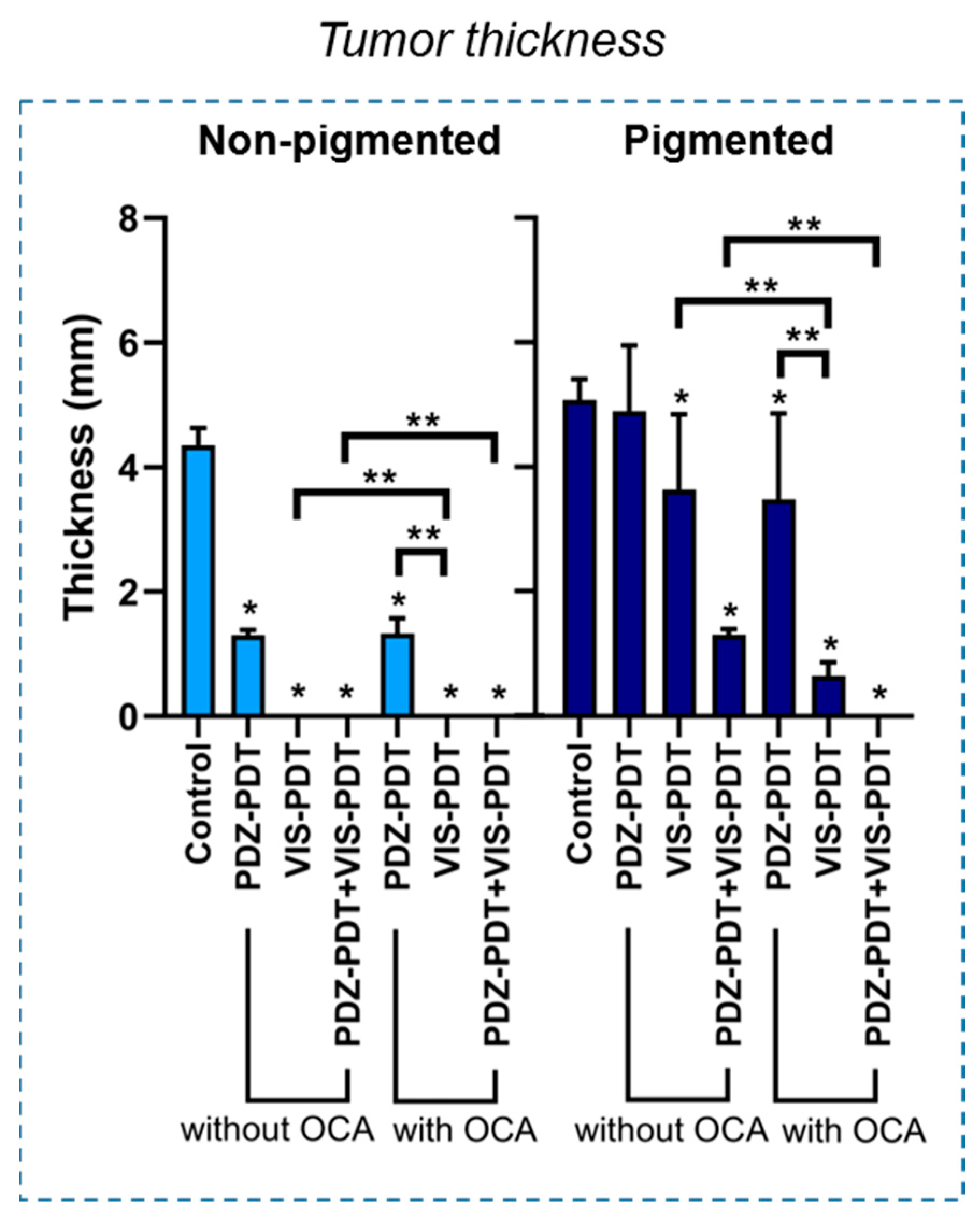
3.2. Intradermal Melanoma Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Street, W. Cancer Facts & Figures 2020; American Cancer Society: Atlanta, GA, USA, 2020. [Google Scholar]
- Domingues, B.; Lopes, J.M.; Soares, P.; Pópulo, H. Melanoma treatment in review. Immunotargets Ther. 2018, 7, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.; Valecha, G.; Sahni, A. Role of Anti-PD-1 Antibodies in Advanced Melanoma: The Era of Immunotherapy. Cureus 2018, 10, e3700. [Google Scholar] [CrossRef] [PubMed]
- Koolen, P.G.L.; Matos, T.R.; Ibrahim, A.M.S.; Sun, J.; Lee, B.T.; Frankenthaler, R.A.; Lin, S.J. Recurrence Rates Over 20 Years in the Treatment of Malignant Melanoma: Immediate Versus Delayed Reconstruction. Plast. Reconstr. Surg. Glob. Open 2017, 5. [Google Scholar] [CrossRef]
- McClain, S.E.; Mayo, K.B.; Shada, A.L.; Smolkin, M.E.; Patterson, J.W.; Slingluff, C.L. Amelanotic Melanomas Presenting as Red Skin Lesions: A Diagnostic Challenge with Potentially Lethal Consequences. Int. J. Dermatol. 2012, 51, 420–426. [Google Scholar] [CrossRef]
- Ramirez, D.P.; Kurachi, C.; Inada, N.M.; Moriyama, L.T.; Salvio, A.G.; Vollet Filho, J.D.; Pires, L.; Buzzá, H.H.; de Andrade, C.T.; Greco, C.; et al. Experience and BCC subtypes as determinants of MAL-PDT response: Preliminary results of a national Brazilian project. Photodiagnosis Photodyn. Ther. 2014, 11, 22–26. [Google Scholar] [CrossRef]
- Baldea, I.; Filip, A.G. Photodynamic therapy in melanoma—An update. J. Physiol. Pharmacol. 2012, 63, 109–118. [Google Scholar]
- Castano, A.P.; Mroz, P.; Wu, M.X.; Hamblin, M.R. Photodynamic therapy plus low-dose cyclophosphamide generates antitumor immunity in a mouse model. Proc. Natl. Acad. Sci. USA 2008, 105, 5495–5500. [Google Scholar] [CrossRef]
- Ferrario, A.; Lim, S.; Xu, F.; Luna, M.; Gaffney, K.J.; Petasis, N.A.; Schönthal, A.H.; Gomer, C.J. Enhancement of photodynamic therapy by 2,5-dimethyl celecoxib, a non-cyclooxygenase-2 inhibitor analog of celecoxib. Cancer Lett. 2011, 304, 33–40. [Google Scholar] [CrossRef]
- Pellosi, D.S.; De Jesus, P.d.C.C.; Tedesco, A.C. Spotlight on the delivery of photosensitizers: Different approaches for photodynamic-based therapies. Expert Opin. Drug Deliv. 2017, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Zhang, L.; Qian, M.; Bao, Y.; Wang, J.; Li, Y. Specific light-up pullulan-based nanoparticles with reduction-triggered emission and activatable photoactivity for the imaging and photodynamic killing of cancer cells. J. Colloid. Interface Sci. 2017, 498, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A.; Stockert, J.C.; Cañete, M.; Acedo, P. A new protocol in photodynamic therapy: Enhanced tumour cell death by combining two different photosensitizers. Photochem. Photobiol. Sci. 2010, 9, 295. [Google Scholar] [CrossRef] [PubMed]
