The Role of Palliative Radiotherapy in the Treatment of Spinal Bone Metastases from Head and Neck Tumors—A Multicenter Analysis of a Rare Event
Abstract
1. Introduction
2. Material and methods
2.1. Patient Selection
2.2. Response Assessment
2.3. Treatment
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef]
- Bernier, J.; Domenge, C.; Ozsahin, M.; Matuszewska, K.; Lefebvre, J.L.; Greiner, R.H.; Giralt, J.; Maingon, P.; Rolland, F.; Bolla, M.; et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N. Engl. J. Med. 2004, 350, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Pignon, J.P.; le Maitre, A.; Maillard, E.; Bourhis, J. Group M-NC: Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother. Oncology J. Eur. Soc. Ther. Radiol. Oncol. 2009, 92, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.S.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Campbell, B.H.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M.; et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2004, 350, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Tanakura, M.; Takeda, D.; Sakakibara, A.; Akashi, M.; Minamikawa, T.; Komori, T. Risk factors associated with distant metastasis in patients with oral squamous cell carcinoma. Otolaryngol. Head Neck Surg. 2015, 152, 1053–1060. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, M.J.; Roh, J.L.; Kim, S.B.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Distant metastases and survival prediction in head and neck squamous cell carcinoma. Otolaryngol. Head Neck Surg. 2012, 147, 870–875. [Google Scholar] [CrossRef]
- Kuperman, D.I.; Auethavekiat, V.; Adkins, D.R.; Nussenbaum, B.; Collins, S.; Boonchalermvichian, C.; Trinkaus, K.; Chen, L.; Morgensztern, D. Squamous cell cancer of the head and neck with distant metastasis at presentation. Head Neck 2011, 33, 714–718. [Google Scholar] [CrossRef]
- Studer, G.; Seifert, B.; Glanzmann, C. Prediction of distant metastasis in head neck cancer patients: Implications for induction chemotherapy and pre-treatment staging? Strahlenther. Und Onkol. 2008, 184, 580–585. [Google Scholar] [CrossRef]
