Lectins in Cervical Screening
Abstract
1. Introduction
2. Results
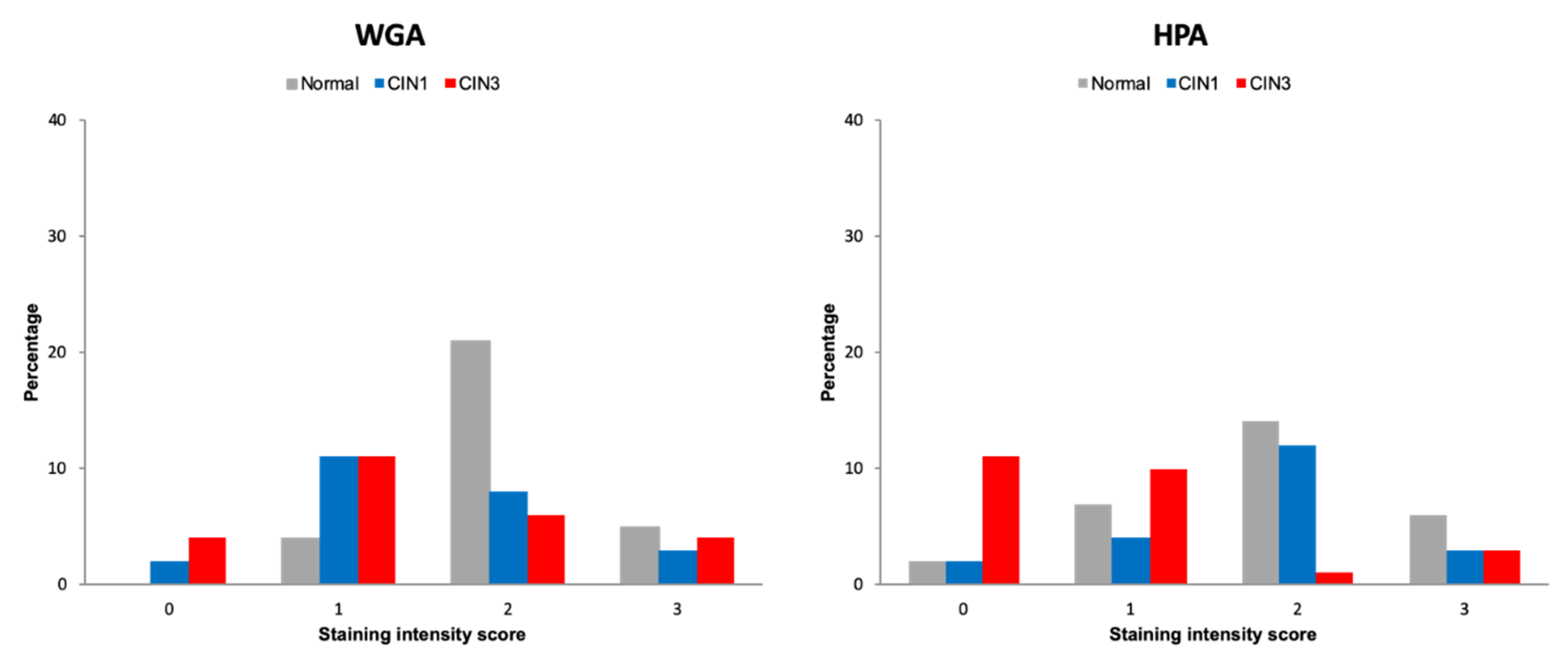
2.1. Discovery Lectin Histochemistry
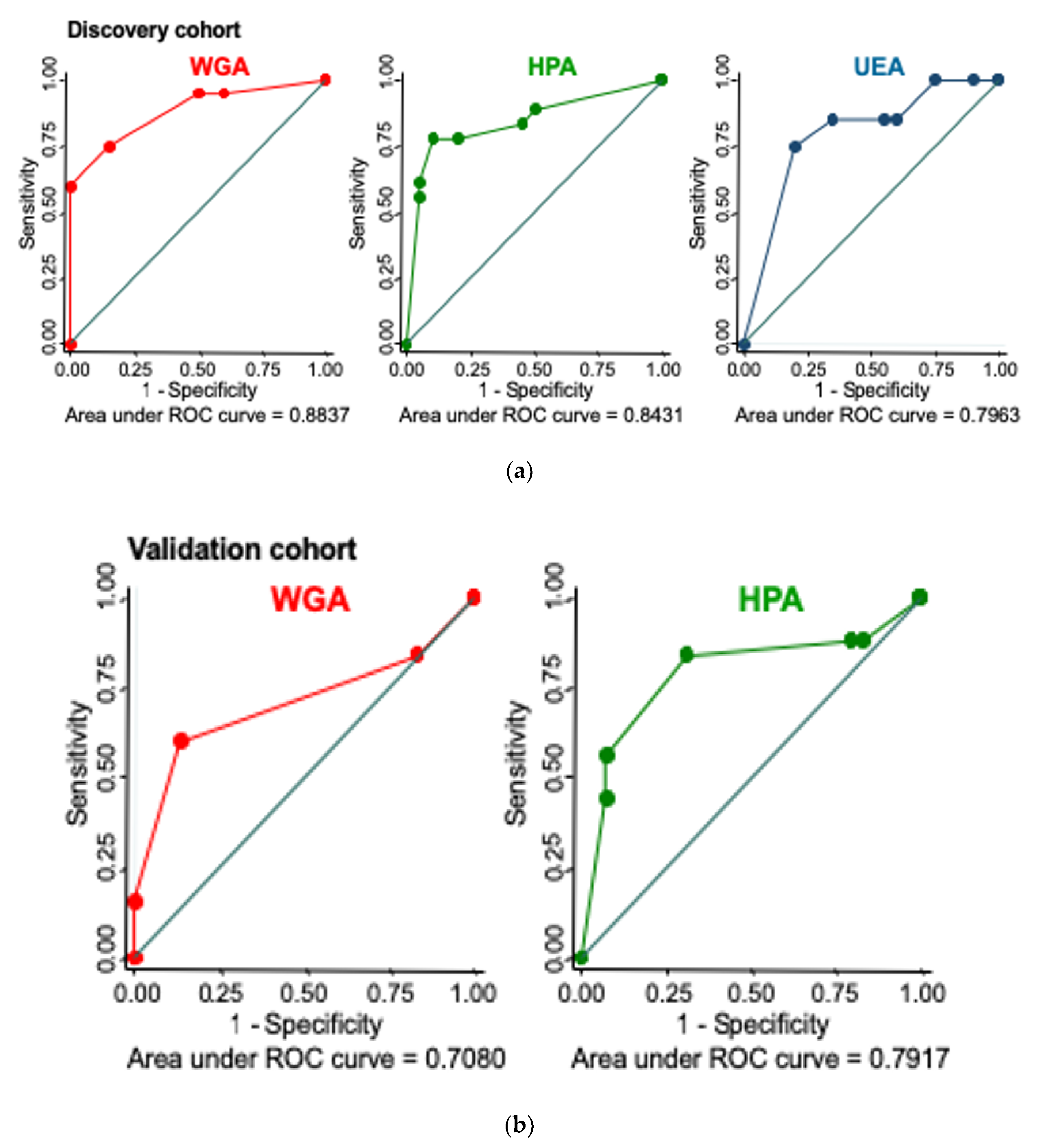
2.2. Validation of Lectin Histochemistry
Lectin Histochemistry Sensitivity and Specificity for CIN3
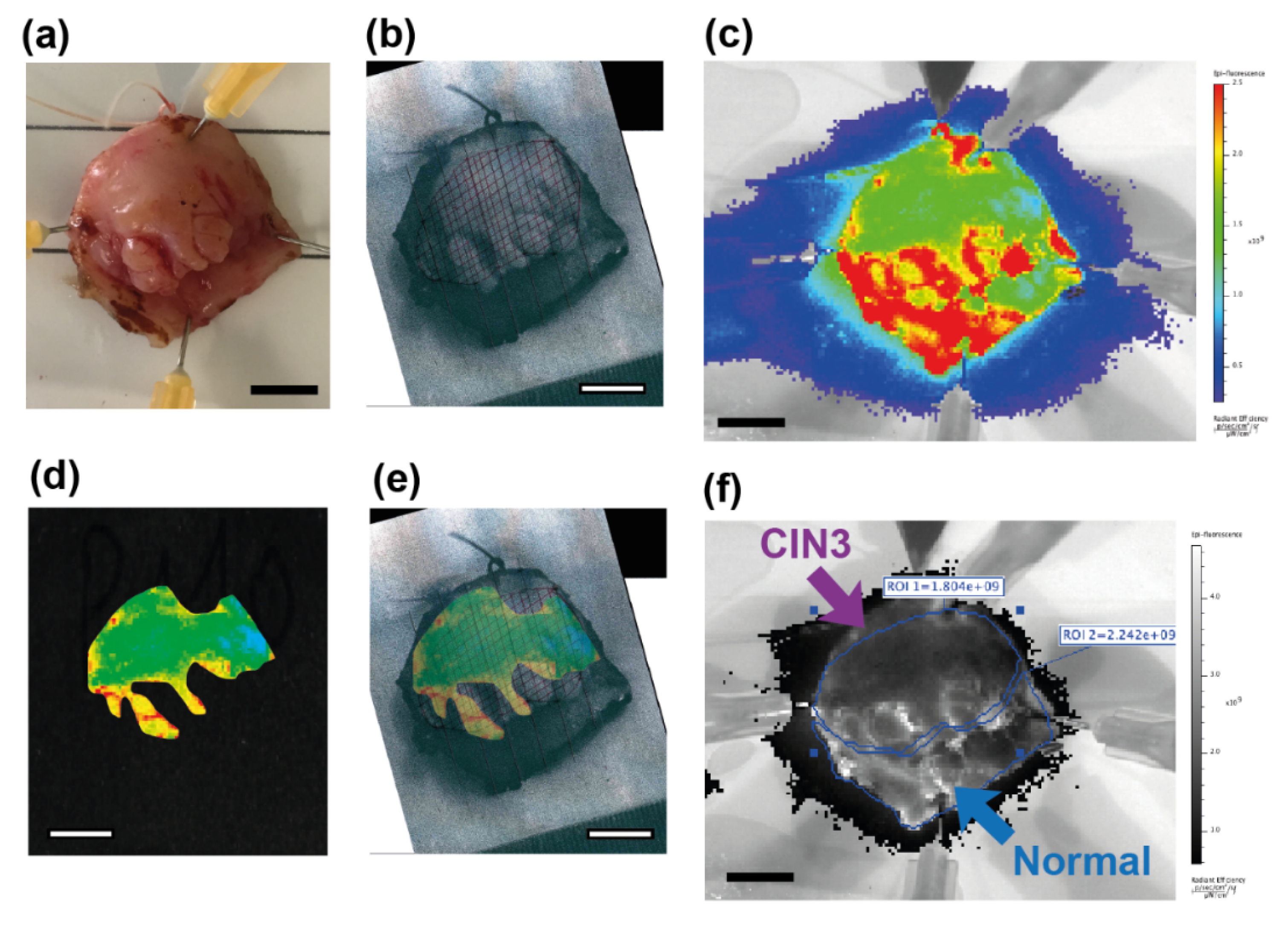
2.3. Molecular Imaging of Topically Applied Fluorescently-Labelled Lectin to Freshly Resected Human Cervix
2.3.1. Whole Specimens
2.3.2. Biopsies
3. Discussion
4. Materials and Methods
4.1. Human Tissue
4.2. Pathological Diagnosis
4.3. Identification of Candidate Lectins
4.4. Lectin Histochemistry
4.4.1. Scoring
4.4.2. Sensitivity and Specificity to High Grade Cervical Disease
4.5. Molecular Imaging of Fluorescently-Labelled Lectin
4.5.1. Staining Protocol
4.5.2. Quantification of NIR Fluorescence Signal for Imaging Data
4.6. Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Budukh, A.M.; Rajkumar, R. Effective screening programmes for cervical cancer in low- and middle-income developing countries. Bull. World Health Organ. 2001, 79, 954–962. [Google Scholar]
- Gallay, C.; Girardet, A.; Viviano, M.; Catarino, R.; Benski, A.-C.; Tran, P.L.; Ecabert, C.; Thiran, J.-P.; Vassilakos, P.; Petignat, P. Cervical cancer screening in low-resource settings: A smartphone image application as an alternative to colposcopy. Int. J. Women’s Health 2017, 9, 455–461. [Google Scholar] [CrossRef]
- Rajaraman, P.; Anderson, B.O.; Basu, P.; Belinson, J.L.; Cruz, A.D.; Dhillon, P.K.; Gupta, P.; Jawahar, T.S.; Joshi, N.; Kailash, U.; et al. Recommendations for screening and early detection of common cancers in India. Lancet Oncol. 2015, 16, e352–e361. [Google Scholar] [CrossRef]
- Almonte, M.; Ferreccio, C.; Luciani, S.; Gonzales, M.; Delgado, J.M.; Santos, C.; Alvarez, M.; Cuzick, J.; Sasieni, P. Visual Inspection after Acetic Acid (VIA) Is Highly Heterogeneous in Primary Cervical Screening in Amazonian Peru. PLoS ONE 2015, 10, e0115355. [Google Scholar] [CrossRef] [PubMed]