- Busetti, A.; Soncin, M.; Jori, G.; Rodgers, M.A.J. High efficiency of benzoporphyrin derivative in the photodynamic therapy of pigmented malignant melanoma. Br. J. Cancer 1999, 79, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zheng, W.; Li, Y.; Zhong, J.; Ji, J.; Shen, P. Apoptosis induced by methylene-blue-mediated photodynamic therapy in melanomas and the involvement of mitochondrial dysfunction revealed by proteomics. Cancer Sci. 2008. [Google Scholar] [CrossRef] [PubMed]
- Mazor, O.; Brandis, A.; Plaks, V.; Neumark, E.; Rosenbach-Belkin, V.; Salomon, Y.; Scherz, A. WST11, A Novel Water-Soluble Bacteriochlorophyll Derivative; Cellular uptake, Pharmacokinetics, Biodistribution, and Vascular Targeted Photodynamic Activity Against Melanoma tumors. Photochem. Photobiol. 2004. [Google Scholar] [CrossRef]
- Pucelik, B.; Arnaut, L.G.; Stochel, G.; Dąbrowski, J.M. Design of Pluronic-Based Formulation for Enhanced Redaporfin-Photodynamic Therapy against Pigmented Melanoma. ACS Appl. Mater. Interfaces 2016, 8, 22039–22055. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Vecchio, D.; Avci, P.; Yin, R.; Garcia-Diaz, M.; Hamblin, M.R. Melanoma resistance to photodynamic therapy: New insights. Boil. Chem. 2013, 394, 239–250. [Google Scholar] [CrossRef]
- Garcia-Uribe, A.; Zou, J.; Duvic, M.; Cho-Vega, J.H.; Prieto, V.G.; Wang, L.V. In Vivo Diagnosis of Melanoma and Nonmelanoma Skin Cancer Using Oblique Incidence Diffuse Reflectance Spectrometry. Cancer Res. 2012, 72, 2738–2745. [Google Scholar] [CrossRef]
- Cicchi, R.; Pavone, F.S.; Massi, D.; Sampson, D.D. Contrast and depth enhancement in two-photon microscopy of human skin ex vivo by use of optical clearing agents. Opt. Express. 2005, 13, 2337. [Google Scholar] [CrossRef]
- Genina, E.A.; Bashkatov, A.N.; Tuchin, V.V. Tissue optical immersion clearing. Expert. Rev. Med. Devices 2010, 7, 825–842. [Google Scholar] [CrossRef]
- Pires, L.; Demidov, V.; Vitkin, I.A.; Bagnato, V.; Kurachi, C.; Wilson, B.C. Optical clearing of melanoma in vivo: Characterization by diffuse reflectance spectroscopy and optical coherence tomography. J. Biomed. Opt. 2016, 21, 081210. [Google Scholar] [CrossRef]