- Garavello, W.; Ciardo, A.; Spreafico, R.; Gaini, R.M. Risk factors for distant metastases in head and neck squamous cell carcinoma. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Di, B.; Shang, Y.; Zhou, Y.; Cheng, J.; He, Z. Clinicopathologic risk factors for distant metastases from head and neck squamous cell carcinomas. Eur. J. Surg. Oncol. 2009, 35, 1348–1353. [Google Scholar] [CrossRef] [PubMed]
- Leon, X.; Quer, M.; Orus, C.; del Prado Venegas, M.; Lopez, M. Distant metastases in head and neck cancer patients who achieved loco-regional control. Head Neck 2000, 22, 680–686. [Google Scholar] [CrossRef]
- Doweck, I.; Robbins, K.T.; Vieira, F. Analysis of risk factors predictive of distant failure after targeted chemoradiation for advanced head and neck cancer. Arch. Otolaryngol. Head Neck Surg. 2001, 127, 1315–1318. [Google Scholar]
- Peters, T.T.; Senft, A.; Hoekstra, O.S.; Castelijns, J.A.; Witte, B.I.; Leemans, C.R.; de Bree, R. Pretreatment screening on distant metastases and head and neck cancer patients: Validation of risk factors and influence on survival. Oral. Oncol. 2015, 51, 267–271. [Google Scholar] [CrossRef]
- Ferlito, A.; Shaha, A.R.; Silver, C.E.; Rinaldo, A.; Mondin, V. Incidence and sites of distant metastases from head and neck cancer. ORL 2001, 63, 202–207. [Google Scholar] [CrossRef]
- Trilling, G.M.; Cho, H.; Ugas, M.A.; Saeed, S.; Katunda, A.; Jerjes, W.; Giannoudis, P. Spinal metastasis in head and neck cancer. Head Neck Oncol. 2012, 4, 36. [Google Scholar] [CrossRef]
- Blanchard, P.; Baujat, B.; Holostenco, V.; Bourredjem, A.; Baey, C.; Bourhis, J.; Pignon, J.P.; Group MACH-CC. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): A comprehensive analysis by tumour site. Radiother. Oncol. 2011, 100, 33–40. [Google Scholar] [CrossRef]
- Bourhis, J.; Overgaard, J.; Audry, H.; Ang, K.K.; Saunders, M.; Bernier, J.; Horiot, J.C.; Le Maitre, A.; Pajak, T.F.; Poulsen, M.G.; et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: A meta-analysis. Lancet 2006, 368, 843–854. [Google Scholar] [CrossRef]
- Semrau, R.; Mueller, R.P.; Stuetzer, H.; Staar, S.; Schroeder, U.; Guntinas-Lichius, O.; Kocher, M.; Eich, H.T.; Dietz, A.; Flentje, M.; et al. Efficacy of intensified hyperfractionated and accelerated radiotherapy and concurrent chemotherapy with carboplatin and 5-fluorouracil: Updated results of a randomized multicentric trial in advanced head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1308–1316. [Google Scholar] [CrossRef]
