Sex and Gender Disparities in Melanoma
Abstract
1. Introduction
2. Sex Steroid Hormone Receptors in Melanoma
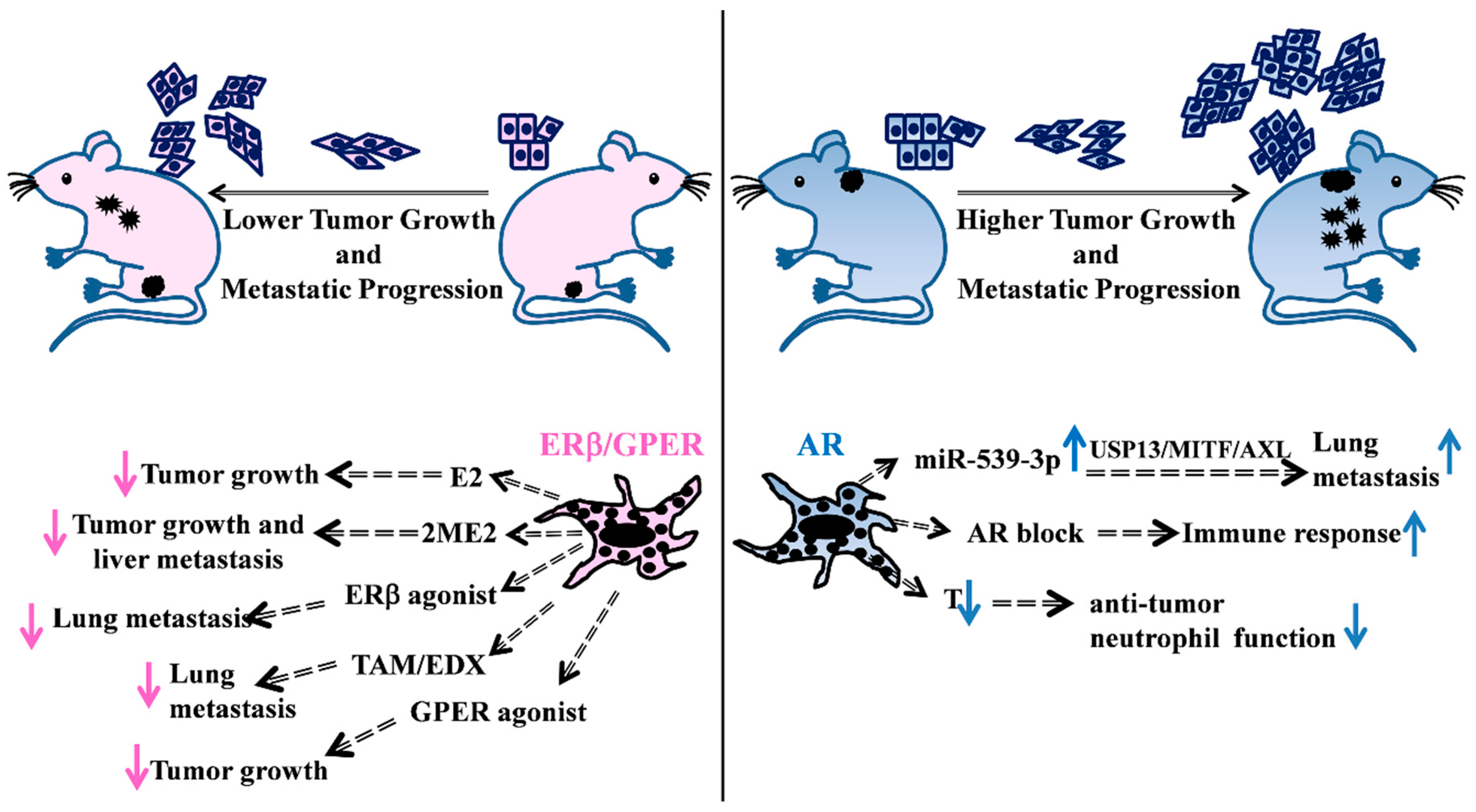
3. Female Hormone Activity
4. Male Hormone Activity
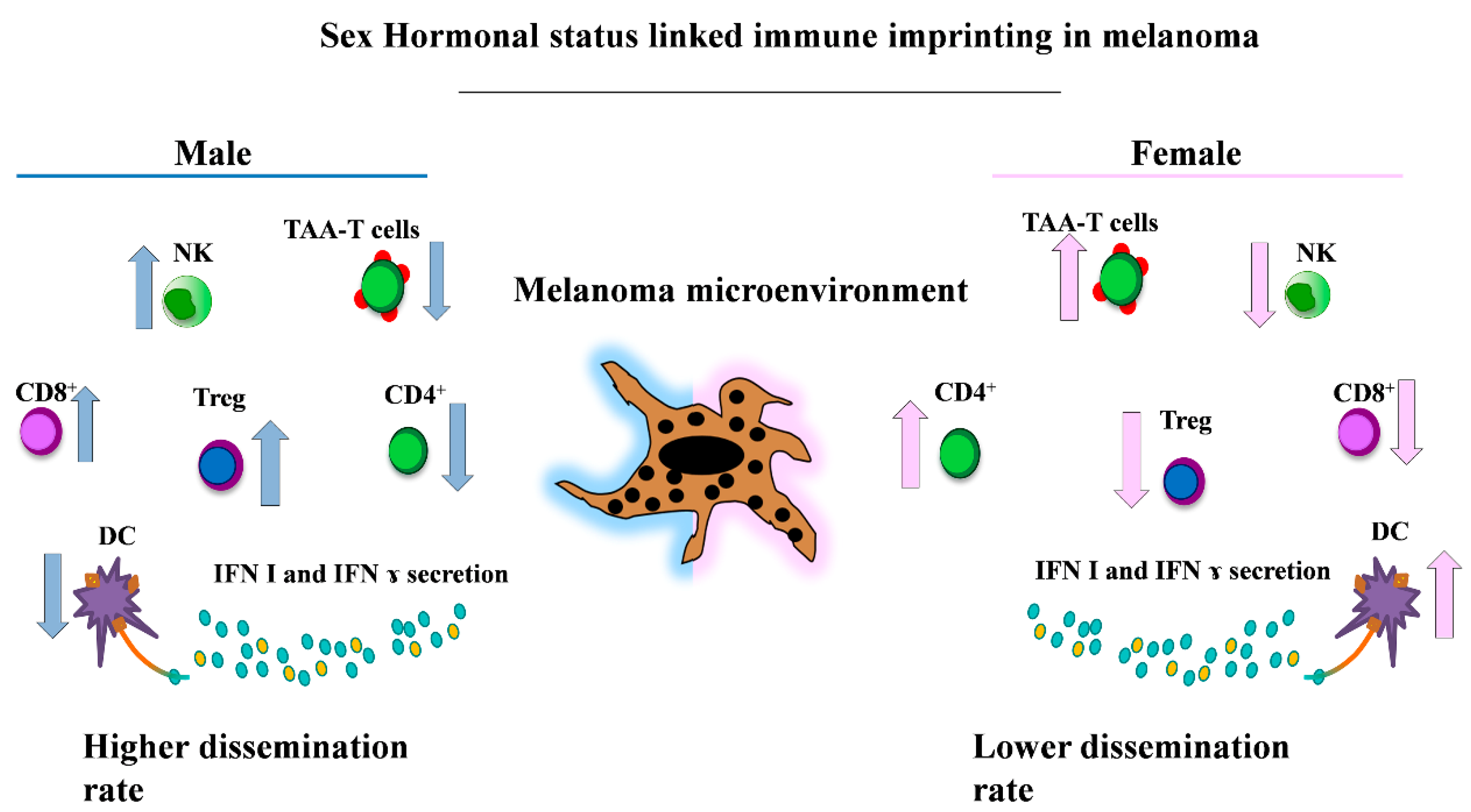
5. Sex and Immunity
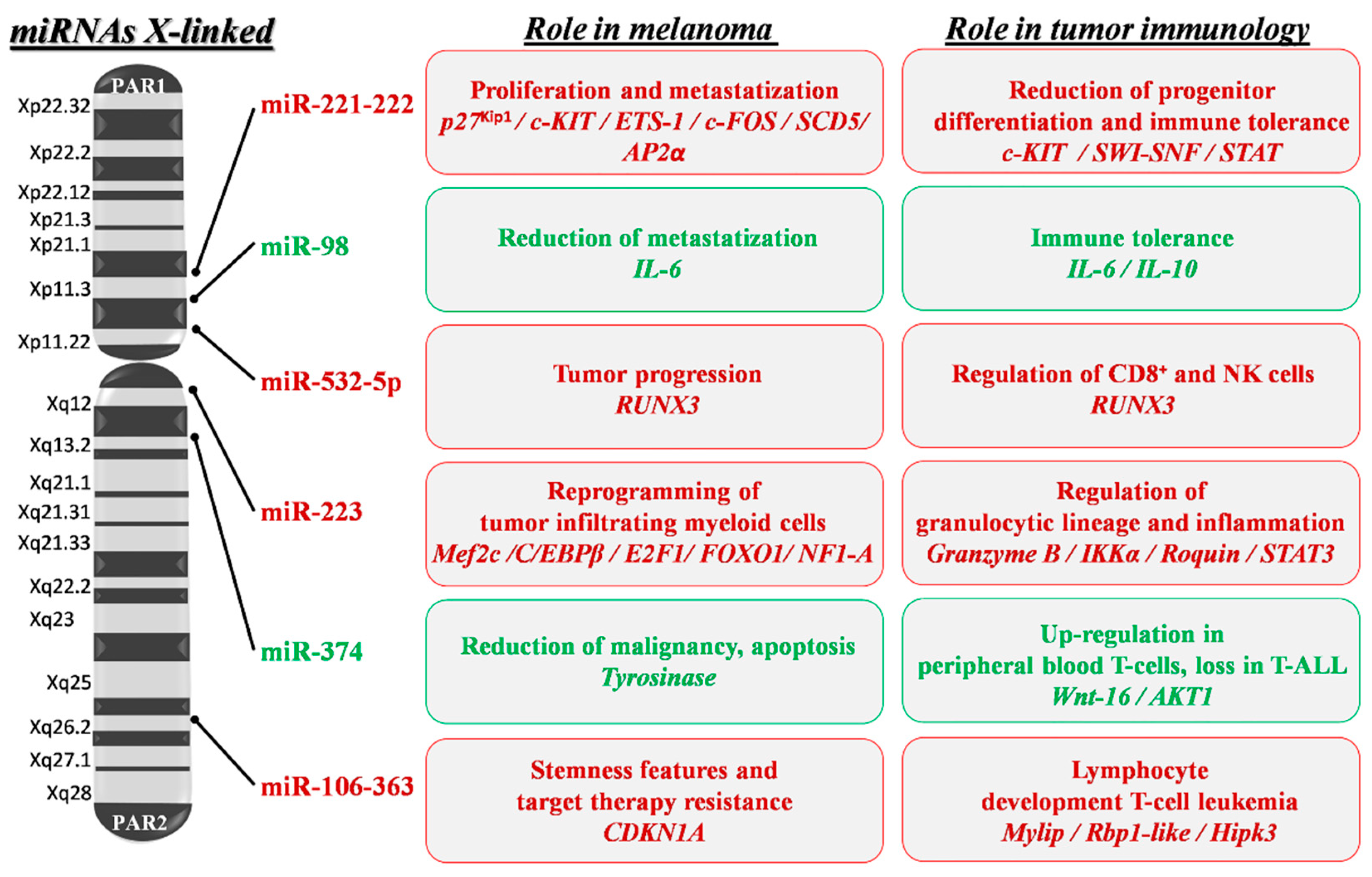
6. Sex Differences and MiRNAs
7. Sex Differences and Response to Therapies
8. Conclusions
Funding
Conflicts of Interest
References
- Italian Association of Medical Oncology (AIOM); Italian Association of Tumor Registries (Airtum). The Numbers of Cancer in Italy 2019. Available online: http://www.salute.gov.it/portale/news/p3_2_1_1_1.jsp?lingua=italiano&menu=notizie&p=dalministero&id=3897 (accessed on 4 July 2020).