- Liopo, A.; Su, R.; Tsyboulski, D.A.; Oraevsky, A.A. Optical clearing of skin enhanced with hyaluronic acid for increased contrast of optoacoustic imaging. J. Biomed. Opt. 2016, 21, 081208. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.H.; Choi, B.; Chess, S.; Kelly, K.M.; McCullough, J.; Nelson, J.S. Optical clearing of in vivo human skin: Implications for light-based diagnostic imaging and therapeutics. Lasers Surg. Med. 2004, 34, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.H.; From, L.; Bernardino, E.A.; Mihm, M.C. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969, 29, 705–727. [Google Scholar]
- Wen, X.; Jacques, S.L.; Tuchin, V.V.; Zhu, D. Enhanced optical clearing of skin in vivo and optical coherence tomography in-depth imaging. J. Biomed. Opt. 2012, 17, 0660221–0660226. [Google Scholar] [CrossRef] [PubMed]
- Olesen, C.M.; Fuchs, C.S.K.; Philipsen, P.A.; Hædersdal, M.; Agner, T.; Clausen, M.-L. Advancement through epidermis using tape stripping technique and Reflectance Confocal Microscopy. Sci. Rep. 2019, 9, 1–6. [Google Scholar] [CrossRef]
- Wilson, B.C.; Patterson, M.S. The physics, biophysics and technology of photodynamic therapy. Phys. Med. Boil. 2008, 53, R61–R109. [Google Scholar] [CrossRef]
- Chen, E.; Brown, D.M.; Wong, T.P.; Benz, M.S.; Kegley, E.; Cox, J.; Fish, R.H.; Kim, R.Y. Lucentis® using Visudyne® study: Determining the threshold-dose fluence of verteporfin photodynamic therapy combined with intravitreal ranibizumab for exudative macular degeneration. Clin. Ophthalmol. 2010, 4, 1073–1079. [Google Scholar] [CrossRef]
- Michy, T.; Massias, T.; Bernard, C.; Vanwonterghem, L.; Henry, M.; Guidetti, M.; Royal, G.; Coll, J.-L.; Texier, I.; Josserand, V.; et al. Verteporfin-Loaded Lipid Nanoparticles Improve Ovarian Cancer Photodynamic Therapy In Vitro and In Vivo. Cancers (Basel) 2019, 11, 1760. [Google Scholar] [CrossRef] [PubMed]
- Mehrara, E.; Forssell-Aronsson, E.; Ahlman, H.; Bernhardt, P. Specific Growth Rate versus Doubling Time for Quantitative Characterization of Tumor Growth Rate. Cancer Res. 2007, 67, 3970–3975. [Google Scholar] [CrossRef]
- Melezinek, I.; Borovansky, J.; Elleder, M. Characterization of s100 proteins of murine melanomas. Med. Sci. Monit. 1999, 5, BR828–BR832. [Google Scholar]
- Ma, X.; Wu, Y.; Zhang, T.; Song, H.; Jv, H.; Guo, W.; Ren, G. Ki67 Proliferation Index as a Histopathological Predictive and Prognostic Parameter of Oral Mucosal Melanoma in Patients without Distant Metastases. J. Cancer 2017, 8, 3828–3837. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; DaCosta, R.S. Optimization of the dorsal skinfold window chamber model and multi-parametric characterization of tumor-associated vasculature. Intravital 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Flueraru, C.; Chang, S.; Popescu, D.P.; Sowa, M. High-Quality Tissue Imaging Using a Catheter-Based Swept-Source Optical Coherence Tomography Systems With an Integrated Semiconductor Optical Amplifier. IEEE Trans. Instrum. Meas. 2011, 60, 3376–3383. [Google Scholar] [CrossRef]
