Improved Prognostic Prediction in Never-Smoker Lung Cancer Patients by Integration of a Systemic Inflammation Marker with Tumor Immune Contexture Analysis
Abstract
1. Introduction
2. Results
2.1. Clinico-Pathological Characteristics According to Patients Inflammatory Profile
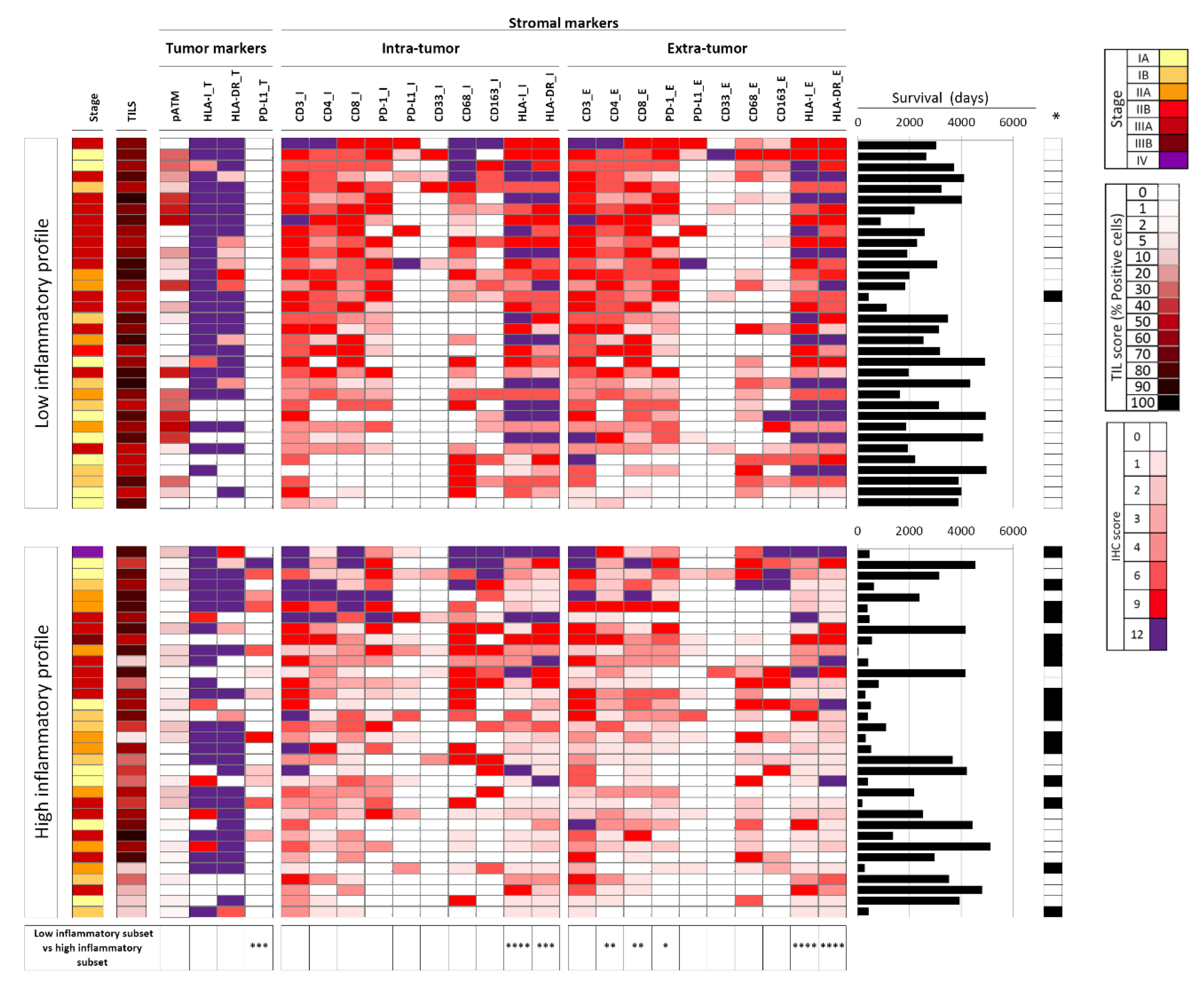
2.2. Tumor Immune Features Are Associated with the Systemic Inflammatory Profile
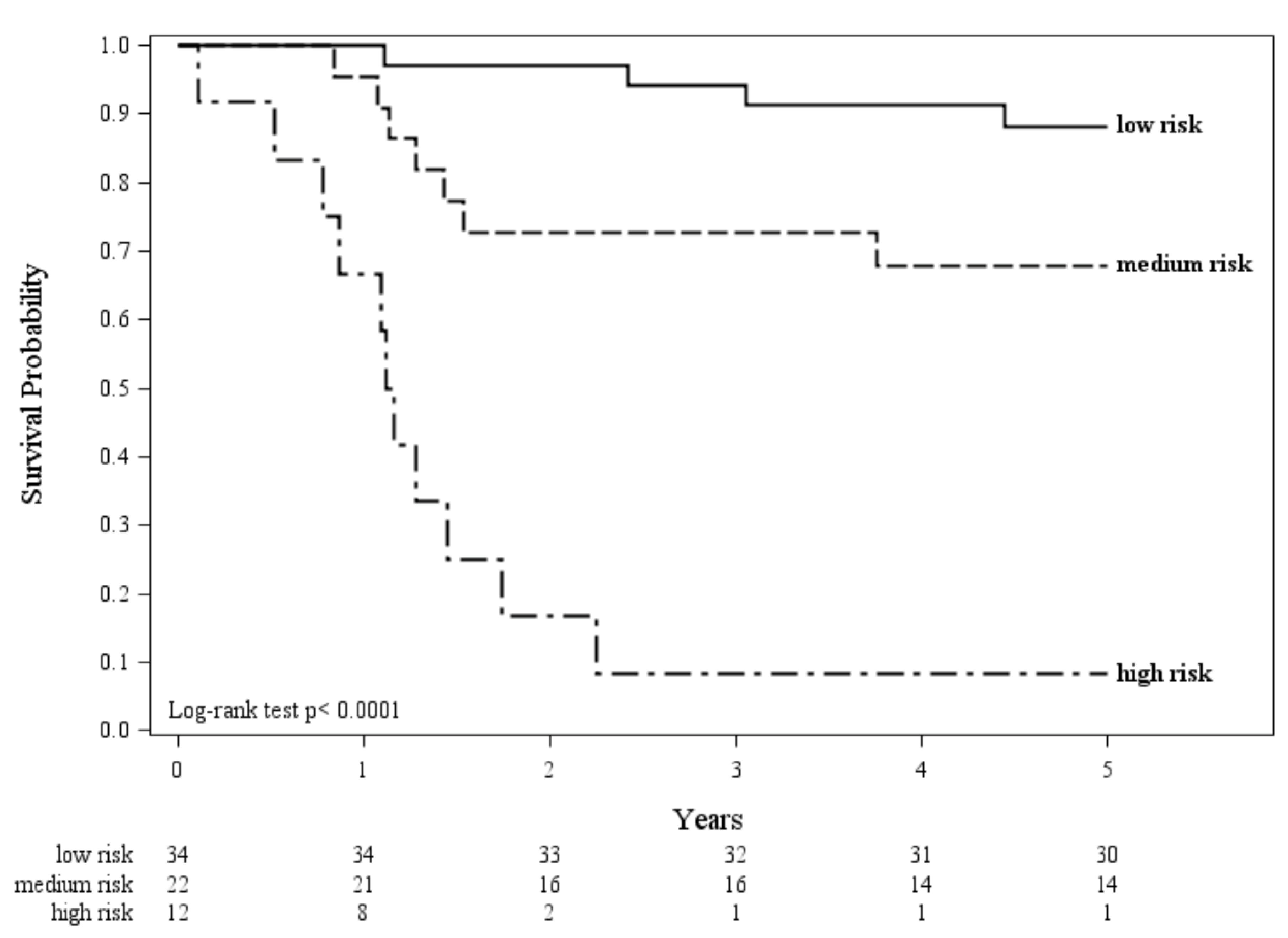
2.3. Combining Immune and Inflammatory Features to Improve the Prognostic Value
2.4. Tumor Mutational Status Across Immune/Inflammatory Profiles
3. Discussion
4. Materials and Methods
4.1. Study Population and Data Collection
4.2. Immunohistochemistry (IHC) Analysis
4.3. Targeted Next Generation Sequencing
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Rivera, G.A.; Wakelee, H. Lung Cancer in Never Smokers. Adv. Exp. Med. Biol. 2016, 893, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Couraud, S.; Souquet, P.J.; Paris, C.; Do, P.; Doubre, H.; Pichon, E.; Dixmier, A.; Monnet, I.; Etienne-Mastroianni, B.; Vincent, M.; et al. BioCAST/IFCT-1002: Epidemiological and molecular features of lung cancer in never-smokers. Eur. Respir. J. 2015, 45, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Stiles, B.M.; Rahouma, M.; Hussein, M.K.; Nasar, A.; Nguyen, A.B.; Harrison, S.; Lee, B.; Port, J.L.; Altorki, N.K. Never smokers with resected lung cancer: Different demographics, similar survival. Eur. J. Cardiothorac. Surg. 2018, 53, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, J.; Wu, P.; Zhou, L.; Lu, B.; Ying, K.; Chen, E.; Lu, Y.; Liu, P. Smoker and non-smoker lung adenocarcinoma is characterized by distinct tumor immune microenvironments. Oncoimmunology 2018, 7, e1494677. [Google Scholar] [CrossRef]
