Elective Neck Dissection or Sentinel Lymph Node Biopsy in Early Stage Oral Cavity Cancer Patients: The Dutch Experience
Abstract
1. Introduction
2. Results
2.1. Floor-of-Mouth Tumours
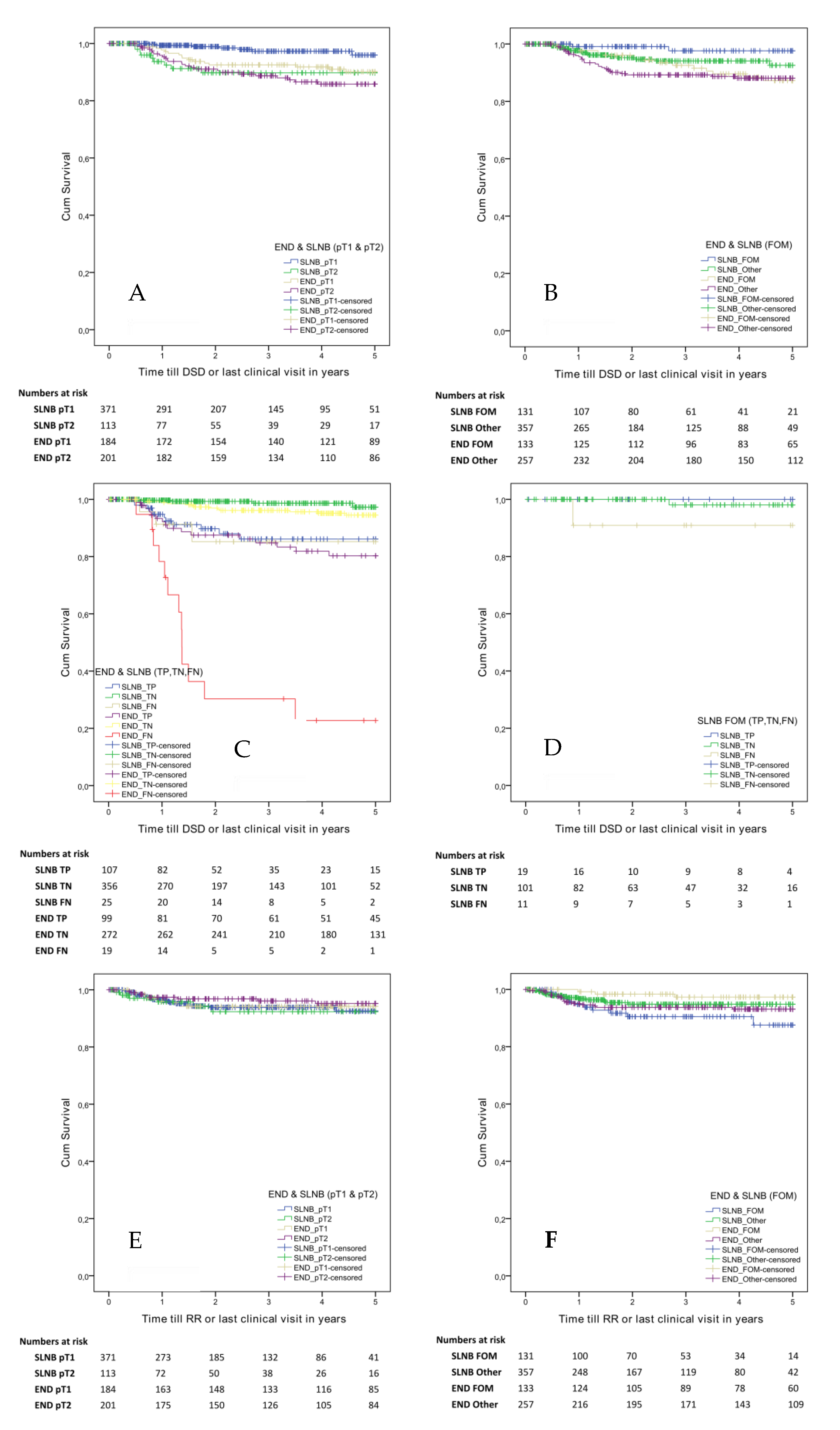
2.2. Five-Year Disease-Specific Survival
2.3. Five-Year Regional Recurrence Free Survival
2.4. Additional Metastasis in the MRND After a Positive SLNB
3. Discussion
4. Materials and Methods
4.1. Ethical Consideration
4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gourin, C.G.; Conger, B.T.; Porubsky, E.S.; Sheils, W.C.; Bilodeau, P.A.; Coleman, T.A. The effect of occult nodal metastases on survival and regional control in patients with head and neck squamous cell carcinoma. Laryngoscope 2008, 118, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.P.; Candela, F.C.; Poddar, A.K. The patterns of cervical lymph node metastases from squamous carcinoma of the oral cavity. Cancer 1990, 66, 109–113. [Google Scholar] [CrossRef]
- Weiss, M.H.; Harrison, L.B.; Isaacs, R.S. Use of decision analysis in planning a management strategy for the stage N0 neck. Arch. Otolaryngol. Head Neck Surg. 1994, 120, 699–702. [Google Scholar] [CrossRef]
- De Bree, R.; Takes, R.P.; Castelijns, J.A.; Medina, J.E.; Stoeckli, S.J.; Mancuso, A.A.; Hunt, J.L.; Rodrigo, J.; Triantafyllou, A.; Teymoortash, A.; et al. Advances in diagnostic modalities to detect occult lymph node metastases in head and neck squamous cell carcinoma. Head Neck 2014, 37, 1829–1839. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Hwang, D.; Lockwood, G.; Goldstein, D.P.; O’Sullivan, B. Predictive value of tumor thickness for cervical lymph-node involvement in squamous cell carcinoma of the oral cavity. Cancer 2009, 115, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Pentenero, M.; Gandolfo, S.; Carrozzo, M. Importance of tumor thickness and depth of invasion in nodal involvement and prognosis of oral squamous cell carcinoma: A review of the literature. Head Neck 2005, 27, 1080–1091. [Google Scholar] [CrossRef]
- D’Cruz, A.K.; Vaish, R.; Kapre, N.; Dandekar, M.; Gupta, S.; Hawaldar, R.; Agarwal, J.; Pantvaidya, G.; Chaukar, D.; Deshmukh, A.D.; et al. Elective versus therapeutic neck dissection in node-negative oral cancer. N. Engl. J. Med. 2015, 373, 521–529. [Google Scholar] [CrossRef]