- Mariampillai, A.; Leung, M.K.K.; Jarvi, M.; Standish, B.A.; Lee, K.; Wilson, B.C.; Vitkin, A.; Yang, V.X.D. Optimized speckle variance OCT imaging of microvasculature. Opt. Lett. 2010, 35, 1257. [Google Scholar] [CrossRef] [PubMed]
- Demidov, V.; Maeda, A.; Sugita, M.; Madge, V.; Sadanand, S.; Flueraru, C.; Vitkin, I.A. Preclinical longitudinal imaging of tumor microvascular radiobiological response with functional optical coherence tomography. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Verteporfin Roundtable 2000 and 2001 Participants; Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group Principal Investigators; Verteporfin in Photodynamic Therapy (VIP) Study Group Principal Investigators. Guidelines for using verteporfin (visudyne) in photodynamic therapy to treat choroidal neovascularization due to age-related macular degeneration and other causes. Retina (Philadelphia, Pa.) 2002, 22, 6–18. [Google Scholar] [CrossRef][Green Version]
- Streit, M.; Detmar, M. Angiogenesis, lymphangiogenesis, and melanoma metastasis. Oncogene 2003, 22, 3172–3179. [Google Scholar] [CrossRef]
- Bedogni, B.; Powell, M.B. Hypoxia, melanocytes and melanoma–survival and tumor development in the permissive microenvironment of the skin. Pigment. Cell Melanoma Res. 2009, 22, 166–174. [Google Scholar] [CrossRef]
- Huber, R.; Meier, B.; Otsuka, A.; Fenini, G.; Satoh, T.; Gehrke, S.; Widmer, D.; Levesque, M.P.; Mangana, J.; Kerl, K.; et al. Tumour hypoxia promotes melanoma growth and metastasis via High Mobility Group Box-1 and M2-like macrophages. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Cordoba, F.; Braathen, L.R.; Weissenberger, J.; Vallan, C.; Kato, M.; Nakashima, I.; Weis, J.; von Felbert, V. 5-aminolaevulinic acid photodynamic therapy in a transgenic mouse model of skin melanoma. Exp. Dermatol. 2005, 14, 429–437. [Google Scholar] [CrossRef]
- Akasov, R.A.; Sholina, N.V.; Khochenkov, D.A.; Alova, A.V.; Gorelkin, P.V.; Erofeev, A.S.; Generalova, A.N.; Khaydukov, E.V. Photodynamic therapy of melanoma by blue-light photoactivation of flavin mononucleotide. Sci. Rep. 2019, 9, 9679. [Google Scholar] [CrossRef] [PubMed]
- Woodburn, K.W.; Fan, Q.; Kessel, D.; Luo, Y.; Young, S.W. Photodynamic Therapy of B16F10 Murine Melanoma with Lutetium Texaphyrin. J. Investig. Dermatol. 1998, 110, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.; Blumenfeld, A.; Siegal, A.; Kaplan, O.; Cohen, M.; Skornick, Y.; Kashtan, H. In vitro and in vivo effects of photodynamic therapy on murine malignant melanoma. Ann. Surg. Oncol. 1998, 5, 241–247. [Google Scholar] [CrossRef]