- Kinoshita, T.; Kudo-Saito, C.; Muramatsu, R.; Fujita, T.; Saito, M.; Nagumo, H.; Sakurai, T.; Noji, S.; Takahata, E.; Yaguchi, T.; et al. Determination of poor prognostic immune features of tumour microenvironment in non-smoking patients with lung adenocarcinoma. Eur. J. Cancer 2017, 86, 15–27. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Govindan, R.; Ding, L.; Griffith, M.; Subramanian, J.; Dees, N.D.; Kanchi, K.L.; Maher, C.A.; Fulton, R.; Fulton, L.; Wallis, J.; et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell 2012, 150, 1121–1134. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Ju, Y.S.; Haase, K.; Van Loo, P.; Martincorena, I.; Nik-Zainal, S.; Totoki, Y.; Fujimoto, A.; Nakagawa, H.; Shibata, T.; et al. Mutational signatures associated with tobacco smoking in human cancer. Science 2016, 354, 618–622. [Google Scholar] [CrossRef]
- Sholl, L.M.; Aisner, D.L.; Varella-Garcia, M.; Berry, L.D.; Dias-Santagata, D.; Wistuba, I.I.; Chen, H.; Fujimoto, J.; Kugler, K.; Franklin, W.A.; et al. Multi-institutional Oncogenic Driver Mutation Analysis in Lung Adenocarcinoma: The Lung Cancer Mutation Consortium Experience. J. Thorac. Oncol. 2015, 10, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Thatcher, N.; Chang, A.; Parikh, P.; Rodrigues Pereira, J.; Ciuleanu, T.; Von Pawel, J.; Thongprasert, S.; Tan, E.H.; Pemberton, K.; Archer, V.; et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: Results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 2005, 366, 1527–1537. [Google Scholar] [CrossRef]
- Paik, P.K.; Johnson, M.L.; D’Angelo, S.P.; Sima, C.S.; Ang, D.; Dogan, S.; Miller, V.A.; Ladanyi, M.; Kris, M.G.; Riely, G.J. Driver mutations determine survival in smokers and never-smokers with stage IIIB/IV lung adenocarcinomas. Cancer 2012, 118, 5840–5847. [Google Scholar] [CrossRef] [PubMed]
- Pine, S.R.; Mechanic, L.E.; Enewold, L.; Chaturvedi, A.K.; Katki, H.A.; Zheng, Y.L.; Bowman, E.D.; Engels, E.A.; Caporaso, N.E.; Harris, C.C. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. J. Natl. Cancer Inst. 2011, 103, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Agusti, A.; Edwards, L.D.; Rennard, S.I.; MacNee, W.; Tal-Singer, R.; Miller, B.E.; Vestbo, J.; Lomas, D.A.; Calverley, P.M.; Wouters, E.; et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: A novel phenotype. PLoS ONE 2012, 7, e37483. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, U.; Morelli, D.; Marchiano, A.; Sestini, S.; Suatoni, P.; Taverna, F.; Boeri, M.; Sozzi, G.; Cantarutti, A.; Corrao, G. Inflammatory status and lung function predict mortality in lung cancer screening participants. Eur. J. Cancer Prev. 2018, 27, 289–295. [Google Scholar] [CrossRef]