- De Bree, R.; Brekel, M.V.D. Elective neck dissection versus observation in the clinically node negative neck in early oral cancer: Do we have the answer yet? Oral Oncol. 2015, 51, 963–965. [Google Scholar] [CrossRef]
- De Bree, R.; Nieweg, O.E. The history of sentinel node biopsy in head and neck cancer: From visualization of lymphatic vessels to sentinel nodes. Oral Oncol. 2015, 51, 819–823. [Google Scholar] [CrossRef]
- Liu, M.; Wang, S.J.; Yang, X.; Peng, H. Diagnostic efficacy of sentinel lymph node biopsy in early oral squamous cell carcinoma: A meta-analysis of 66 studies. PLoS ONE 2017, 12, e0170322. [Google Scholar] [CrossRef]
- Boeve, K.; Schepman, K.; Schuuring, E.; Roodenburg, J.; Halmos, G.B.; Boorsma, R.; De Visscher, J.; Brouwers, A.; Van Der Vegt, B.; Witjes, M.J.H.; et al. High sensitivity and negative predictive value of sentinel lymph node biopsy in a retrospective early stage oral cavity cancer cohort in the Northern Netherlands. Clin. Otolaryngol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Toom, I.J.D.; Heuveling, D.; Flach, G.B.; Van Weert, S.; Karagozoglu, K.H.; Van Schie, A.; Bloemena, E.; Leemans, C.; De Bree, R. Sentinel node biopsy for early-stage oral cavity cancer: The VU University Medical Center experience. Head Neck 2014, 37, 573–578. [Google Scholar] [CrossRef]
- Flach, G.B.; Bloemena, E.; Klop, W.M.C.; Van Es, R.J.; Schepman, K.-P.; Hoekstra, O.S.; Castelijns, J.A.; Leemans, C.; De Bree, R. Sentinel lymph node biopsy in clinically N0 T1–T2 staged oral cancer: The Dutch multicenter trial. Oral Oncol. 2014, 50, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Schilling, C.; Shaw, R.; Schache, A.; McMahon, J.; Chegini, S.; Kerawala, C.; McGurk, M. Sentinel lymph node biopsy for oral squamous cell carcinoma. Where are we now? Br. J. Oral Maxillofac. Surg. 2017, 55, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.D.; Sridharan, S.; Ferris, R.L.; Duvvuri, U.; Samant, S. Sentinel lymph node biopsy versus elective neck dissection for stage I to II oral cavity cancer. Laryngoscope 2018, 129, 162–169. [Google Scholar] [CrossRef] [PubMed]
- De Bree, R.; Takes, R.P.; Shah, J.P.; Hamoir, M.; Kowalski, L.P.; Robbins, K.T.; Rodrigo, J.P.; Sanabria, A.; Medina, J.E.; Rinaldo, A.; et al. Elective neck dissection in oral squamous cell carcinoma: Past, present and future. Oral Oncol. 2019, 90, 87–93. [Google Scholar] [CrossRef]