- Coleman, R.E. Skeletal complications of malignancy. Cancer 1997, 80 (Suppl. 8), 1588–1594. [Google Scholar] [CrossRef]
- Chow, E.; Harris, K.; Fan, G.; Tsao, M.; Sze, W.M. Palliative radiotherapy trials for bone metastases: A systematic review. J. Clin. Oncol. 2007, 25, 1423–1436. [Google Scholar] [CrossRef]
- Hartsell, W.F.; Scott, C.B.; Bruner, D.W.; Scarantino, C.W.; Ivker, R.A.; Roach, M., 3rd; Suh, J.H.; Demas, W.F.; Movsas, B.; Petersen, I.A.; et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J. Natl. Cancer Inst. 2005, 97, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Rief, H.; Forster, R.; Rieken, S.; Bruckner, T.; Schlampp, I.; Bostel, T.; Debus, J. The influence of orthopedic corsets on the incidence of pathological fractures in patients with spinal bone metastases after radiotherapy. BMC Cancer 2015, 15, 745. [Google Scholar] [CrossRef]
- Rief, H.; Bischof, M.; Bruckner, T.; Welzel, T.; Askoxylakis, V.; Rieken, S.; Lindel, K.; Combs, S.; Debus, J. The stability of osseous metastases of the spine in lung cancer—A retrospective analysis of 338 cases. Radiat. Oncol. 2013, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Foerster, R.; Habermehl, D.; Bruckner, T.; Bostel, T.; Schlampp, I.; Welzel, T.; Debus, J.; Rief, H. Spinal bone metastases in gynecologic malignancies: A retrospective analysis of stability, prognostic factors and survival. Radiat. Oncol. 2014, 9, 194. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schlampp, I.; Rieken, S.; Habermehl, D.; Bruckner, T.; Forster, R.; Debus, J.; Rief, H. Stability of spinal bone metastases in breast cancer after radiotherapy: A retrospective analysis of 157 cases. Strahlenther. Und Onkol. 2014, 190, 792–797. [Google Scholar] [CrossRef]
- Rades, D.; Janssen, S.; Kasmann, L.; Bolm, L.; Schild, S.E. Outcomes After Irradiation of Epidural Spinal Cord Compression Due to Metastatic Thyroid Cancer. Anticancer Res. 2016, 36, 2035–2039. [Google Scholar]
- Rades, D.; Bajrovic, A.; Bartscht, T. Predictive Factors and a Survival Score for Patients Irradiated for Metastatic Spinal Cord Compression from Carcinoma of the Salivary Glands. Anticancer Res. 2017, 37, 7011–7015. [Google Scholar]