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Holman, D.M.; Ding, H.; Guy, G.P.; Watson, M.; Hartman, A.M.; Perna, F.M. Prevalence of sun protection use and sunburn and association of demographic and behaviorial characteristics with sunburn among US adults. JAMA Dermatol. 2018, 154, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Courtenay, W.H. Behavioral Factors Associated with Disease, Injury, and Death among Men: Evidence and Implications for Prevention. J. Men Stud. 2000, 9, 81–142. [Google Scholar] [CrossRef]
- Paddock, L.E.; Lu, S.E.; Bandera, E.V.; Rhoads, G.G.; Fine, J.; Paine, S.; Barnhill, R.; Berwick, M. Skin self-examination and long-term melanoma survival. Melanoma Res. 2016, 26, 401–408. [Google Scholar] [CrossRef]
- Joosse, A.; Collette, S.; Suciu, S.; Nijsten, T.; Lejeune, F.; Kleeberg, U.R.; Coebergh, J.W.; Eggermont, A.M.; de Vries, E. Superior outcome of women with stage I/II cutaneous melanoma: Pooled analysis of four European organization for research and treatment of cancer phase III trials. J. Clin. Oncol. 2012, 30, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Joosse, A.; de Vries, E.; Eckel, R.; Nijsten, T.; Eggermont, A.M.; Hölze, L.D.; Coebergh, J.W.; Engel, J. Munich Melanoma Group. Gender Differences in Melanoma Survival: Female Patients Have a Decreased Risk of Metastasis. J. Investig. Dermatol. 2011, 131, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Joosse, A.; Collette, S.; Suciu, S.; Nijsten, T.; Patel, P.M.; Keilholz, U.; Eggermont, A.M.; Coebergh, J.W.; de Vries, E. Sex is an independent prognostic indicator for survival and relapse/progression free-survival in metastasized stage III to stage IV melanoma: A pooled analysis of five European organization for research and treatment on cancer randomized controlled trials. J. Clin. Oncol. 2013, 31, 2337–2346. [Google Scholar] [CrossRef]
- Balaton, B.P.; Dixon-McDougal, T.; Peeters, S.B.; Brown, C.J. The eXceptional nature of the X chromosome. Hum. Mol. Genet. 2018, 27, R242–R249. [Google Scholar] [CrossRef]
- Gupta, S.; Artomov, M.; Goggins, W.; Daly, M.; Tsao, H. Gender Disparity and Mutation Burden in Metastatic Melanoma. J. Natl. Cancer Inst. 2015, 107, djv221. [Google Scholar] [CrossRef]
- Hernando, B.; Ibarrola-Villava, M.; Fernandez, L.P.; Peña-Chilet, M.; Llorca-Cardeñosa, M.; Oltra, S.S.; Alonso, S.; Boyano, M.D.; Martinez-Cadenas, C.; Ribas, G. Sex-specific genetic effects associated with pigmentation, sensitivity to sunlight, and melanoma in a population of Spanish origin. Biol. Sex Differ. 2016, 7, 17. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Slominski, A.; Zbytek, B.; Nikolakis, G.; Manna, P.R.; Skobowiat, C.; Zmijewski, M.; Li, W.; Janjetovic, Z.; Postlethwaite, A.; Zouboulis, C.C.; et al. Steroidogenesis in the skin: Implications for local immune functions. J. Steroid Biochem. Mol. Biol. 2013, 137, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Dahlman-Wright, K.; Gustafsson, J.Å. Estrogen receptor alpha and beta in health and disease. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 557–568. [Google Scholar] [CrossRef]
- Zhou, J.H.; Kim, K.B.; Myers, J.N.; Fox, P.S.; Ning, J.; Bassett, R.L.; Hasanein, H.; Prieto, V.G. Immunohistochemical expression of hormone receptors in melanoma of pregnant women, nonpregnant women, and men. Am. J. Derm. 2014, 36, 74–79. [Google Scholar] [CrossRef]
- De Giorgi, V.; Gori, A.; Gandini, S.; Papi, F.; Grazzini, M.; Rossari, S.; Simoni, A.; Maio, V.; Massi, D. Oestrogen receptor beta and melanoma: A comparative study. Br. J. Dermatol. 2013, 168, 513–519. [Google Scholar] [CrossRef]
- Ohata, C.; Tadokoro, T.; Itami, S. Expression of estrogen receptor beta in normal skin, melanocytic nevi and malignant melanomas. J. Dermatol. 2008, 35, 215–221. [Google Scholar] [CrossRef]
- Schmidt, A.N.; Nanney, L.B.; Boyd, A.S.; King, L.E., Jr.; Ellis, D.L. Oestrogen receptor beta expression in melanocytic lesions. Exp. Dermatol. 2006, 15, 971–980. [Google Scholar] [CrossRef]
- De Giorgi, V.; Mavilia, C.; Massi, D.; Gozzini, A.; Aragona, P.; Tanini, A.; Sestini, S.; Paglierani, M.; Boddi, V.; Brandi, M.L.; et al. Estrogen receptor expression in cutaneous melanoma: A real-time reverse transcriptase-polymerase chain reaction and immunohistochemical study. Arch. Dermatol. 2009, 145, 30–36. [Google Scholar] [CrossRef]
- Mori, T.; Martinez, S.R.; O’Day, S.J.; Morton, D.L.; Umetani, N.; Kitago, M.; Tanemura, A.; Nguyen, S.L.; Tran, A.N.; Wang, H.J.; et al. Estrogen receptor-alpha methylation predicts melanoma progression. Cancer Res. 2006, 66, 6692–6698. [Google Scholar] [CrossRef]
- Yu, Z.; Gao, W.; Jiang, E.; Lu, F.; Zhang, L.; Shi, Z.; Wang, X.; Chen, L.; Lv, T. Interaction between IGF-IR and ER induced by E2 and IGF-I. PLoS ONE 2013, 8, e62642. [Google Scholar] [CrossRef]
- Yuan, T.-A.; Yourk, V.; Farhat, A.; Guo, K.L.; Garcia, A.; Meyskens, F.L.; Liu-Smith, F. A Possible Link of Genetic Variations in ER/IGF1R Pathway and Risk of Melanoma. Int. J. Mol. Sci. 2020, 21, 1776. [Google Scholar] [CrossRef]
- Liu, M.; Du, Y.; Li, H.; Wang, L.; Ponikwicka-Tyszko, D.; Lebiedzinska, W.; Pilaszewicz-Puza, A.; Liu, H.; Zhou, L.; Fan, H.; et al. Cyanidin-3-o-Glucoside Pharmacologically Inhibits Tumorigenesis via Estrogen Receptor β in Melanoma Mice. Front. Oncol. 2019, 9, 1110. [Google Scholar] [CrossRef]
- Marzagalli, M.; Casati, L.; Moretti, R.M.; Montagnani Marelli, M.; Limonta, P. Estrogen Receptor β Agonists Differentially Affect the Growth of Human Melanoma Cell Lines. PLoS ONE 2015, 10, e0134396. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.P.C.; Santos, A.E.; Custódio, J.B.A. The activation of the G protein-coupled estrogen receptor (GPER) inhibits the proliferation of mouse melanoma K1735-M2 cells. Chem. Biol. Interact. 2017, 277, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Fábián, M.; Rencz, F.; Krenács, T.; Brodszky, V.; Hársing, J.; Németh, K.; Balogh, P.; Kárpáti, S. Expression of G protein-coupled oestrogen receptor in melanoma and in pregnancy-associated melanoma. J. Eur. Acad. Derm. Venereol. 2017, 31, 1453–1461. [Google Scholar] [CrossRef]
- Contrò, V.; Basile, J.R.; Proia, P. Sex steroid hormone receptors, their ligands, and nuclear and non-nuclear pathways. AIMS Mol.Sci. 2015, 2, 294–310. [Google Scholar] [CrossRef]
- Mitkov, M.; Joseph, R.; Copland, J., III. Steroid hormone influence on melanomagenesis. Mol. Cell. Endocrinol. 2015, 417, 94–102. [Google Scholar] [CrossRef]
- Morvillo, V.; Lüthy, I.A.; Bravo, A.I.; Capurro, M.I.; Portel, P.; Calandra, R.S.; Mordoh, J. Androgen receptors in human melanoma cell lines IIB-MEL-LES and IIB-MEL-IAN and in human melanoma metastases. Melanoma Res. 2002, 12, 529–538. [Google Scholar] [CrossRef]
- Still, R.; Brennecke, S. Melanoma in pregnancy. Obstet. Med. 2017, 10, 107–112. [Google Scholar] [CrossRef]
- Donley, G.M.; Liu, W.T.; Pfeiffer, R.M.; McDonald, E.C.; Peters, K.O.; Tucker, M.A.; Cahoon, E.K. Reproductive factors, exogenous hormone use and incidence of melanoma among women in the United States. Br. J. Cancer 2019, 120, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Kemeny, M.M.; Busch, E.; Stewart, A.K.; Menck, H.R. Superior survival of young women with malignant melanoma. Am. J. Surg. 1998, 175, 437–444. [Google Scholar] [CrossRef]