- Lim, D.-S.; Ko, S.-H.; Lee, W.-Y. Silkworm-pheophorbide a mediated photodynamic therapy against B16F10 pigmented melanoma. J. Photochem. Photobiol. B: Boil. 2004, 74, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Carmello, J.C.; Alves, F.; Basso, F.G.; de Souza Costa, C.A.; Bagnato, V.S.; Mima, E.G.; Pavarina, A.C. Treatment of Oral Candidiasis Using Photodithazine®-Mediated Photodynamic Therapy In Vivo. PLoS ONE 2016, 11, e0156947. [Google Scholar] [CrossRef]
- Han, S.J.; Wen, L.Y.; Bae, S.M.; Hong, Y.-S.; Jang, H.-S.; Lee, J.W.; Ahn, W.S. Photodynamic effects of Photodithazine on cervical cancer model. In Photodynamic Therapy: Back to the Future; International Society for Optics and Photonics: Bellingham, WA, USA, 2009; Volume 7380, p. 73805Z. [Google Scholar]
- Bressler, N.M.; Bressler, S.B. Photodynamic therapy with verteporfin (Visudyne): Impact on ophthalmology and visual sciences. Investig. Ophthalmol. Vis. Sci. 2000, 41, 624–628. [Google Scholar]
- Acedo, P.; Stockert, J.C.; Canete, M. Villanueva, Ángeles Two combined photosensitizers: A goal for more effective photodynamic therapy of cancer. Cell Death Dis. 2014, 5, e1122. [Google Scholar] [CrossRef]
- 17th International Photodynamic Association World Congress, Conference Details. Available online: https://spie.org/PDT/conferencedetails/17th-international-photodynamic-association-world-congress?SSO=1 (accessed on 5 February 2020).
- Lajis, A.F.B.; Hamid, M.; Ariff, A.B.; Lajis, A.F.B.; Hamid, M.; Ariff, A.B. Depigmenting Effect of Kojic Acid Esters in Hyperpigmented B16F1 Melanoma Cells. J. Biomed. Biotechnol. 2012, 2012, e952452. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Singh, T.R.R.; Morrow, D.I.J.; Woolfson, A.D. Transdermal Drug Delivery. In Microneedle-Mediated Transdermal and Intradermal Drug Delivery; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 1–19. ISBN 978-1-119-95968-7. [Google Scholar]
- Reginato, E.; Wolf, P.; Hamblin, M.R. Immune response after photodynamic therapy increases anti-cancer and anti-bacterial effects. World J. Immunol. 2014, 4, 1–11. [Google Scholar] [CrossRef]
- Ananth, A.A.; Tai, L.-H.; Lansdell, C.; Alkayyal, A.A.; Baxter, K.E.; Angka, L.; Zhang, J.; De Souza, C.T.; Stephenson, K.B.; Parato, K.; et al. Surgical Stress Abrogates Pre-Existing Protective T Cell Mediated Anti-Tumor Immunity Leading to Postoperative Cancer Recurrence. PLoS ONE 2016, 11, e0155947. [Google Scholar] [CrossRef]
| Group | OCA | Metrics | Non-Pigmented Melanoma | Pigmented Melanoma |
|---|---|---|---|---|
| PDZ-PDT | Without OCA | SGR reduced >80% | Yes | No |
| Thickness <1 mm | No | No | ||