- Fisher, C.G.; Versteeg, A.L.; Schouten, R.; Boriani, S.; Varga, P.P.; Rhines, L.D.; Heran, M.K.; Kawahara, N.; Fourney, D.; Reynolds, J.J.; et al. Reliability of the spinal instability neoplastic scale among radiologists: An assessment of instability secondary to spinal metastases. AJR Am. J. Roentgenol. 2014, 203, 869–874. [Google Scholar] [CrossRef]
- Fisher, C.G.; Schouten, R.; Versteeg, A.L.; Boriani, S.; Varga, P.P.; Rhines, L.D.; Kawahara, N.; Fourney, D.; Weir, L.; Reynolds, J.J.; et al. Reliability of the Spinal Instability Neoplastic Score (SINS) among radiation oncologists: An assessment of instability secondary to spinal metastases. Radiat. Oncol. 2014, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Bollen, L.; Groenen, K.; Pondaag, W.; van Rijswijk, C.S.P.; Fiocco, M.; Van der Linden, Y.M.; Dijkstra, S.P.D. Clinical Evaluation of the Spinal Instability Neoplastic Score in Patients Treated With Radiotherapy for Symptomatic Spinal Bone Metastases. Spine (Phila Pa 1976) 2017, 42, E956–E962. [Google Scholar] [CrossRef] [PubMed]
- Grisanti, S.; Bianchi, S.; Locati, L.D.; Triggiani, L.; Vecchio, S.; Bonetta, A.; Bergamini, C.; Conte, P.; Airoldi, M.; Merlano, M.; et al. Bone metastases from head and neck malignancies: Prognostic factors and skeletal-related events. PLoS ONE 2019, 14, e0213934. [Google Scholar] [CrossRef] [PubMed]
- Bostel, T.; Forster, R.; Schlampp, I.; Wolf, R.; Serras, A.F.; Mayer, A.; Bruckner, T.; Welzel, T.; Schmidberger, H.; Debus, J.; et al. Stability, prognostic factors and survival of spinal bone metastases in malignant melanoma patients after palliative radiotherapy. Tumori 2016, 102, 156–161. [Google Scholar] [CrossRef]
- Schlampp, I.; Lang, H.; Forster, R.; Wolf, R.; Bostel, T.; Bruckner, T.; Debus, J.; Rief, H. Stability of spinal bone metastases and survival analysis in renal cancer after radiotherapy. Tumori 2015, 101, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Bostel, T.; Forster, R.; Schlampp, I.; Sprave, T.; Bruckner, T.; Nicolay, N.H.; Welte, S.E.; Debus, J.; Rief, H. Spinal bone metastases in colorectal cancer: A retrospective analysis of stability, prognostic factors and survival after palliative radiotherapy. Radiat Oncol. 2017, 12, 115. [Google Scholar] [CrossRef]
- Morii, K.; Aoyama, Y.; Nakamura, S.; Okushin, H. Synergistic anti-tumor effects of zoledronic acid and radiotherapy against metastatic hepatocellular carcinoma. Intern. Med. 2015, 54, 2609–2613. [Google Scholar] [CrossRef]