- Shiels, M.S.; Katki, H.A.; Freedman, N.D.; Purdue, M.P.; Wentzensen, N.; Trabert, B.; Kitahara, C.M.; Furr, M.; Li, Y.; Kemp, T.J.; et al. Cigarette smoking and variations in systemic immune and inflammation markers. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Pastorino, U.; Morelli, D.; Leuzzi, G.; Gisabella, M.; Suatoni, P.; Taverna, F.; Bertocchi, E.; Boeri, M.; Sozzi, G.; Cantarutti, A.; et al. Baseline and postoperative C-reactive protein levels predict mortality in operable lung cancer. Eur. J. Cancer 2017, 79, 90–97. [Google Scholar] [CrossRef]
- Shinohara, S.; Otsuki, R.; Onitsuka, T.; Machida, K.; Matsuo, M.; Nakagawa, M.; Sugaya, M. Postoperative C-reactive Protein Is a Predictive Biomarker for Survival After Non-small Cell Lung Cancer Resection. Anticancer Res. 2019, 39, 2193–2198. [Google Scholar] [CrossRef]
- Dong, Z.Y.; Zhang, J.T.; Liu, S.Y.; Su, J.; Zhang, C.; Xie, Z.; Zhou, Q.; Tu, H.Y.; Xu, C.R.; Yan, L.X.; et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology 2017, 6, e1356145. [Google Scholar] [CrossRef] [PubMed]
- Mouw, K.W.; Goldberg, M.S.; Konstantinopoulos, P.A.; D’Andrea, A.D. DNA Damage and Repair Biomarkers of Immunotherapy Response. Cancer Discov. 2017, 7, 675–693. [Google Scholar] [CrossRef] [PubMed]
- Milione, M.; Miceli, R.; Barretta, F.; Pellegrinelli, A.; Spaggiari, P.; Tagliabue, G.; Centonze, G.; Paolino, C.; Mangogna, A.; Kankava, K.; et al. Microenvironment and tumor inflammatory features improve prognostic prediction in gastro-entero-pancreatic neuroendocrine neoplasms. J. Pathol. Clin. Res. 2019, 5, 217–226. [Google Scholar] [CrossRef]