- Hernando, J.; Villarreal, P.; Marcos, F.Á.; Gallego, L.; Garcia-Consuegra, L.; Junquera, L. Comparison of related complications: Sentinel node biopsy versus elective neck dissection. Int. J. Oral Maxillofac. Surg. 2014, 43, 1307–1312. [Google Scholar] [CrossRef]
- Schiefke, F.; Akdemir, M.; Weber, A.; Akdemir, D.; Singer, S.; Frerich, B. Function, postoperative morbidity, and quality of life after cervical sentinel node biopsy and after selective neck dissection. Head Neck 2009, 31, 503–512. [Google Scholar] [CrossRef]
- Murer, K.; Huber, G.F.; Haile, S.R.; Stoeckli, S.J. Comparison of morbidity between sentinel node biopsy and elective neck dissection for treatment of the n0 neck in patients with oral squamous cell carcinoma. Head Neck 2010, 33, 1260–1264. [Google Scholar] [CrossRef]
- Hernando, J.; Villarreal, P.; Marcos, F.Á.; Consuegra, L.G.; Gallego, L.; Junquera, L. Sentinel node biopsy versus elective neck dissection. Which is more cost-effective? A prospective observational study. J. Cranio-Maxillofac. Surg. 2016, 44, 550–556. [Google Scholar] [CrossRef]
- Govers, T.; Takes, R.P.; Karakullukcu, B.; Hannink, G.; Merkx, M.; Grutters, J.P.C.; Rovers, M.M. Management of the N0 neck in early stage oral squamous cell cancer: A modeling study of the cost-effectiveness. Oral Oncol. 2013, 49, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Van Der Linden, N.; Flach, G.B.; De Bree, R.; Groot, C.A.U.-D. Cost–utility of sentinel lymph node biopsy in cT1–T2N0 oral cancer. Oral Oncol. 2016, 53, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Alkureishi, L.W.T.; Ross, G.L.; Shoaib, T.; Soutar, D.S.; Robertson, A.G.; Thompson, R.; Hunter, K.D.; Sørensen, J.A.; Thomsen, J.; Krogdahl, A.; et al. Sentinel node biopsy in head and neck squamous cell cancer: 5-year follow-up of a European Multicenter Trial. Ann. Surg. Oncol. 2010, 17, 2459–2464. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.J.; Jensen, D.H.; Hedbäck, N.; Frendø, M.; Kiss, K.; Lelkaitis, G.; Mortensen, J.; Christensen, A.; Specht, L.; Von Buchwald, C. Staging of early lymph node metastases with the sentinel lymph node technique and predictive factors in T1/T2 oral cavity cancer: A retrospective single-center study. Head Neck 2015, 38, E1033–E1040. [Google Scholar] [CrossRef]
- Stoeckli, S.J.; Huebner, T.; Huber, G.F.; Broglie, M.A. Technique for reliable sentinel node biopsy in squamous cell carcinomas of the floor of mouth. Head Neck 2016, 38, 1367–1372. [Google Scholar] [CrossRef]