- Lis, H.; Sharon, N. Lectins as molecules and as tools. Annu. Rev. Biochem. 1986, 55, 35–67. [Google Scholar] [CrossRef] [PubMed]
- Munkley, J.; Elliott, D. Hallmarks of glycosylation in cancer. Oncotarget 2016, 7, 35478–35489. [Google Scholar] [CrossRef]
- Hashim, O.H.; Jayapalan, J.J.; Lee, C.-S. Lectins: An effective tool for screening of potential cancer biomarkers. PeerJ 2017, 5, e3784. [Google Scholar] [CrossRef]
- Bird-Lieberman, E.; Neves, A.; Lao-Sirieix, P.; O’Donovan, M.; Novelli, M.; Lovat, L.; Eng, W.S.; Mahal, L.K.; Brindle, K.M.; Fitzgerald, R.C. Molecular imaging using fluorescent lectins permits rapid endoscopic identification of dysplasia in Barrett’s esophagus. Nat. Med. 2012, 18, 315–321. [Google Scholar] [CrossRef]
- Munkley, J. The glycosylation landscape of pancreatic cancer. Oncol. Lett. 2019, 17, 2569–2575. [Google Scholar] [CrossRef]
- Louis, C.J.; Wyllie, R.G.; Chou, S.T.; Sztynda, T. Lectin-binding Affinities of Human Epidermal Tumors and Related Conditions. Am. J. Clin. Pathol. 1981, 75, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, A.; Duarte, H.; Reis, C.A. Aberrant Glycosylation in Cancer: A Novel Molecular Mechanism Controlling Metastasis. Cancer Cell 2017, 31, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Kawai, T.; Suzuki, M. Lectin binding and expression of blood group-related antigens in carcinoma-in-situ and invasive carcinoma of urinary bladder. Histopathology 1993, 23, 153–158. [Google Scholar] [CrossRef]
- Ohyama, C. Glycosylation in bladder cancer. Int. J. Clin. Oncol. 2008, 13, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhan, T.; Deng, Z.; Li, Q.; Liu, Y.; Yang, S.; Ji, D.; Li, Y. Glycan analysis of colorectal cancer samples reveals stage-dependent changes in CEA glycosylation patterns. Clin. Proteom. 2018, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Macartney, J.C. Fucose-containing antigens in normal and neoplastic human gastric mucosa: A comparative study using lectin histochemistry and blood group immunohistochemistry. J. Pathol. 1987, 152, 23–30. [Google Scholar] [CrossRef]