- Wiegand, S.; Zimmermann, A.; Wilhelm, T.; Werner, J.A. Survival After Distant Metastasis in Head and Neck Cancer. Anticancer Res. 2015, 35, 5499–5502. [Google Scholar]
- Calhoun, K.H.; Fulmer, P.; Weiss, R.; Hokanson, J.A. Distant metastases from head and neck squamous cell carcinomas. Laryngoscope 1994, 104, 1199–1205. [Google Scholar] [CrossRef]
- Bollen, L.; Jacobs, W.C.H.; Van der Linden, Y.M.; Van der Hel, O.; Taal, W.; Dijkstra, P.D.S. A systematic review of prognostic factors predicting survival in patients with spinal bone metastases. Eur. Spine J. 2018, 27, 799–805. [Google Scholar] [CrossRef]
- Bollen, L.; Wibmer, C.; Van der Linden, Y.M.; Pondaag, W.; Fiocco, M.; Peul, W.C.; Marijnen, C.A.; Nelissen, R.G.; Leithner, A.; Dijkstra, S.P. Predictive Value of Six Prognostic Scoring Systems for Spinal Bone Metastases: An Analysis Based on 1379 Patients. Spine (Phila Pa 1976) 2016, 41, E155–E162. [Google Scholar] [CrossRef] [PubMed]
- Bostel, T.; Forster, R.; Schlampp, I.; Sprave, T.; Akbaba, S.; Wollschlager, D.; Debus, J.; Mayer, A.; Schmidberger, H.; Rief, H.; et al. Stability and survival analysis of elderly patients with osteolytic spinal bone metastases after palliative radiotherapy: Results from a large multicenter cohort. Strahlenther. Und Onkol. 2019, 195, 1074–1085. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.C.; Lin, C.Y.; Pai, P.C.; Tseng, C.K.; Hsieh, C.E.; Chang, K.P.; Hsu, C.L.; Liao, C.T.; Wang, C.C.; Chin, S.C.; et al. Dose-escalated radiation therapy is associated with better overall survival in patients with bone metastases from solid tumors: A propensity score-matched study. Cancer Med. 2017, 6, 2087–2097. [Google Scholar] [CrossRef] [PubMed]
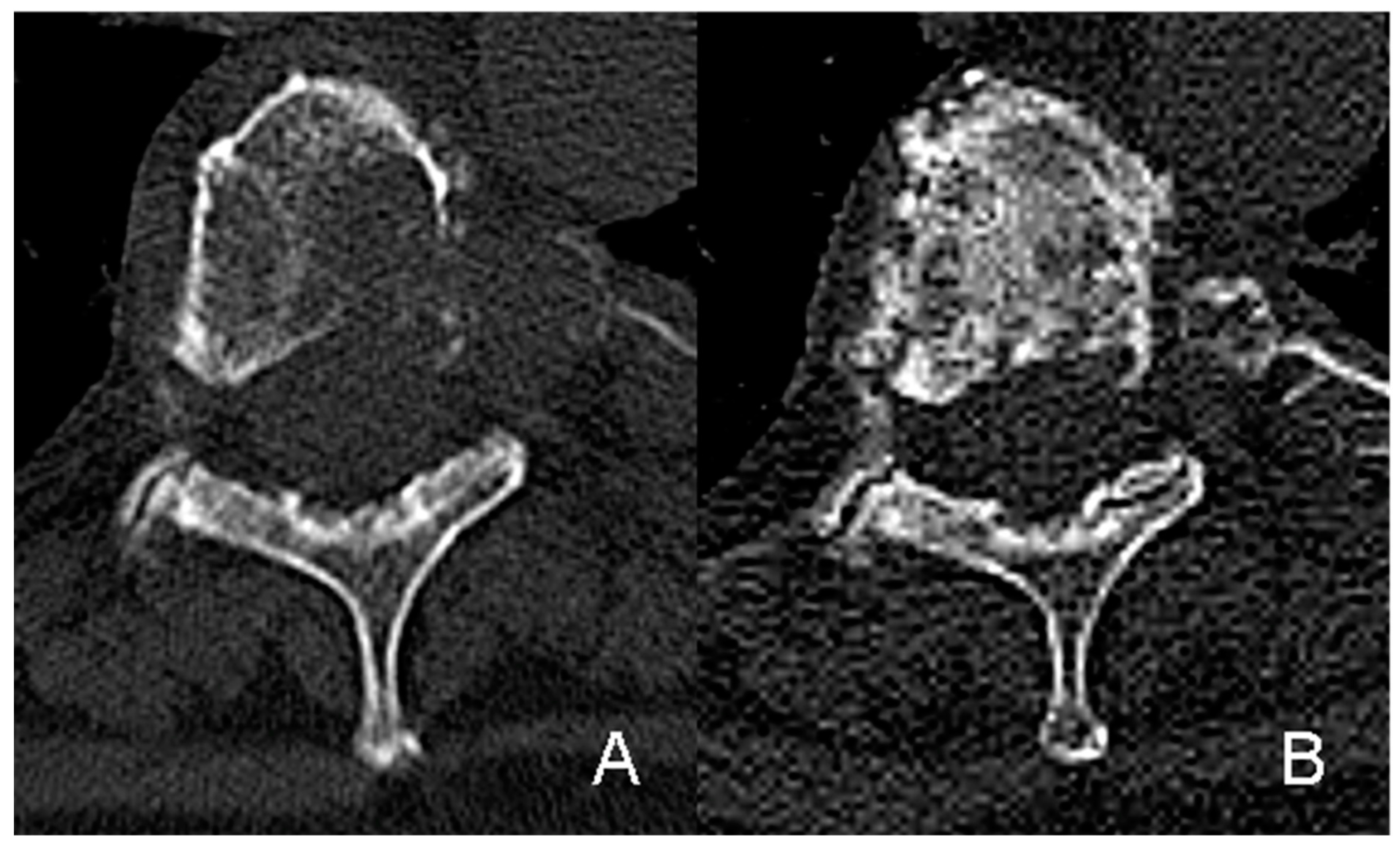
| Characteristics | Value | % |
|---|---|---|
| Age at start of RT (years) | ||
| Median (range) 60.8 (22.2–80.0) | ||
| Gender | ||
| Female | 13 | 19.7 |
| Male | 53 | 80.3 |
| Karnofsky PS | ||
| <70% | 47 | 58.0 |
| ≥70% | 34 | 42.0 |
| Number of bone metastases | ||
| Median (range) 2.5 (1–11) | ||
| Solitary | 36 | 37.9 |
| Multiple | 59 | 62.1 |
| Spine involvement | ||
| Cervical | 9 | 9.5 |
| Cervicothoracic | 8 | 8.4 |
| Thoracic | 38 | 40.0 |
| Thoracolumbar | 17 | 17.9 |
| Lumbar | 15 | 15.8 |