- Busico, A.; Maisonneuve, P.; Prinzi, N.; Pusceddu, S.; Centonze, G.; Garzone, G.; Pelligrinelli, A.; Giacomelli, L.; Mangogna, A.; Paolino, C.; et al. Gastroenteropancreatic High-Grade Neuroendocrine Neoplasms (H-NENs): Histology and molecular analysis, two sides of the same coin. Neuroendocrinology 2019. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Classification and Regression Trees; CRC Press: New York, NY, USA, 2017. [Google Scholar]
- Hochholzer, W.; Trenk, D.; Fromm, M.F.; Valina, C.M.; Stratz, C.; Bestehorn, H.P.; Buttner, H.J.; Neumann, F.J. Impact of cytochrome P450 2C19 loss-of-function polymorphism and of major demographic characteristics on residual platelet function after loading and maintenance treatment with clopidogrel in patients undergoing elective coronary stent placement. J. Am. Coll. Cardiol. 2010, 55, 2427–2434. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, U.; Berg-Ribbe, I.; Wingen, L.U.; Brakensiek, K.; Becker, T.; Klempnauer, J.; Schlegelberger, B.; Kreipe, H.; Flemming, P. Distinct methylation patterns of benign and malignant liver tumors revealed by quantitative methylation profiling. Clin. Cancer Res. 2005, 11, 3654–3660. [Google Scholar] [CrossRef]

| Patients Characteristics | Low Inflammatory Profile | High Inflammatory Profile | p-Value | ||
|---|---|---|---|---|---|
| (N = 34) | (N = 34) | ||||
| At baseline | |||||
| Matching variables | |||||
| Age - mean (SD) | 66 | (9.7) | 67 | (12.2) | 0.7761 |
| Female Gender - nr. (%) | 22 | (64.7) | 20 | (58.8) | 0.9064 |
| Stage of disease - nr. (%) | 0.8353 | ||||
| I | 14 | (41.2) | 13 | (38.2) | |
| II | 6 | (17.7) | 8 | (23.5) | |
| III-IV | 14 | (41.2) | 13 | (38.2) | |
| No-matching variables | |||||
| FEV1 (%) - median (IQ range) | 96.5 | (50) | 74 | (47) | 0.0070 |
| BMI - median (IQ range) | 24.5 | (5) | 25 | (3) | 0.3486 |
| During follow-up | |||||
| Two-year mortality | |||||
| deaths - nr. (%) | 1 | (2.9) | 16 | (47.1) | <0.0001 |
| person-years - mean (SD) | 1.9 | (0.2) | 1.6 | (0.5) | |
| mortality rate - x100 person-years | 1.5 | 29.9 | |||
| Five-year mortality | |||||
| deaths - nr. (%) | 4 | (11.8) | 18 | (52.3) | 0.0006 |
| person-years - mean (SD) | 4.7 | (0.8) | 3 | (1.9) | |