- Narita, T.; Numao, H. Lectin binding patterns in normal, metaplastic, and neoplastic gastric mucosa. J. Histochem. Cytochem. 1992, 40, 681–687. [Google Scholar] [CrossRef]
- Li, X.; Guan, F.; Li, D.; Tan, Z.; Yang, G.; Wu, Y.; Huang, Z. Identification of aberrantly expressed glycans in gastric cancer by integrated lectin microarray and mass spectrometric analyses. Oncotarget 2016, 7, 87284–87300. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Kim, S.C.; Kim, H.J.; Ju, W.; Kim, Y.H.; Kim, H.-J. A lectin-based diagnostic system using circulating antibodies to detect cervical intraepithelial neoplasia and cervical cancer. Glycobiology 2015, 26, 100–107. [Google Scholar] [CrossRef]
- Kim, Y.H.; Ju, W.; Yin, S.Y. Aberrant sialylation and fucosylation of intracellular proteins in cervical tissue are critical markers of cervical carcinogenesis. Oncol. Rep. 2013, 31, 1417–1422. [Google Scholar] [CrossRef][Green Version]
- Neves, A.; Di Pietro, M.; O’Donovan, M.; Waterhouse, D.J.; Bohndiek, S.E.; Brindle, K.M.; Fitzgerald, R.C. Detection of early neoplasia in Barrett’s esophagus using lectin-based near-infrared imaging: An ex vivo study on human tissue. Endoscopy 2018, 50, 618–625. [Google Scholar] [CrossRef]
- Kuo, J.; Ibrahim, A.E.K.; Dawson, S.; Parashar, D.; Howat, W.J.; Guttula, K.; Miller, R.; Fearnhead, N.S.; Winton, U.J.; Neves, A.; et al. Detection of colorectal dysplasia using fluorescently labelled lectins. Sci. Rep. 2016, 6, 24231. [Google Scholar] [CrossRef]
- Baeten, J.; Johnson, A.; Kuriakose, M.A.; Suresh, A.; Birur, P.; Uma, K.; Kadamani, D. Chairside molecular imaging of aberrant glycosylation in subjects with suspicious oral lesions using fluorescently labeled wheat germ agglutinin. Head Neck 2017, 40, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Baeten, J.; Suresh, A.; Johnson, A.; Patel, K.; Kuriakose, M.; Flynn, A.; Kademani, D. Molecular imaging of oral premalignant and malignant lesions using fluorescently labeled lectins. Transl. Oncol. 