| Lumbosacral | 2 | 2.1 |
| Sacral | 6 | 6.3 |
| Histology | ||
| Adenocarcinoma | 3 | 4.5 |
| Adenoid cystic carcinoma | 13 | 19.7 |
| Squamous cell carcinoma | 46 | 69.7 |
| Sarcoma | 2 | 3.0 |
| Transitional cell carcinoma | 1 | 1.5 |
| Neuroblastoma | 1 | 1.5 |
| Distant extraskeletal metastases | ||
| Brain | 6 | 7.4 |
| Lung | 53 | 65.4 |
| Liver | 17 | 30.9 |
| Adrenal gland | 2 | 2.5 |
| Visceral | 57 | 70.4 |
| Non-visceral | 24 | 29.6 |
| Characteristics | Value | % |
|---|---|---|
| RT completed | ||
| Yes | 86 | 90.5 |
| No | 9 | 9.5 |
| Radiation dose | ||
| RT completed | ||
| Cumulative dose | ||
| Median | 30 | |
| Range | 8–60 | |
| RT discontinued | ||
| Cumulative dose | ||
| Median | 15 | |
| Range | 3–27 | |
| Indications for palliative RT | ||
| Pain | 72 | 75.8 |
| Instability | 64 | 67.4 |
| Neurological impairment | 21 | 22.1 |
| Postoperative | 13 | 13.7 |
| Other treatments | ||
| Orthopedic corset | 25 | 26.3 |
| Bisphosphonates | 35 | 43.2 |
| Chemotherapy | 55 | 67.9 |
| Prior to RT | 42 | 51.9 |
| After RT | 46 | 56.8 |
| Cetuximab | 34 | 42.0 |
| Prior to RT | 27 | 33.3 |
| After RT | 25 | 30.9 |
| Immunotherapy | 13 | 16.0 |
| Prior to RT | 5 | 6.2 |
| After RT | 12 | 14.8 |
| Surgery | 16 | 16.8 |
| Prior to RT | 13 | 13.7 |
| Laminectomy | 6 | 6.3 |
| Spondylodesis | 5 | 5.3 |
| Both | 2 | 2.1 |
| After RT | 3 | 3.2 |
| Laminectomy | 1 | 1.1 |
| Spondylodesis | 2 | 2.1 |
| Parameter | n | % |
|---|---|---|
| Frankel classification before RT | ||
| No deficit (E) | 74 | 77.9 |
| Minor motor or sensory deficit (D) | 18 | 18.9 |
| Major motor or sensory deficit (A, B, C) | 3 | 3.2 |
| Frankel classification after RT | ||
| No deficit (E) | 83 | 87.4 |
| Minor motor or sensory deficit (D) | 7 | 7.4 |
| Major motor or sensory deficit (A, B, C) | 3 | 3.2 |
| NA | 2 | 2.1 |
| Parameter | n | % |
|---|---|---|
| Stability before RT | ||
| Unstable | 64 | 67.4 |
| Stable | 31 | 32.6 |
| Stability after 3 months | ||
| Unstable | 23 | 24.2 |
| Stable | 32 | 33.7 |
| NA | 40 | 42.1 |
| Stability after 6 months | ||
| Unstable | 10 | 10.5 |
| Stable | 20 | 21.1 |
| NA | 65 | 68.4 |
| Predictor | p-Value | OR | CL |
|---|---|---|---|
| Karnofsky PS ≥ 70% | |||
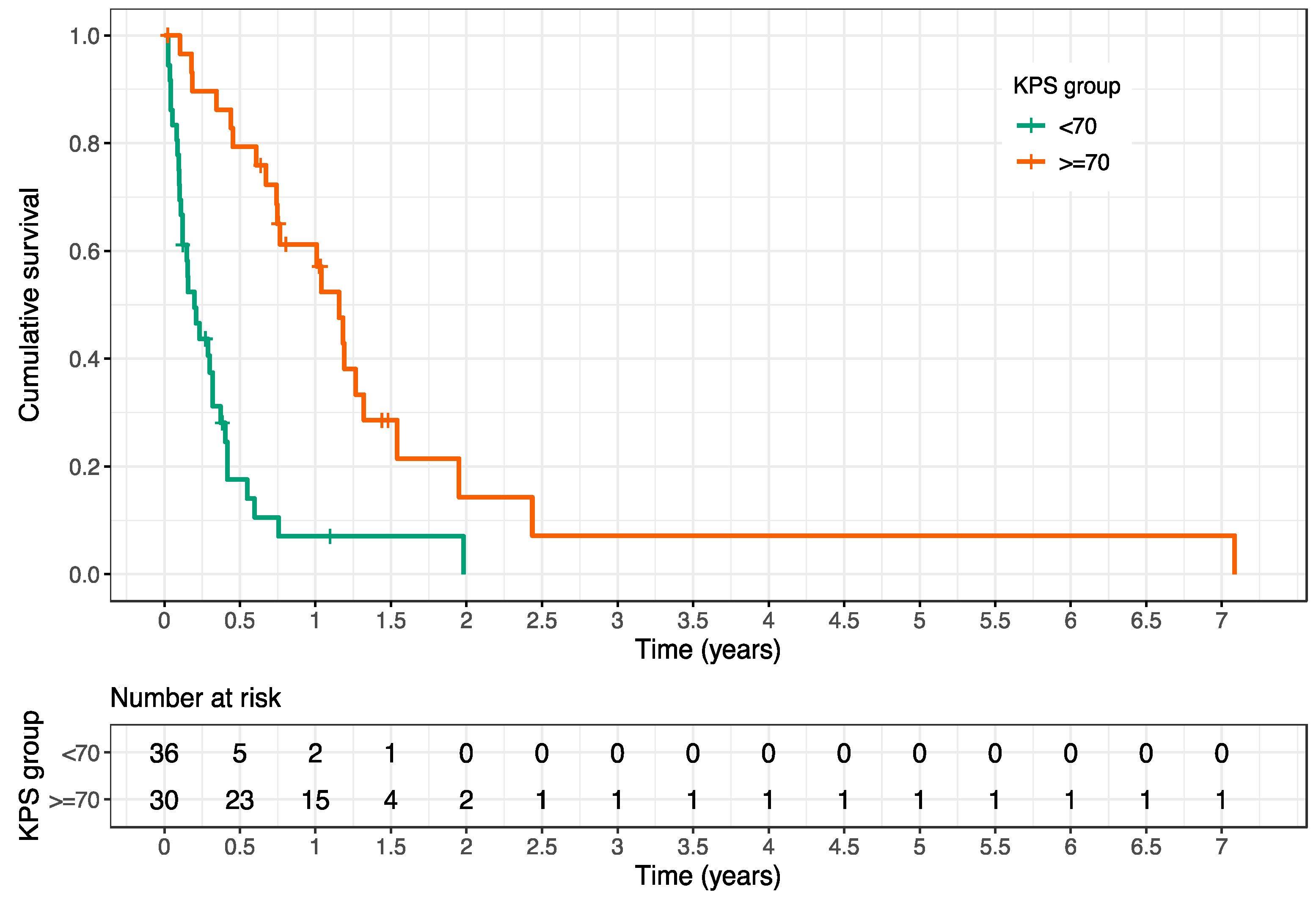
| - Stable SBM pre-RT | <0.001 | 4.16 | 1.81–9.57 |