- Lasithiotakis, K.; Leiter, U.; Meier, F.; Eigentler, T.; Metzler, G.; Moehrle, M.; Breuninger, H.; Garbe, C. Age and gender are significant independent predictors of survival in primary cutaneous melanoma. Cancer 2008, 112, 1795–1804. [Google Scholar] [CrossRef] [PubMed]
- Mervic, L.; Leiter, U.; Meier, F.; Eigentler, T.; Forschner, A.; Metzler, G.; Bartenje, I.; Büttner, P.; Garbe, C. Sex differences in survival of cutaneous melanoma are age dependent: An analysis of 7338 patients. Melanoma Res. 2011, 21, 244–252. [Google Scholar] [CrossRef]
- Cervenka, I.; Mahamat-Saleh, Y.; Savoye, I.; Dartois, L.; Boutron-Ruault, M.C.; Fournier, A.; Kvaskoff, M. Oral contraceptive use and cutaneous melanoma risk: A French prospective cohort study. Int. J. Cancer 2018, 143, 2390–2399. [Google Scholar] [CrossRef] [PubMed]
- Botteri, E.; Støer, N.C.; Sakshaug, S.; Graff-Iversen, S.; Vangen, S.; Hofvind, S.; Ursin, G.; Weiderpass, E. Menopausal hormone therapy and risk of melanoma: Do estrogens and progestins have a different role? Int. J. Cancer 2017, 141, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Simin, J.; Tamimi, R.; Lagergren, J.; Adami, H.O.; Brusselaers, N. Menopausal hormone therapy and cancer risk: An overestimated risk? Eur. J. Cancer 2017, 84, 60–68. [Google Scholar] [CrossRef]
- Hicks, B.M.; Kristensen, K.B.; Pedersen, S.A.; Hölmich, L.R.; Pottegård, A. Hormone replacement therapy and the risk of melanoma in post-menopausal women. Hum. Reprod. 2019, 34, 2418–2429. [Google Scholar] [CrossRef]
- Botteri, E.; Støer, N.C.; Weiderpass, E.; Pukkala, E.; Ylikorkala, O.; Lyytinen, H. Menopausal Hormone Therapy and Risk of Melanoma: A Nationwide Register-Based Study in Finland. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1857–1860. [Google Scholar] [CrossRef]
- Cervenka, I.; Rahmoun, M.A.; Mahamat-Saleh, Y.; Boutron-Ruault, M.C.; Fournier, A.; Kvaskoff, M. Premenopausal Use of Progestogens and Cutaneous Melanoma Risk: A French Prospective Cohort Study. Am. J. Epidemiol. 2019. [Google Scholar] [CrossRef]
- Roh, M.R.; Eliades, P.; Gupta, S.; Grant-Kels, J.M.; Tsao, H. Cutaneous melanoma in women. Int. J. Womens Dermatol. 2017, 3, S11–S15. [Google Scholar] [CrossRef]
- Byrom, L.; Olsen, C.M.; Knight, L.; Khosrotehrani, K.; Green, A.C. Does pregnancy after a diagnosis of melanoma affect prognosis? Systematic review and meta-analysis. Dermatol. Surg. 2015, 41, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Kyrgidis, A.; Lallas, A.; Moscarella, E.; Longo, C.; Alfano, R.; Argenziano, G. Does pregnancy influence melanoma prognosis? A meta-analysis. Melanoma Res. 2017, 27, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Richardson, B.; Price, A.; Wagner, M.; Williams, V.; Lorigan, P.; Browne, S.; Miller, J.G.; Mac Neil, S. Investigation of female survival benefit in metastatic melanoma. Br. J. Cancer 1999, 80, 2025–2033. [Google Scholar] [CrossRef] [PubMed]
- Kanda, N.; Watanabe, S. 17beta-estradiol, progesterone, and dihydrotestosterone suppress the growth of human melanoma by inhibiting interleukin-8 production. J. Investig. Dermatol. 2001, 117, 274–283. [Google Scholar] [CrossRef]
- Sarti, M.S.; Visconti, M.A.; Castrucci, A.M. Biological activity and binding of estradiol to SK-Mel 23 human melanoma cells. Braz. J. Med. Biol. Res. 2004, 37, 901–905. [Google Scholar] [CrossRef]
- Feucht, K.A.; Walker, M.J.; Das Gupta, T.K.; Beattie, C.W. Effect of 17 beta-estradiol on the growth of estrogen receptor positive human melanoma in vitro and in athymic mice. Cancer Res. 1988, 48, 7093–7101. [Google Scholar]
- Ghosh, R.; Ott, A.M.; Seetharam, D.; Slaga, T.J.; Kumar, A.P. Cell cycle block and apoptosis induction in a human melanoma cell line following treatment with 2-methoxyostradiol: Therapeutic implications? Melanoma Res. 2003, 13, 119–127. [Google Scholar] [CrossRef]
- Dobos, J.; Timar, J.; Bocsi, J.; Burian, Z.; Nagy, K.; Barna, G.; Petak, I.; Ladanyi, A. In vitro and in vivo antitumor effect of 2-Methoxyestradiol on human melanoma. Int. J. Cancer 2004, 112, 771–776. [Google Scholar] [CrossRef]
- Dobos, J. Endocrine factors influencing melanoma progression. MagyOnkol 2009, 53, 47–50. [Google Scholar]
- Ladányi, A.; Tímár, J.; Bocsi, J.; Tóvári, J.; Lapis, K. Sex-dependent liver metastasis of human melanoma lines in SCID mice. Melanoma Res. 1995, 5, 83–86. [Google Scholar] [CrossRef]
- Vantyghem, S.A.; Postenka, C.O.; Chambers, A.F. Estrous cycle influences organ-specific metastasis of B16F10 melanoma cells. Cancer Res. 2003, 63, 4763–4765. [Google Scholar] [PubMed]
- Massaro, R.R.; Faião-Flores, F.; Rebecca, V.W.; Sandri, S.; Alves-Fernandes, D.K.; Pennacchi, P.C.; Smalley, K.S.M.; Maria-Engler, S.S. Inhibition of proliferation and invasion in 2D and 3D models by 2-methoxyestradiol in human melanoma cells. Pharmacol. Res. 2017, 119, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Natale, C.A.; Li, J.; Zhang, J.; Dahal, A.; Dentchev, T.; Stanger, B.Z.; Ridky, T.W. Activation of G protein-coupled estrogen receptor signaling inhibits melanoma and improves response to immune checkpoint blockade. eLife 2018, 7, e31770. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Huang, S.; Mei, S.; Yang, Z.; Xu, L.; Zhou, N.; Yang, Q.; Shen, Q.; Wang, W.; Le, X.; et al. Pharmacological activation of estrogen receptor beta augments innate immunity to suppress cancer metastasis. Proc. Natl. Acad. Sci. USA 2018, 115, E3673–E3681. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Tsubaki, M.; Yamazoe, Y.; Ogaki, M.; Satou, T.; Itoh, T.; Kusunoki, T.; Nishida, S. Tamoxifen inhibits tumor cell invasion and metastasis in mouse melanoma through suppression of PKC/MEK/ERK and PKC/PI3K/Akt pathways. Exp. Cell. Res. 2009, 315, 2022–2032. [Google Scholar] [CrossRef]
- Chen, P.; Sheikh, S.; Ahmad, A.; Ali, S.M.; Ahmad, M.U.; Ahmad, I. Orally administered endoxifen inhibits tumor growth in melanoma-bearing mice. Cell. Mol. Biol. Lett. 2018, 23, 3. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, Z.; Sun, Y.; Yeh, S.; Wang, X.; Long, J.; Chang, C. Androgen receptor promotes melanoma metastasis via altering the miRNA-539-3p/USP13/MITF/AXL signals. Oncogene 2017, 36, 1644–1654. [Google Scholar] [CrossRef]
- Hsueh, E.C.; Gupta, R.K.; Lefor, A.; Reyzin, G.; Ye, W.; Morton, D.L. Androgen blockade enhances response to melanoma vaccine. J. Surg. Res. 2003, 110, 393–398. [Google Scholar] [CrossRef]
- Markman, J.L.; Porritt, R.A.; Wakita, D.; Lane, M.E.; Martinon, D.; Noval Rivas, M.; Luu, M.; Posadas, E.M.; Crother, T.R.; Arditi, M. Loss of testosterone impairs anti-tumor neutrophil function. Nat. Commun. 2020, 11, 1613. [Google Scholar] [CrossRef]
- Marzagalli, M.; Montagnani Marelli, M.; Casati, L.; Fontana, F.; Moretti, R.M.; Limonta, P. Estrogenreceptor β in melanoma: From molecularinsights to potentialclinical utility. Front. Endocrinol. (Lausanne) 2016, 7, 140. [Google Scholar] [CrossRef]
- Shagufta; Ahmad, I. Tamoxifen a pioneering drug: An update on the therapeutic potential of tamoxifen derivatives. Eur. J. Med. Chem. 2018, 143, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Kanter-Lewensohn, L.; Girnita, L.; Girnita, A.; Dricu, A.; Olsson, G.; Leech, L.; Nilsson, G.; Hilding, A.; Wejde, J.; Brismar, K.; et al. Tamoxifen-induced cell death in malignant melanoma cells: Possible involvement of the insulin-like growth factor-1 (IGF-1) pathway. Mol. Cell. Endocrinol. 2000, 165, 131–137. [Google Scholar] [CrossRef]