| Ki-67 positive fraction <99% | No | No | ||
| With OCA | SGR reduced >80% | Yes | No | |
| Thickness <1 mm | No | No | ||
| Ki-67 positive fraction <99% | No | No | ||
| VIS-PDT | Without OCA | SGR reduced >80% | Yes | No |
| Thickness <1 mm | Yes | No | ||
| Ki-67 positive fraction <99% | Yes | No | ||
| With OCA | SGR reduced >80% | Yes | Yes | |
| Thickness <1 mm | Yes | Yes | ||
| Ki-67 positive fraction <99% | Yes | No | ||
| PDZ-PDT+ VIS-PDT | Without OCA | SGR reduced >80% | Yes | Yes |
| Thickness <1 mm | Yes | No | ||
| Ki-67 positive fraction <99% | Yes | No | ||
| With OCA | SGR reduced >80% | Yes | Yes | |
| Thickness <1 mm | Yes | Yes | ||
| Ki-67 positive fraction <99% | Yes | Yes |
| Specific Growth Rate (SGR) and Doubling Time (DT) | ||||
|---|---|---|---|---|
| Non-Pigmented | Pigmented | |||
| SGR (mm3/day) | DT (day) | SGR (mm3/day) | DT (day) | |
| Control | 0.50 ± 0.15 | 1.34 ± 0.35 | 0.68 ± 0.09 | 1.03 ± 0.13 |
| PDZ-PDT | 0.09 ± 0.04 * | 9.90 ± 6.38 | 0.62 ± 0.08 | 1.12 ± 0.15 |
| OCA+PDZ-PDT | 0.13 ± 0.09 * | 9.71 ± 10.10 | −0.04 ± 0.09 ** | −1.40 ± 12.32 |
| VIS-PDT | 0.11 ± 0.04 * | 6.72 ± 2.88 | 0.32 ± 0.07 * | 2.22 ± 0.49 |
| OCA+VIS-PDT | 0.02 ± 0.17 * | 2.61 ± 5.60 | −0.25 ± 0.10 ** | −3.12 ± 1.36 |
| PDZ-PDT+VIS-PDT | −0.15 ± 0.00 * | −4.79 ± 0.07 | 0.07 ± 0.02 * | 10.15 ± 1.94 |
| OCA+(PDZ-PDT+VIS-PDT) | −0.27 ± 0.001 * | −2.58 ± 0.001 | −0.24 ± 0.01 ** | −2.92 ± 0.46 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires, L.; Demidov, V.; Wilson, B.C.; Salvio, A.G.; Moriyama, L.; Bagnato, V.S.; Vitkin, I.A.; Kurachi, C. Dual-Agent Photodynamic Therapy with Optical Clearing Eradicates Pigmented Melanoma in Preclinical Tumor Models. Cancers 2020, 12, 1956. https://doi.org/10.3390/cancers12071956
Pires L, Demidov V, Wilson BC, Salvio AG, Moriyama L, Bagnato VS, Vitkin IA, Kurachi C. Dual-Agent Photodynamic Therapy with Optical Clearing Eradicates Pigmented Melanoma in Preclinical Tumor Models. Cancers. 2020; 12(7):1956. https://doi.org/10.3390/cancers12071956
Chicago/Turabian StylePires, Layla, Valentin Demidov, Brian C. Wilson, Ana Gabriela Salvio, Lilian Moriyama, Vanderlei S. Bagnato, I. Alex Vitkin, and Cristina Kurachi. 2020. "Dual-Agent Photodynamic Therapy with Optical Clearing Eradicates Pigmented Melanoma in Preclinical Tumor Models" Cancers 12, no. 7: 1956. https://doi.org/10.3390/cancers12071956
APA StylePires, L., Demidov, V., Wilson, B. C., Salvio, A. G., Moriyama, L., Bagnato, V. S., Vitkin, I. A., & Kurachi, C. (2020). Dual-Agent Photodynamic Therapy with Optical Clearing Eradicates Pigmented Melanoma in Preclinical Tumor Models. Cancers, 12(7), 1956. https://doi.org/10.3390/cancers12071956