- Ganly, I.; Goldstein, D.; Carlson, D.L.; Patel, S.G.; O’Sullivan, B.; Lee, N.; Gullane, P.; Shah, J.P. Long-term regional control and survival in patients with “low-risk,” early stage oral tongue cancer managed by partial glossectomy and neck dissection without postoperative radiation. Cancer 2012, 119, 1168–1176. [Google Scholar] [CrossRef]
- Mizrachi, A.; Migliacci, J.C.; Montero, P.H.; McBride, S.; Shah, J.P.; Patel, S.G.; Ganly, I. Neck recurrence in clinically node-negative oral cancer: 27-year experience at a single institution. Oral Oncol. 2018, 78, 94–101. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, T.; Yu, C.; Guo, X.; Li, C.; Li, L. Elective neck dissection versus wait-and-watch policy for oral cavity squamous cell carcinoma in early stage: A systematic review and meta-analysis based on survival data. J. Oral Maxillofac. Surg. 2019, 77, 2154–2167. [Google Scholar] [CrossRef]
- Rinaldo, A.; Devaney, K.O.; Ferlito, A. Immunohistochemical studies in the identification of lymph node micrometastases in patients with squamous cell carcinoma of the head and neck. ORL 2004, 66, 38–41. [Google Scholar] [CrossRef]
- Brekel, M.W.V.D.; Castelijns, J.A.; Stel, H.V.; Luth, W.J.; Valk, J.; Van Der Waal, I.; Snow, G.B. Occult metastatic neck disease: Detection with US and US-guided fine-needle aspiration cytology. Radiology 1991, 180, 457–461. [Google Scholar] [CrossRef]
- Schilling, C.; Stoeckli, S.J.; Vigili, M.G.; De Bree, R.; Lai, S.Y.; Alvarez, J.; Christensen, A.; Cognetti, D.M.; D’Cruz, A.K.; Frerich, B.; et al. Surgical consensus guidelines on sentinel node biopsy (SNB) in patients with oral cancer. Head Neck 2019, 41, 2655–2664. [Google Scholar] [CrossRef]
- Berg, N.S.V.D.; Brouwer, O.R.; Klop, W.M.C.; Karakullukçu, B.; Zuur, C.L.; Tan, I.B.; Balm, A.J.M.; Brekel, M.W.M.V.D.; Olmos, R.A.V.; Van Leeuwen, F.W.B. Concomitant radio- and fluorescence-guided sentinel lymph node biopsy in squamous cell carcinoma of the oral cavity using ICG-99mTc-nanocolloid. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.; Juhl, K.; Charabi, B.; Mortensen, J.; Kiss, K.; Kjaer, A.; Von Buchwald, C. Feasibility of real-time near-infrared fluorescence tracer imaging in sentinel node biopsy for oral cavity cancer patients. Ann. Surg. Oncol. 2016, 23, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Civantos, F.J.; Brumund, K.T.; Chepeha, D.B.; Hall, N.C.; Carroll, W.R.; Smith, R.B.; Zitsch, R.P.; Lee, W.T.; Shnayder, Y.; et al. [(99m)Tc]Tilmanocept accurately detects sentinel lymph nodes and predicts node pathology status in patients with oral squamous cell carcinoma of the head and neck: Results of a phase III multi-institutional trial. Ann. Surg. Oncol. 2015, 22, 3708–3715. [Google Scholar] [CrossRef] [PubMed]