2014, 7, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Baeten, J.; Patel, K.; Killian, M.; Sunny, S.; Suresh, A.; Uma, K.; Birur, P.; Kuriakose, M.; Kadamani, D. Evaluation of a Lectin-Based Imaging System for the Chairside Detection of Oral Dysplasia and Malignancy. J. Oral Maxillofac. Surg. 2019, 77, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Kademani, J.; Baeten, J.; Johnson, A.; Claiborne, S.; Idle, M.; Patel, K.; Kuriakose, M.; Kademani, D. The use of fluorescent lecthins to detect oral cancer and dysplasia. Int. J. Oral Maxillofac. Surg. 2017, 46, 206. [Google Scholar] [CrossRef][Green Version]
- Novikova, T. Optical techniques for cervical neoplasia detection. Beilstein J. Nanotechnol. 2017, 8, 1844–1862. [Google Scholar] [CrossRef]
- Zhu, H.; Yaglidere, O.; Su, T.-W.; Tseng, D.; Ozcan, A. Wide-field fluorescent microscopy on a cell-phone. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011. [Google Scholar]
- Vuitton, D.-A.; Rance, F.; Paquin, M.-L.; Adessi, B.; Vigan, M.; Gomot, A.; Dutau, G. Cross-reactivity between terrestrial snails (Helix species) and house-dust mite (Dermatophagoides pteronyssinus). I. In vivo study. Allergy 1998, 53, 144–150. [Google Scholar] [CrossRef]
- Goldstein, I.J.; Winter, H.C.; Poretz, R.D. Plant lectins: Tools for the study of complex carbohydrates. In New Comprehensive Biochemistry; Montreuil, J.F.G.V.J., Schachter, H., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; pp. 403–474. [Google Scholar]
- Byrne, P.; Williams, A.; Rollason, T. Studies of lectin binding to the human cervix uteri: II. Cervical intraepithelial neoplasia and invasive squamous carcinoma. J. Mol. Histol. 1989, 21, 323–336. [Google Scholar] [CrossRef]
- Byrne, P.; Williams, A.; Rollason, T. Studies of lectin binding to the human cervix uteri: I. Normal cervix. Histochem. J. 1989, 21, 311–322. [Google Scholar] [CrossRef]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; De Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- Shield, K.D.; Ferlay, J.; Jemal, A.; Sankaranarayanan, R.; Chaturvedi, A.K.; Bray, F.; Soerjomataram, I. The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J. Clin. 