| - Stable SBM 3 mo. post-RT | 0.29 | 1.84 | 0.60–5.64 |
| - Stable SBM 6 mo. post-RT | 0.006 | 6.09 | 1.68–22.05 |
| Chemotherapy (yes) | |||
| - Stable SBM pre-RT | 0.088 | 2.42 | 0.88–6.71 |
| - Stable SBM 3 mo. post-RT | 0.099 | 3.53 | 0.79–15.75 |
| - Stable SBM 6 mo. post-RT | 0.75 | 0.73 | 0.10–5.13 |
| Immunotherapy (yes) | |||
| - Stable SBM pre-RT | 0.002 | 4.92 | 1.82–13.28 |
| - Stable SBM 3 mo. post-RT | 0.74 | 2.86 | 0.55–14.90 |
| - Stable SBM 6 mo. post-RT | 0.61 | v | 0.26–9.57 |
| Cetuximab (yes) | |||
| - Stable SBM pre-RT | 0.008 | 3.25 | 1.37–7.72 |
| - Stable SBM 3 mo. post-RT | 0.36 | 1.23 | 0.36–4.17 |
| - Stable SBM 6 mo. post-RT | 0.56 | 0.64 | 0.14–2.86 |
| Bone-modifying therapy (yes) | |||
| - Stable SBM pre-RT | 0.23 | 1.74 | 0.70–4.32 |
| - Stable SBM 3 mo. post-RT | 0.58 | 0.73 | 0.24–2.22 |
| - Stable SBM 6 mo. post-RT | 0.38 | 0.46 | 0.09–2.54 |
| Total dose of palliative RT | |||
| - Stable SBM pre-RT | 0.05 | 1.06 | 1.00–1.12 |
| - Stable SBM 3 mo. post-RT | 0.10 | 1.06 | 0.99–1.15 |
| - Stable SBM 6 mo. post-RT | 0.16 | 1.07 | 0.97–1.18 |
| Liver metastases (yes) | |||
| - Stable SBM pre-RT | 0.35 | 0.61 | 0.21–1.71 |
| - Stable SBM 3 mo. post-RT | 0.64 | 1.35 | 0.39–4.69 |
| - Stable SBM 6 mo. post-RT | 0.18 | 3.92 | 0.52–29.59 |
| Lung metastases (yes) | |||
| - Stable SBM pre-RT | 0.58 | 1.30 | 0.52–3.26 |
| - Stable SBM 3 mo. post-RT | 0.79 | 1.17 | 0.37–3.73 |
| - Stable SBM 6 mo. post-RT | 0.73 | 1.30 | 0.29–5.85 |
| Brain metastases (yes) | |||
| - Stable SBM pre-RT | 0.64 | 0.72 | 0.18–2.90 |
| - Stable SBM 3 mo. post-RT | NA | NA | NA |
| - Stable SBM 6 mo. post-RT | NA | NA | NA |
| Visceral metastases (yes) | |||
| - Stable SBM pre-RT | 0.68 | 1.23 | 0.47–3.17 |
| - Stable SBM 3 mo. post-RT | 0.59 | 1.38 | 0.43–4.46 |
| - Stable SBM 6 mo. post-RT | 0.48 | 1.74 | 0.38–8.09 |
| Non visceral metastases (yes) | |||
| - Stable SBM pre-RT | 0.95 | 1.03 | 0.40–2.64 |
| - Stable SBM 3 mo. post-RT | 0.44 | 1.71 | 0.44–6.62 |
| Parameter | p-Value | HR | CL |
|---|---|---|---|
| Karnofsky PS | <0.001 | 0.197 | 0.11–0.35 |
| (≥70% vs. <70%) | |||
| Chemotherapy | 0.45 | 0.79 | 0.44–1.45 |
| (yes vs. no) | |||
| Immunotherapy | 0.13 | 0.45 | 0.16–1.27 |
| (yes vs. no) | |||
| Cetuximab | 0.46 | 1.24 | 0.45–1.44 |