- Toom, I.J.D.; Bloemena, E.; Van Weert, S.; Karagozoglu, K.H.; Hoekstra, O.S.; De Bree, R. Additional non-sentinel lymph node metastases in early oral cancer patients with positive sentinel lymph nodes. Eur. Arch. Oto-Rhino-Laryngol. 2016, 274, 961–968. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA A Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.; Wittekind, C. UICC TNM Classification of Malignant Tumours, 8th ed.; Wiley: Chichester, UK, 2017. [Google Scholar]
- Ebrahimi, A.; Gil, Z.; Amit, M.; Yen, T.-C.; Liao, C.-T.; Chaturvedi, P.; Agarwal, J.; Kowalski, L.P.; Kreppel, M.; Cernea, C.R.; et al. Primary Tumor Staging for Oral Cancer and a Proposed Modification Incorporating Depth of Invasion. JAMA Otolaryngol. Neck Surg. 2014, 140, 1138–1148. [Google Scholar] [CrossRef]
- Lydiatt, W.M.; Patel, S.G.; O’Sullivan, B.; Brandwein, M.S.; Ridge, J.A.; Migliacci, J.C.; Loomis, A.M.; Shah, J.P. Head and neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA A Cancer J. Clin. 2017, 67, 122–137. [Google Scholar] [CrossRef]
- Boeve, K.; Melchers, L.J.; Schuuring, E.; Roodenburg, J.L.; Halmos, G.B.; Dijk, B.A.; Van Der Vegt, B.; Witjes, M.J. Addition of tumour infiltration depth and extranodal extension improves the prognostic value of the pathological TNM classification for early-stage oral squamous cell carcinoma. Histopathology 2019, 75, 329–337. [Google Scholar] [CrossRef]
- Toom, I.J.D.; Janssen, L.M.; Es, R.J.; Karagozoglu, K.H.; Keizer, B.; Van Weert, S.; Willems, S.M.; Bloemena, E.; Leemans, C.R.; De Bree, R. Depth of invasion in patients with early stage oral cancer staged by sentinel node biopsy. Head Neck 2019, 41, 2100–2106. [Google Scholar] [CrossRef]
- Alkureishi, L.W.T.; Burak, Z.; Alvarez, J.A.; Ballinger, J.; Bilde, A.; Britten, A.J.; Calabrese, L.; Chiesa, C.; Chiti, A.; De Bree, R.; et al. Joint practice guidelines for radionuclide lymphoscintigraphy for sentinel node localization in oral/oropharyngeal squamous cell carcinoma. Ann. Surg. Oncol. 2009, 16, 3190–3210. [Google Scholar] [CrossRef] [PubMed]