- Lens, M.B.; Reiman, T.; Husain, A.F. Use of tamoxifen in the treatment of malignant melanoma. Cancer 2003, 98, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Beguerie, J.R.; Xingzhong, J.; Valdez, R.P. Tamoxifen vs. non-tamoxifen treatment for advanced melanoma: A meta-analysis. Int. J. Dermatol. 2010, 49, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.P.; Santos, A.E.; Custódio, J.B. Rethinking tamoxifen in the management of melanoma: New answers for an old question. Eur. J. Pharmacol. 2015, 764, 372–378. [Google Scholar] [CrossRef]
- Madeira, M.; Mattar, A.; Logullo, A.F.; Soares, F.A.; Gebrim, L.H. Estrogen receptor alpha/beta ratio and estrogen receptor beta as predictors of endocrine therapy responsiveness-a randomized neoadjuvant trial comparison between anastrozole and tamoxifen for the treatment of postmenopausal breast cancer. BMC Cancer 2013, 13, 425. [Google Scholar] [CrossRef]
- Horner-Glister, E.; Maleki-Dizaji, M.; Guerin, C.; Johnson, S.; Styles, J.; White, I. Influence of oestradiol and tamoxifen on oestrogen receptors-α and -β protein degradation and non-genomic signalling pathways in uterine and breast carcinoma cells. J. Mol. Endocrinol. 2005, 35, 421–432. [Google Scholar] [CrossRef]
- Sun, M.; Xie, H.F.; Tang, Y.; Lin, S.Q.; Li, J.M.; Sun, S.N.; Hu, X.L.; Huang, Y.X.; Shi, W.; Jian, D. G protein-coupled estrogen receptor enhances melanogenesis via cAMP-protein kinase (PKA) by upregulating microphthalmia-related transcription factor-tyrosinase in melanoma. J. Steroid Biochem. Mol. Biol. 2017, 165, 236–246. [Google Scholar] [CrossRef]
- Natale, C.A.; Duperret, E.K.; Zhang, J.; Sadeghi, R.; Dahal, A.; O’Brien, K.T.; Cookson, R.; Winkler, J.D.; Ridky, T.W. Sex steroids regulate skin pigmentation through nonclassical membrane-bound receptors. eLife 2016, 5, e15104. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, X.; Zhou, M.; Li, J. Effects of Progesterone on the Growth Regulation in Classical Progesterone Receptor-negative Malignant Melanoma Cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2010, 30, 231–234. [Google Scholar] [CrossRef]
- Ramaraj, P.; Cox, J.L. In vitro effect of progesterone on human melanoma (BLM) cell growth. Int. J. Clin. Exp. Med. 2014, 7, 3941–3953. [Google Scholar]
- Leder, D.C.; Brown, J.R.; Ramaraj, P. In-vitro rescue and recovery studies of human melanoma (BLM) cell growth, adhesion and migration functions after treatment with progesterone. Int. J. Clin. Exp. Med. 2015, 8, 12275–12285. [Google Scholar]
- Ramaraj, P.; Cox, J. In-Vitro Effect of Sex Steroids on Mouse Melanoma (B16F10) Cell Growth. CellBio 2014, 3, 60–71. [Google Scholar] [CrossRef]
- Moroni, G.; Gaziano, R.; Buè, C.; Agostini, M.; Perno, C.F.; Sinibaldi-Vallebona, P.; Pica, F. Progesterone and Melanoma Cells: An Old Story Suspended between Life and Death. J. Steroids Horm. Sci. 2015, S13, 158. [Google Scholar]
- Morvillo, V.; Luthy, I.A.; Bravo, A.I.; Capurro, M.I.; Donaldson, M.; Quintans, C.; Calandra, R.S.; Mordoh, J. Atypical androgen receptor in the human melanoma cell line IIB-MEL-J. Pigment Cell Res. 1995, 8, 135–141. [Google Scholar] [CrossRef]
- Schmidt, K.; Carroll, J.S.; Yee, E.; Thomas, D.D.; Wert-Lamas, L.; Neier, S.C.; Sheynkman, G.; Ritz, J.; Novina, C.D. The lncRNA SLNCR Recruits the Androgen Receptor to EGR1-Bound Genes in Melanoma and Inhibits Expression of Tumor Suppressor p21. Cell Rep. 2019, 27, 2493–2507. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Fang, D.; Xu, H.; Wang, Q.; Xia, H. The androgen receptor expression and association with patient’s survival in different cancers. Genomics 2020, 112, 1926–1940. [Google Scholar] [CrossRef]
- Clocchiatti, A.; Cora, E.; Zhang, Y.; Dotto, G.P. Sexual dimorphism in cancer. Nat. Rev. Cancer 2016, 16, 330–339. [Google Scholar] [CrossRef]
- Conforti, F.; Pala, L.; Bagnardi, V.; De Pas, T.; Martinetti, M.; Viale, G.; Gelber, R.D.; Goldhirsch, A. Cancer immunotherapy efficacy and patients’ sex: A systematic review and meta-analysis. Lancet Oncol. 2018, 19, 737–746. [Google Scholar] [CrossRef]
- Wallis, C.J.D.; Butaney, M.; Satkunasivam, R.; Freedland, S.J.; Patel, S.P.; Hamid, O.; Pal, S.K.; Klaassen, Z. Association of PatientSex With Efficacy of Immune Checkpoint Inhibitors and Overall Survival in Advanced Cancers: A Systematic Review and Meta-analysis. JAMA Oncol. 2019, 5, 529–536. [Google Scholar] [CrossRef]
- Khan, D.; Ansar Ahmed, S. The ImmuneSystem Is a Natural Target for Estrogen Action: Opposing Effects of Estrogen in Two Prototypical Autoimmune Diseases. Front. Immunol. 2016, 6, 635. [Google Scholar] [CrossRef] [PubMed]
- Kovats, S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015, 294, 63–69. [Google Scholar] [CrossRef]
- Pierdominici, M.; Maselli, A.; Colasanti, T.; Giammarioli, A.M.; Delunardo, F.; Vacirca, D.; Sanchez, M.; Giovannetti, A.; Malorni, W.; Ortona, E. Estrogen receptor profiles in human peripheral blood lymphocytes. Immunol. Lett. 2010, 132, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.; Trejo, T.; Luthra, H.; Griffiths, M.; David, C.S.; Taneja, V. Mechanism by which HLA-DR4 regulates sex-bias of arthritis in humanized mice. J. Autoimmun. 2010, 35, 1–9. [Google Scholar] [CrossRef]
- Kovats, S.; Carreras, E. Regulation of dendritic cell differentiation and function by estrogen receptor ligands. Cell Immunol. 2008, 252, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Capone, I.; Marchetti, P.; Ascierto, P.A.; Malorni, W.; Gabriele, L. Sexual Dimorphism of Immune Responses: A New Perspective in Cancer Immunotherapy. Front. Immunol. 2018, 9, 552. [Google Scholar] [CrossRef] [PubMed]
- Barry, K.C.; Hsu, J.; Broz, M.L.; Cueto, F.J.; Binnewies, M.; Combes, A.J.; Nelson, A.E.; Loo, K.; Kumar, R.; Rosenblum, M.D.; et al. A naturalkiller-dendriticcell axis defines checkpoint therapy-responsive tumor microenvironments. Nat. Med. 2018, 24, 1178–1191. [Google Scholar] [CrossRef]
- De Giorgi, V.; Gori, A.; Grazzini, M.; Rossari, S.; Scarfì, F.; Corciova, S.; Verdelli, A.; Lotti, T.; Massi, D. Estrogens, estrogen receptors and melanoma. Expert Rev. Anticancer Ther. 2011, 11, 739–747. [Google Scholar] [CrossRef]
- Krahn-Bertil, E.; Dos Santos, M.; Damour, O.; Andre, V.; Bolzinger, M.A. Expression of estrogen-related receptor beta (ERRβ) in human skin. Eur. J. Dermatol. 2010, 20, 719–723. [Google Scholar]
- Seillet, C.; Laffont, S.; Trémollières, F.; Rouquié, N.; Ribot, C.; Arnal, J.F.; Douin-Echinard, V.; Gourdy, P.; Guéry, J.C. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor α signaling. Blood 2012, 119, 454–464. [Google Scholar] [CrossRef]
- Laffont, S.; Rouquié, N.; Azar, P.; Seillet, C.; Plumas, J.; Aspord, C.; Guéry, J.C. X-Chromosome complement and estrogen receptor signaling independently contribute to the enhanced TLR7-mediated IFN-α production of plasmacytoid dendritic cells from women. J. Immunol. 2014, 193, 5444–5452. [Google Scholar] [CrossRef] [PubMed]
- Berghöfer, B.; Frommer, T.; Haley, G.; Fink, L.; Bein, G.; Hackstein, H. TLR7 Ligands Induce Higher IFN-alpha Production in Females. J. Immunol. 2006, 177, 2088–2096. [Google Scholar] [CrossRef] [PubMed]