| (yes vs. no) | |||
| Bone-modifying therapy | 0.4 | 0.79 | 0.45–1.37 |
| (yes vs. no) | |||
| Total dose | 0.016 | 0.95 | 0.92–0.99 |
| (palliative RT) | |||
| Liver metastases | 0.075 | 1.66 | 0.95–2.88 |
| (yes vs. no) | |||
| Lung metastases | 0.21 | 1.44 | 0.81–2.55 |
| (yes vs. no) | |||
| Brain metastases | 0.45 | 1.4 | 0.59–3.30 |
| (yes vs. no) | |||
| Visceral metastases | 0.098 | 1.67 | 0.91–3.07 |
| (yes vs. no) | |||
| Non visceral metastases | 0.79 | 1.09 | 0.57–2.11 |
| (yes vs. no) | |||
| Pathologic fracture | 0.71 | 0.9 | 0.65–1.90 |
| (yes vs. no) | |||
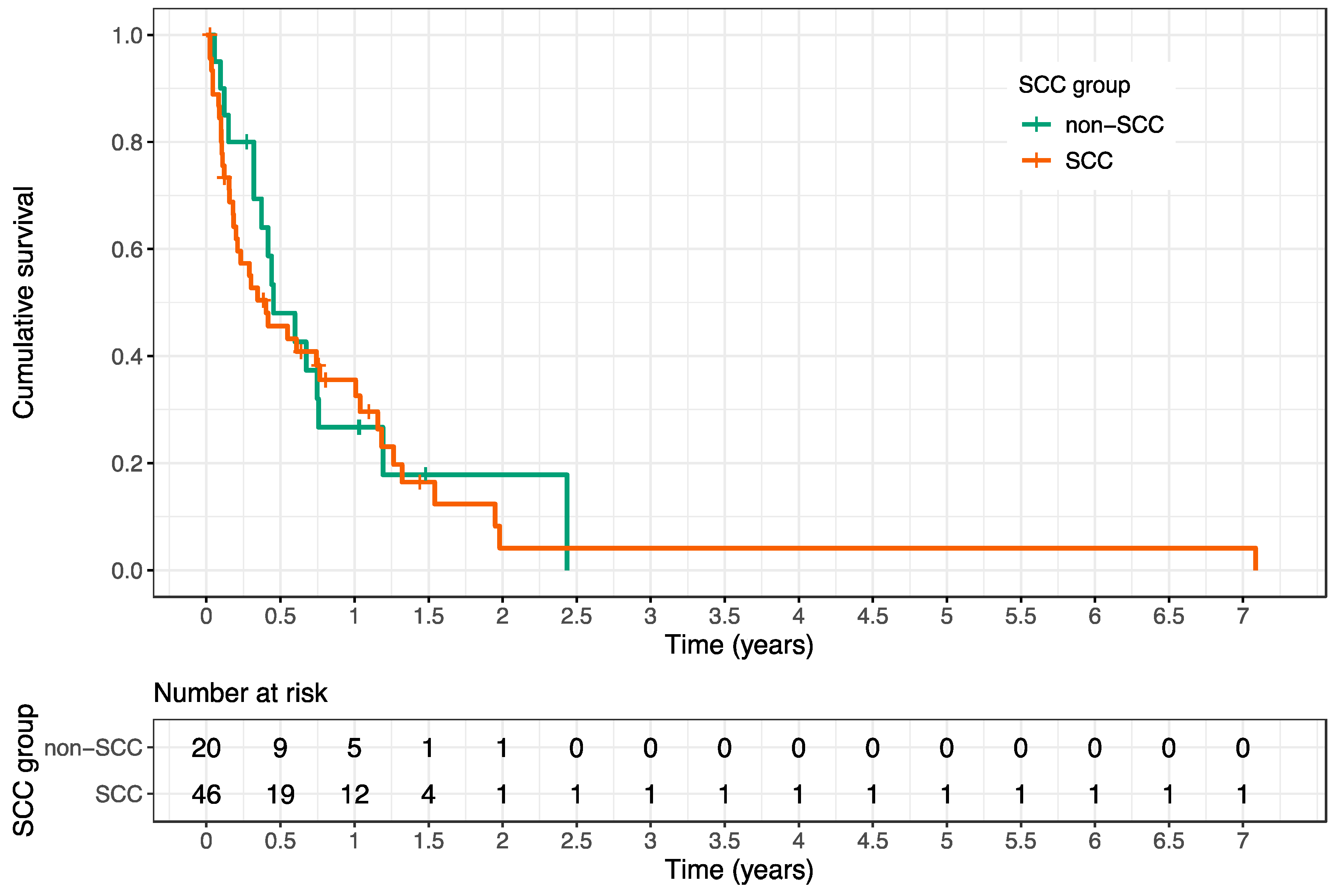
| Histology SCC | 0.67 | 1.13 | 0.65–1.98 |
| (yes vs. no) | |||
| Salivary gland tumors | 0.35 | 0.76 | 0.43–1.35 |
| (yes vs. no) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bostel, T.; Rühle, A.; Rackwitz, T.; Mayer, A.; Klodt, T.; Oebel, L.; Förster, R.; Schlampp, I.; Wollschläger, D.; Rief, H.; et al. The Role of Palliative Radiotherapy in the Treatment of Spinal Bone Metastases from Head and Neck Tumors—A Multicenter Analysis of a Rare Event. Cancers 2020, 12, 1950. https://doi.org/10.3390/cancers12071950
Bostel T, Rühle A, Rackwitz T, Mayer A, Klodt T, Oebel L, Förster R, Schlampp I, Wollschläger D, Rief H, et al. The Role of Palliative Radiotherapy in the Treatment of Spinal Bone Metastases from Head and Neck Tumors—A Multicenter Analysis of a Rare Event. Cancers. 2020; 12(7):1950. https://doi.org/10.3390/cancers12071950
Chicago/Turabian StyleBostel, Tilman, Alexander Rühle, Tilmann Rackwitz, Arnulf Mayer, Tristan Klodt, Laura Oebel, Robert Förster, Ingmar Schlampp, Daniel Wollschläger, Harald Rief, and et al. 2020. "The Role of Palliative Radiotherapy in the Treatment of Spinal Bone Metastases from Head and Neck Tumors—A Multicenter Analysis of a Rare Event" Cancers 12, no. 7: 1950. https://doi.org/10.3390/cancers12071950
APA StyleBostel, T., Rühle, A., Rackwitz, T., Mayer, A., Klodt, T., Oebel, L., Förster, R., Schlampp, I., Wollschläger, D., Rief, H., Sprave, T., Debus, J., Grosu, A.-L., Schmidberger, H., Akbaba, S., & Nicolay, N. H. (2020). The Role of Palliative Radiotherapy in the Treatment of Spinal Bone Metastases from Head and Neck Tumors—A Multicenter Analysis of a Rare Event. Cancers, 12(7), 1950. https://doi.org/10.3390/cancers12071950