- Giammarile, F.; Schilling, C.; Gnanasegaran, G.; Bal, C.; Oyen, W.; Rubello, D.; Schwarz, T.; Tartaglione, G.; Miller, R.N.; Paez, D.; et al. The EANM practical guidelines for sentinel lymph node localisation in oral cavity squamous cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2018, 46, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Hermanek, P.; Hutter, R.V.; Sobin, L.H.; Wittekind, C. International Union Against Cancer. Classification of isolated tumor cells and micrometastasis. Cancer 1999, 86, 2668–2673. [Google Scholar] [CrossRef]
- Melchers, L.; Schuuring, E.; Van Dijk, B.; De Bock, G.; Witjes, M.J.H.; Van Der Laan, B.F.; Van Der Wal, J.; Roodenburg, J.; De Bock, G. Tumour infiltration depth ≥4 mm is an indication for an elective neck dissection in pT1cN0 oral squamous cell carcinoma. Oral Oncol. 2012, 48, 337–342. [Google Scholar] [CrossRef] [PubMed]
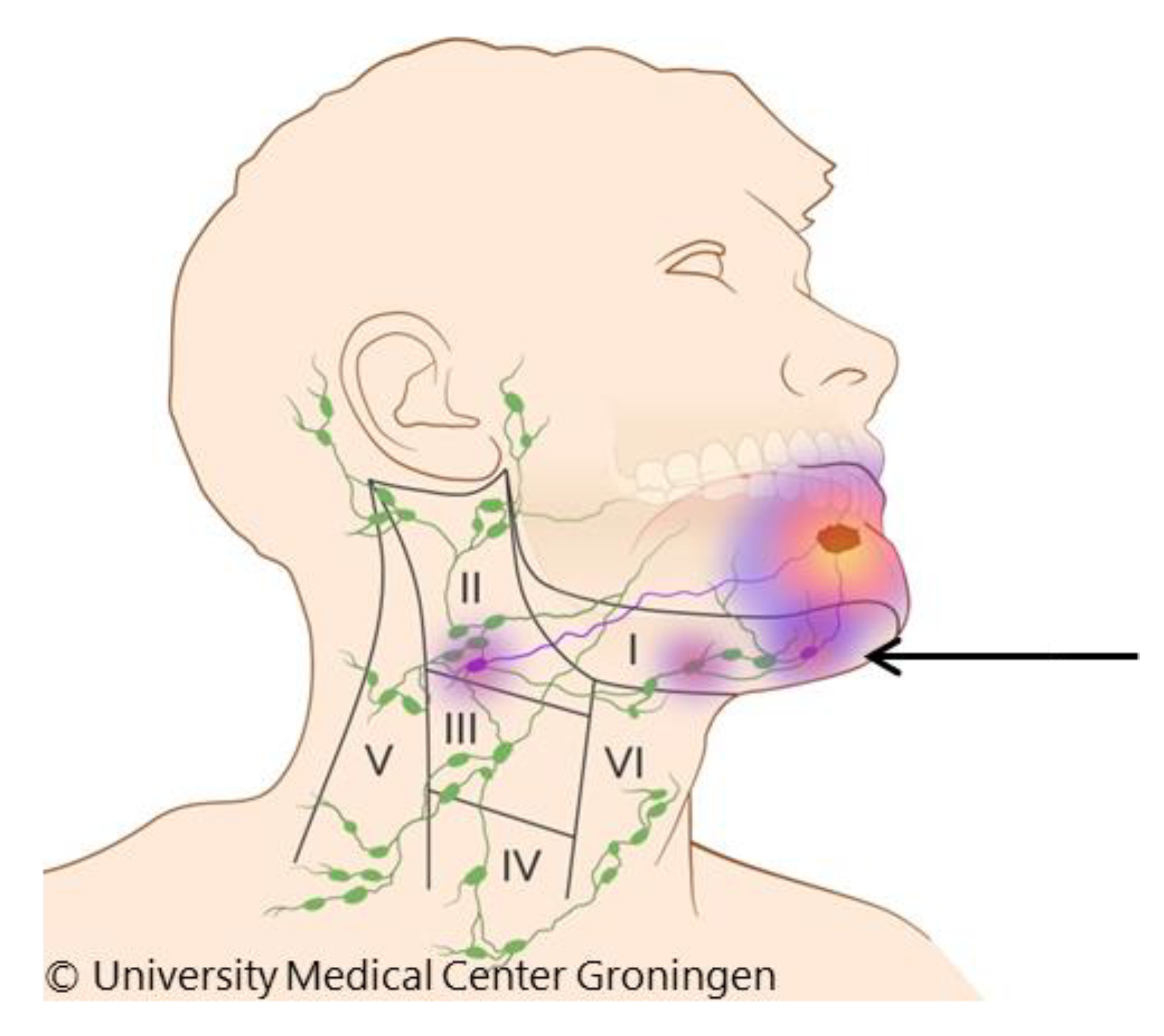
| Characteristic | Category | SLNB | END | |||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | p-Value | ||
| Total | 488 | (56) | 390 | (44) | ||
| Gender | Female | 237 | (49) | 178 | (46) | 0.377 |
| Male | 250 | (51) | 212 | (54) | ||
| Age at treatment (years) | Median (IQR) | 63 | (55 to 69) | 62 | (53 to 70) | 0.767 |
| Range | 20 to 93 | 22 to 95 | ||||
| cT (7th) | T1 | 335 | (69) | 136 | (35) | <0.001 |
| T2 | 153 | (31) | 254 | (65) | ||
| pT (7th) | T1 | 371 | (76) | 184 | (47) | <0.001 |
| T2 | 113 | (23) | 201 | (51) | ||
| T3 | 4 | (1) | 3 | (1) | ||
| T4 | 0 | (0) | 2 | (1) | ||
| pN | Negative | 381 | (78) | 291 | (75) | 0.264 |
| Positive | 107 | (22) | 99 | (25) | ||
| Metastasis size | ITC (<0.2 mm) | 15 | ||||
| Micro (0.2 to 2.0 mm) | 31 | |||||
| Macro (>2 mm) | 61 | |||||
| Postoperative RTx | Yes | 52 | (11) | 131 | (34) | <0.001 |
| No | 436 | (89) | 259 | (66) | ||
| Location | Tongue | 302 | (62) | 196 | (50) | 0.007 |
| FOM | 131 | (27) | 133 | (34) | ||
| Cheek/Buccal/Trigonum | 34 | (7) | 35 | (9) | ||
| Others | 21 | (4) | 26 | (7) | ||
| END levels | I to III | NA | 300 | (77) | NA | |
| I to IV | NA | 16 | (4) | |||
| I to V | NA | 74 | (19) | |||
| Follow-up | Time in years median (IQR) | 2.2 | (1.0 to 4.1) | 4.5 | (2.5 to 7.3) | <0.001 |
| Range (years) | 0.0 to 9.7 | 0.0 to 20.8 | ||||
| Regional recurrences | 25 | (5) | 19 | (5) | 1.000 | |
| Deceased | 52 | (11) | 140 | (36) | <0.001 | |
| Deceased by disease | 18 | (4) | 45 | (11) | <0.001 | |