- Song, P.I.; Park, Y.M.; Abraham, T.; Harten, B.; Zivony, A.; Neparidze, N.; Armstrong, C.A.; Ansel, J.C. Human keratinocytes express functional CD14 and toll-like receptor 4. J. Investig. Dermatol. 2002, 119, 424–432. [Google Scholar] [CrossRef]
- Jin, S.H.; Kang, H.Y. Activation of Toll-like Receptors 1, 2, 4, 5, and 7 on Human Melanocytes Modulate Pigmentation. Ann. Dermatol. 2010, 22, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Arigami, T.; Kitago, M.; Nguyen, S.L.; Narita, N.; Ferrone, S.; Morton, D.L.; Irie, R.F.; Hoon, D.S. Activation of Toll-like receptors 2, 3, and 4 on human melanoma cells induces inflammatory factors. Mol. Cancer Ther. 2008, 7, 3642–3653. [Google Scholar] [CrossRef]
- Saint-Jean, M.; Knol, A.C.; Nguyen, J.M.; Khammari, A.; Dréno, B. TLR expression in human melanoma cells. Eur. J. Dermatol. 2011, 21, 899–905. [Google Scholar] [CrossRef]
- Mittal, D.; Saccheri, F.; Vénéreau, E.; Pusterla, T.; Bianchi, M.E.; Rescigno, M. TLR4-mediated skin carcinogenesis is dependent on immune and radioresistant cells. EMBO J. 2010, 29, 2242–2252. [Google Scholar] [CrossRef]
- Hemmi, H.; Kaisho, T.; Takeuchi, O.; Sato, S.; Sanjo, H.; Hoshino, K.; Horiuchi, T.; Tomizawa, H.; Takeda, K.; Akira, S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 2002, 3, 196–200. [Google Scholar] [CrossRef]
- Stockfleth, E.; Trefzer, U.; Garcia-Bartels, C.; Wegner, T.; Schmook, T.; Sterry, W. The use of Toll-like receptor-7 agonist in the treatment of basal cell carcinoma: An overview. Br. J. Dermatol. 2003, 149, 53–56. [Google Scholar] [CrossRef]
- Schön, M.P.; Schön, M. Immune modulation and apoptosis induction: Two sides of the antitumoral activity of imiquimod. Apoptosis 2004, 9, 291–298. [Google Scholar] [CrossRef]
- Stiff, A.; Trikha, P.; Wesolowski, R.; Kendra, K.; Hsu, V.; Uppati, S.; McMichael, E.; Duggan, M.; Campbell, A.; Keller, K.; et al. Myeloid-Derived Suppressor Cells Express Bruton’s Tyrosine Kinase and Can Be Depleted in Tumor-Bearing Hosts by IbrutinibTreatment. Cancer Res. 2016, 76, 2125–2136. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Geng, D.; Liu, Y.; Zheng, L.; Li, Z.; Joseph, M.A.; McKenna, C.; Bansal, N.; Ochoa, A.; Davila, E. Augmentation of therapeutic responses in melanoma by inhibition of IRAK-1,-4. Cancer Res. 2012, 72, 6209–6216. [Google Scholar] [CrossRef]
- Romano, S.; Xiao, Y.; Nakaya, M.; D’Angelillo, A.; Chang, M.; Jin, J.; Hausch, F.; Masullo, M.; Feng, X.; Romano, M.F.; et al. FKBP51 employs both scaffold and isomerase functions to promote NF-κB activation in melanoma. Nucleic Acids Res. 2015, 43, 6983–6993. [Google Scholar] [CrossRef]
- Touil, Y.; Segard, P.; Ostyn, P.; Begard, S.; Aspord, C.; El Machhour, R.; Masselot, B.; Vandomme, J.; Flamenco, P.; Idziorek, T.; et al. Melanoma dormancy in a mouse model is linked to GILZ/FOXO3A-dependent quiescence of disseminated stem-like cells. Sci. Rep. 2016, 6, 30405. [Google Scholar] [CrossRef] [PubMed]
- Aydin, E.; Johansson, J.; Nazir, F.H.; Hellstrand, K.; Martner, A. Role of NOX2-Derived Reactive Oxygen Species in NK Cell-Mediated Control of Murine Melanoma Metastasis. Cancer Immunol. Res. 2017, 5, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Sander, C.S.; Hamm, F.; Elsner, P.; Thiele, J.J. Oxidative stress in malignant melanoma and non-melanoma skin cancer. Br. J. Dermatol. 2003, 148, 913–922. [Google Scholar] [CrossRef]
- Joosse, A.; De Vries, E.; van Eijck, C.H.; Eggermont, A.M.; Nijsten, T.; Coebergh, J.W. Reactive oxygen species and melanoma: An explanation for gender differences in survival? Pigment Cell Melanoma Res. 2010, 23, 352–364. [Google Scholar] [CrossRef]
- Obrador, E.; Liu-Smith, F.; Dellinger, R.W.; Salvador, R.; Meyskens, F.L.; Estrela, J.M. Oxidative stress and antioxidants in the pathophysiology of malignant melanoma. Biol. Chem. 2019, 400, 589–612. [Google Scholar] [CrossRef]
- Malorni, W.; Straface, E.; Matarrese, P.; Ascione, B.; Coinu, R.; Canu, S.; Galluzzo, P.; Marino, M.; Franconi, F. Redox state and gender differences in vascular smooth muscle cells. FEBS Lett. 2008, 582, 635–642. [Google Scholar] [CrossRef]
- Miller, A.A.; De Silva, T.M.; Jackman, K.A.; Sobey, C.G. Effect of gender and sex hormones on vascular oxidativestress. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1037–1043. [Google Scholar] [CrossRef]
- Cheng, G.C.; Schulze, P.C.; Lee, R.T.; Sylvan, J.; Zetter, B.R.; Huang, H. Oxidative stress and thioredoxin-interacting protein promote intravasation of melanoma cells. Exp. Cell Res. 2004, 300, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M. Reactive oxygen species in tumor metastasis. Cancer Lett. 2008, 266, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Offner, F.A.; Wirtz, H.C.; Schiefer, J.; Bigalke, I.; Klosterhalfen, G.; Bittinger, F.; Mittermayer, C.; Kirkpatrick, C.J. Interaction of Human Malignant Melanoma (ST-ML-12) Tumor Spheroids with Endothelial Cell Monolayers. Damage to Endothelium by Oxygen-Derived Free Radicals. Am. J. Pathol. 1992, 141, 601–610. [Google Scholar] [PubMed]
- Mendez, R.; Aptsiauri, N.; Del Campo, A.; Maleno, I.; Cabrera, T.; Ruiz-Cabello, F.; Garrido, F.; Garcia-Lora, A. HLA and melanoma: Multiple alterations in HLA class I and II expression in human melanoma cell lines from ESTDAB cell bank. Cancer Immunol. Immunother. 2009, 58, 1507–1515. [Google Scholar] [CrossRef]
- Garrido, F.; Algarra, I.; García-Lora, A.M. The escape of cancer from T lymphocytes: Immunoselection of MHC class I loss variants harboring structural-irreversible “hard” lesions. Cancer Immunol. Immunother. 2010, 59, 1601–1606. [Google Scholar] [CrossRef]
- Hölsken, O.; Miller, M.; Cerwenka, A. Exploiting natural killer cells for therapy of melanoma. J. Dtsch. Dermatol. Ges. 2015, 13, 23–29. [Google Scholar] [CrossRef]
- Castelli, C.; Rivoltini, L.; Andreola, G.; Carrabba, M.; Renkvist, N.; Parmiani, G. T-cell recognition of melanoma-associated antigens. J. Cell. Physiol. 2000, 182, 323–331. [Google Scholar] [CrossRef]
- Epping, M.T.; Bernards, R. A causal role for the human tumor antigen preferentially expressed antigen of melanoma in cancer. Cancer Res. 2006, 66, 10639–10642. [Google Scholar] [CrossRef]
- Lupetti, R.; Pisarra, P.; Verrecchia, A.; Farina, C.; Nicolini, G.; Anichini, A.; Bordignon, C.; Sensi, M.; Parmiani, G.; Traversari, C. Translation of a retained intron in tyrosinase-related protein (TRP) 2 mRNA generates a new cytotoxic T lymphocyte (CTL)-defined and shared human melanoma antigen not expressed in normal cells of the melanocytic lineage. J. Exp. Med. 1998, 188, 1005–1016. [Google Scholar] [CrossRef]
- Scanlan, M.J.; Gure, A.O.; Jungbluth, A.A.; Old, L.J.; Chen, Y.T. Cancer/testis antigens: An expanding family of targets for cancer immunotherapy. Immunol. Rev. 2002, 188, 22–32. [Google Scholar] [CrossRef]
- Passarelli, A.; Mannavola, F.; Stucci, L.S.; Tucci, M.; Silvestris, F. Immune system and melanoma biology: A balance between immunosurveillance and immune escape. Oncotarget 2017, 8, 106132–106142. [Google Scholar] [CrossRef]
- Spitzer, J.A. Gender differences in some host defense mechanisms. Lupus 1999, 8, 380–383. [Google Scholar] [CrossRef]