2016, 67, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Nagarajan, V.K.; Ferris, D.G. Mobile Fiber-Optic Sensor for Detection of Oral and Cervical Cancer in the Developing World. Adv. Struct. Saf. Stud. 2014, 1256, 155–170. [Google Scholar]
- Ricard-Gauthier, D.; Wisniak, A.; Catarino, R.; Van Rossum, A.F.; Meyer-Hamme, U.; Negulescu, R.; Scaringella, S.; Jinoro, J.; Vassilakos, P.; Petignat, P. Use of Smartphones as Adjuvant Tools for Cervical Cancer Screening in Low-Resource Settings. J. Low. Genit. Tract Dis. 2015, 19, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Bedell, S.L.; Goldstein, L.S.; Goldstein, A.R.; Goldstein, A. Cervical Cancer Screening: Past, Present, and Future. Sex. Med. Rev. 2020, 8, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Purwoto, G.; Dianika, H.D.; Putra, A.; Purbadi, S.; Nuranna, L. Modified Cervicography and Visual Inspection With Acetic Acid as an Alternative Screening Method for Cervical Precancerous Lesions. J. Cancer Prev. 2017, 22, 254–259. [Google Scholar] [CrossRef][Green Version]
- Tran, P.L.; Benski, C.; Viviano, M.; Petignat, P.; Combescure, C.; Jinoro, J.; Herinianasolo, J.L.; Vassilakos, P. Performance of smartphone-based digital images for cervical cancer screening in a low-resource context. Int. J. Technol. Assess. Health Care 2018, 34, 337–342. [Google Scholar] [CrossRef]
- Hu, L.; Bell, D.; Antani, S.; Xue, Z.; Yu, K.; Horning, M.P.; Gachuhi, N.; Wilson, B.; Jaiswal, M.S.; Befano, B.; et al. An Observational Study of Deep Learning and Automated Evaluation of Cervical Images for Cancer Screening. J. Natl. Cancer Inst. 2019, 111, 923–932. [Google Scholar] [CrossRef]
- Wright, C.S. Crystal structure of a wheat germ agglutinin/glycophorin-sialoglycopeptide receptor complex. Structural basis for cooperative lectin-cell binding. J. Boil. Chem. 1992, 267, 14345–14352. [Google Scholar]