| mortality rate - x100 person-years | 2.5 | 17.8 | |||
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| HR (95% CI) * | HR (95% CI) * | HR (95% CI) * | HR (95% CI) * | |
| High inflammatory profile | 6.8 (2.3–20.2) | 3.4 (1.0–11.8) | ||
| High risk immune profile | 12.1 (4.9–28.5) | 6.6 (2.5–17.6) | ||
| Inflammatory and immune profile | ||||
| Low risk | 1.0 (reference) | |||
| Medium risk | 3.4 (1.0–11.7) | |||
| High risk | 22.5 (6.8–74.4) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milione, M.; Boeri, M.; Cantarutti, A.; Centonze, G.; Busico, A.; Suatoni, P.; Garzone, G.; Cattaneo, L.; Tamborini, E.; Perrone, F.; et al. Improved Prognostic Prediction in Never-Smoker Lung Cancer Patients by Integration of a Systemic Inflammation Marker with Tumor Immune Contexture Analysis. Cancers 2020, 12, 1828. https://doi.org/10.3390/cancers12071828
Milione M, Boeri M, Cantarutti A, Centonze G, Busico A, Suatoni P, Garzone G, Cattaneo L, Tamborini E, Perrone F, et al. Improved Prognostic Prediction in Never-Smoker Lung Cancer Patients by Integration of a Systemic Inflammation Marker with Tumor Immune Contexture Analysis. Cancers. 2020; 12(7):1828. https://doi.org/10.3390/cancers12071828
Chicago/Turabian StyleMilione, Massimo, Mattia Boeri, Anna Cantarutti, Giovanni Centonze, Adele Busico, Paola Suatoni, Giovanna Garzone, Laura Cattaneo, Elena Tamborini, Federica Perrone, and et al. 2020. "Improved Prognostic Prediction in Never-Smoker Lung Cancer Patients by Integration of a Systemic Inflammation Marker with Tumor Immune Contexture Analysis" Cancers 12, no. 7: 1828. https://doi.org/10.3390/cancers12071828
APA StyleMilione, M., Boeri, M., Cantarutti, A., Centonze, G., Busico, A., Suatoni, P., Garzone, G., Cattaneo, L., Tamborini, E., Perrone, F., Mangogna, A., Corrao, G., Pruneri, G., Sozzi, G., Anichini, A., & Pastorino, U. (2020). Improved Prognostic Prediction in Never-Smoker Lung Cancer Patients by Integration of a Systemic Inflammation Marker with Tumor Immune Contexture Analysis. Cancers, 12(7), 1828. https://doi.org/10.3390/cancers12071828