- Weinstein, Y.; Ran, S.; Segal, S. Sex-associated Differences in the Regulation of Immune Responses Controlled by the MHC of the Mouse. J. Immunol. 1984, 132, 656–661. [Google Scholar]
- Orstavik, K.H. X chromosome inactivation in clinical practice. Hum. Genet. 2009, 126, 363–373. [Google Scholar] [CrossRef]
- Migeon, B.R. The role of X inactivation and cellular mosaicism in women’s health and sex-specific diseases. JAMA 2006, 295, 1428–1433. [Google Scholar] [CrossRef]
- Libert, C.; Dejager, L.; Pinheiro, I. The X chromosome in immune functions: When a chromosome makes the difference. Nat. Rev. Immunol. 2010, 10, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, S.K.; Bermas, B.; Costenbader, K.H. Sexual disparities in the incidence and course of SLE and RA. Clin. Immunol. 2013, 149, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Ortona, E.; Pierdominici, M.; Maselli, A.; Veroni, C.; Aloisi, F.; Shoenfeld, Y. Sex-based differences in autoimmune diseases. Ann. Ist. Super. Sanita 2016, 52, 205–212. [Google Scholar] [PubMed]
- Abdullah, M.; Chai, P.S.; Chong, M.Y.; Tohit, E.R.; Ramasamy, R.; Pei, C.P.; Vidyadaran, S. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell Immunol. 2012, 272, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Uppal, S.S.; Verma, S.; Dhot, P.S. Normal values of CD4 and CD8 lymphocyte subsets in healthy indian adults and the effects of sex, age, ethnicity, and smoking. Cytom. B Clin. Cytom. 2003, 52, 32–36. [Google Scholar] [CrossRef]
- Afshan, G.; Afzal, N.; Qureshi, S. CD4+CD25(hi) Regulatory T Cells in Healthy Males and Females Mediate Gender Difference in the Prevalence of Autoimmune Diseases. Clin. Lab. 2012, 58, 567–571. [Google Scholar]
- Wesa, A.K.; Mandic, M.; Taylor, J.L.; Moschos, S.; Kirkwood, J.M.; Kwok, W.W.; Finke, J.H.; Storkus, W.J. Circulating Type-1 Anti-Tumor CD4(+) T Cells are Preferentially Pro-Apoptotic in Cancer Patients. Front. Oncol. 2014, 4, 266. [Google Scholar] [CrossRef]
- Knutson, K.L.; Disis, M.L. Tumor antigen-specific Thelper cells in cancer immunity and immunotherapy. Cancer Immunol. Immunother. 2005, 54, 721–728. [Google Scholar] [CrossRef]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef]
- Wang, C.; Dehghani, B.; Li, Y.; Kaler, L.J.; Vandenbark, A.A.; Offner, H. Oestrogen modulates experimental autoimmune encephalomyelitis and interleukin-17 production via programmed death 1. Immunology 2009, 126, 329–335. [Google Scholar] [CrossRef]
- Curiel, T.J.; Wei, S.; Dong, H.; Alvarez, X.; Cheng, P.; Mottram, P.; Krzysiek, R.; Knutson, K.L.; Daniel, B.; Zimmermann, M.C.; et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med. 2003, 9, 562–567. [Google Scholar] [CrossRef]
- Dong, H.; Chen, L. B7-H1 pathway and its role in the evasion of tumor immunity. J. Mol. Med. (Berl.) 2003, 81, 281–287. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, Q.; Zhou, S.; Hu, Z.; Lan, R. Costimulatory molecule B7-H1 on the immune escape of bladder cancer and its clinical significance. J. Huazhong Univ. Sci. Technol. Med. Sci. 2009, 29, 77–79. [Google Scholar] [CrossRef]
- Lin, P.Y.; Sun, L.; Thibodeaux, S.R.; Ludwig, S.M.; Vadlamudi, R.K.; Hurez, V.J.; Bahar, R.; Kious, M.J.; Livi, C.B.; Wall, S.R.; et al. B7-H1-dependent sex-related differences in tumor immunity and immunotherapy responses. J. Immunol. 2010, 185, 2747–2753. [Google Scholar] [CrossRef]
- Zeng, Y. Principles of micro-RNA production and maturation. Oncogene 2006, 25, 6156–6162. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Kim, V.N.; Han, J.; Siomi, M.C. Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell Biol. 2009, 10, 126–139. [Google Scholar] [CrossRef]
- Jansson, M.D.; Lund, A.H. MicroRNA and cancer. Mol. Oncol. 2012, 6, 590–610. [Google Scholar] [CrossRef]
- Calin, G.A.; Sevignani, C.; Dumitru, C.D.; Hyslop, T.; Noch, E.; Yendamuri, S.; Shimizu, M.; Rattan, S.; Bullrich, F.; Negrini, M.; et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl. Acad. Sci. USA 2004, 101, 2999–3004. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Croce, C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer 2006, 6, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Fattore, L.; Costantini, S.; Malpicci, D.; Ruggiero, C.F.; Ascierto, P.A.; Croce, C.M.; Mancini, R.; Ciliberto, G. MicroRNAs in melanoma development and resistance to target therapy. Oncotarget 2017, 8, 22262–22278. [Google Scholar] [CrossRef]
- Collins, L.C.; Marotti, J.D.; Gelber, S.; Cole, K.; Ruddy, K.; Kereakoglow, S.; Brachtel, E.F.; Schapira, L.; Come, S.E.; Winer, E.P.; et al. Pathologic features and molecular phenotype by patient age in a large cohort of young women with breast cancer. Breast Cancer Res. Treat. 2012, 131, 1061–1066. [Google Scholar] [CrossRef]
- Maillot, G.; Lacroix-Triki, M.; Pierredon, S.; Gratadou, L.; Schmidt, S.; Benes, V.; Roche, H.; Dalenc, F.; Auboeuf, D.; Millevoi, S.; et al. Widespread estrogen-dependent repression of microRNAs involved in breast tumor cell growth. Cancer Res. 2009, 69, 8332–8340. [Google Scholar] [CrossRef]
- Iorio, M.V.; Ferracin, M.; Liu, C.G.; Veronese, A.; Spizzo, R.; Sabbioni, S.; Magri, E.; Pedriali, M.; Fabbri, M.; Campiglio, M.; et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005, 65, 7065–7070. [Google Scholar] [CrossRef]
- Bottner, M.; Thelen, P.; Jarry, H. Estrogen receptor beta: Tissue distribution and the still largely enigmatic physiological function. J. Steroid Biochem. Mol. Biol. 2014, 139, 245–251. [Google Scholar] [CrossRef]
- Di Leva, G.; Gasparini, P.; Piovan, C.; Ngankeu, A.; Garofalo, M.; Taccioli, C.; Iorio, M.V.; Li, M.; Volinia, S.; Alder, H. MicroRNA Cluster 221-222 and Estrogen Receptor α Interactions in Breast Cancer. J. Natl. Cancer Inst. 2010, 102, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Che, Q.; Qiu, H.; Bao, W.; Chen, X.; Lu, W.; Li, B.; Wan, X. Elevated MiR-222-3p Promotes Proliferation and Invasion of Endometrial Carcinoma via Targeting ERα. PLoS ONE 2014, 9, e87563. [Google Scholar] [CrossRef] [PubMed]
- Di Leva, G.; Cheung, D.G.; Croce, C.M. miRNA clusters as therapeutic targets for hormone-resistant breast cancer. Expert Rev. Endocrinol. Metab. 2015, 10, 607–617. [Google Scholar] [CrossRef]
- Zhao, J.J.; Lin, J.; Yang, H.; Kong, W.; He, L.; Ma, X.; Coppola, D.; Cheng, J.Q. MicroRNA-221/222 negatively regulates estrogen receptor alpha and is associated with tamoxifen resistance in breast cancer. J. Biol. Chem. 2008, 283, 31079–31086. [Google Scholar] [CrossRef]
- Felicetti, F.; Errico, M.C.; Bottero, L.; Segnalini, P.; Stoppacciaro, A.; Biffoni, M.; Felli, N.; Mattia, G.; Petrini, M.; Colombo, M.P.; et al. The promyelocyticleukemiazinc finger-microRNA-221/-222 pathwaycontrols melanoma progressionthrough multiple oncogenicmechanisms. Cancer Res. 2008, 68, 2745–2754. [Google Scholar] [CrossRef] [PubMed]
- Mattia, G.; Errico, M.C.; Felicetti, F.; Petrini, M.; Bottero, L.; Tomasello, L.; Romania, P.; Boe, A.; Segnalini, P.; Di Virgilio, A.; et al. Constitutive activation of the ETS-1-miR-222 circuitry in metastatic melanoma. Pigment Cell Melanoma Res. 2011, 24, 953–965. [Google Scholar] [CrossRef] [PubMed]
- Errico, M.C.; Felicetti, F.; Bottero, L.; Mattia, G.; Boe, A.; Felli, N.; Petrini, M.; Bellenghi, M.; Pandha, H.S.; Calvaruso, M.; et al. The abrogation of the HOXB7/PBX2 complex induces apoptosis in melanoma through the miR-221&222-c-FOS pathway. Int. J. Cancer 2013, 133, 879–892. [Google Scholar]