- Jeronimo, J.; Schiffman, M. Colposcopy at a crossroads. Am. J. Obstet. Gynecol. 2006, 195, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Milne, D.S.; Wadehra, V.; Mennim, D.; Wagstaff, T.I. A prospective follow up study of women with colposcopically unconfirmed positive cervical smears. BJOG Int. J. Obstet. Gynaecol. 1999, 106, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, R.G.; Zhang, W.-H.; Belinson, J.L.; Huang, M.-N.; Wu, L.-Y.; Zhang, X.; Qiao, Y. Colposcopically directed biopsy, random cervical biopsy, and endocervical curettage in the diagnosis of cervical intraepithelial neoplasia II or worse. Am. J. Obstet. Gynecol. 2004, 191, 430–434. [Google Scholar] [CrossRef]
- Ambros, R.A.; Kurman, R.J. Association of Ulex Europaeus Agglutinin I Binding with Invasion in Endometrial Carcinoma. Int. J. Gynecol. Pathol. 1993, 12, 301–306. [Google Scholar] [CrossRef]
- Banerjee, S.; Robson, P.; Soutter, W.; Foster, C.S. Modulated expression of glycoprotein oligosaccharides identifies phenotypic differentiation in squamous carcinomas of the human cervix. Hum. Pathol. 1995, 26, 1005–1013. [Google Scholar] [CrossRef]
- Bychkov, V.; Toto, P.D. Lectin Binding to Normal, Dysplastic, and Neoplastic Cervical Epithelium. Am. J. Clin. Pathol. 1986, 85, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.; Goldberg, I.; Gotlieb, W.H.; Lerner-Geva, L.; Ben-Baruch, G.; Kopolovic, J. Ulex Europaeus Lectin and Anti-CD31 Staining in Squamous Cell Carcinoma of the Uterine Cervix. Int. J. Gynecol. Pathol. 1998, 17, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Davina, J.H.; Stadhouders, A.M.; Van Haelst, U.J.; De Graaf, R.; Kenemans, P. Feasibility of a concanavalin A-peroxidase labeling method to detect cancerous and precancerous lesions of the uterine cervix. Cancer Res. 1986, 46, 1539–1543. [Google Scholar] [PubMed]
- Di Loreto, C.; De Nictolis, M.; Stramazzotti, D.; Beltrami, C.A. Different binding to squamous and columnar epithelium of the uterine cervix as a marker of epithelial differentiation. Basic Appl. Histochem. 1987, 31, 143–152. [Google Scholar] [PubMed]