| (Sub)Group | SLNB | END | ||||
|---|---|---|---|---|---|---|
| % | (95% CI) | % | (95% CI) | p-Value | ||
| Overall | Sensitivity | 81 | (73 to 87) | 84 | (76 to 90) | 0.612 |
| NPV | 93 | (91 to 95) | 93 | (90 to 95) | 1.000 | |
| pT1 * | Sensitivity | 76 | (65 to 85) | 70 | (51 to 84) | 0.637 |
| NPV | 94 | (91 to 96) | 94 | (90 to 96) | 1.000 | |
| pT2 * | Sensitivity | 88 | (77 to 96) | 90 | (82 to 96) | 0.776 |
| NPV | 91 | (83 to 96) | 94 | (88 to 97) | 0.565 | |
| FOM ** | Sensitivity | 63 | (44 to 80) | 92 | (79 to 98) | 0.006 |
| NPV | 90 | (85 to 94) | 97 | (91 to 99) | 0.057 | |
| Other locations ** | Sensitivity | 86 | (78 to 92) | 80 | (70 to 88) | 0.315 |
| NPV | 95 | (92 to 97) | 92 | (88 to 95) | 0.250 | |
| Yes | No | Other | Total | ||
|---|---|---|---|---|---|
| SLNB | ITC | 1 | 12 | 2 | 15 |
| MiM | 1 | 26 | 4 | 31 | |
| MaM | 17 | 35 | 9 | 61 | |
| Total | 19 | 73 | 15 | 107 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
den Toom, I.J.; Boeve, K.; Lobeek, D.; Bloemena, E.; Donswijk, M.L.; de Keizer, B.; Klop, W.M.C.; Leemans, C.R.; Willems, S.M.; Takes, R.P.; et al. Elective Neck Dissection or Sentinel Lymph Node Biopsy in Early Stage Oral Cavity Cancer Patients: The Dutch Experience. Cancers 2020, 12, 1783. https://doi.org/10.3390/cancers12071783
den Toom IJ, Boeve K, Lobeek D, Bloemena E, Donswijk ML, de Keizer B, Klop WMC, Leemans CR, Willems SM, Takes RP, et al. Elective Neck Dissection or Sentinel Lymph Node Biopsy in Early Stage Oral Cavity Cancer Patients: The Dutch Experience. Cancers. 2020; 12(7):1783. https://doi.org/10.3390/cancers12071783
Chicago/Turabian Styleden Toom, Inne J., Koos Boeve, Daphne Lobeek, Elisabeth Bloemena, Maarten L. Donswijk, Bart de Keizer, W. Martin C. Klop, C. René Leemans, Stefan M. Willems, Robert P. Takes, and et al. 2020. "Elective Neck Dissection or Sentinel Lymph Node Biopsy in Early Stage Oral Cavity Cancer Patients: The Dutch Experience" Cancers 12, no. 7: 1783. https://doi.org/10.3390/cancers12071783
APA Styleden Toom, I. J., Boeve, K., Lobeek, D., Bloemena, E., Donswijk, M. L., de Keizer, B., Klop, W. M. C., Leemans, C. R., Willems, S. M., Takes, R. P., Witjes, M. J. H., & de Bree, R. (2020). Elective Neck Dissection or Sentinel Lymph Node Biopsy in Early Stage Oral Cavity Cancer Patients: The Dutch Experience. Cancers, 12(7), 1783. https://doi.org/10.3390/cancers12071783