- Puglisi, R.; Bellenghi, M.; Pontecorvi, G.; Gulino, A.; Petrini, M.; Felicetti, F.; Bottero, L.; Mattia, G.; Carè, A. SCD5 restored expression favors differentiation and epithelial-mesenchymal reversion in advanced melanoma. Oncotarget 2018, 9, 7567–7581. [Google Scholar] [CrossRef][Green Version]
- Felli, N.; Errico, M.C.; Pedini, F.; Petrini, M.; Puglisi, R.; Bellenghi, M.; Boe, A.; Felicetti, F.; Mattia, G.; De Feo, A.; et al. AP2α controls the dynamic balance between miR-126&126* and miR-221&222 during melanoma progression. Oncogene 2016, 35, 3016–3026. [Google Scholar]
- Ohno, S.; Labhart, A.; Mann, T.; Samuels, L.T.; Zander, J. (Eds.) Sex Chromosomes and Sex-Linked Genes. In Monographs in Endocrinology; Springer: Berlin, Germany, 1967. [Google Scholar]
- Matarrese, P.; Tieri, P.; Anticoli, S.; Ascione, B.; Conte, M.; Franceschi, C.; Malorni, W.; Salvioli, S.; Ruggieri, A. X-chromosome-linked miR548am-5p is a key regulator of sex disparity in the susceptibility to mitochondria-mediated apoptosis. Cell Death Dis. 2019, 10, 673–685. [Google Scholar] [CrossRef]
- Pearce, M.S.; Parker, L. Childhood cancer registrations in the developing world: Still more boys than girls. Int. J. Cancer 2001, 91, 402–406. [Google Scholar] [CrossRef]
- Cartwright, R.A.; Gurney, K.A.; Moorman, A.V. Sex ratios and the risks of haematological malignancies. Brit. J. Haematol. 2002, 118, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.B.; Dawsey, S.M.; Freedman, N.D.; Inskip, P.D.; Wichner, S.M.; Quraishi, S.M.; Devesa, S.S.; McGlynn, K.A. Sex disparities in cancer incidence by period and age. Cancer Epidem. Biomar. 2009, 18, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, M.A. microRNAs and the immune response. Trends Immunol. 2008, 29, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.L.; Rao, D.S.; Boldin, M.P.; Taganov, K.D.; O’Connell, R.M.; Baltimore, D. NF-kappa B dysregulation in microRNA-146a-deficient mice drives the development of myeloid malignancies. Proc. Natl. Acad. Sci. USA 2011, 108, 9184–9189. [Google Scholar] [CrossRef]
- Song, Q.; An, Q.; Niu, B.; Lu, X.; Zhang, N.; Cao, X. Role of miR-221/222 in Tumor Development and the Underlying Mechanism. J. Oncol. 2019, 2019, e7252013. [Google Scholar] [CrossRef]
- Urbich, C.; Kuehbacher, A.; Dimmeler, S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc. Res. 2008, 79, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, M.; Heo, H.R.; Ha, K.S.; Han, E.T.; Park, W.S.; Yang, S.R.; Hong, S.H. Inhibition of MicroRNA-221 and 222 Enhances Hematopoietic Differentiation from Human Pluripotent Stem Cells via c-KIT Upregulation. Mol. Cells 2018, 41, 971–978. [Google Scholar]
- Pontecorvi, G.; Bellenghi, M.; Puglisi, R.; Carè, A.; Mattia, G. Tumor-derived extracellular vesicles and microRNAs: Functional roles, diagnostic, prognostic and therapeutic options. Cytokine Growth Factor Rev. 2020, 51, 75–83. [Google Scholar] [CrossRef]
- Felicetti, F.; De Feo, A.; Coscia, C.; Puglisi, R.; Pedini, F.; Pasquini, L.; Bellenghi, M.; Errico, M.C.; Pagani, E.; Carè, A. Exosome-mediated transfer of miR-222 is sufficient to increase tumor malignancy in melanoma. J. Transl. Med. 2016, 24, 56. [Google Scholar] [CrossRef]
- Korabecna, M.; Koutova, L.; Tesarova, P. The potential roles of vesicle-enclosed miRNAs in communication between macrophages and cancer cells in tumor microenvironment. Neoplasma 2017, 64, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Gruszka, R.; Zakrzewska, M. The Oncogenic Relevance of miR-17-92 Cluster and Its Paralogous miR-106b-25 and miR-106a-363 Clusters in Brain Tumors. Int. J. Mol. Sci. 2018, 19, 879. [Google Scholar] [CrossRef] [PubMed]
- Audrito, V.; Serra, S.; Stingi, A.; Orso, F.; Gaudino, F.; Bologna, C.; Neri, F.; Garaffo, G.; Nassini, R.; Baroni, G.; et al. PD-L1 up-regulation in melanoma increases disease aggressiveness and is mediated through miR-17-5p. Oncotarget 2017, 8, 15894–15911. [Google Scholar] [CrossRef]
- Landais, S.; Landry, S.; Legault, P.; Rassart, E. Oncogenic potential of the miR-106-363 cluster and its implication in human T-cell leukemia. Cancer Res. 2007, 67, 5699–5707. [Google Scholar] [CrossRef]
- Hao, T.; Li, C.X.; Ding, X.Y.; Xing, X.J. MicroRNA-363-3p/p21 (Cip1/Waf1) Axis Is Regulated by HIF-2α in Mediating Stemness of Melanoma Cells. Neoplasma 2019, 23, 66–427. [Google Scholar] [CrossRef] [PubMed]
- Johnnidis, J.B.; Harris, M.H.; Wheeler, R.T.; Stehling, S.S.; Lam, M.H.; Kirak, O.; Brummelkamp, T.R.; Fleming, M.D.; Camargo, F.D. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature 2008, 451, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Moschos, S.A.; Williams, A.E.; Perry, M.M.; Birrell, M.A.; Belvisi, M.G.; Lindsay, M.A. Expression profiling in vivo demonstrates rapid changes in lung microRNA levels following lipopolysaccharide induced inflammation but not in the anti-inflammatory action of glucocorticoids. BMC Genom. 2007, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Mathsyaraja, H.; Thies, K.; Taffany, D.A.; Deighan, C.; Liu, T.; Yu, L.; Fernandez, S.A.; Shapiro, C.; Otero, J.; Timmers, C. CSF1-ETS2 Induced microRNA in Myeloid Cells Promote Metastatic Tumor Growth. Oncogene 2015, 34, 3651–3661. [Google Scholar] [CrossRef]
- Haneklaus, M.; Gerlic, M.; O’Neill, L.A.; Masters, S.L. miR-223: Infection, inflammation and cancer. J. Intern. Med. 2013, 274, 215–226. [Google Scholar] [CrossRef]
- Kitago, M.; Martinez, S.R.; Nakamura, T.; Sim, M.S.; Hoon, D.S. Regulation of RUNX3 Tumor Suppressor Gene Expression in Cutaneous Melanoma. Clin. Cancer Res. 2009, 15, 2988–2994. [Google Scholar] [CrossRef]
- Chen, M.L.; Sun, A.; Cao, W.; Eliason, A.; Mendez, K.M.; Getzler, A.J.; Tsuda, S.; Diao, H.; Mukori, C.; Bruno, N.E. Physiological expression and function of the MDR1 transporter in cytotoxic T lymphocytes. J. Exp. Med. 2020, 217, e201991388. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Li, Z.F.; Xu, Y.Y.; Han, Z.; Liu, Z.J. microRNA-374 inhibits proliferation and promotes apoptosis of mouse melanoma cells by inactivating the Wnt signalling pathway through its effect on tyrosinase. J. Cell Mol. Med. 2019, 238, 4991–5005. [Google Scholar] [CrossRef] [PubMed]
- Rajasekhar, M.; Schmitz, U.; Flamant, S.; Wong, J.J.-L.; Bailey, C.G.; Ritchie, W.; Hols, J.; Rasko, J.E.J. Identifying microRNA determinants of human myelopoiesis. Sci. Rep. 2018, 8, 7264. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Chen, K.; Deng, H.; Rao, H.; Huang, H.; Liao, Y.; Sun, X.; Lu, S.; Yuan, Z.; Xie, D. MicroRNA-374b suppresses proliferation and promotes apoptosis in T-cell lymphoblastic lymphoma by repressing AKT1 and Wnt-16. Clin. Cancer Res. 2015, 21, 4881–4891. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Feng, R.; Wang, Y.Z.; Sun, H.W.; Zou, Q.M.; Li, H.B. Toll-like receptors: Triggers of regulated cell death and promising targets for cancer therapy. Immunol. Lett. 2020, 17. [Google Scholar] [CrossRef]
- Li, F.; Li, X.J.; Qiao, L.; Shi, F. miR-98 suppresses melanoma metastasis through a negative feedback loop with its target gene IL-6. Exp. Mol. Med. 2014, 46, e116. [Google Scholar] [CrossRef]