- Hirao, T.; Sakamoto, Y.; Kamada, M.; Hamada, S.-I.; Aono, T. Tn antigen, a marker of potential for metastasis of uterine cervix cancer cells. Cancer 1993, 72, 154–159. [Google Scholar] [CrossRef]
- Li, Z.H. Distribution of lectin-receptors in normal, dysplastic and neoplastic cervical epithelium. Zhonghua Bing Li Xue Za Zhi Chin. J. Pathol. 1991, 20, 284–287. [Google Scholar]
- López-Morales, D.; Reyes-Leyva, J.; Santos-López, G.; Zenteno, E.; Vallejo-Ruiz, V. Increased expression of sialic acid in cervical biopsies with squamous intraepithelial lesions. Diagn. Pathol. 2010, 5, 74. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; Souberbielle, B.; Cowan, M.; Allen, C.; Luesley, D.; Mould, J.; Blackledge, G.; Skinner, G. Glycoprotein patterns in normal and malignant cervical tissue. Cancer Lett. 1991, 58, 247–254. [Google Scholar] [CrossRef]
- Pillai, K.R.; Remani, P.; Kannan, S.; Mathew, A.; Sujathan, K.; Vijayakumar, T.; Nair, M.K. Jack fruit lectin-specific glycoconjugate expression during the progression of cervical intraepithelial neoplasia: A study on exfoliated cells. Diagn. Cytopathol. 1994, 10, 342–346. [Google Scholar] [CrossRef]
- Reddi, A.L.; Sankaranarayanan, K.; Arulraj, H.S.; Devaraj, N.; Devaraj, H. Enzyme-linked PNA lectin-binding assay of serum T-antigen in patients with SCC of the uterine cervix. Cancer Lett. 2000, 149, 207–211. [Google Scholar] [CrossRef]
- Remani, P.; Joy, A.; Vijayan, K.K.; Ravindran, A.; Beevi, V.M.H.; Vasudevan, D.M.; Vijayakumar, T. Jack fruit lectin binding pattern in carcinoma of the uterine cervix. J. Exp. Pathol. 1990, 5, 89–96. [Google Scholar] [PubMed]
- Remani, P.; Pillai, K.R.; Haseenabeevi, V.M.; Ankathil, R.; Bhattathiri, V.N.; Nair, M.K.; Vijayakumar, T. Lectin cytochemistry in the exfoliative cytology of uterine cervix. Neoplasma 1994, 41, 39–42. [Google Scholar] [PubMed]
- Santaella-Verdejo, A.; Gallegos, B.; Pérez-Campos, E.; Hernandez, P.; Zenteno, E. Use of Amaranthus leucocarpus Lectin to Differentiate Cervical Dysplasia (CIN). Prep. Biochem. Biotechnol. 2007, 37, 219–228. [Google Scholar] [CrossRef]