- Li, L.; Sun, P.; Zhang, C.; Li, Z.; Zhou, W. MiR-98 suppresses the effects of tumor-associated macrophages on promoting migration and invasion of hepatocellular carcinoma cells by regulating IL-10. Biochimie 2018, 150, 23–30. [Google Scholar] [CrossRef]
- Seeley, J.J.; Baker, R.G.; Mohamed, G.; Bruns, T.; Hayden, M.S.; Deshmukh, S.D.; Freedberg, D.E.; Ghosh, S. Induction of innate immune memory via microRNA targeting of chromatin remodelling factors. Nature 2018, 559, 114–119. [Google Scholar] [CrossRef]
- Clark, W.H.; From, L.; Bernardino, E.A.; Mihm, M.C. The histogenesis and biological behavior of primary human malignant melanomas of the skin. Cancer Res. 1969, 29, 705–727. [Google Scholar]
- Hieken, T.J.; Glasgow, A.E.; Enninga, E.A.L.; Kottschade, L.A.; Dronca, R.S.; Markovic, S.N.; Block, M.S.; Habermann, E.B. Sex-Based Differences in Melanoma Survival in a Contemporary Patient Cohort. J. Womens Health (Larchmt) 2020. [Google Scholar] [CrossRef]
- Yakerson, A. Women in clinical trials: A review of policy development and health equity in the Canadian context. Int. J. Equity Health 2019, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Olsen, C.M.; Pandeya, N.; Thompson, B.S.; Dusingize, J.C.; Webb, P.M.; Green, A.C.; Neale, R.E.; Whiteman, D.C. QSkin Study. Risk Stratification for Melanoma: Models Derived and Validated in a Purpose-Designed Prospective Cohort. J. Natl. Cancer Inst. 2018, 110, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Avery, E.; Clark, J. Sex-related reporting in randomised controlled trials in medical journals. Lance 2016, 388, 2839–2840. [Google Scholar] [CrossRef]
- Heidari, S.; Babor, T.F.; De Castro, P.; Tort, S.; Curno, M. Sex and Gender Equity in Research: Rationale for the SAGER guidelines and recommended use. Res. Integr. Peer. Rev. 2016, 1, 2. [Google Scholar] [CrossRef]
- Italian Official Gazette GU Series General n.25. Delegation to the Government Concerning Clinical Trials of Medicinal Products and Provisions for the Reorganization of the Health Professions and for the Healthcare Officials of the Ministry of Health. 2018. Available online: https://www.gazzettaufficiale.it/eli/gu/2018/01/31/25/sg/pdf (accessed on 4 July 2020).
- Unger, J.M.; Vaidya, R.; Albain, K.S.; LeBlanc, M.L.; Minasian, L.M.; Gotay, C.; Henry, N.L.; Fisch, M.J.; Ramsey, S.D.; Blanke, C.D.; et al. Sex differences in adverse event reporting in SWOG chemotherapy, biologic/immunotherapy, and targeted agent cancer clinical trials. J. Clin. Oncol. 2019, 37, 11588. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, S.N.; Hahn, H.J.; Lee, Y.W.; Choe, Y.B.; Ahn, K.J. Meta-analysis of BRAF mutations and clinicopathologic characteristics in primary melanoma. J. Am. Acad. Dermatol. 2015, 72, 1036–1046. [Google Scholar] [CrossRef]
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Ferrucci, P.F.; Fisher, R.; Del Vecchio, M.; Atkinson, V.; Schmidt, H.; Schachter, J.; Queirolo, P.; Long, G.V.; Di Giacomo, A.M.; et al. Dabrafenib, trametinib and pembrolizumab or placebo in BRAF-mutant melanoma. Nat. Med. 2019, 25, 941–946. [Google Scholar] [CrossRef]
- Broman, K.K.; Dossett, L.A.; Sun, J.; Eroglu, Z.; Zager, J.S. Update on BRAF and MEK inhibition for treatment of melanoma in metastatic, unresectable, and adjuvant settings. Expert Opin. Drug. Saf. 2019, 18, 381–392. [Google Scholar] [CrossRef]
- Khalil, D.N.; Smith, E.L.; Brentjens, R.J.; Wolchok, J.D. The future of cancer treatment: Immunomodulation, CARs and combination immunotherapy. Nat. Rev. Clin. Oncol. 2016, 13, 273–290. [Google Scholar] [CrossRef]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; ChiarionSileni, V.; Schachter, J.; Garbe, C.; Bondarenko, I.; et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N. Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Oertelt-Prigione, S. The influence of women sex and gender on the immune response. Autoimmun. Rev. 2012, 11, A479–A485. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, A.; Onesti, C.E.; Zizzari, I.; Cerbelli, B.; Sciattella, P.; Occhipinti, M.; Roberto, M.; Di Pietro, F.; Bonifacino, A.; Ghidini, M.; et al. The sexist behaviour of immune checkpoint inhibitors in cancer therapy? Oncotarget 2017, 8, 99336–99346. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.S. Treg and CTLA-4: Two intertwining pathways to immune tolerance. J. Autoimmun. 2013, 45, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ju, Q.; Jia, K.; Yu, J.; Shi, H.; Wu, H.; Jiang, M. Correlation between sex and efficacy of immune checkpoint inhibitors (PD-1 and CTLA-4 inhibitors). Int. J. Cancer 2018, 143, 45–51. [Google Scholar] [CrossRef]
- Grassadonia, A.; Sperduti, I.; Vici, P.; Iezzi, L.; Brocco, D.; Gamucci, T.; Pizzuti, L.; Maugeri-Saccà, M.; Marchetti, P.; Cognetti, G.; et al. Effect of Gender on the Outcome of Patients Receiving Immune Checkpoint Inhibitors for Advanced Cancer: A Systematic Review and Meta-Analysis of Phase III Randomized Clinical Trials. J. Clin. Med. 2018, 7, 542. [Google Scholar] [CrossRef]
- McQuade, J.L.; Daniel, C.R.; Hess, K.R.; Mak, C.; Wang, D.Y.; Rai, R.R.; Park, J.J.; Haydu, L.E.; Spencer, C.; Wongchenko, M.; et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: A retrospective, multicohort analysis. Lancet Oncol. 2018, 19, 310–322. [Google Scholar] [CrossRef]
- Grasa, M.D.M.; Gulfo, J.; Camps, N.; Alcalà, R.; Monserrat, L.; Moreno-Navarrete, J.M.; Ortega, F.J.; Esteve, M.; Remesar, X.; Fernàndez-Lòpez, J.A.; et al. Modulation of SHBG Binding to Testosterone and Estradiol by Sex and Morbid Obesity. Eur. J. Endocrinol. 2017, 176, 393–404. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y. Pharmacogenomics of sex difference in chemotherapeutic toxicity. Curr. Drug. Discov. Technol. 2007, 4, 59–68. [Google Scholar] [CrossRef]
- Ozdemir, B.C.; Coukos, G.; Wagner, A.D. Immune-related adverse events of immune checkpoint inhibitors and the impact of sex-what we know and what we need to learn. Ann. Oncol. 2018, 29, 1067. [Google Scholar] [CrossRef]
- Wang, S.; Cowley, L.A.; Liu, X.S. Sex Differences in Cancer Immunotherapy Efficacy, Biomarkers, and Therapeutic Strategy. Molecules 2019, 24, 3214. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Morgan, R. The impact of sex and gender on immunotherapy outcomes. Biol. Sex Differ. 2020, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Olsen, C.M.; Thompson, F.C.; Pandeya, N.; Whiteman, D.C. Evaluation of Sex-Specific Incidence of Melanoma. JAMA Dermatol. 2020, 156, 553–560. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellenghi, M.; Puglisi, R.; Pontecorvi, G.; De Feo, A.; Carè, A.; Mattia, G. Sex and Gender Disparities in Melanoma. Cancers 2020, 12, 1819. https://doi.org/10.3390/cancers12071819
Bellenghi M, Puglisi R, Pontecorvi G, De Feo A, Carè A, Mattia G. Sex and Gender Disparities in Melanoma. Cancers. 2020; 12(7):1819. https://doi.org/10.3390/cancers12071819
Chicago/Turabian StyleBellenghi, Maria, Rossella Puglisi, Giada Pontecorvi, Alessandra De Feo, Alessandra Carè, and Gianfranco Mattia. 2020. "Sex and Gender Disparities in Melanoma" Cancers 12, no. 7: 1819. https://doi.org/10.3390/cancers12071819
APA StyleBellenghi, M., Puglisi, R., Pontecorvi, G., De Feo, A., Carè, A., & Mattia, G. (2020). Sex and Gender Disparities in Melanoma. Cancers, 12(7), 1819. https://doi.org/10.3390/cancers12071819