- Sen, U. Concanavalin A—Horse radish peroxidase (Con A-HRP) labelling technique in detection and prognosis of cancer of uterine cervix. Eur. J. Gynaecol. Oncol. 1989, 10, 289–291. [Google Scholar]


| Patient Number | Age (Years) | Pathological Diagnosis | Mean Fluorescent Intensity MFI (SD) | Contrast (%) 1 | Dysplasia, % of Area | |
|---|---|---|---|---|---|---|
| Normal | CIN3 | |||||
| 101 | 53 | Normal | 0.111 (0.009) | N/E | N/D | 0 |
| 104 | 38 | CIN3 | 0.119 (0.008) | 0.090 (0.008) | 24.6 | 4.8 |
| 105 | 44 | CIN3 | 0.063 (0.006) | 0.0584 (0.004) | 7.6 | 26.7 |
| 106 | 42 | CIN3 | 0.184 (0.021) | 0.146 (0.030) | 20.8 | 18.6 |
| 107 | 28 | CIN3 | 0.048 (0.006) | 0.038 (0.007) | 21.7 | 10.1 |
| 108 | 25 | CIN3 | 0.153 (0.055) | 0.115 (0.039) | 24.8 | 49.3 |
| 110 | 25 | CIN3 | 0.224 (0.073) | 0.180 (0.055) | 19.5 | 33.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, A.W.; Neves, A.A.; Lam Shang Leen, S.; Lao-Sirieix, P.; Bird-Lieberman, E.; Singh, N.; Sheaff, M.; Hollingworth, T.; Brindle, K.; Sasieni, P. Lectins in Cervical Screening. Cancers 2020, 12, 1928. https://doi.org/10.3390/cancers12071928
Lim AW, Neves AA, Lam Shang Leen S, Lao-Sirieix P, Bird-Lieberman E, Singh N, Sheaff M, Hollingworth T, Brindle K, Sasieni P. Lectins in Cervical Screening. Cancers. 2020; 12(7):1928. https://doi.org/10.3390/cancers12071928
Chicago/Turabian StyleLim, Anita WW, André A. Neves, Sarah Lam Shang Leen, Pierre Lao-Sirieix, Elizabeth Bird-Lieberman, Naveena Singh, Michael Sheaff, Tony Hollingworth, Kevin Brindle, and Peter Sasieni. 2020. "Lectins in Cervical Screening" Cancers 12, no. 7: 1928. https://doi.org/10.3390/cancers12071928
APA StyleLim, A. W., Neves, A. A., Lam Shang Leen, S., Lao-Sirieix, P., Bird-Lieberman, E., Singh, N., Sheaff, M., Hollingworth, T., Brindle, K., & Sasieni, P. (2020). Lectins in Cervical Screening. Cancers, 12(7), 1928. https://doi.org/10.3390/cancers12071928





